Methodological Study to Investigate the Potential of Ultrasound-Based Elastography and Texture as Biomarkers to Monitor Liver Tumors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Data Analysis Methods
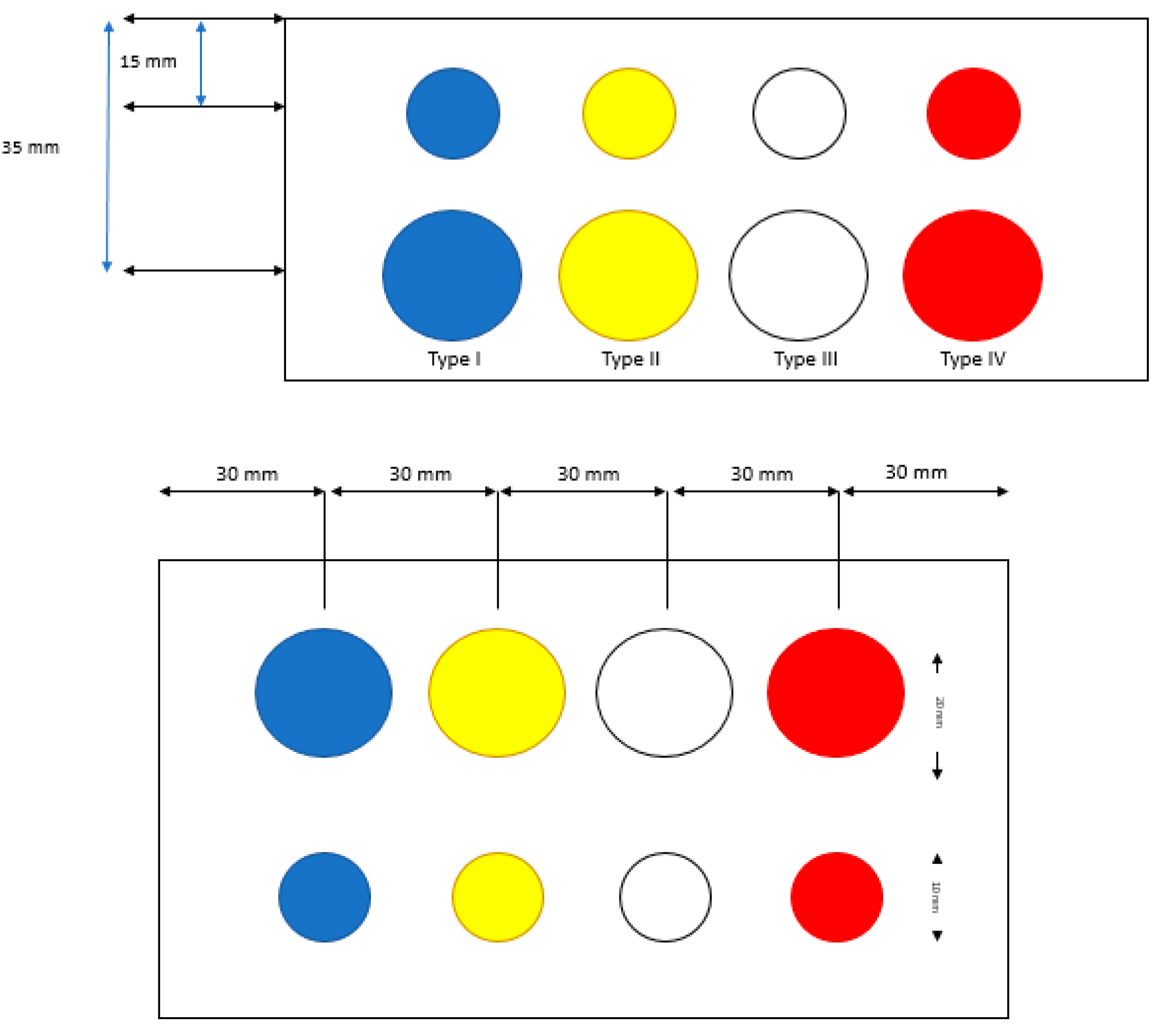
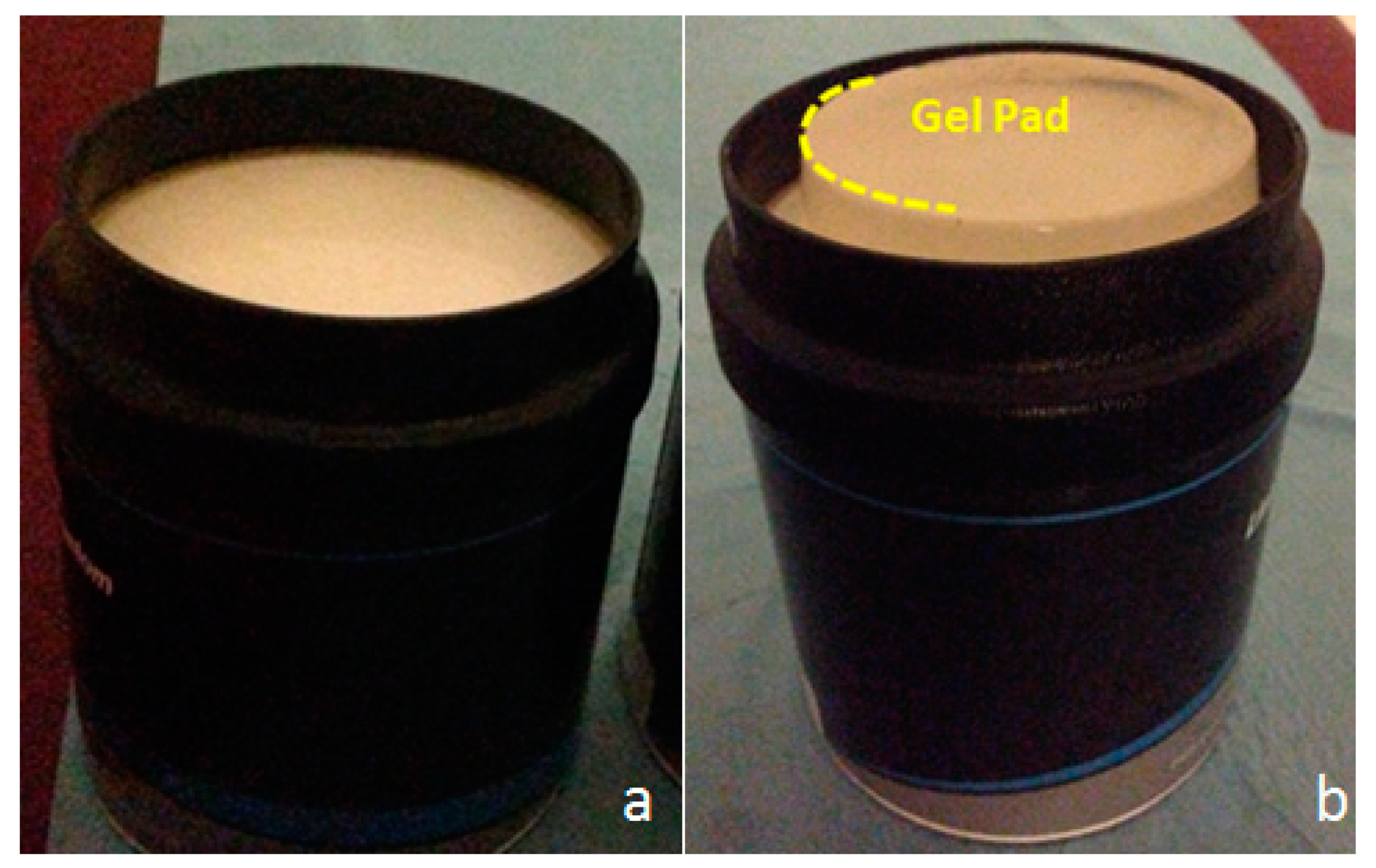
2.2. Ultrasonic-Based Elastography Phantoms
2.3. In Vivo Study: Healthy Volunteer Livers
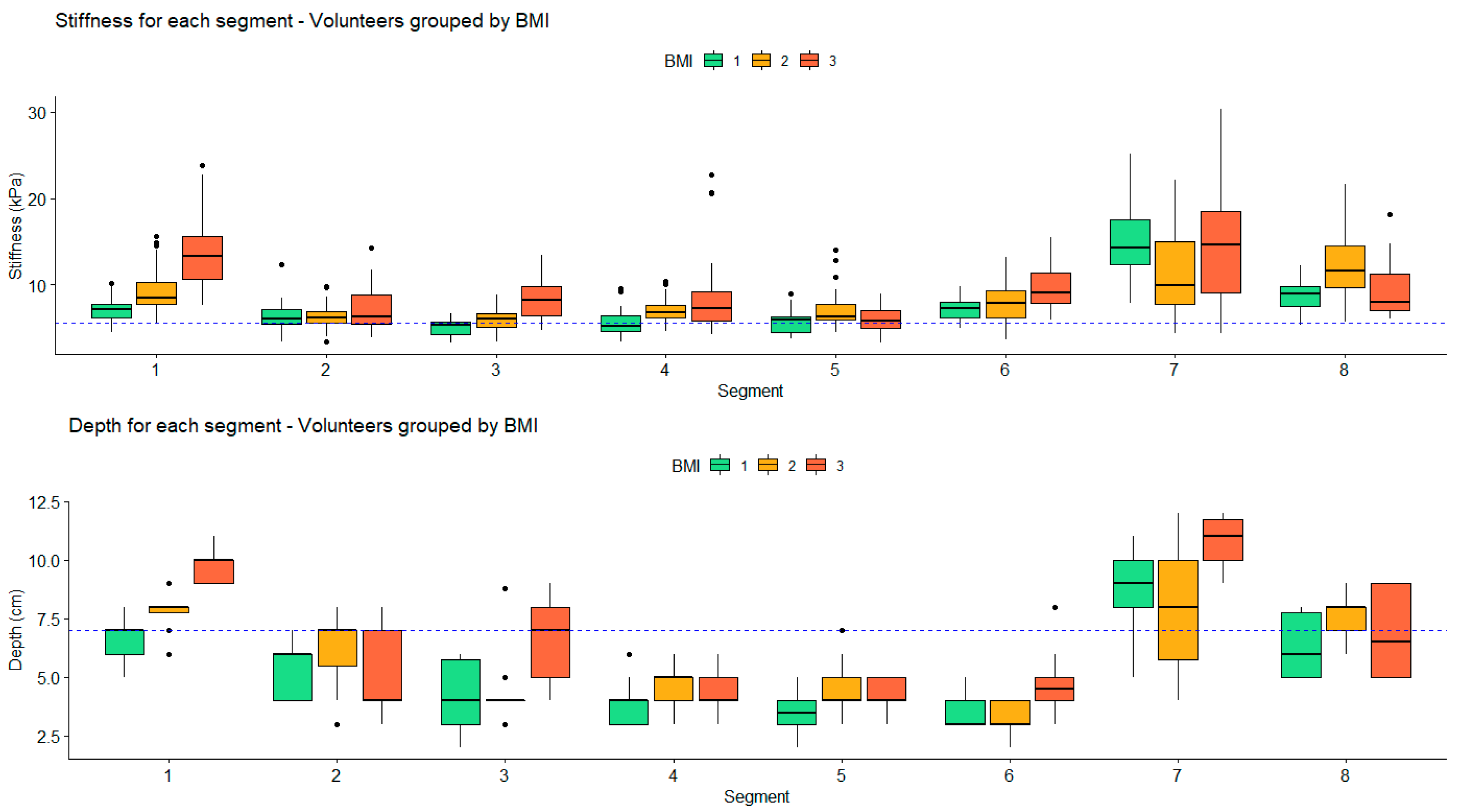
Impact of Depth and Acquisition Protocol on Hepatic Segment Stiffness Measurements
2.4. Evaluation of Texture Feature Variability
3. Results
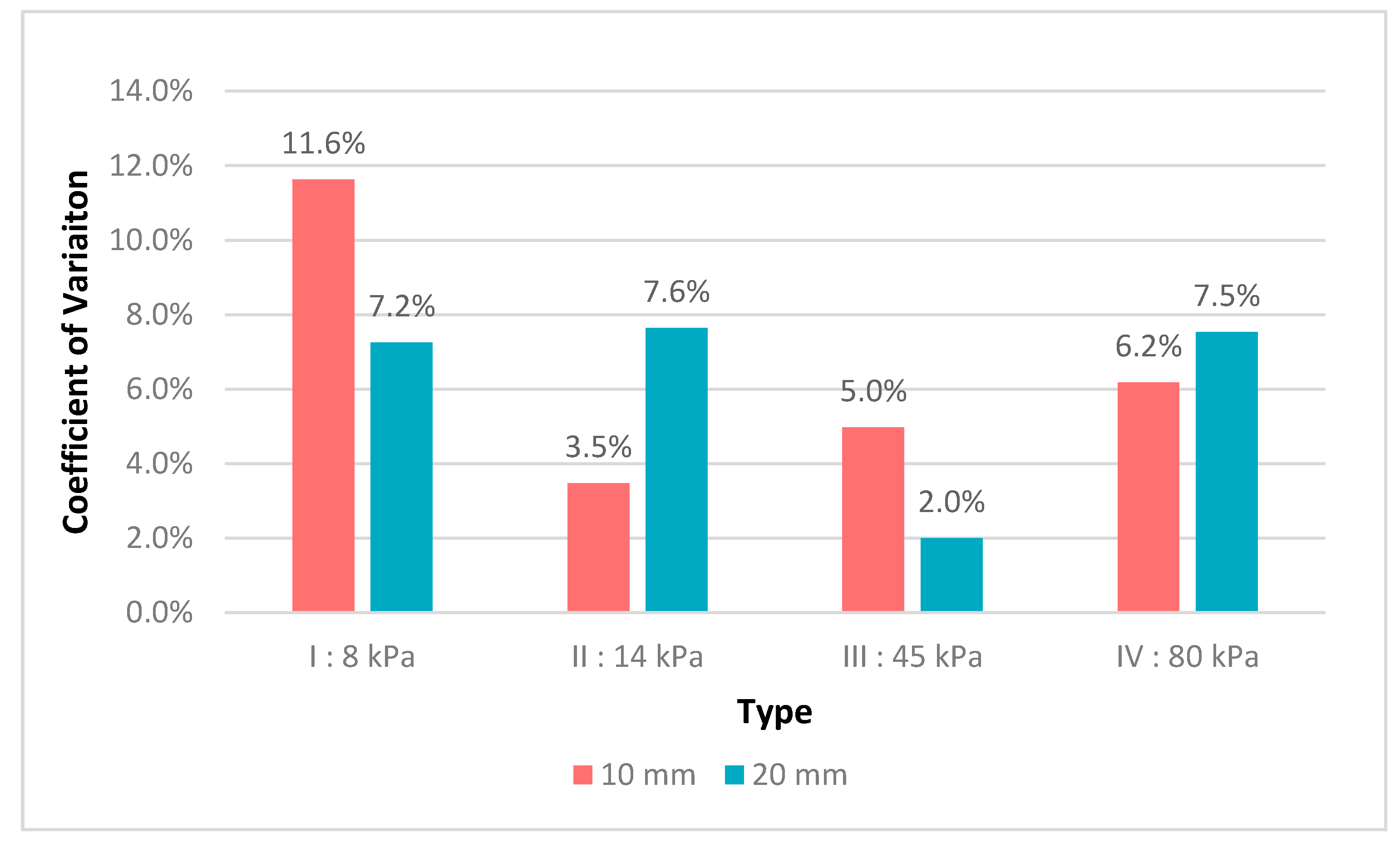
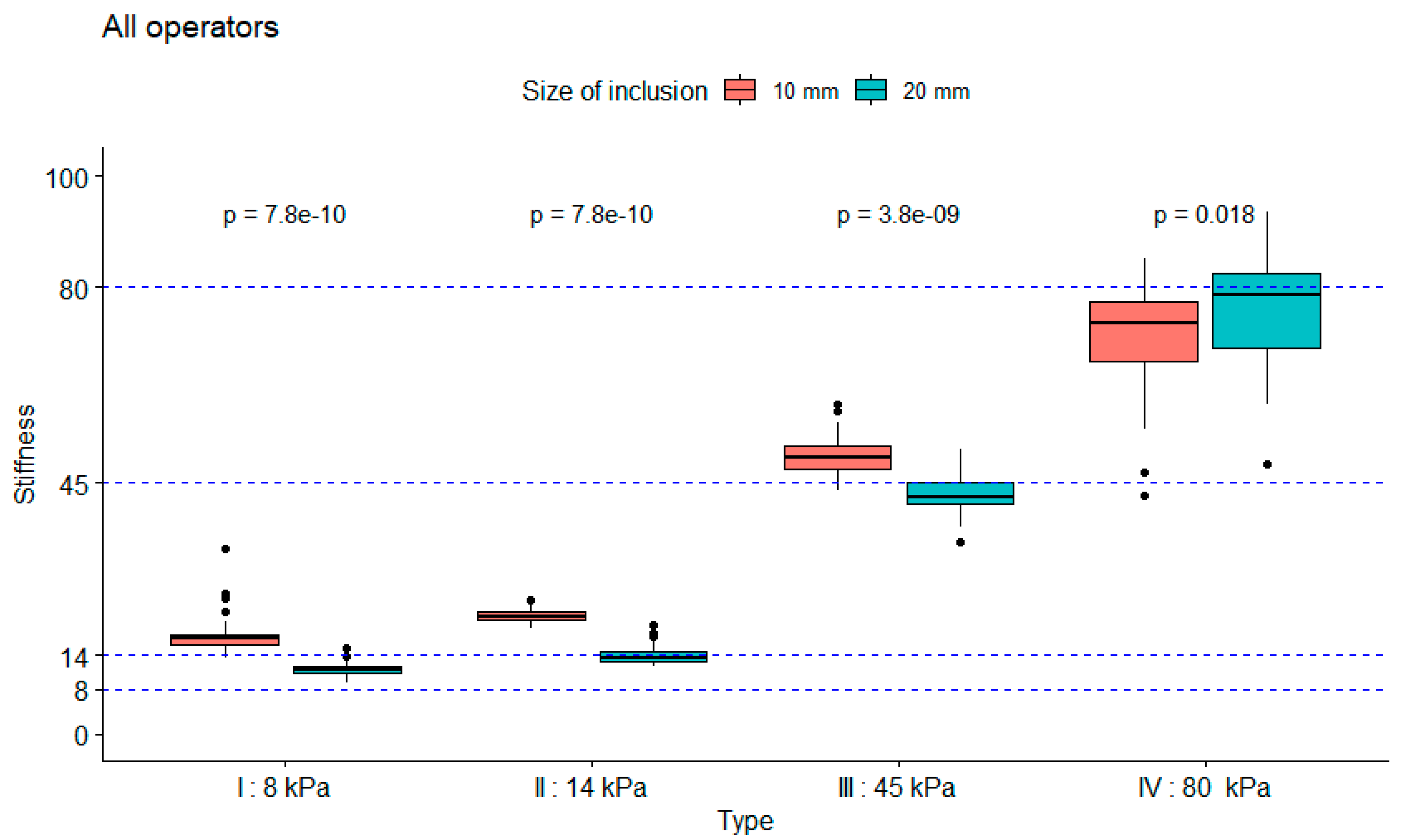
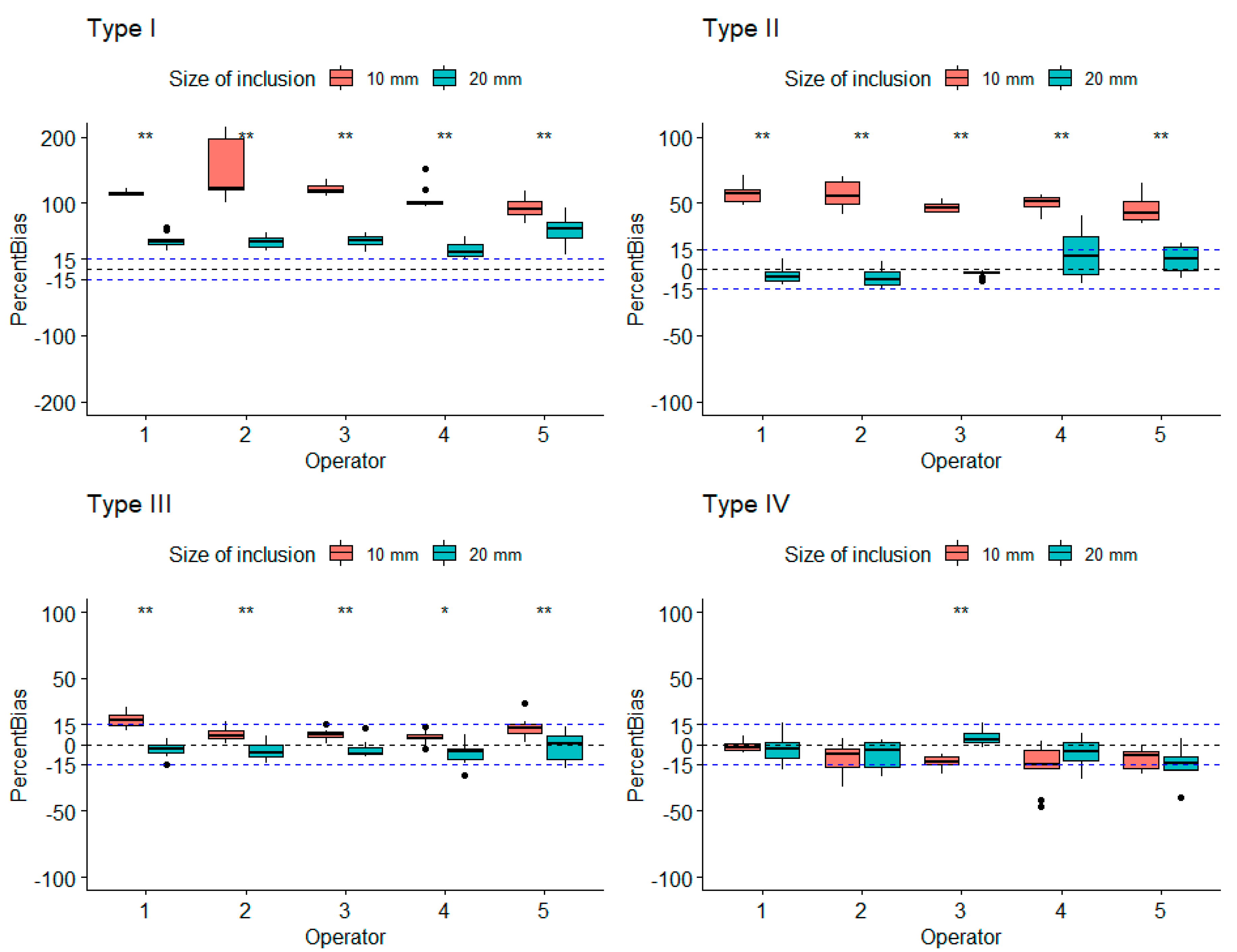
3.1. Robustness Evaluation Regarding Size and Type of Inclusions
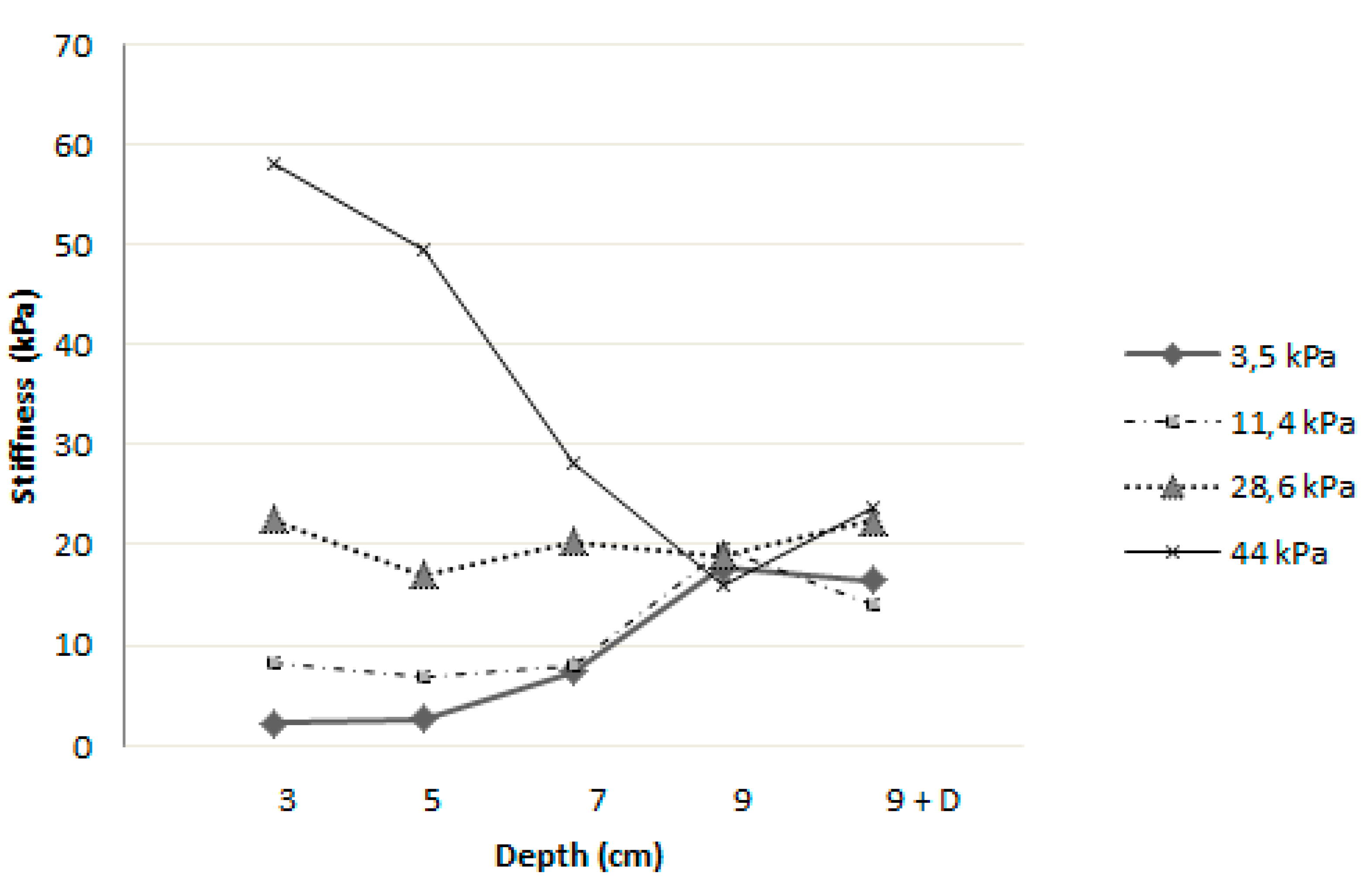
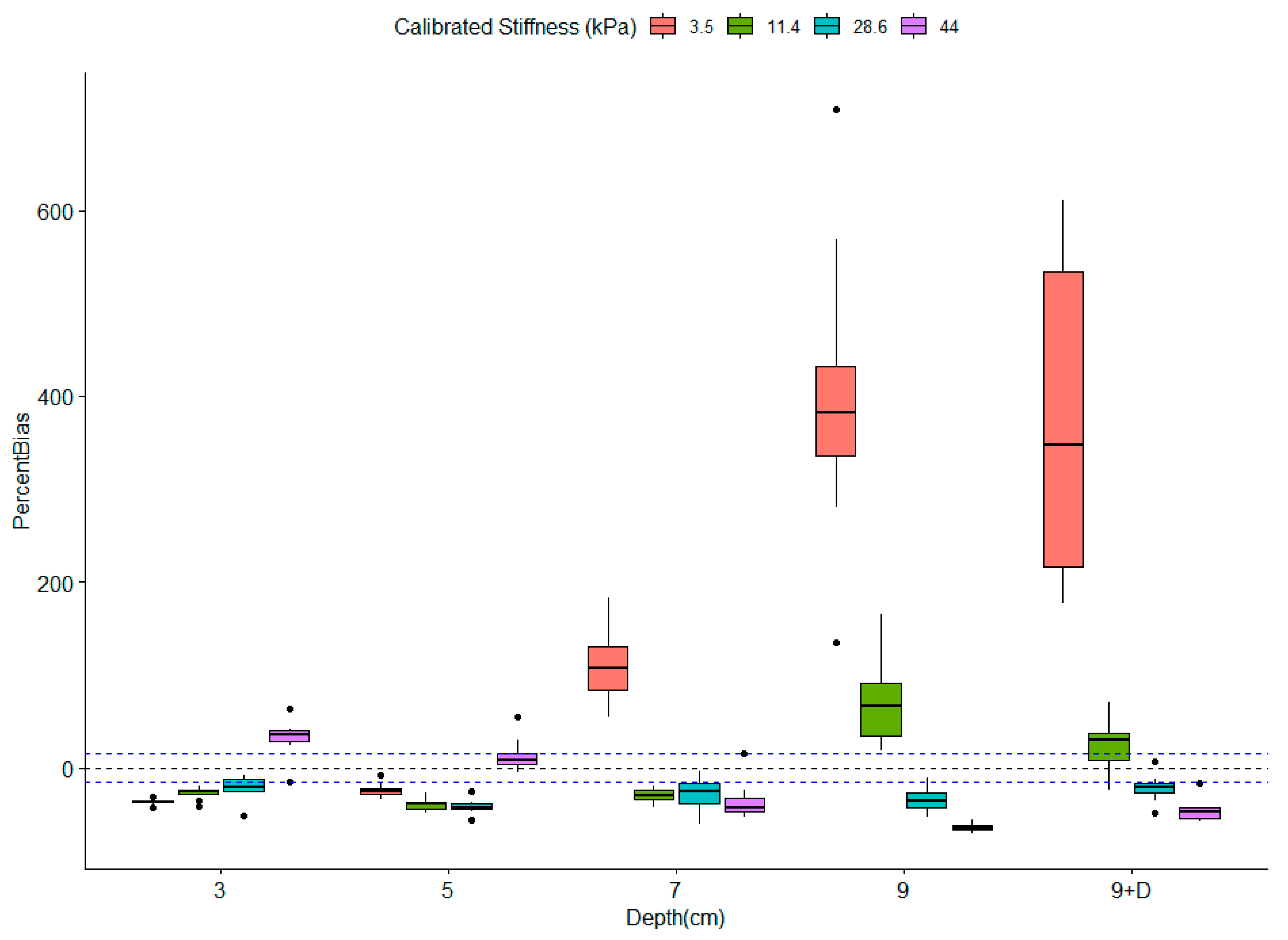
3.2. Influence of Depth on Measured Stiffness
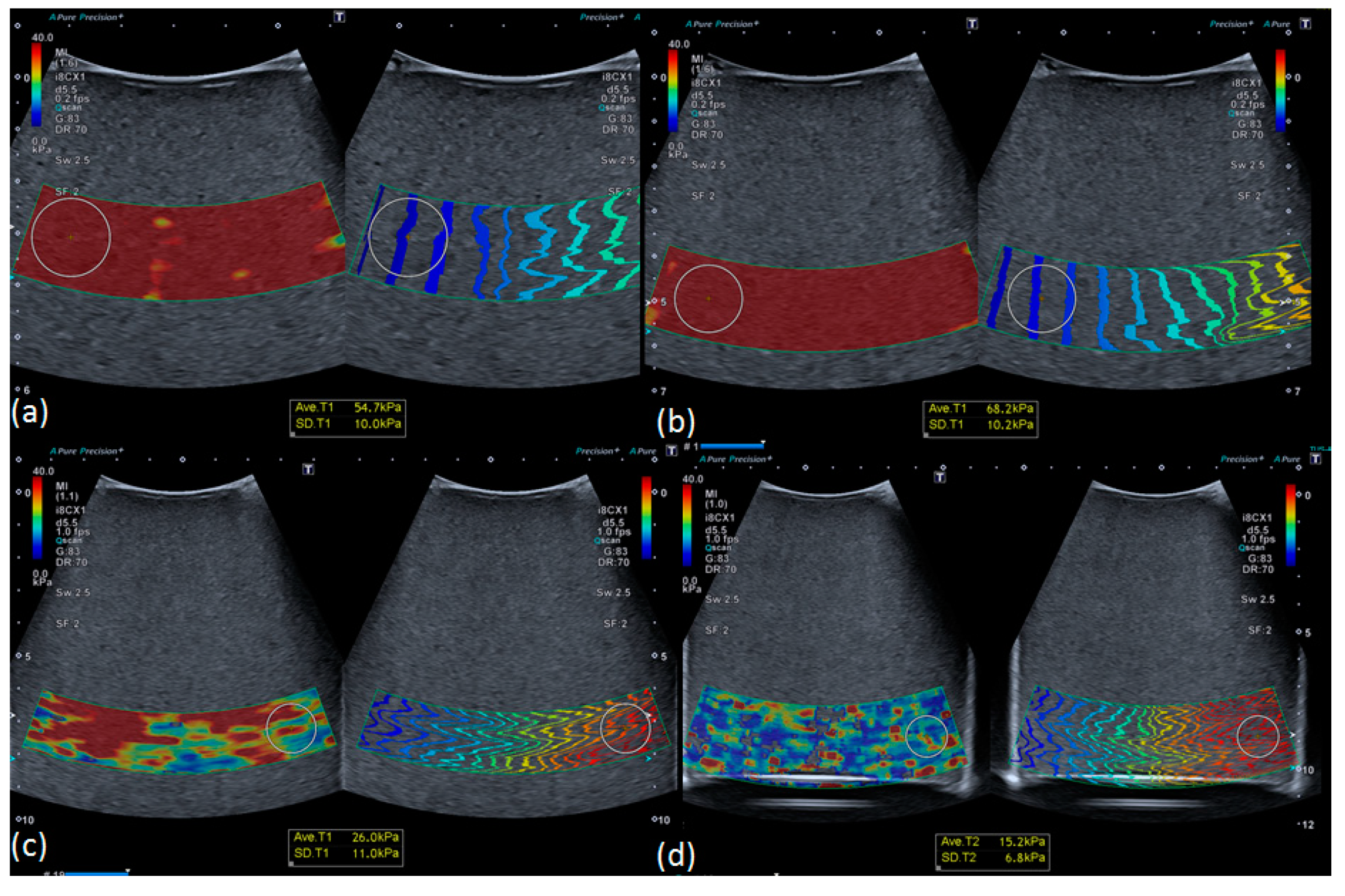
3.3. Robustness of Texture Features in Healthy Livers
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GLNU | Gray Level Non-Uniformity for run |
| HGRE | High Gray-level Run Emphasis |
| LGRE | Low Gray-level Run Emphasis |
| LRE | Long Run Emphasis |
| LRHGE | Long Run High Gray-Level Emphasis |
| LRLGE | Long Run Low Gray-level Emphasis |
| RLNU | Run Length Non-Uniformity |
| RP | Run Percentage |
| SRE | Short Run Emphasis |
| SRHGE | Short Run High Gray-level Emphasis |
| SRLGE | Short Run Low Gray-level Emphasis |
| HGZE | High Gray-level Zone Emphasis |
| LGZE | Low Gray-level Zone Emphasis |
| LZE | Long Zone Emphasis |
| LZHGE | Long Zone High Gray-Level Emphasis |
| SZE | Short Zone Emphasis |
| SZHGE | Short Zone High Gray-Level Emphasis |
| SZLGE | Short Zone Low Gray-Level Emphasis |
| ZLNU | Zone Length Non-Uniformity |
| ZP | Zone Percentage |
References
- Ollivier, L.; Leclère, J.; Thiesse, P.; Di Stefano, D.; Vincent, C. Measurement of tumour response to cancer treatment: Morphologic imaging role. Bull Cancer 2007, 94, 171–177. [Google Scholar] [PubMed]
- Ammari, S.; Thiam, R.; Cuenod, C.-A.; Oudard, S.; Hernigou, A.; Grataloup, C.; Siauve, N.; Medioni, J.; Fournier, L.S. Évaluation radiologique de la réponse au traitement: Application aux cancers du rein métastatiques sous traitement anti-angiogénique. J. Radiol. Diagn. Interv. 2014, 95, 527–540. [Google Scholar] [CrossRef]
- Nishino, M. Tumor Response Assessment for Precision Cancer Therapy: Response Evaluation Criteria in Solid Tumors and Beyond. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, R.S.; Choi, H.; Macapinlac, H.A.; Burgess, M.A.; Patel, S.R.; Chen, L.L.; Podoloff, D.A.; Charnsangavej, C. We Should Desist Using RECIST, at Least in GIST. J. Clin. Oncol. 2007, 25, 1760–1764. [Google Scholar] [CrossRef]
- Lassau, N.; Bonastre, J.; Kind, M.; Vilgrain, V.; Lacroix, J.; Cuinet, M.; Taied, S.; Aziza, R.; Sarran, A.; Labbe-Devilliers, C.; et al. Validation of dynamic contrast-enhanced ultrasound in predicting outcomes of antiangiogenic therapy for solid tumors: The French multicenter support for innovative and expensive techniques study. Investig. Radiol. Déc. 2014, 49, 794–800. [Google Scholar] [CrossRef] [Green Version]
- Sigrist, R.M.S.; Liau, J.; Kaffas, A.E.; Chammas, M.C.; Willmann, J.K. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef]
- Ferraioli, G.; Wong, V.W.-S.; Castera, L.; Berzigotti, A.; Sporea, I.; Dietrich, C.F.; Choi, B.; Wilson, S.; Kud, M.; Barr, R. Liver Ultrasound Elastography: An Update to the World Federation for Ultrasound in Medicine and Biology Guidelines and Recommendations. Ultrasound. Med. Biol. 2018, 44, 2419–2440. [Google Scholar] [CrossRef] [Green Version]
- Săftoiu, A.; Gilja, O.H.; Sidhu, P.S.; Dietrich, C.F.; Cantisani, V.; Amy, D.; Bachmann-Nielsen, M.; Bob, F.; Bojunga, J.; Brock, M.; et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Elastography in Non-Hepatic Applications: Update 2018. Ultraschall. Med. Eur. J. Ultrasound 2019, 40, 425–453. [Google Scholar] [CrossRef] [Green Version]
- Gerber, L.; Fitting, D.; Srikantharajah, K.; Weiler, N.; Kyriakidou, G.; Bojunga, J.; Schulze, F.; Bon, D.; Zeuzmen, S.; Rust, M. Evaluation of 2D- Shear Wave Elastography for Characterisation of Focal Liver Lesions. J. Gastrointestin. Liver. Dis. 2017, 26, 283–290. [Google Scholar] [CrossRef]
- Tian, W.-S.; Lin, M.-X.; Zhou, L.-Y.; Pan, F.-S.; Huang, G.-L.; Wang, W.; Lu, M.; Xie, X. Maximum Value Measured by 2-D Shear Wave Elastography Helps in Differentiating Malignancy from Benign Focal Liver Lesions. Ultrasound. Med. Biol. 2016, 42, 2156–2166. [Google Scholar] [CrossRef]
- Huang, Z.; Zheng, W.; Zhang, Y.-J.; Xu, L.; Chen, J.-B.; Chen, J.-C.; Chen, M.-S.; Zhou, Z. Assessing Hepatic Fibrosis Using 2-D Shear Wave Elastography in Patients with Liver Tumors: A Prospective Single-Center Study. Ultrasound. Med. Biol. 2017, 43, 2522–2529. [Google Scholar] [CrossRef] [PubMed]
- Choong, K.L.; Wong, Y.H.; Yeong, C.H.; Gnanasuntharam, G.K.; Goh, K.L.; Yoong, B.K.; Pongnapang, N.; Abdullah, B.-J.-J. Elasticity Characterization of Liver Cancers Using Shear Wave Ultrasound Elastography: Comparison Between Hepatocellular Carcinoma and Liver Metastasis. J. Diagn. Med. Sonogr. 2017, 33, 481–488. [Google Scholar] [CrossRef] [Green Version]
- Nioche, C.; Orlhac, F.; Boughdad, S.; Reuzé, S.; Goya-Outi, J.; Robert, C.; Pellot-Barakat, C.; Soussan, M.; Frouin, F.; Buvat, I. LIFEx: A Freeware for Radiomic Feature Calculation in Multimodality Imaging to Accelerate Advances in the Characterization of Tumor Heterogeneity. Cancer Res. 2018, 78, 4786–4789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlsen, J.F.; Ewertsen, C.; Săftoiu, A.; Lönn, L.; Nielsen, M.B. Accuracy of visual scoring and semi-quantification of ultrasound strain elastography--a phantom study. PLoS ONE 2014, 9, e88699. [Google Scholar] [CrossRef]
- Yamanaka, N.; Kaminuma, C.; Taketomi-Takahashi, A.; Tsushima, Y. Reliable Measurement by Virtual Touch Tissue Quantification with Acoustic Radiation Force Impulse Imaging: Phantom Study. J. Ultrasound. Med. 2012, 31, 1239–1244. [Google Scholar] [CrossRef]
- Havre, R.; Waage, J.; Gilja, O.; Ødegaard, S.; Nesje, L. Real-Time Elastography: Strain Ratio Measurements Are Influenced by the Position of the Reference Area. Ultraschall. Med. Eur. J. Ultrasound 2012, 33, 559–568. [Google Scholar] [CrossRef]
- Wang, C.-Z.; Zheng, J.; Huang, Z.-P.; Xiao, Y.; Song, D.; Zeng, J.; Zheng, H.-R.; Zheng, R.-Q. Influence of Measurement Depth on the Stiffness Assessment of Healthy Liver with Real-Time Shear Wave Elastography. Ultrasound. Med. Biol. Mars. 2014, 40, 461–469. [Google Scholar] [CrossRef]
- Carlsen, J.F.; Pedersen, M.R.; Ewertsen, C.; Săftoiu, A.; Lönn, L.; Rafaelsen, S.R.; Nielsen, M.-B. A Comparative Study of Strain and Shear-Wave Elastography in an Elasticity Phantom. Am. J. Roentgenol. 2015, 204, W236–W242. [Google Scholar] [CrossRef]
- Wang, J.; Ning, C.; Fang, S.; Fu, W. Thyroid tuberculosis mimics cancer on grayscale sonography and elastography: LETTER TO THE EDITOR. J. Clin. Ultrasound. 2014, 42, 291–292. [Google Scholar] [CrossRef]
- Huang, Z.-P.; Zhang, X.-L.; Zeng, J.; Zheng, J.; Wang, P.; Zheng, R.-Q. Study of detection times for liver stiffness evaluation by shear wave elastography. World J. Gastroenterol 2014, 20, 9578–9584. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Bamber, J.; Berzigotti, A.; Bota, S.; Cantisani, V.; Castera, L.; Cosgrove, D.; Ferraioli, G.; Friedrich-Rust, M.; Gilja, O.H.; et al. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography. Ultraschall. Med. Stuttg. Ger. 2017, 38, e16–e47. [Google Scholar]
- Jian, Z.-C.; Long, J.-F.; Liu, Y.-J.; Hu, X.-D.; Liu, J.-B.; Shi, X.-Q.; Li, W.-S.; Qian, L.-X. Diagnostic value of two dimensional shear wave elastography combined with texture analysis in early liver fibrosis. World J. Clin. Cases 2019, 7, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Caramella, C.; Allorant, A.; Orlhac, F.; Bidault, F.; Asselain, B.; Ammari, S.; Jaranowski, P.; Moussier, A.; Balleyguier, C.; Lassau, N.; et al. Can we trust the calculation of texture indices of CT images? A phantom study. Med. Phys. 2018, 45, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Carles, M.; Torres-Espallardo, I.; Alberich-Bayarri, A.; Olivas, C.; Bello, P.; Nestle, U.; Martí-Bonmatí, L. Evaluation of PET texture features with heterogeneous phantoms: Complementarity and effect of motion and segmentation method. Phys. Med. Biol. 2017, 62, 652–668. [Google Scholar] [CrossRef]
- Abe, C.; Kahn, C.; Doi, K.; Katsuragawa, S. Computed-Aided Detection of dffusion liver disease in ultrasound images. Investig. Radiol. 1992, 27, 71–77. [Google Scholar] [CrossRef]


| Feature | Number of Segments with CV ≤ 15% B-Mode/Stiffness Map | Mean CV B-Mode/Stiffness Map |
|---|---|---|
| SRE | 8 | 2% |
| RP | 8 | 3% |
| Correlation | 8/5 | 3%/15% |
| Entropy (GLCM) | 8 | 6% |
| Entropy (histogram) | 8 | 6% |
| RLNU | 8 | 7% |
| SZE | 8 | 8% |
| Homogeneity | 8/6 | 10%/11% |
| LRE | 7 | 11% |
| ZP | 6 | 11% |
| GLNU | 5 | 15% |
| Dissimilarity | 4 | 15% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moalla, S.; Girot, C.; Franchi-Abella, S.; Ammari, S.; Balleyguier, C.; Lassau, N.; Pitre-Champagnat, S. Methodological Study to Investigate the Potential of Ultrasound-Based Elastography and Texture as Biomarkers to Monitor Liver Tumors. Diagnostics 2020, 10, 811. https://doi.org/10.3390/diagnostics10100811
Moalla S, Girot C, Franchi-Abella S, Ammari S, Balleyguier C, Lassau N, Pitre-Champagnat S. Methodological Study to Investigate the Potential of Ultrasound-Based Elastography and Texture as Biomarkers to Monitor Liver Tumors. Diagnostics. 2020; 10(10):811. https://doi.org/10.3390/diagnostics10100811
Chicago/Turabian StyleMoalla, Salma, Charly Girot, Stéphanie Franchi-Abella, Samy Ammari, Corinne Balleyguier, Nathalie Lassau, and Stéphanie Pitre-Champagnat. 2020. "Methodological Study to Investigate the Potential of Ultrasound-Based Elastography and Texture as Biomarkers to Monitor Liver Tumors" Diagnostics 10, no. 10: 811. https://doi.org/10.3390/diagnostics10100811
APA StyleMoalla, S., Girot, C., Franchi-Abella, S., Ammari, S., Balleyguier, C., Lassau, N., & Pitre-Champagnat, S. (2020). Methodological Study to Investigate the Potential of Ultrasound-Based Elastography and Texture as Biomarkers to Monitor Liver Tumors. Diagnostics, 10(10), 811. https://doi.org/10.3390/diagnostics10100811





