Efficacy and Safety of Non-Anesthesiologist Administration of Propofol Sedation in Endoscopic Ultrasound: A Propensity Score Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Sedation
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Patients
3.2. Safety and Efficacy
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Early, D.S.; Lightdale, J.R.; Vargo, J.J.; Acosta, R.D.; Chandrasekhara, V.; Chathadi, K.V.; Evans, J.A.; Fisher, D.A.; Fonkalsrud, L.; Hwang, J.H.; et al. Guidelines for sedation and anesthesia in GI endoscopy. Gastrointest. Endosc. 2018, 87, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.R.; Jagannath, S.; Baron, T.H.; Anderson, M.A.; Banerjee, S.; Dominitz, J.A.; Fanelli, R.D.; Gan, S.I.; Harrison, M.E.; Ikenberry, S.O.; et al. RETRACTED: Sedation and anesthesia in GI endoscopy. Gastrointest. Endosc. 2008, 68, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Rex, D.K.; Deenadayalu, V.P.; Eid, E.; Imperiale, T.F.; Walker, J.A.; Sandhu, K.; Clarke, A.C.; Hillman, L.C.; Horiuchi, A.; Cohen, L.B.; et al. Endoscopist-Directed Administration of Propofol: A Worldwide Safety Experience. Gastroenterol. 2009, 137, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.B.; Hightower, C.D.; Wood, A.D.; Miller, K.M.; Aisenberg, J. Moderate level sedation during endoscopy: A prospective study using low-dose propofol, meperidine/fentanyl, and midazolam. Gastrointest. Endosc. 2004, 59, 795–803. [Google Scholar] [CrossRef]
- Hassan, C.; Rex, D.K.; Cooper, G.S.; Benamouzig, R. Endoscopist-directed propofol administration versus anesthesiologist assistance for colorectal cancer screening: A cost-effectiveness analysis. Endoscopy 2012, 44, 456–464. [Google Scholar] [CrossRef]
- American Society of Anesthesiologists (ASA). AANA-ASA Joint Statement Regarding Propofol Administration. Available online: https://www.asahq.org/standards-and-guidelines/statement-of-granting-privileges-for-administration-of-moderate-sedation-to-practitioners (accessed on 13 August 2020).
- American Society of Anesthesiologists (ASA). ASA House of Delegates Statement on Safe Use of Propofol. Available online: https://www.asahq.org/standards-and-guidelines/statement-of-granting-privileges-for-administration-of-moderate-sedation-to-practitioners (accessed on 13 August 2020).
- Triantafillidis, J.K. Sedation in gastrointestinal endoscopy: Current issues. World J. Gastroenterol. 2013, 19, 463–481. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, T.; Suzuki, H. Propofol for gastrointestinal endoscopy. United Eur. Gastroenterol. J. 2018, 6, 801–805. [Google Scholar] [CrossRef]
- Conigliaro, R.; Fanti, L.; Manno, M.; Brosolo, P. Italian Society of Digestive Endoscopy (SIED) position paper on the non-anaesthesiologist administration of propofol for gastrointestinal endoscopy. Dig. Liver Dis. 2017, 49, 1185–1190. [Google Scholar] [CrossRef]
- Facciorusso, A. Endoscopic ultrasound-guided tissue sampling of pancreatic lesions. Minerva Gastroenterol. Dietol. 2020, 66. [Google Scholar] [CrossRef]
- Facciorusso, A.; Wani, S.; Triantafyllou, K.; Tziatzios, G.; Cannizzaro, R.; Muscatiello, N.; Singh, S. Comparative accuracy of needle sizes and designs for EUS tissue sampling of solid pancreatic masses: A network meta-analysis. Gastrointest. Endosc. 2019, 90, 893–903. [Google Scholar] [CrossRef]
- Facciorusso, A.; Sunny, S.P.; Del Prete, V.; Antonino, M.; Muscatiello, N. Comparison between fine-needle biopsy and fine-needle aspiration for EUS-guided sampling of subepithelial lesions: A meta-analysis. Gastrointest. Endosc. 2020, 91, 14–22. [Google Scholar] [CrossRef]
- Facciorusso, A.; Del Prete, V.; Buccino, V.R.; Purohit, P.; Setia, P.; Muscatiello, N. Diagnostic yield of Franseen and Fork-Tip biopsy needles for endoscopic ultrasound-guided tissue acquisition: A meta-analysis. Endosc. Int. Open 2019, 7, E1221–E1230. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Pinto, E.; Baron, T.H. Evaluation of the AXIOS stent for the treatment of pancreatic fluid collections. Expert Rev. Med. Devices 2016, 13, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Di Maso, M.; Serviddio, G.; Larghi, A.; Costamagna, G.; Muscatiello, N. Echoendoscopic ethanol ablation of tumor combined with celiac plexus neurolysis in patients with pancreatic adenocarcinoma. J. Gastroenterol. Hepatol. 2017, 32, 439–445. [Google Scholar] [CrossRef]
- Facciorusso, A.; Del Prete, V.; Antonino, M.; Buccino, V.R.; Muscatiello, N. Response to repeat echoendoscopic celiac plexus neurolysis in pancreatic cancer patients: A machine learning approach. Pancreatology 2019, 19, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Buccino, R.V.; Muscatiello, N. How to measure quality in endoscopic ultrasound. Ann. Transl. Med. 2018, 6, 266. [Google Scholar] [CrossRef] [PubMed]
- Pagano, N.; Arosio, M.; Romeo, F.; Rando, G.; Del Conte, G.; Carlino, A.; Strangio, G.; Vitetta, E.; Malesci, A.; Repici, A. Balanced Propofol Sedation in Patients Undergoing EUS-FNA: A Pilot Study to Assess Feasibility and Safety. Diagn. Ther. Endosc. 2011, 2011, 1–5. [Google Scholar] [CrossRef]
- Facciorusso, A.; Buccino, V.R.; Turco, A.; Antonino, M.; Muscatiello, N. Antibiotics Do Not Decrease the Rate of Infection After Endoscopic Ultrasound Fine-Needle Aspiration of Pancreatic Cysts. Dig. Dis. Sci. 2019, 64, 2308–2315. [Google Scholar] [CrossRef]
- Facciorusso, A.; Mohan, B.P.; Tacelli, M.; Crinò, S.F.; Antonini, F.; Fantin, A.; Barresi, L. Use of antibiotic prophylaxis is not needed for endoscopic ultrasound-guided fine-needle aspiration of pancreatic cysts: A meta-analysis. Expert Rev. Gastroenterol. Hepatol. 2020, 25, 1–7. [Google Scholar] [CrossRef]
- Facciorusso, A.; Buccino, V.R.; Del Prete, V.; Antonino, M.; Contaldo, A.; Muscatiello, N. Statins decrease the risk of acute pancreatitis after endoscopic ultrasound fine-needle aspiration of pancreatic cysts. Hepatobiliary Pancreat. Dis. Int. 2020, 19, 74–79. [Google Scholar] [CrossRef]
- Facciorusso, A.; Buccino, V.R.; Del Prete, V.; Antonino, M.; Muscatiello, N. Cirrhosis Is a Predictor of Adverse Events in Endoscopic Ultrasound Fine-Needle Aspiration: A Propensity-Score Analysis. Dig. Dis. 2019, 38, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.T.; Hornslet, P.; Konge, L.; Møller, A.M.; Vilmann, P. High efficacy with deep nurse-administered propofol sedation for advanced gastroenterologic endoscopic procedures. Endosc. Int. Open 2015, 4, E107–E111. [Google Scholar] [CrossRef] [PubMed]
- DeWitt, J.; McGreevy, K.; Sherman, S.; Imperiale, T.F. Nurse-administered propofol sedation compared with midazolam and meperidine for EUS: A prospective, randomized trial. Gastrointest. Endosc. 2008, 68, 499–509. [Google Scholar] [CrossRef]
- Austin, P.C. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat. Med. 2008, 27, 2037–2049. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef]
- Facciorusso, A.; Di Maso, M.; Antonino, M.; Del Prete, V.; Panella, C.; Barone, M.; Muscatiello, N. Polidocanol injection decreases the bleeding rate after colon polypectomy: A propensity score analysis. Gastrointest. Endosc. 2015, 82, 350–358.e2. [Google Scholar] [CrossRef]
- Burtea, D.E.; Dimitriu, A.; Maloş, A.E.; Saftoiu, A. Current role of non-anesthesiologist administered propofol sedation in advanced interventional endoscopy. World J. Gastrointest. Endosc. 2015, 7, 981–986. [Google Scholar] [CrossRef]
- Delgado, A.A.D.A.; De Moura, D.T.H.; Ribeiro, I.B.; Bazarbashi, A.N.; Dos Santos, M.E.L.; Bernardo, W.M.; De Moura, E.G.H. Propofol vs traditional sedatives for sedation in endoscopy: A systematic review and meta-analysis. World J. Gastrointest. Endosc. 2019, 11, 573–588. [Google Scholar] [CrossRef]
- Fanti, L.; Massimo, A.; Marco, G.; Giulia, G.; Antonio, F.; Mario, G.; Giorgio, T.; Alberto, T.P.; Agostoni, M.; Gemma, M.; et al. Remifentanil vs. Meperidine for Patient-Controlled Analgesia During Colonoscopy: A Randomized Double-Blind Trial. Am. J. Gastroenterol. 2009, 104, 1119–1124. [Google Scholar] [CrossRef]
- Slagelse, C.; Vilmann, P.; Hornslet, P.; Hammering, A.; Mantoni, T. Nurse-administered propofol sedation for gastrointestinal endoscopic procedures: First Nordic results from implementation of a structured training program. Scand. J. Gastroenterol. 2011, 46, 1503–1509. [Google Scholar] [CrossRef]
- New Italian Professional Ethics Code May 2014. Nuovo Codice Deontologia Medica.pdf. Available online: www.privacy.it/2014 (accessed on 13 August 2020).
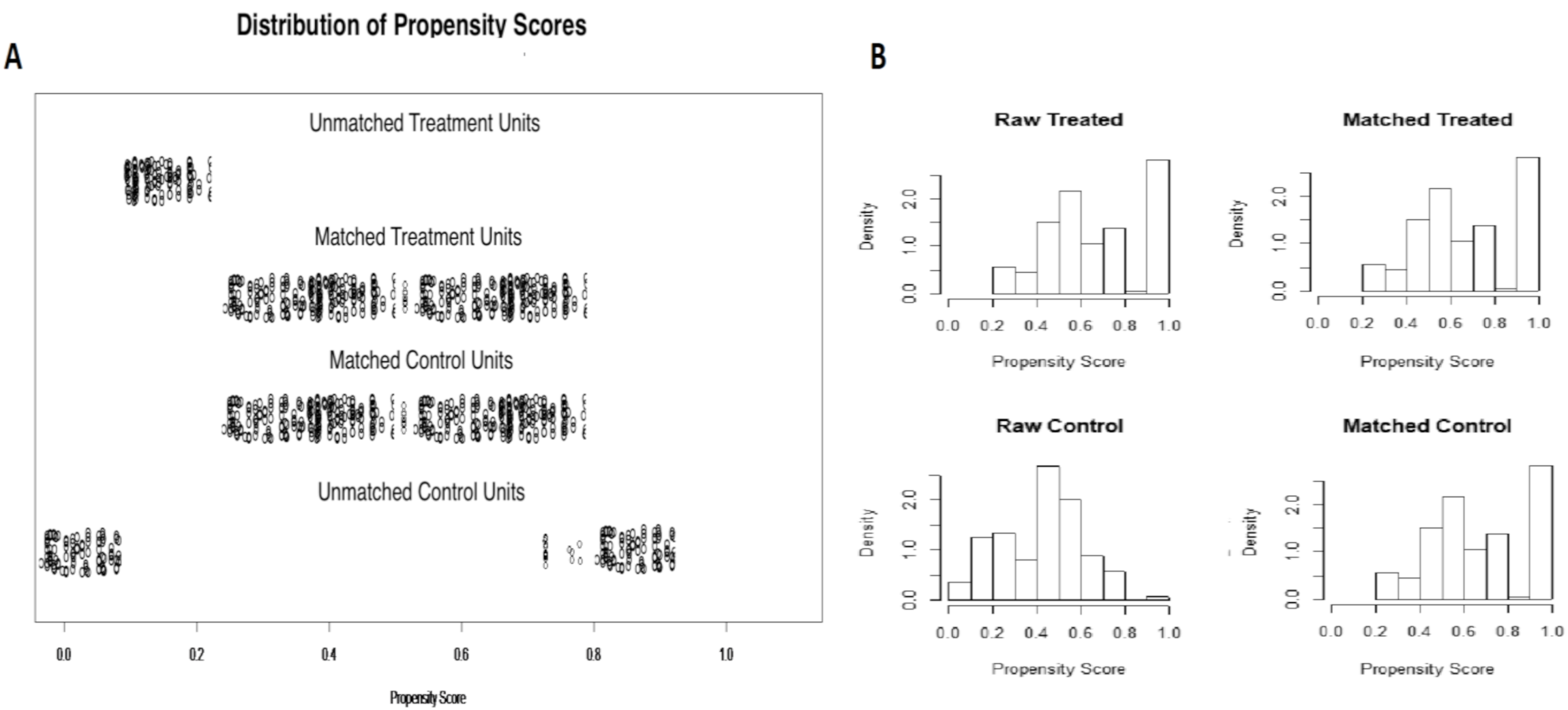
| Variable | NAAP (n = 395) | Controls (n = 437) | p Value |
|---|---|---|---|
| Age (years) | 68 ± 9 | 65 ± 7 | 0.54 |
| Gender: Male | 229 (57.9%) | 279 (63.8%) | 0.08 |
| Female | 166 (42.1%) | 158(36.2%) | |
| Comorbidities | 128 (32.4%) | 143 (32.7%) | 0.77 |
| ASA score ≤II | 304 (76.9%) | 361 (82.6%) | 0.05 |
| EUS-FNA/FNB | 315 (79.7%) | 345 (78.9%) | 0.77 |
| Other EUS procedures | 80 (20.3%) | 92 (21.1%) | |
| Number of previous endoscopies | 1.3 ± 1.2 | 2.1 ± 1.5 | 0.65 |
| Location: Pancreas | 305 (77.2%) | 352 (80.5%) | 0.26 |
| Other | 90 (22.8%) | 85 (19.5%) |
| Variable | NAAP (n = 305) | Controls (n = 305) | p Value |
|---|---|---|---|
| Age (years) | 67 ± 3 | 66 ± 9 | 0.91 |
| Gender: Male | 183 (60%) | 183 (60%) | 1.0 |
| Female | 122 (40%) | 122 (40%) | |
| Comorbidities | 97 (31.8%) | 97 (31.8%) | 1.0 |
| ASA score ≤2 | 252 (82.6%) | 250 (81.9%) | 0.89 |
| EUS-FNA/FNB | 248 (81.3%) | 250 (81.9%) | 0.93 |
| Other EUS procedures | 57 (18.7%) | 55 (18.1%) | |
| Number of previous endoscopies | 1.2 ± 1 | 1.3 ± 1.3 | 0.85 |
| Location: Pancreas | 250 (81.9%) | 248 (81.3%) | 0.93 |
| Other | 55 (18.1%) | 57 (18.7%) |
| NAAP (305 Patients) | Controls (305 Patients) | p-Value a | |
|---|---|---|---|
| Safety | |||
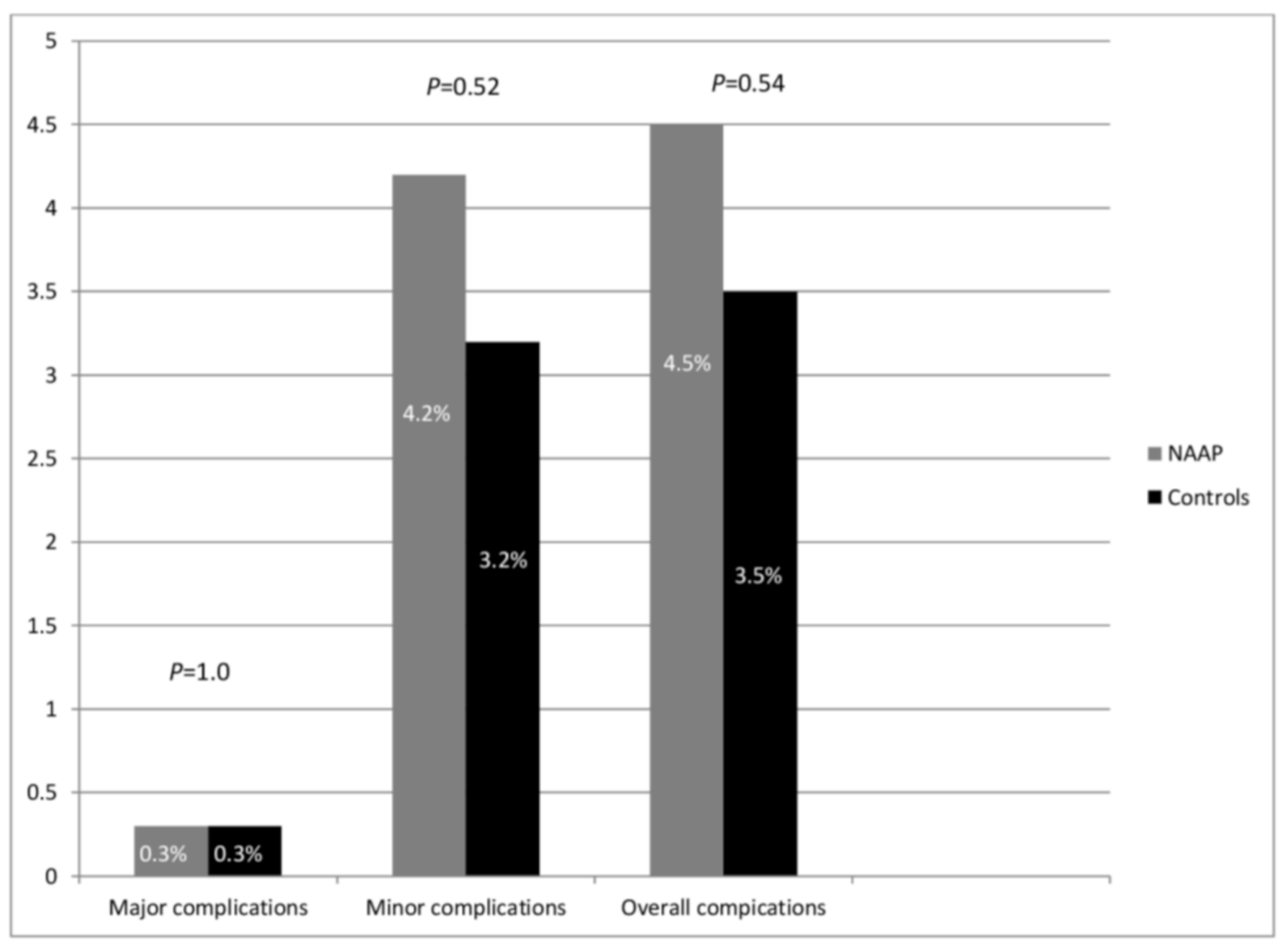
| • Any major complications * | 1 (0.3%) | 1 (0.3%) | 1.0 |
| • Any minor complications + | 13 (4.2%) | 10 (3.2%) | 0.52 |
| • Mean propofol dose (mg) | 85.4 ± 25.5 | 92.4 ± 30.5 | 0.24 |
| Efficacy | |||
| • Patient satisfaction with the procedure § | 8.5 ± 1.5 | 8.3 ± 1.5 | 0.88 |
| • Endoscopist satisfaction with the procedure § | 8.3 ± 2 | 8.2 ± 1.8 | 0.75 |
| • Quality of EUS § | 9 ± 1 | 9 ± 0.8 | 1.0 |
| • Overall pain during the procedure § | 2.3 ± 1 | 1.8 ± 1 | 0.67 |
| • Pain or discomfort upon awakening § | 1 ± 0.5 | 1 ± 0.5 | 0.72 |
| • Mean procedure duration (min) | 20 ± 23 | 23 ± 18 | 0.62 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Facciorusso, A.; Turco, A.; Barnabà, C.; Longo, G.; Dipasquale, G.; Muscatiello, N. Efficacy and Safety of Non-Anesthesiologist Administration of Propofol Sedation in Endoscopic Ultrasound: A Propensity Score Analysis. Diagnostics 2020, 10, 791. https://doi.org/10.3390/diagnostics10100791
Facciorusso A, Turco A, Barnabà C, Longo G, Dipasquale G, Muscatiello N. Efficacy and Safety of Non-Anesthesiologist Administration of Propofol Sedation in Endoscopic Ultrasound: A Propensity Score Analysis. Diagnostics. 2020; 10(10):791. https://doi.org/10.3390/diagnostics10100791
Chicago/Turabian StyleFacciorusso, Antonio, Antonio Turco, Carlo Barnabà, Grazia Longo, Graziano Dipasquale, and Nicola Muscatiello. 2020. "Efficacy and Safety of Non-Anesthesiologist Administration of Propofol Sedation in Endoscopic Ultrasound: A Propensity Score Analysis" Diagnostics 10, no. 10: 791. https://doi.org/10.3390/diagnostics10100791
APA StyleFacciorusso, A., Turco, A., Barnabà, C., Longo, G., Dipasquale, G., & Muscatiello, N. (2020). Efficacy and Safety of Non-Anesthesiologist Administration of Propofol Sedation in Endoscopic Ultrasound: A Propensity Score Analysis. Diagnostics, 10(10), 791. https://doi.org/10.3390/diagnostics10100791






