Simplified Submucosal Tunneling Biopsy Using Clip-With-Line Traction and Closure for Gastric Subepithelial Lesion
Abstract
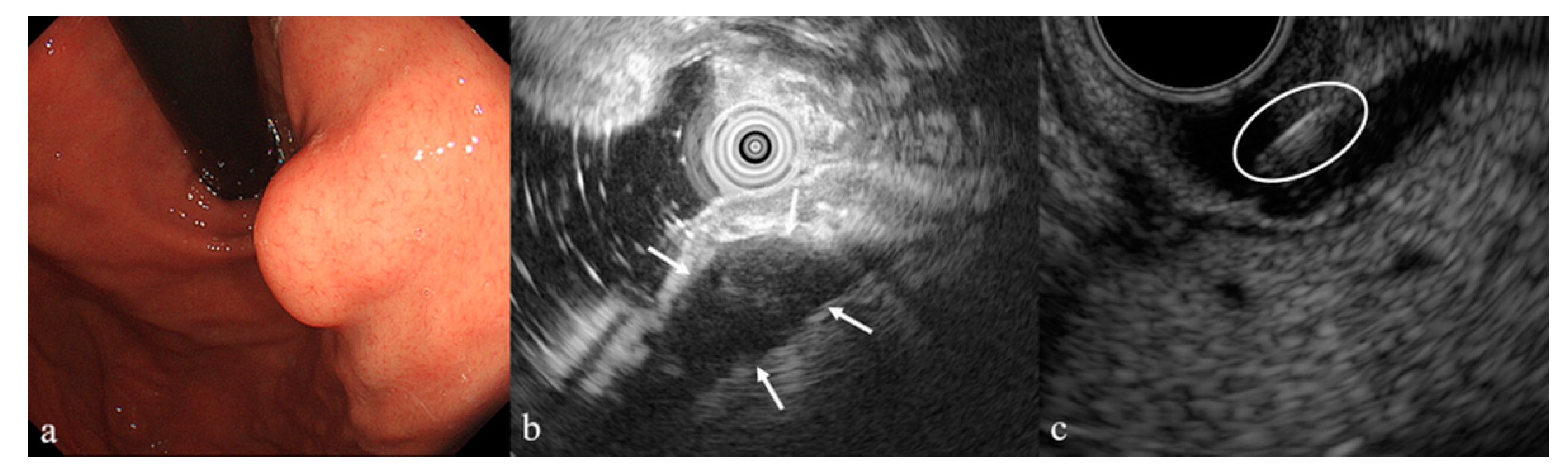
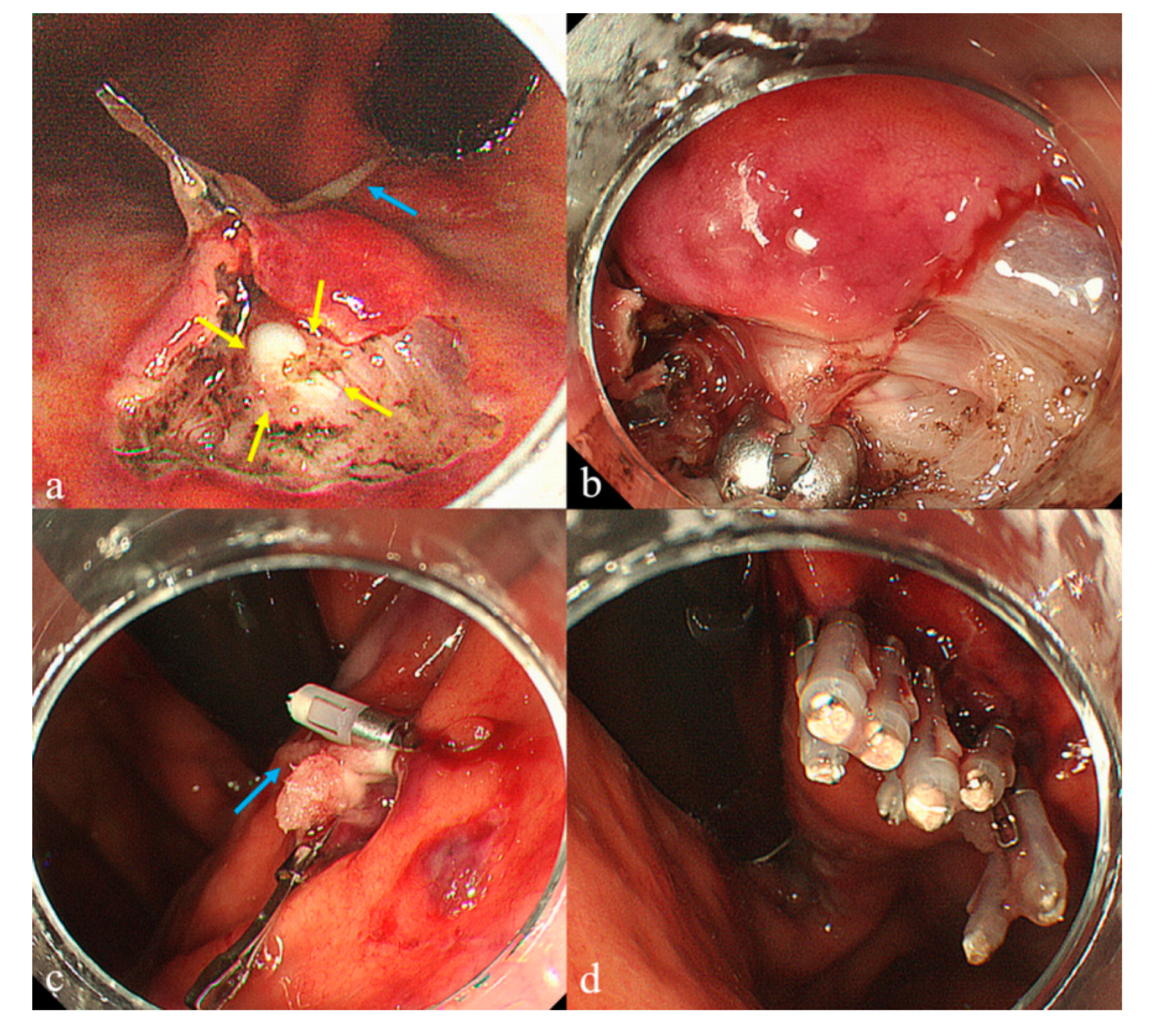
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Consent for Publication
References
- Kobara, H.; Mori, H.; Nishimoto, N.; Fujihara, S.; Nishiyama, N.; Ayaki, M.; Yachida, T.; Matsunaga, T.; Chiyo, T.; Kobayashi, N.; et al. Comparison of Submucosal Tunneling Biopsy versus EUS-guided FNA for Gastric Subepithelial Lesions: A Prospective Study with Crossover Design. Endosc. Int. Open 2017, 5, E695–E705. [Google Scholar] [CrossRef] [PubMed]
- Sumiyama, K.; Gostout, C.J.; Rajan, E.; Bakken, T.A.; Knipschield, M.A.; Marler, R.J. Submucosal Endoscopy with Mucosal Flap Safety Valve. Gastrointest. Endosc. 2007, 65, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Kobara, H.; Mori, H.; Fujihara, S.; Nishiyama, N.; Kobayashi, M.; Kamata, H.; Masaki, T. Bloc Biopsy by Using Submucosal Endoscopy with a Mucosal Flap Method for Gastric Subepithelial Tumor Tissue Sampling (with Video). Gastrointest. Endosc. 2013, 77, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Jeon, W.J.; You, I.Y.; Chae, H.B.; Park, S.M.; Youn, S.J. A New Technique for Gastric Endoscopic Submucosal Dissection: Peroral Traction-Assisted Endoscopic Submucosal Dissection. Gastrointest. Endosc. 2009, 69, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Parra-Blanco, A. Traction is Most Important for the Widespread Use of Endoscopic Submucosal Dissection, Especially in Procedures Presenting Particular Difficulty. Endoscopy 2020, 52, 328–329. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-F.; Cheng, S.-W.; Chang, C.C.; Kang, Y.-N. Efficacy and Safety of Traction-Assisted Endoscopic Submucosal Dissection: A Meta-Regression of Randomized Clinical Trials. Endoscopy 2020, 52, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Kobara, H.; Mori, H.; Fujihara, S.; Nishiyama, N.; Tani, J.; Morishita, A.; Oryu, M.; Tsutsui, K.; Masaki, T. Endoscopically Visualized Features of Gastric Submucosal Tumors on Submucosal Endoscopy. Endoscopy 2014, 46, E660–E661. [Google Scholar] [CrossRef] [PubMed]
- Kobara, H.; Mori, H.; Rafiq, K.; Matsunaga, T.; Fujihara, S.; Nishiyama, N.; Ayaki, M.; Yachida, T.; Tani, J.; Miyoshi, H.; et al. Evaluation of Gastric Submucosal Tumors by Endoscopic Visualized Features on Submucosal Endoscopy. Oncol Lett. 2014, 8, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, Y.; Takeuchi, Y.; Iwatsubo, T.; Kato, M.; Hamada, K.; Tonai, Y.; Matsuura, N.; Kanesaka, T.; Yamashina, T.; Arao, M.; et al. Line-Assisted Complete Closure for a Large Mucosal Defect after Colorectal Endoscopic Submucosal Dissection Decreased Post-Electrocoagulation Syndrome. Dig. Endosc. 2018, 30, 633–641. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobara, H.; Kobayashi, N.; Nishiyama, N.; Tada, N.; Fujihara, S.; Masaki, T. Simplified Submucosal Tunneling Biopsy Using Clip-With-Line Traction and Closure for Gastric Subepithelial Lesion. Diagnostics 2020, 10, 690. https://doi.org/10.3390/diagnostics10090690
Kobara H, Kobayashi N, Nishiyama N, Tada N, Fujihara S, Masaki T. Simplified Submucosal Tunneling Biopsy Using Clip-With-Line Traction and Closure for Gastric Subepithelial Lesion. Diagnostics. 2020; 10(9):690. https://doi.org/10.3390/diagnostics10090690
Chicago/Turabian StyleKobara, Hideki, Nobuya Kobayashi, Noriko Nishiyama, Naoya Tada, Shintaro Fujihara, and Tsutomu Masaki. 2020. "Simplified Submucosal Tunneling Biopsy Using Clip-With-Line Traction and Closure for Gastric Subepithelial Lesion" Diagnostics 10, no. 9: 690. https://doi.org/10.3390/diagnostics10090690
APA StyleKobara, H., Kobayashi, N., Nishiyama, N., Tada, N., Fujihara, S., & Masaki, T. (2020). Simplified Submucosal Tunneling Biopsy Using Clip-With-Line Traction and Closure for Gastric Subepithelial Lesion. Diagnostics, 10(9), 690. https://doi.org/10.3390/diagnostics10090690






