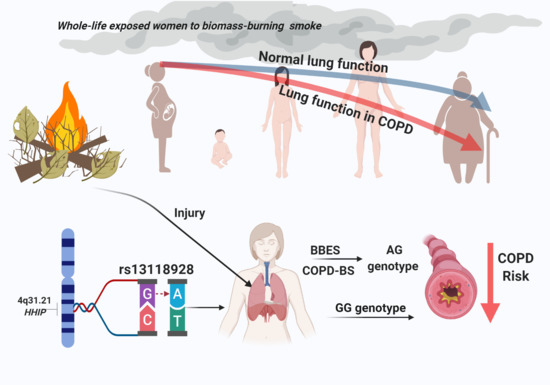
Participation of HHIP Gene Variants in COPD Susceptibility, Lung Function, and Serum and Sputum Protein Levels in Women Exposed to Biomass-Burning Smoke
Abstract
:1. Introduction
2. Materials and Methods
2.1. Case and Control Groups
2.2. Ethics Approval and Informed Consent
2.3. Blood Sample Processing and DNA Extraction
2.4. Sputum Induction and Sample Preparation
2.5. SNP Selection
2.6. SNP Genotyping
2.7. Serum and Sputum HHIP Protein Level Measurement
2.8. Statistical Assessment
2.9. Drugs′ Metabolism in Silico Analysis for COPD and Its Interaction with HHIP
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Hardy-Weinberg Equilibrium and Genotype Frequencies and Genetic Susceptibility
3.3. Genetic Models
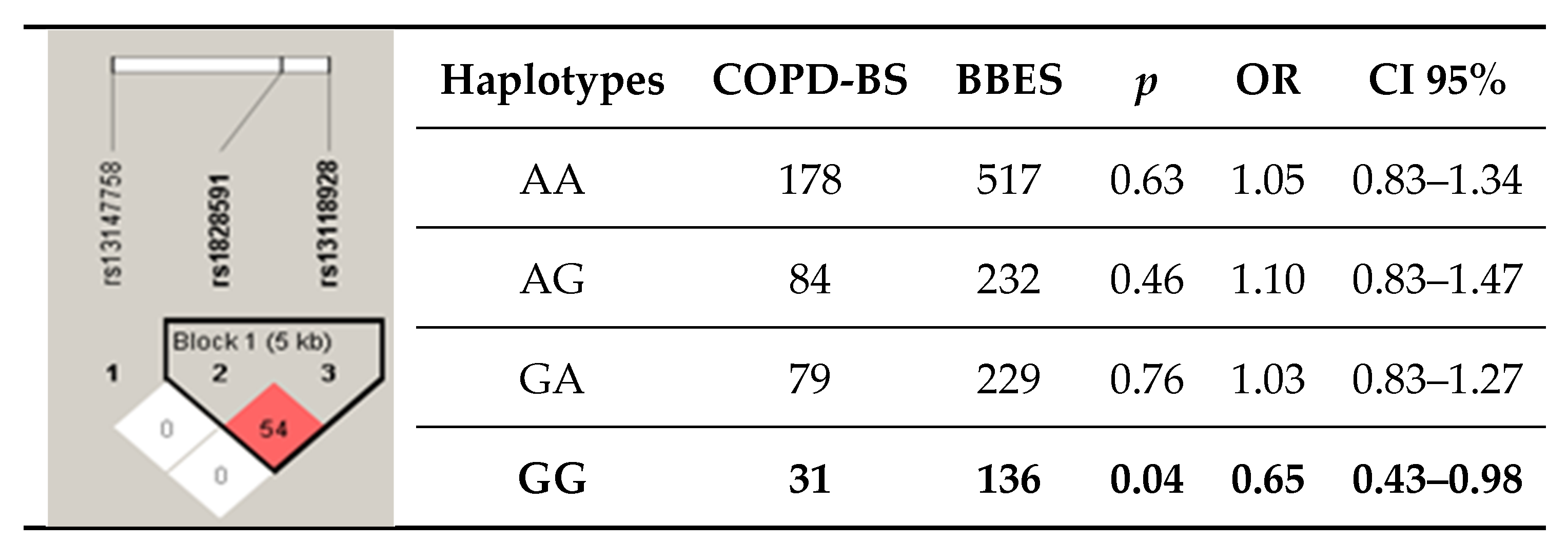
3.4. Haplotypes
3.5. Severity in COPD and Genetic Analysis
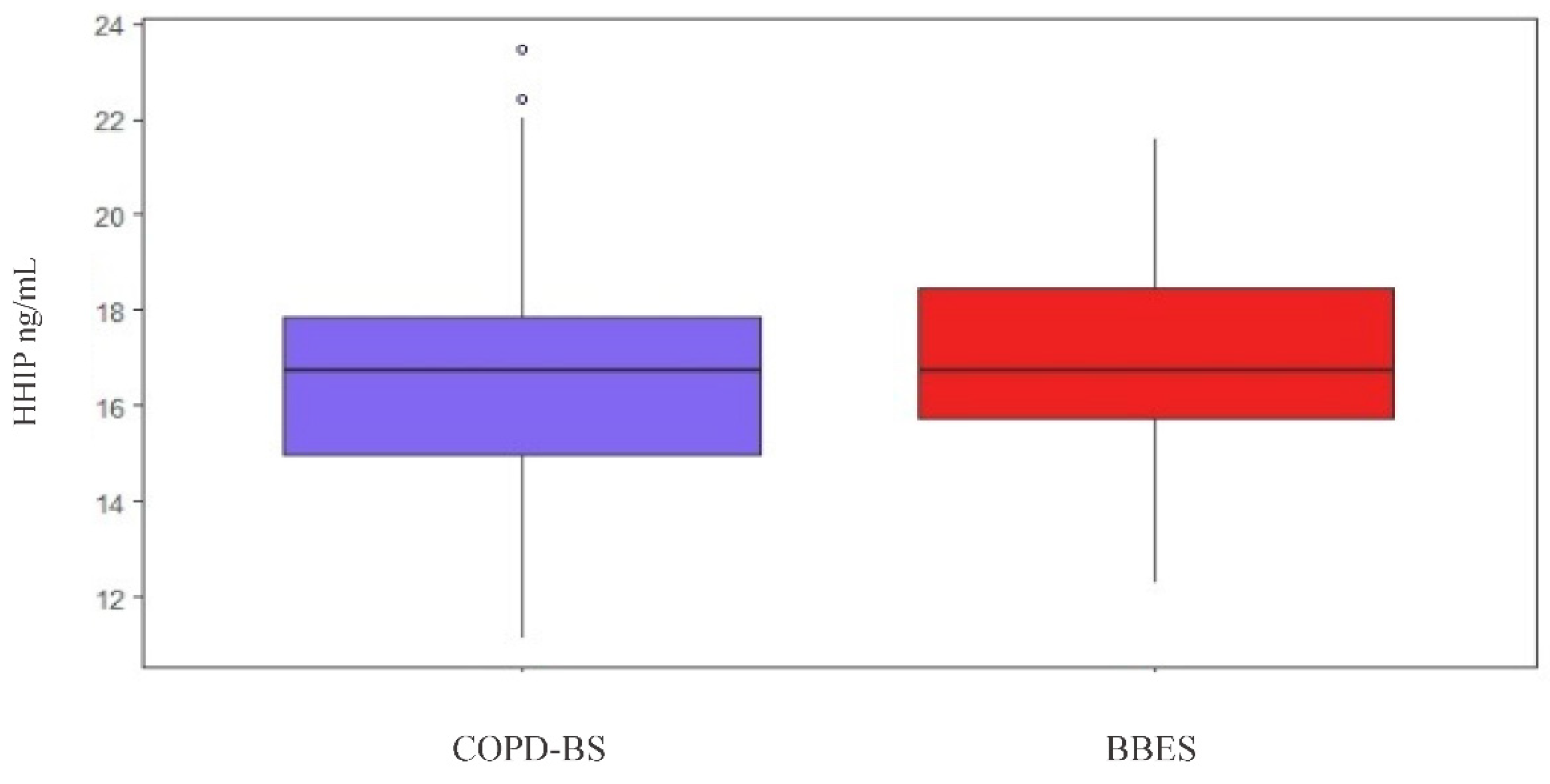
3.6. HHIP Serum Levels
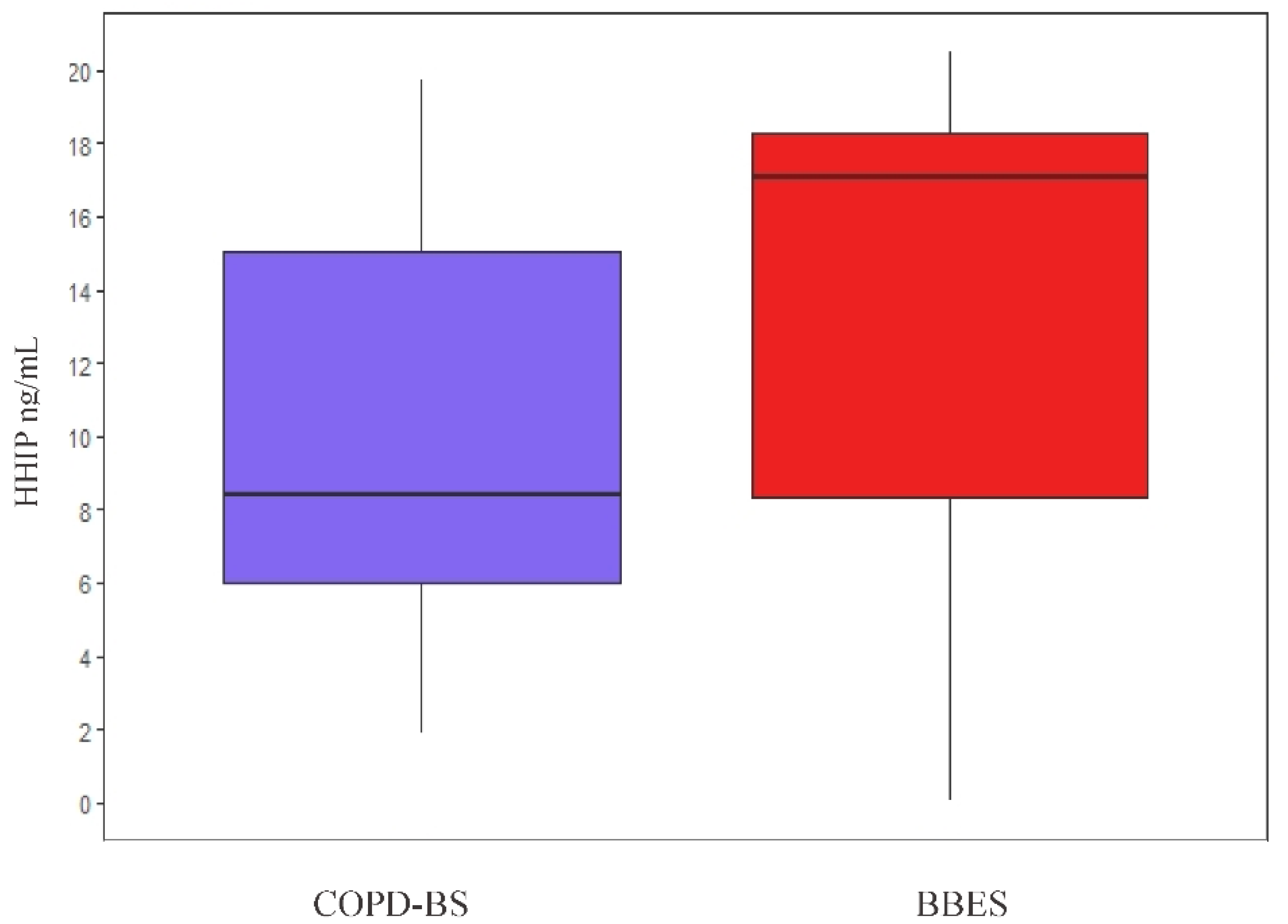
3.6.1. HHIP Serum Level Comparison
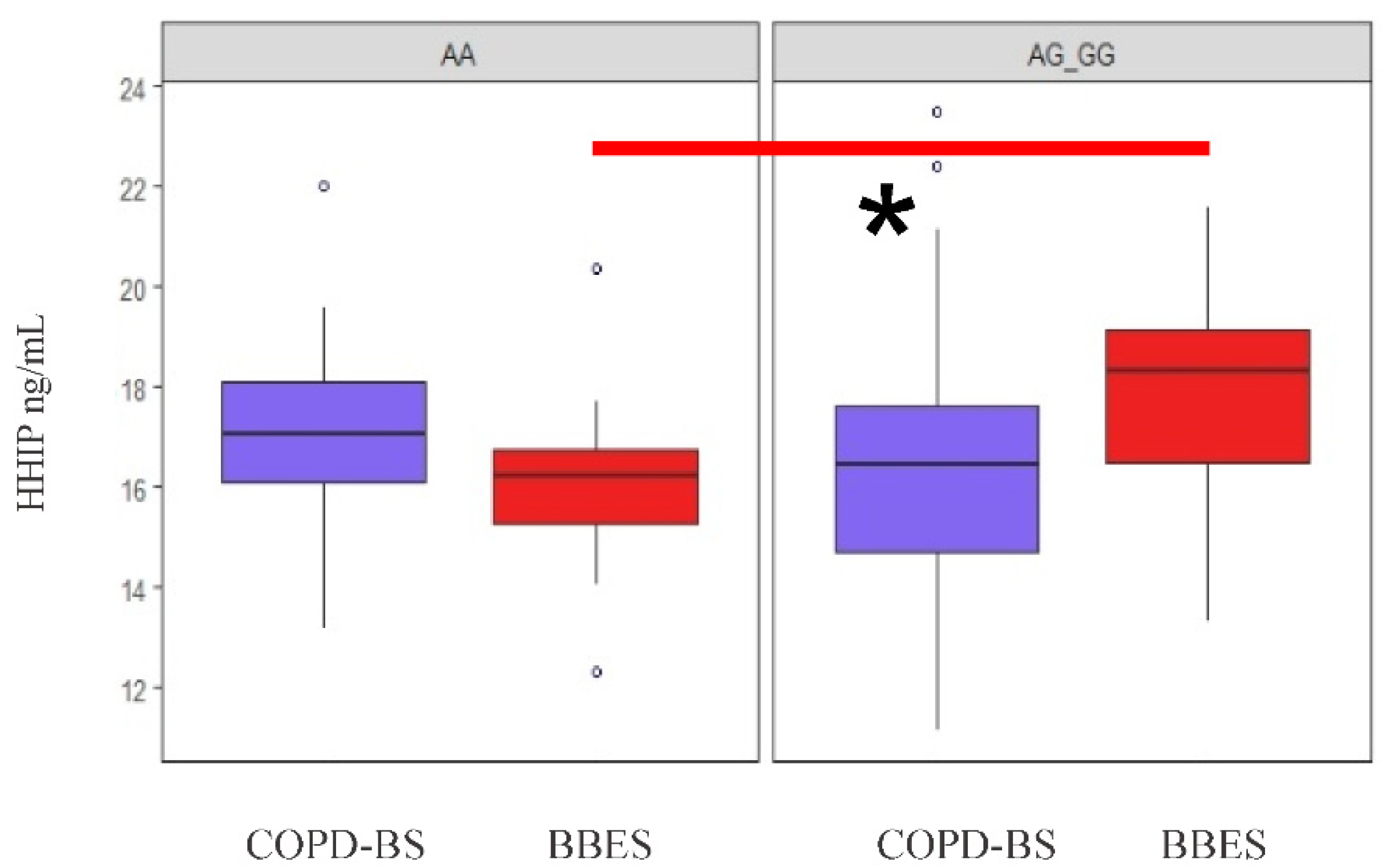
3.6.2. Analysis of Serum Protein Levels by Applying Genetic Models
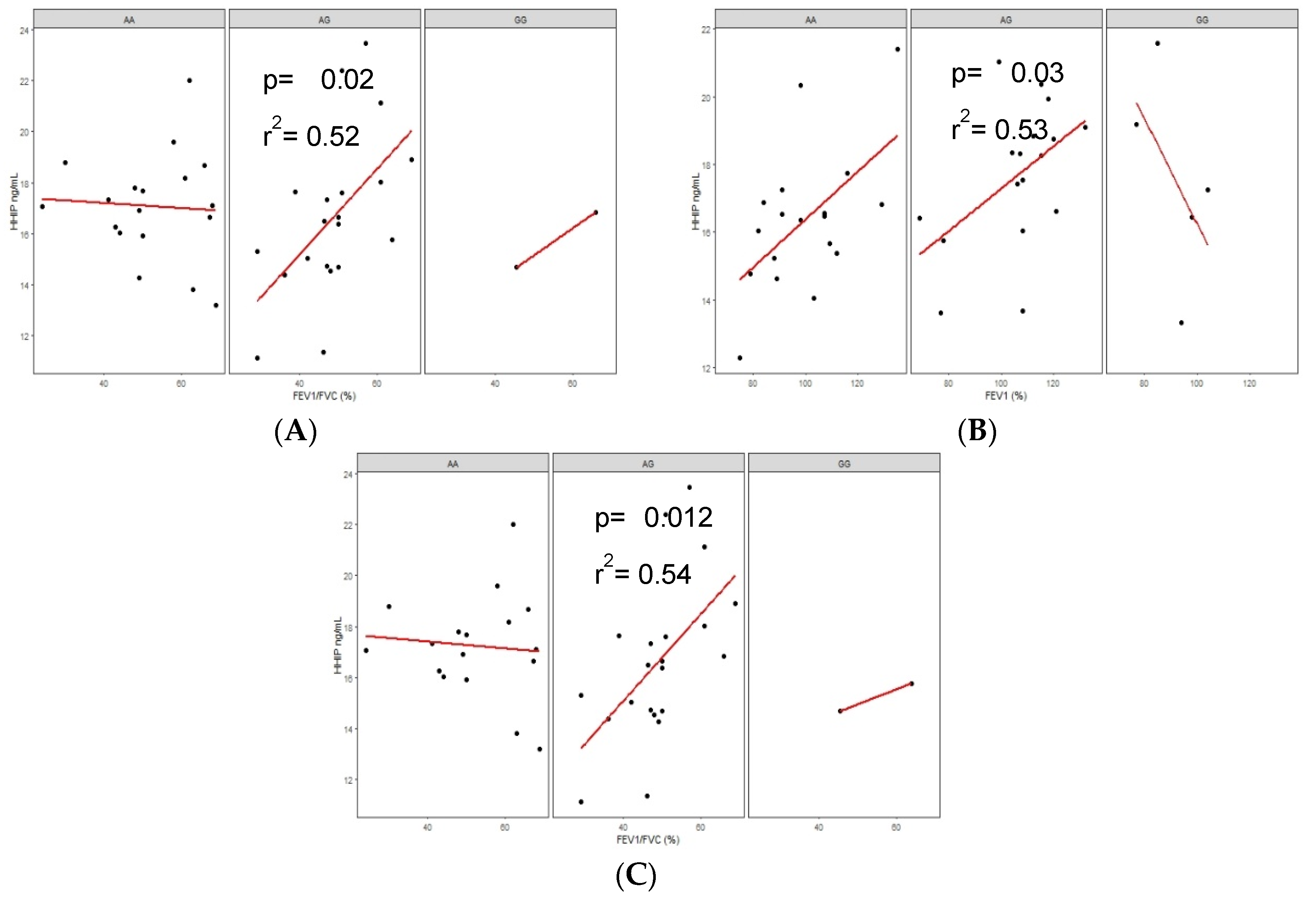
3.6.3. Correlations of Serum Protein Levels in COPD-BS and BBES with Lung Function
3.7. HHIP Levels in Supernatant Sputum of Smoke Biomass Burning
Protein Level Comparison in Sputum Supernatant
3.8. HHIP Levels and Metabolism of Drugs for COPD
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Data Availability Statement
References
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2018 Report); Global Initiative for Chronic Obstructive Lung Disease, 2018. Available online: http://www.goldcopd.org (accessed on 1 January 2019).
- Montes de Oca, M.; Zabert, G.; Moreno, D.; Laucho-Contreras, M.E.; Lopez Varela, M.V.; Surmont, F. Smoke, biomass exposure, and COPD risk in the primary care setting: The PUMA study. Respir. Care 2018, 62, 1058–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannino, D.M.; Buist, A.S. Global burden of COPD: Risk factors, prevalence, and future trends. Lancet 2007, 370, 765–773. [Google Scholar] [CrossRef]
- Ramírez-Venegas, A.; Velázquez-Uncal, M.; Pérez-Hernández, R.; Guzmán-Bouilloud, N.E.; Falfán-Valencia, R.; Mayar-Maya, M.E.; Aranda-Chávez, A.; Sansores, R.H. Prevalence of COPD and respiratory symptoms associated with biomass smoke exposure in a suburban area. Int. J. COPD 2018, 13, 1727–1734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Padilla, R.; Fernandez, R.; Lopez Varela, M.V.; Montes de Oca, M.; Muiño, A.; Tálamo, C.; Brito Jardim, J.R.; Valdivia, G.; Baptista Menezes, A.M. Airflow Obstruction in Never Smokers in Five Latin American Cities: The PLATINO Study. Arch. Med. Res. 2012, 43, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Perez-Rubio, G.; Cordoba-Lanus, E.; Cupertino, P.; Cartujano-Barrera, F.; Campos, M.A.; Falfan-Valencia, R. Role of genetic susceptibility in nicotine addiction and chronic obstructive pulmonary disease. Rev. Investig. Clin. 2019, 71, 36–54. [Google Scholar] [CrossRef] [Green Version]
- Pillai, S.G.; Ge, D.; Zhu, G.; Kong, X.; Shianna, K.V. A Genome-Wide Association Study in Chronic Obstructive Pulmonary Disease (COPD): Identification of Two Major Susceptibility Loci. PLoS Genet 2009, 5, 1000421. [Google Scholar] [CrossRef] [Green Version]
- Wilk, J.B.; Chen, T.H.; Gottlieb, D.J.; Walter, R.E.; Nagle, M.W.; Brandler, B.J.; Myers, R.H.; Borecki, I.B.; Silverman, E.K.; Weiss, S.T.; et al. A genome-wide association study of pulmonary function measures in the framingham heart study. PLoS Genet. 2009, 5, 1000421. [Google Scholar] [CrossRef]
- Murone, M.; Rosenthal, A.; De Sauvage, F.J. Hedgehog signal transduction: From flies to vertebrates. Exp. Cell Res. 1999, 253, 25–33. [Google Scholar] [CrossRef] [Green Version]
- HGNC SUB1P1 Gene Symbol Report. HUGO Gene Nomenclature Committee. Available online: https://www.genenames.org/data/gene-symbol-report/#!/hgnc_id/HGNC:14866 (accessed on 1 March 2020).
- Bak, M.; Hansen, C.; Friis Henriksen, K.; Tommerup, N. The human hedgehog-interacting protein gene: Structure and chromosome mapping to 4q31.21-->q31.3. Cytogenet. Cell Genet. 2001, 92, 300–303. [Google Scholar] [CrossRef]
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global Strategy for The Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (Updated 2013). 2013. Available online: https://goldcopd.org/ (accessed on 1 January 2020).
- Pérez-Padilla, R.; Valdivia, G.; Muiño, A.; López, M.V.; Márquez, M.N.; Montes de Oca, M.; Tálamo, C.; Lisboa, C.; Pertuzé, J.; Jardim, J.R.B.; et al. Spirometric Reference Values in 5 Large Latin American Cities for Subjects Aged 40 Years or Over. Arch. Bronconeumol. (Engl. Ed.) 2006, 42, 317–325. [Google Scholar] [CrossRef]
- Little, J.; Higgins, J.P.T.; Ioannidis, J.P.A.; Moher, D.; Gagnon, F.; von Elm, E.; Khoury, M.J.; Cohen, B.; Davey-Smith, G.; Grimshaw, J.; et al. STrengthening the REporting of Genetic Association studies (STREGA)--an extension of the STROBE statement. Eur. J. Clin. Investig. 2009, 39, 247–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambrocio-Ortiz, E.; Pérez-Rubio, G.; Ramírez-Venegas, A.; Hernández-Zenteno, R.; Del Angel-Pablo, A.D.; Pérez-Rodríguez, M.E.; Salazar, A.M.; Abarca-Rojano, E.; Falfán-Valencia, R. Effect of SNPs in HSP Family Genes, Variation in the mRNA and Intracellular Hsp Levels in COPD Secondary to Tobacco Smoking and Biomass-Burning Smoke. Front. Genet. 2020, 10, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zerbino, D.R.; Achuthan, P.; Akanni, W.; Amode, M.R.; Barrell, D.; Bhai, J.; Billis, K.; Cummins, C.; Gall, A.; Girón, C.G.; et al. Ensembl 2018. Nucleic Acids Res. 2017, 46, D754–D761. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [Green Version]
- Henschke, H. De Finetti Diagram. Available online: https://web.archive.org/web/20110719103301/https://finetti.meb.uni-bonn.de/downloads/finetti_3.0.5_windows.zip (accessed on 2 June 2020).
- CDC Epi Info Epi InfoTM 7. Available online: https://www.cdc.gov/epiinfo/esp/es_pc.html (accessed on 18 April 2020).
- de Galicia, X. Organización Panamericana de la Salud EPIDAT, Versión 3.1. 2006. Available online: https://extranet.sergas.es/EPIWB/EPIWB/SolicitudeEpidat.aspx?IdPaxina=62715&idv=1&lng=es (accessed on 25 April 2020).
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef] [Green Version]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [Green Version]
- Torres-Duque, C.A.; García-Rodriguez, M.C.; González-García, M. Enfermedad pulmonar obstructiva crónica por humo de leña: ¿un fenotipo diferente o una entidad distinta? Arch. Bronconeumol. 2016, 52, 425–431. [Google Scholar] [CrossRef]
- Pérez-Padilla, R.; Ramirez-Venegas, A.; Sansores-Martinez, R. Clinical Characteristics of Patients With Biomass Smoke-Associated COPD and Chronic Bronchitis, 2004–2014. Chronic Obstr. Pulm. Dis. J. COPD Found. 2014, 1, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Regalado, J.; Pérez-Padilla, R.; Sansores, R.; Ramirez, J.I.P.; Brauer, M.; Paré, P.; Vedal, S. The effect of biomass burning on respiratory symptoms and lung function in rural Mexican women. Am. J. Respir. Crit. Care Med. 2006, 174, 901–905. [Google Scholar] [CrossRef]
- Ramírez-Venegas, A.; Sansores, R.H.; Pérez-Padilla, R.; Regalado, J.; Velázquez, A.; Sánchez, C.; Eugenia Mayar, M.; Mayar, M.E. Survival of patients with chronic obstructive pulmonary disease due to biomass smoke and tobacco. Am. J. Respir. Crit. Care Med. 2006, 173, 393–397. [Google Scholar] [CrossRef]
- Silva, R.; Oyarzún, M.; Olloquequi, J. Mecanismos patogénicos en la enfermedad pulmonar obstructiva crónica causada por exposición a humo de biomasa. Arch. Bronconeumol. 2015, 51, 285–292. [Google Scholar] [CrossRef]
- Moran-Mendoza, O.; Pérez-Padilla, J.R.; Salazar-Flores, M.; Vazquez-Alfaro, F.; Moran-Mendoza, O. Wood smoke-associated lung disease: A clinical, functional, radiological and pathological description. Int. J. Tuberc. Lung Dis. 2008, 12, 1092–1098. [Google Scholar]
- Kim, W.J.; Oh, Y.M.; Lee, J.H.; Park, C.S.; Park, S.W.; Park, J.S.; Lee, S. Do Genetic variants in HHIP are associated with FEV1 in subjects with chronic obstructive pulmonary disease. Respirology 2013, 18, 1202–1209. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Zheng, Z.; Chen, X.; Zeng, X.; Zhang, Y.; Li, D.; Shu, J.; Yang, K.; Lai, N.; et al. Genetic Variants in the Hedgehog Interacting Protein Gene Are Associated with the FEV1/FVC Ratio in Southern Han Chinese Subjects with Chronic Obstructive Pulmonary Disease. Biomed Res. Int. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Xu, G.; Gao, X.; Zhang, S.; Wang, Y.; Ding, M.; Liu, W.; Shen, J.; Sun, D. Comparison of the role of HHIP SNPs in susceptibility to chronic obstructive pulmonary disease between Chinese Han and Mongolian populations. Gene 2017, 637, 50–56. [Google Scholar] [CrossRef]
- Pérez-Rubio, G.; Ambrocio-Ortiz, E.; López-Flores, L.A.; Juárez-Martín, A.I.; Jiménez-Valverde, L.O.; Zoreque-Cabrera, S.; Galicia-Negrete, G.; Ramírez-Díaz, M.E.; Cruz-Vicente, F.; de Castillejos-López, M.J.; et al. Heterozygous genotype rs17580 AT (PiS) in SERPINA1 is associated with COPD secondary to biomass-burning and tobacco smoking: A case-control and populational study. Int. J. COPD 2020, 15, 1181–1190. [Google Scholar] [CrossRef]
- Ortega-Martínez, A.; Pérez-Rubio, G.; Ambrocio-Ortiz, E.; Nava-Quiroz, K.; Hernández-Zenteno, R.; Abarca-Rojano, E.; Rodríguez-Llamazares, S.; Hernández-Pérez, A.; García-Gómez, L.; Ramírez-Venegas, A.; et al. The SNP rs13147758 in the HHIP gene is associated with COPD susceptibility, serum and sputum protein levels in smokers. Front. Genet. 2020, 11, 882. [Google Scholar]
- Wan, E.S.; Li, Y.; Lao, T.; Qiu, W.; Jiang, Z.; Mancini, J.D.; Owen, C.A.; Clish, C.; Demeo, D.L.; Silverman, E.K.; et al. Metabolomic profiling in a Hedgehog Interacting Protein (Hhip) murine model of chronic obstructive pulmonary disease. Sci. Rep. 2017, 7, 2504. [Google Scholar] [CrossRef]
- Lao, T.; Jiang, Z.; Yun, J.; Qiu, W.; Guo, F.; Huang, C.; Mancini, J.D.; Gupta, K.; Laucho-Contreras, M.E.; Naing, Z.Z.C.; et al. Hhip haploinsufficiency sensitizes mice to age-related emphysema. Proc. Natl. Acad. Sci. USA 2016, 113, E4681–E4687. [Google Scholar] [CrossRef] [Green Version]
| Variables | COPD-BS (n = 186) | BBES (n = 557) | p |
|---|---|---|---|
| Age (Years) | 73 (47–93) | 62 (45–98) | <0.001 |
| BMI | 26.47 (17.81–45.04) | 27.83 (15.67–56.25) | 0.020 |
| Years of exposure to smoke biomass-burning | 50 (10–80) | 43 (10–87) | 0.005 |
| Hours/day exposure | 8 (2–24) | 7 (2–24) | 0.003 |
| BBEI | 320 (108–1116) | 240 (105–1050) | <0.001 |
| Lung function post-bronchodilator | |||
| FEV1 (%) | 64 (18–119) | 98 (55–187) | <0.001 |
| FVC (%) | 83 (35–146) | 94 (53–193) | <0.001 |
| FEV1/FVC (%) | 58 (21.53–69.7) | 83 (70–138) | <0.001 |
| GOLD | |||
| GOLD I (%) | 46 (24.7) | NA | - |
| GOLD II (%) | 103 (55.4) | NA | - |
| GOLD III (%) | 27 (14.5) | NA | - |
| GOLD IV (%) | 10 (5.4) | NA | - |
| Genotype | COPD-BS | BBES | p | OR | CI 95% | * p | ||
|---|---|---|---|---|---|---|---|---|
| n = 186 | % | n = 557 | % | |||||
| rs13147758 | ||||||||
| AA | 87 | 46.78 | 243 | 43.63 | 0.49 | 0.13 | 0.89–1.28 | |
| AG | 80 | 43.01 | 243 | 43.63 | 0.93 | 0.97 | 0.69–1.36 | |
| GG | 19 | 10.21 | 71 | 12.74 | 0.43 | 0.80 | 0.49–1.29 | 0.42 |
| rs1828591 | ||||||||
| AA | 90 | 48.38 | 257 | 46.16 | 0.61 | 1.09 | 0.78–1.52 | |
| AG | 77 | 41.41 | 232 | 41.64 | 1 | 0.98 | 0.70–1.38 | |
| GG | 19 | 10.21 | 68 | 12.20 | 0.51 | 0.81 | 0.47–1.40 | 0.13 |
| rs13118928 | ||||||||
| AA | 88 | 47.32 | 258 | 46.32 | 0.86 | 1.04 | 0.74–1.45 | |
| AG | 86 | 46.23 | 233 | 41.83 | 0.30 | 1.19 | 0.85–1.66 | |
| GG | 12 | 6.45 | 66 | 11.85 | 0.038 | 0.51 | 0.27–0.97 | 0.021 |
| Model/Genotype | COPD-BS | BBES | p | OR | CI 95% | * p | ||
|---|---|---|---|---|---|---|---|---|
| n = 186 | % | n = 557 | % | |||||
| Codominant | ||||||||
| AA | 88 | 47.32 | 258 | 46.32 | 0.25 | 1 (ref.) | 0.36 | |
| AG | 86 | 46.23 | 233 | 41.83 | 1.08 | 0.76–1.52 | ||
| GG | 12 | 6.45 | 66 | 11.85 | 0.53 | 0.27–1.03 | ||
| Recessive | ||||||||
| GG AA + AG | 12 | 6.45 | 66 | 11.85 | 0.038 | 0.51 | 0.27–0.97 | 0.0023 |
| 174 | 93.55 | 491 | 88.15 | 1.94 | 1.02–3.69 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Martínez, A.; Pérez-Rubio, G.; Ramírez-Venegas, A.; Ramírez-Díaz, M.E.; Cruz-Vicente, F.; Martínez-Gómez, M.d.L.; Ramos-Martínez, E.; Abarca-Rojano, E.; Falfán-Valencia, R. Participation of HHIP Gene Variants in COPD Susceptibility, Lung Function, and Serum and Sputum Protein Levels in Women Exposed to Biomass-Burning Smoke. Diagnostics 2020, 10, 734. https://doi.org/10.3390/diagnostics10100734
Ortega-Martínez A, Pérez-Rubio G, Ramírez-Venegas A, Ramírez-Díaz ME, Cruz-Vicente F, Martínez-Gómez MdL, Ramos-Martínez E, Abarca-Rojano E, Falfán-Valencia R. Participation of HHIP Gene Variants in COPD Susceptibility, Lung Function, and Serum and Sputum Protein Levels in Women Exposed to Biomass-Burning Smoke. Diagnostics. 2020; 10(10):734. https://doi.org/10.3390/diagnostics10100734
Chicago/Turabian StyleOrtega-Martínez, Alejandro, Gloria Pérez-Rubio, Alejandra Ramírez-Venegas, María Elena Ramírez-Díaz, Filiberto Cruz-Vicente, María de Lourdes Martínez-Gómez, Espiridión Ramos-Martínez, Edgar Abarca-Rojano, and Ramcés Falfán-Valencia. 2020. "Participation of HHIP Gene Variants in COPD Susceptibility, Lung Function, and Serum and Sputum Protein Levels in Women Exposed to Biomass-Burning Smoke" Diagnostics 10, no. 10: 734. https://doi.org/10.3390/diagnostics10100734
APA StyleOrtega-Martínez, A., Pérez-Rubio, G., Ramírez-Venegas, A., Ramírez-Díaz, M. E., Cruz-Vicente, F., Martínez-Gómez, M. d. L., Ramos-Martínez, E., Abarca-Rojano, E., & Falfán-Valencia, R. (2020). Participation of HHIP Gene Variants in COPD Susceptibility, Lung Function, and Serum and Sputum Protein Levels in Women Exposed to Biomass-Burning Smoke. Diagnostics, 10(10), 734. https://doi.org/10.3390/diagnostics10100734