Application of Artificial Intelligence in Early Diagnosis of Spontaneous Preterm Labor and Birth
Abstract
1. Introduction
1.1. Preterm Birth
1.2. Artificial Intelligence
1.3. Aims of Study
1.4. Methods of Study
2. Application of Machine Learning in Early Diagnosis of Spontaneous Preterm Labor and Birth
2.1. Duke University Medical Center Study
2.2. Korea University Anam Hospital Study
2.3. U.S. Center for Disease Control Study
2.4. Ljubljana University Medical Center Study
3. Application of Deep Learning in Early Diagnosis of Spontaneous Preterm Labor and Birth
4. Summary of Study
5. Current Limitations and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Magro Malosso, E.R.; Saccone, G.; Simonetti, B.; Squillante, M.; Berghella, V. US trends in abortion and preterm birth. J. Matern. Fetal Neonatal Med. 2018, 31, 2463–2467. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Oza, S.; Hogan, D.; Chu, Y.; Perin, J.; Zhu, J.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, regional, and national causes of under-5 mortality in 2000-15: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016, 388, 3027–3035. [Google Scholar] [CrossRef]
- World Health Organization. News: Preterm Birth. Available online: http://www.who.int/news-room/fact-sheets/detail/preterm-birth (accessed on 1 September 2020).
- Harrison, M.S.; Goldenberg, R.L. Global burden of prematurity. Semin. Fetal Neonatal Med. 2016, 21, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Feinberg, R.F.; Kliman, H.J.; Lockwood, C.J. Is oncofetal fibronectin a trophoblast glue for human implantation? Am. J. Pathol. 1991, 138, 537–543. [Google Scholar]
- Kim, Y.J.; Lee, B.E.; Park, H.S.; Kang, J.G.; Kim, J.O.; Ha, E.H. Risk factors for preterm birth in Korea: A multicenter prospective study. Gynecol. Obstet. Investig. 2005, 60, 206–212. [Google Scholar] [CrossRef]
- Conde-Agudelo, A.; Papageorghiou, A.T.; Kennedy, S.H.; Villar, J. Novel biomarkers for the prediction of the spontaneous preterm birth phenotype: A systematic review and meta-analysis. BJOG 2011, 118, 1042–1054. [Google Scholar] [CrossRef]
- Di Renzo, G.C.; Giardina, I.; Rosati, A.; Clerici, G.; Torricelli, M.; Petraglia, F.; the Italian Preterm Network Study Group. Maternal risk factors for preterm birth: A country-based population analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 159, 342–346. [Google Scholar] [CrossRef]
- Boghossian, N.S.; Yeung, E.; Albert, P.S.; Mendola, P.; Laughon, S.K.; Hinkle, S.N.; Zhang, C. Changes in diabetes status between pregnancies and impact on subsequent newborn outcomes. Am. J. Obstet. Gynecol. 2014, 210. [Google Scholar] [CrossRef]
- Moroz, L.A.; Simhan, H.N. Rate of sonographic cervical shortening and biologic pathways of spontaneous preterm birth. Am. J. Obstet. Gynecol. 2014, 210, 555.e1–555.e5. [Google Scholar] [CrossRef]
- Premkumar, A.; Henry, D.E.; Moghadassi, M.; Nakagawa, S.; Norton, M.E. The interaction between maternal race/ethnicity and chronic hypertension on preterm birth. Am. J. Obstet. Gynecol. 2016, 215, 787.e1–787.e8. [Google Scholar] [CrossRef] [PubMed]
- Cavoretto, P.; Candiani, M.; Giorgione, V.; Inversetti, A.; Abu-Saba, M.M.; Tiberio, F.; Sigismondi, C.; Farina, A. Risk of spontaneous preterm birth in singleton pregnancies conceived after IVF/ICSI treatment: meta-analysis of cohort studies. Ultrasound. Obstet. Gynecol. 2018, 51, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Micheline, K. Data Mining: Concepts and Techniques, 2nd ed.; Elsevier: San Francisco, CA, USA, 2006. [Google Scholar]
- Abiodun, O.I.; Jantan, A.; Omolara, A.E.; Dada, K.V.; Mohamed, N.A.; Arshad, H. State-of-the-art in artificial neural network applications: A survey. Heliyon 2018, 23, e00938. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Mitnitski, A.; Cox, J.; Rockwood, K. Comparison of machine learning techniques with classical statistical models in predicting health outcomes. Stud. Health Technol. Inform. 2004, 7, 736–740. [Google Scholar]
- Goodwin, L.K.; Maher, S. Data mining for preterm birth prediction. In Proceedings of the 2000 ACM Symposium on Applied Computing, Como, Italy, 19–21 March 2000; pp. 46–51. [Google Scholar]
- Goodwin, L.K.; Iannacchione, M.A.; Hammond, W.E.; Crockett, P.; Maher, S.; Schlitz, K. Data mining methods find demographic predictors of preterm birth. Nurs. Res. 2001, 50, 340–345. [Google Scholar] [CrossRef]
- Goodwin, L.K.; Iannacchione, M.A. Data mining methods for improving birth outcomes prediction. Outcomes Manag. 2002, 6, 80–85. [Google Scholar]
- Lee, K.S.; Ahn, K.H. Artificial neural network analysis of spontaneous preterm labor and birth and its major determinants. J. Korean Med. Sci. 2019, 34, e128. [Google Scholar] [CrossRef]
- Lee, K.S.; Song, I.S.; Kim, E.S.; Ahn, K.H. Determinants of spontaneous preterm labor and birth including gastroesophageal reflux disease and periodontitis. J. Korean Med. Sci. 2020, 35, e105. [Google Scholar] [CrossRef]
- Parker, M.G.; Ouyang, F.; Pearson, C.; Gillman, M.W.; Belfort, M.B.; Hong, X.; Wang, G.; Heffner, L.; Zuckerman, B.; Wang, X. Prepregnancy body mass index and risk of preterm birth: Association heterogeneity by preterm subgroups. BMC Pregnancy Childbirth 2014, 14, 153. [Google Scholar] [CrossRef]
- Heude, B.; Thiébaugeorges, O.; Goua, V.; Forhan, A.; Kaminski, M.; Foliguet, B.; Schweitzer, M.; Magnin, G.; Charles, M.-A.; EDEN Mother-Child Cohort Study group. Pre-pregnancy body mass index and weight gain during pregnancy: Relations with gestational diabetes and hypertension, and birth outcomes. Matern. Child Health J. 2012, 16, 355–363. [Google Scholar] [CrossRef]
- Shin, D.; Song, W.O. Prepregnancy body mass index is an independent risk factor for gestational hypertension, gestational diabetes, preterm labor, and small- and large-for-gestational-age infants. J. Matern. Fetal Neonatal Med. 2015, 28, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Sibai, B.M.; Caritis, S.N.; Hauth, J.C.; MacPherson, C.; Van Dorsten, J.P.; Klebanoff, M.; Landon, M.; Paul, R.H.; Meis, P.J.; Miodovnik, M.; et al. Preterm delivery in women with pregestational diabetes mellitus or chronic hypertension relative to women with uncomplicated pregnancies. The National institute of Child health and Human Development Maternal- Fetal Medicine Units Network. Am. J. Obstet. Gynecol. 2000, 183, 1520–1524. [Google Scholar] [CrossRef] [PubMed]
- Hedderson, M.M.; Ferrara, A.; Sacks, D.A. Gestational diabetes mellitus and lesser degrees of pregnancy hyperglycemia: Association with increased risk of spontaneous preterm birth. Obstet. Gynecol. 2003, 102, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Villar, J.; Sun, W.; Merialdi, M.; Abdel-Aleem, H.; Mathai, M.; Ali, M.; Yu, K.F.; Zavaleta, N.; Purwar, M.; et al. Blood pressure dynamics during pregnancy and spontaneous preterm birth. Am. J. Obstet. Gynecol. 2007, 197, 162.e1–162.e6. [Google Scholar] [CrossRef]
- O’Hara, S.; Zelesco, M.; Sun, Z. Cervical length for predicting preterm birth and a comparison of ultrasonic measurement techniques. Australas. J. Ultrasound Med. 2013, 16, 124–134. [Google Scholar] [CrossRef]
- Society for Maternal-Fetal Medicine; McIntosh, J.; Feltovich, H.; Berghella, V.; Manuck, T. The role of routine cervical length screening in selected high- and low-risk women for preterm birth prevention. Am. J. Obstet. Gynecol. 2016, 215, B2–B7. [Google Scholar] [CrossRef]
- Berghella, V.; Pereira, L.; Gariepy, A.; Simonazzi, G. Prior cone biopsy: Prediction of preterm birth by cervical ultrasound. Am. J. Obstet. Gynecol. 2004, 191, 1393–1397. [Google Scholar] [CrossRef]
- Bevis, K.S.; Biggio, J.R. Cervical conization and the risk of preterm delivery. Am. J. Obstet. Gynecol. 2011, 205, 19–27. [Google Scholar] [CrossRef]
- Pinborg, A.; Ortoft, G.; Loft, A.; Rasmussen, S.C.; Ingerslev, H.J. Cervical conization doubles the risk of preterm and very preterm birth in assisted reproductive technology twin pregnancies. Hum. Reprod. 2015, 30, 197–204. [Google Scholar] [CrossRef]
- Cho, S.H.; Park, K.H.; Jung, E.Y.; Joo, J.K.; Jang, J.A.; Yoo, H.N. Maternal characteristics, short mid-trimester cervical length, and preterm delivery. J. Korean Med. Sci. 2017, 32, 488–494. [Google Scholar] [CrossRef]
- Eke, P.I.; Dye, B.A.; Wei, L.; Slade, G.D.; Thornton-Evans, G.O.; Borgnakke, W.S.; Taylor, G.W.; Page, R.C.; Beck, J.D.; Genco, R.J. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J. Periodontol. 2015, 86, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Puertas, A.; Magan-Fernandez, A.; Blanc, V.; Revelles, L.; O’Valle, F.; Pozo, E.; León, R.; Mesa, F. Association of periodontitis with preterm birth and low birth weight: A comprehensive review. J. Matern. Fetal Neonatal Med. 2018, 31, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Vakil, N.; van Zanten, S.V.; Kahrilas, P.; Dent, J.; Jones, R.; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: A global evidence-based consensus. Am. J. Gastroenterol. 2006, 101, 1900–1920. [Google Scholar] [CrossRef] [PubMed]
- Patrick, L. Gastroesophageal reflux disease (GERD): A review of conventional and alternative treatments. Altern. Med. Rev. 2011, 16, 116–133. [Google Scholar]
- Vinesh, E.; Masthan, K.; Kumar, M.S.; Jeyapriya, S.M.; Babu, A.; Thinakaran, M. A clinicopathologic study of oral changes in gastroesophageal reflux disease, gastritis, and ulcerative colitis. J. Contemp. Dent. Pract. 2016, 17, 943–947. [Google Scholar]
- Deppe, H.; Mücke, T.; Wagenpfeil, S.; Kesting, M.; Rozej, A.; Bajbouj, M.; Sculean, A. Erosive esophageal reflux vs. non erosive esophageal reflux: Oral findings in 71 patients. BMC Oral Health 2015, 15, 84. [Google Scholar] [CrossRef]
- Ali, R.A.; Egan, L.J. Gastroesophageal reflux disease in pregnancy. Best Pract. Res. Clin. Gastroenterol. 2007, 21, 793–806. [Google Scholar] [CrossRef]
- Koivu, A.; Sairanen, M. Predicting risk of stillbirth and preterm pregnancies with machine learning. Health Inf. Sci. Syst. 2020, 8, 14. [Google Scholar] [CrossRef]
- Fergus, P.; Cheung, P.; Hussain, A.; Al-Jumeily, D.; Dobbins, C.; Iram, S. Prediction of preterm deliveries from EHG signals using machine learning. PLoS ONE 2013, 8, e77154. [Google Scholar] [CrossRef]
- Sadi-Ahmed, N.; Kacha, B.; Taleb, H.; Kedir-Talha, M. Relevant features selection for automatic prediction of preterm deliveries from pregnancy ElectroHysterograhic (EHG) records. J. Med. Syst. 2017, 41, 204. [Google Scholar] [CrossRef]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. ImageNet classification with deep convolutional neural networks. In Proceedings of the 25th International Conference on Neural Information Processing Systems, Siem Reap, Cambodia, 13–16 December 2018; pp. 1097–1105. [Google Scholar]
- Lee, K.S.; Park, K.W. Social determinants of association among cerebrovascular disease, hearing loss and cognitive impairment in a middle-aged or old population: Recurrent-neural-network analysis of the Korean Longitudinal Study of Aging (2014–2016). Geriatr. Gerontol. Int. 2019, 19, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Osmundson, S.; Velez Edwards, D.R.; Jackson, G.P.; Malin, B.A.; Chen, Y. Deep learning predicts extreme preterm birth from electronic health records. J. Biomed. Inform. 2019, 100, 103334. [Google Scholar] [CrossRef] [PubMed]
- Grigorescu, I.; Cordero-Grande, L.; Edwards, A.D.; Hajnal, J.; Modat, M.; Deprez, M. Interpretable convolutional neural networks for preterm birth classification. arXiv 2019, arXiv:1910.00071. [Google Scholar]
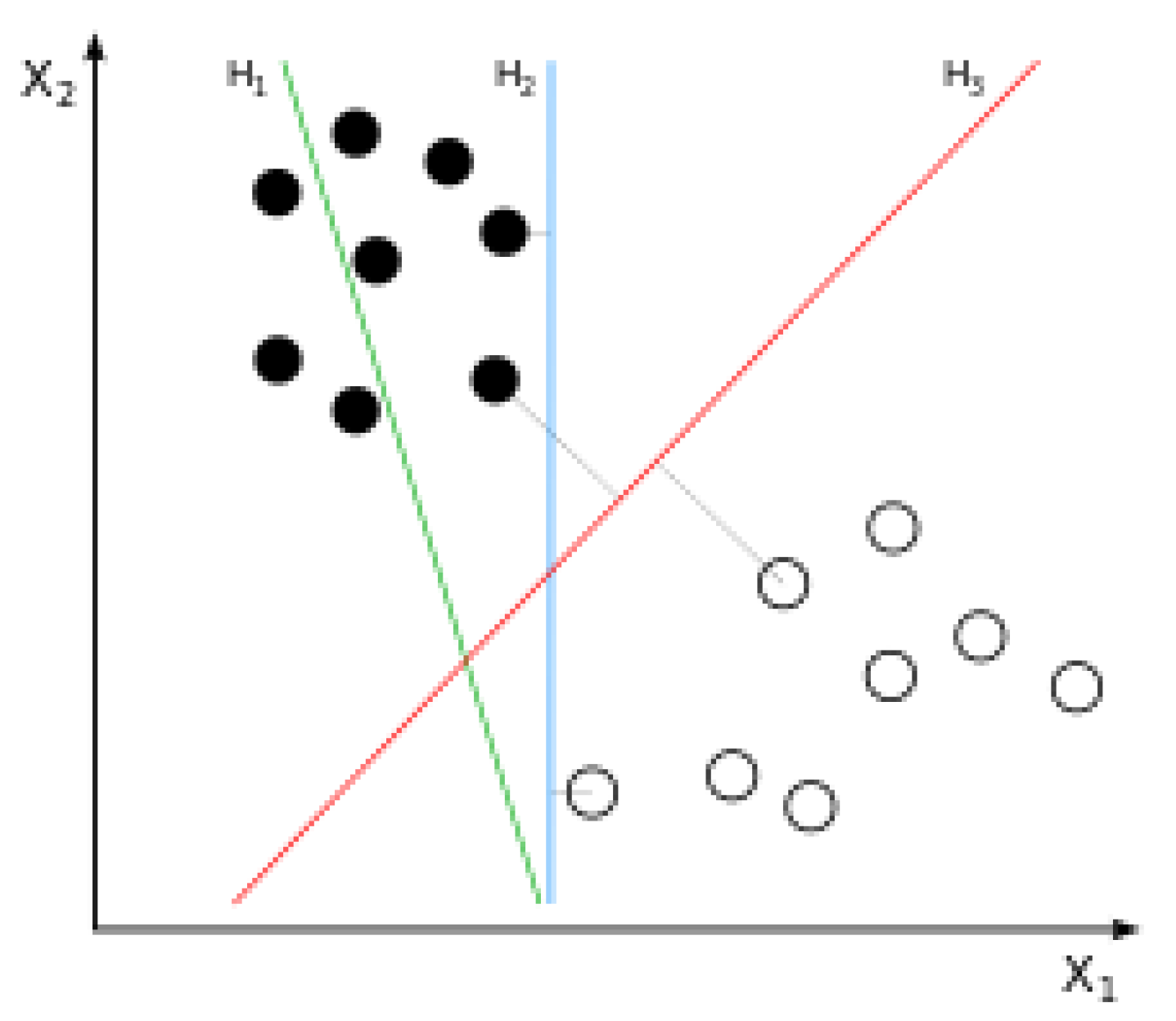
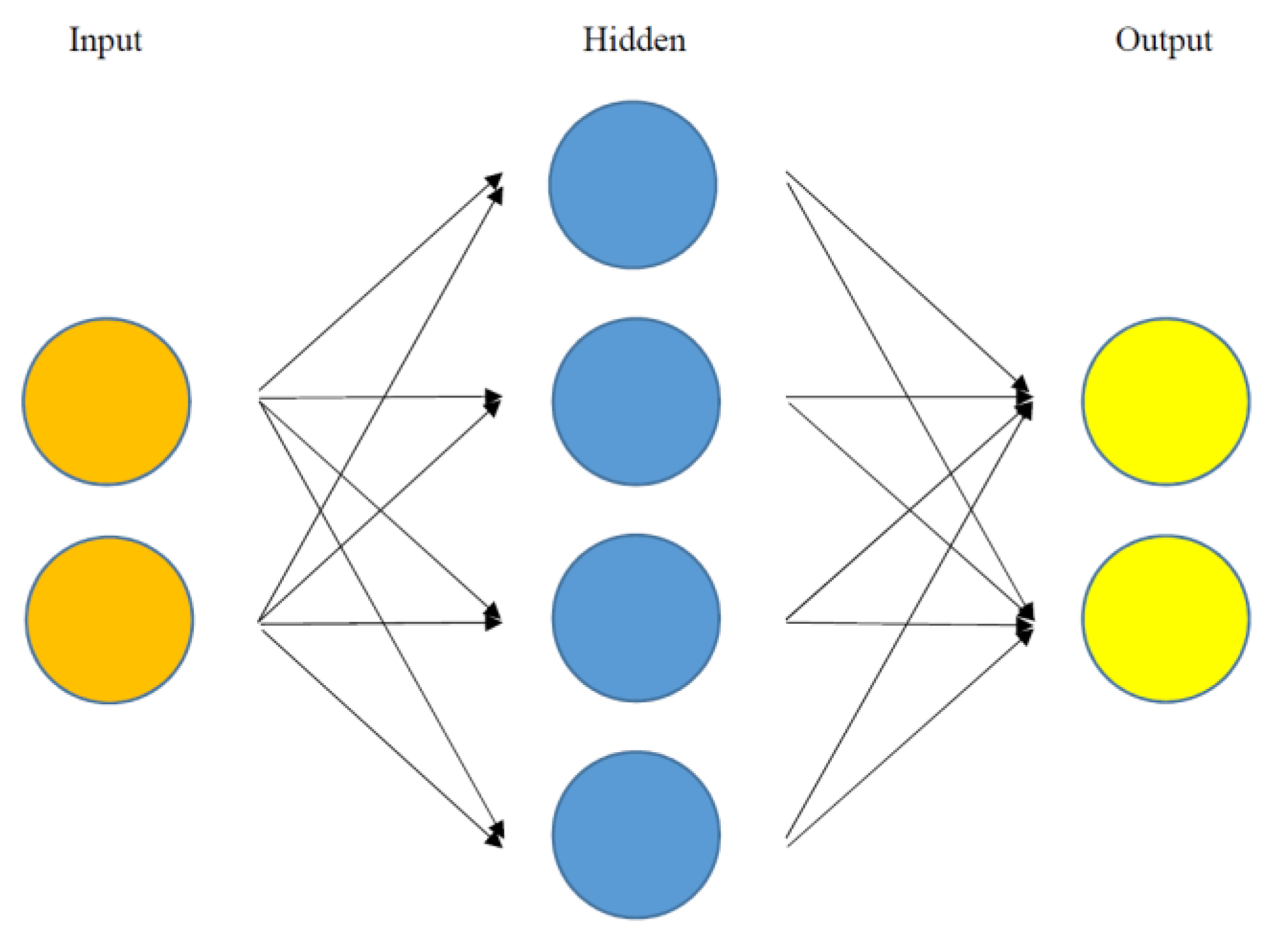
| Publication | Method | Sample Size | Data Type | Performance | Important Predictors |
|---|---|---|---|---|---|
| [18] | ANN * DT LR | 19,970 | Numeric | AUC 0.65–0.68 | Age, race, region, religion, education, insurance, marriage |
| [20] | ANN * DT LR * NB RF * SVM * | 596 | Numeric | Accuracy 0.89–0.92 AUC 0.62–0.64 | Body mass index, hypertension, diabetes mellitus, prior cone biopsy, parity, cervical length, age, prior preterm birth, myomas & adenomyosis ** |
| [21] | ANN DT LR * NB RF * SVM | 731 | Numeric | Accuracy 0.79–0.87 AUC 0.54–0.76 | Delivery and pregestational body mass index, age, parity, predelivery systolic and diastolic blood pressure, twin, below high school graduation, infant sex, prior preterm birth, progesterone medication history, upper gastrointestinal tract symptom, gastroesophageal reflux disease, Helicobacter pylori, urban region, calcium-channel-blocker medication history, gestational diabetes mellitus ** |
| [41] | ANN * DT LR | 16,340,661 | Numeric | Sensitivity 0.22-0.24 AUC 0.62–0.64 | Demographic (age, race, marital status, education, previous terminations, Special Nutritional Program for Women, Infants and Children, pre-pregnancy smoking, body mass index, weight). Obstetric (parity, pre-pregnancy diabetes, gestational diabetes, pre-pregnancy hypertension, gestational hypertension, hypertension eclampsia, previous preterm birth, infertility treatment, infertility medication, Assisted Reproductive Technology, previous cesarean section, Gonorrhea, Syphilis, Chlamydia, Hepatitis C). |
| [42] | DT LR SVM * | 300 | Electrohysterogram | Specificity 0.86-1.00 AUC 0.60–0.61 | Uterine electrical signals (root mean squares, peak frequency, median frequency, sample entropy) |
| [46] | LR RNN * SVM | 25,689 | Text (5,602,792 Medical Concepts) | Sensitivity 0.66-0.97 AUC 0.73–0.83 | Twin pregnancy, short cervical length, hypertensive disorder, systemic lupus erythematosus, hydroxychloroquine sulfate |
| [47] | CNN | 157 | Magnetic Resonance Imaging | Accuracy 0.94 | Increased cerebrospinal fluid and reduced cortical folding due to impaired brain growth |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-S.; Ahn, K.H. Application of Artificial Intelligence in Early Diagnosis of Spontaneous Preterm Labor and Birth. Diagnostics 2020, 10, 733. https://doi.org/10.3390/diagnostics10090733
Lee K-S, Ahn KH. Application of Artificial Intelligence in Early Diagnosis of Spontaneous Preterm Labor and Birth. Diagnostics. 2020; 10(9):733. https://doi.org/10.3390/diagnostics10090733
Chicago/Turabian StyleLee, Kwang-Sig, and Ki Hoon Ahn. 2020. "Application of Artificial Intelligence in Early Diagnosis of Spontaneous Preterm Labor and Birth" Diagnostics 10, no. 9: 733. https://doi.org/10.3390/diagnostics10090733
APA StyleLee, K.-S., & Ahn, K. H. (2020). Application of Artificial Intelligence in Early Diagnosis of Spontaneous Preterm Labor and Birth. Diagnostics, 10(9), 733. https://doi.org/10.3390/diagnostics10090733






