Survivability of Anhydrobiotic Cyanobacteria in Salty Ice: Implications for the Habitability of Icy Worlds
Abstract
1. Introduction
2. Materials and Methods
2.1. Cyanobacterial Strains and Culture Conditions
2.2. Salty-Ice Environments
2.3. Exposure of Cyanobacteria to Salty-Icy Environments
2.4. Evaluation of the Effects of Salt Solutions and Ddh2o on Cell Viability
2.5. Exposure of Air-Dried Cyanobacteria to 233 K and 193 K
2.6. Cell Viability
3. Results
3.1. Salty-Ice Environments
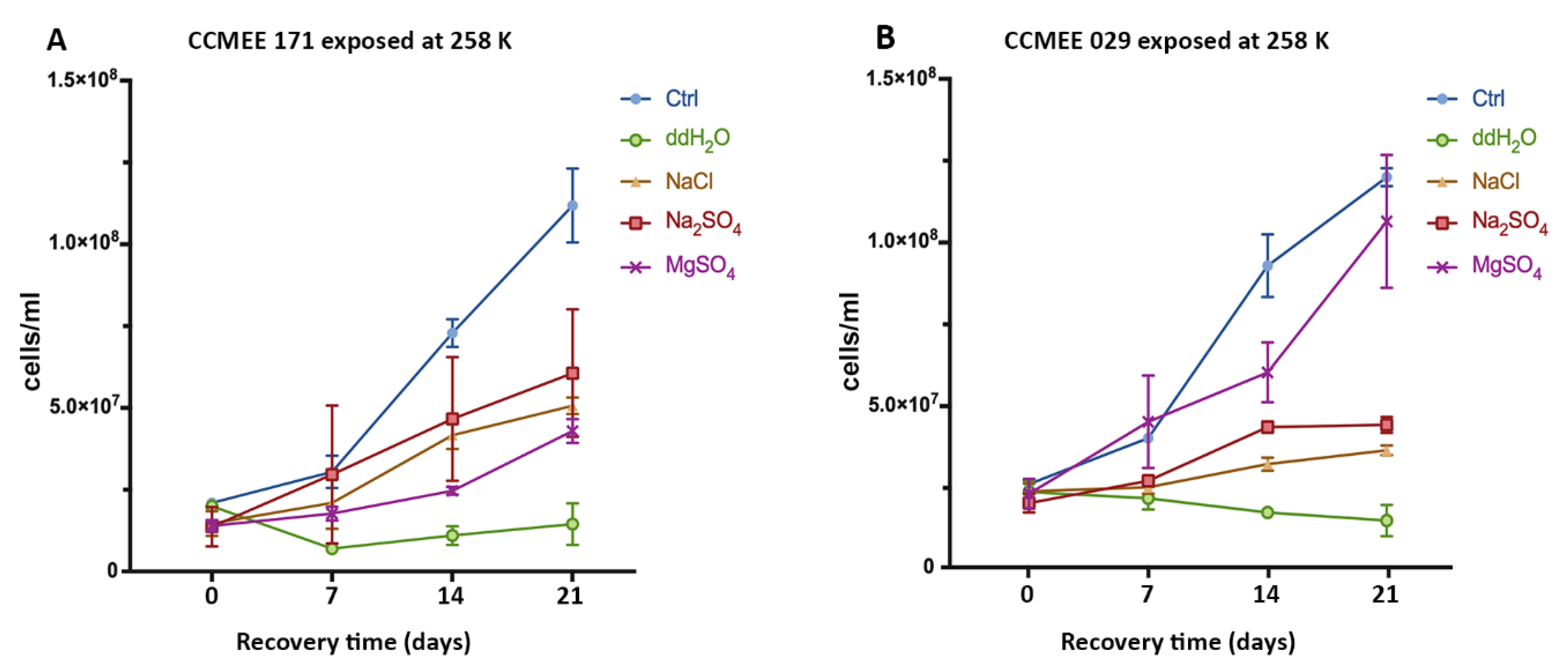
3.2. Cold And Hot Desert Strains Survived in Salty-Icy Conditions at 258 K
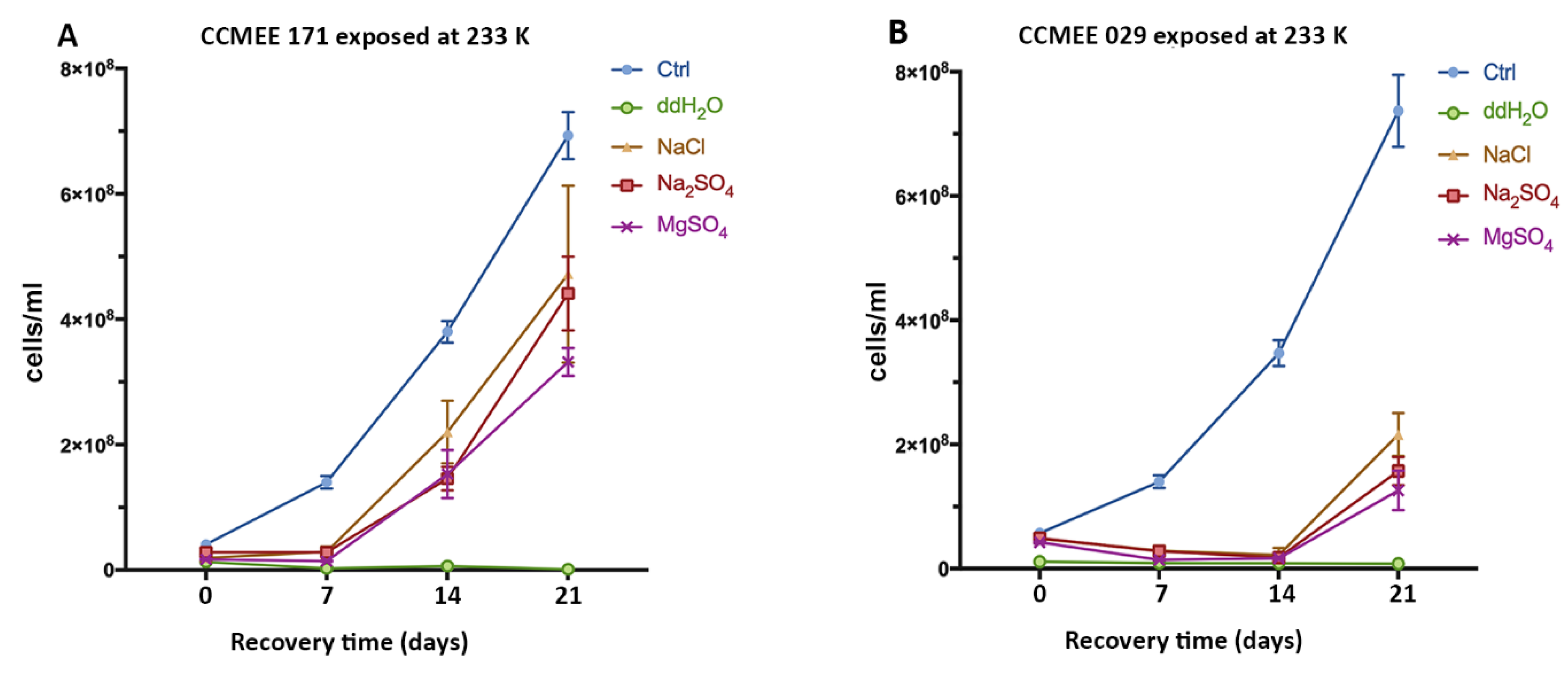
3.3. Enhanced Surival of the Cold Desert Strain in Salty-Icy Conditions at 233 K
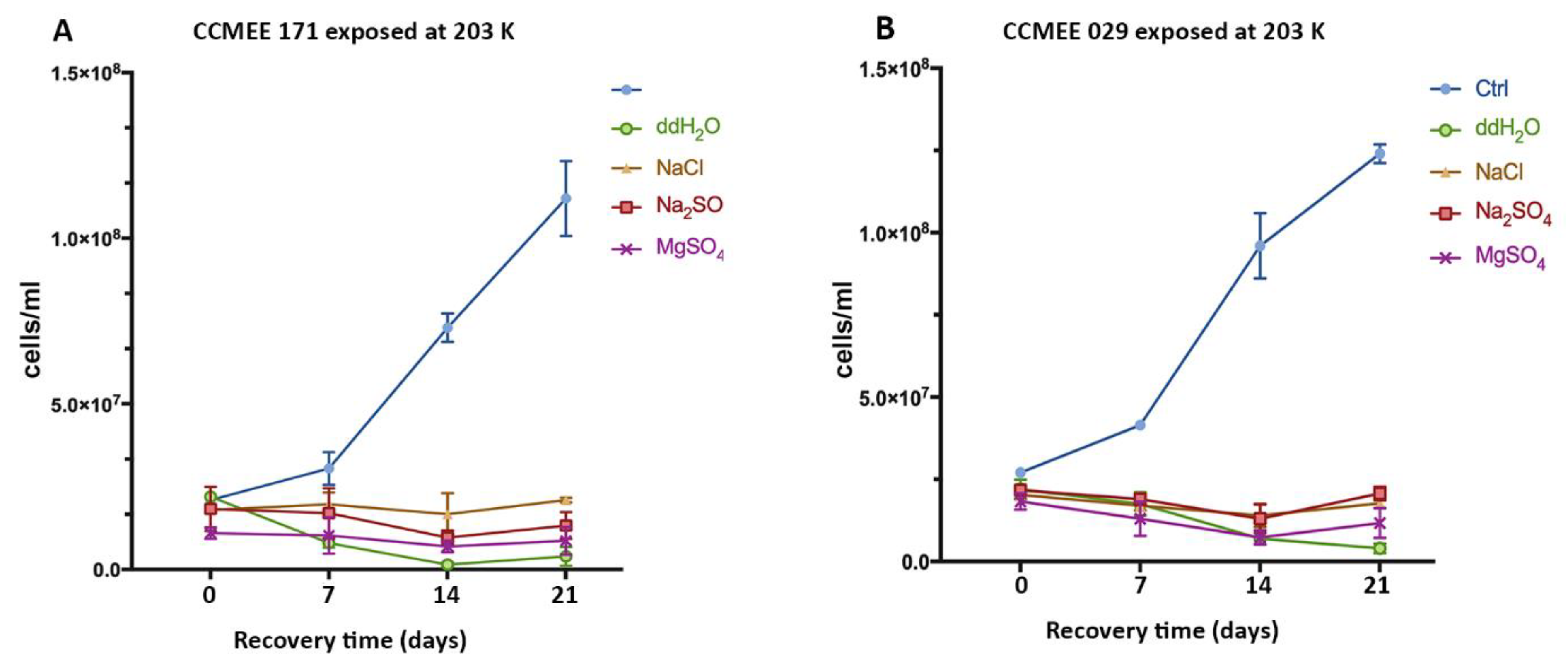
3.4. Cold and Hot Desert Strains Died in Salty-Icy Conditions at 203 K
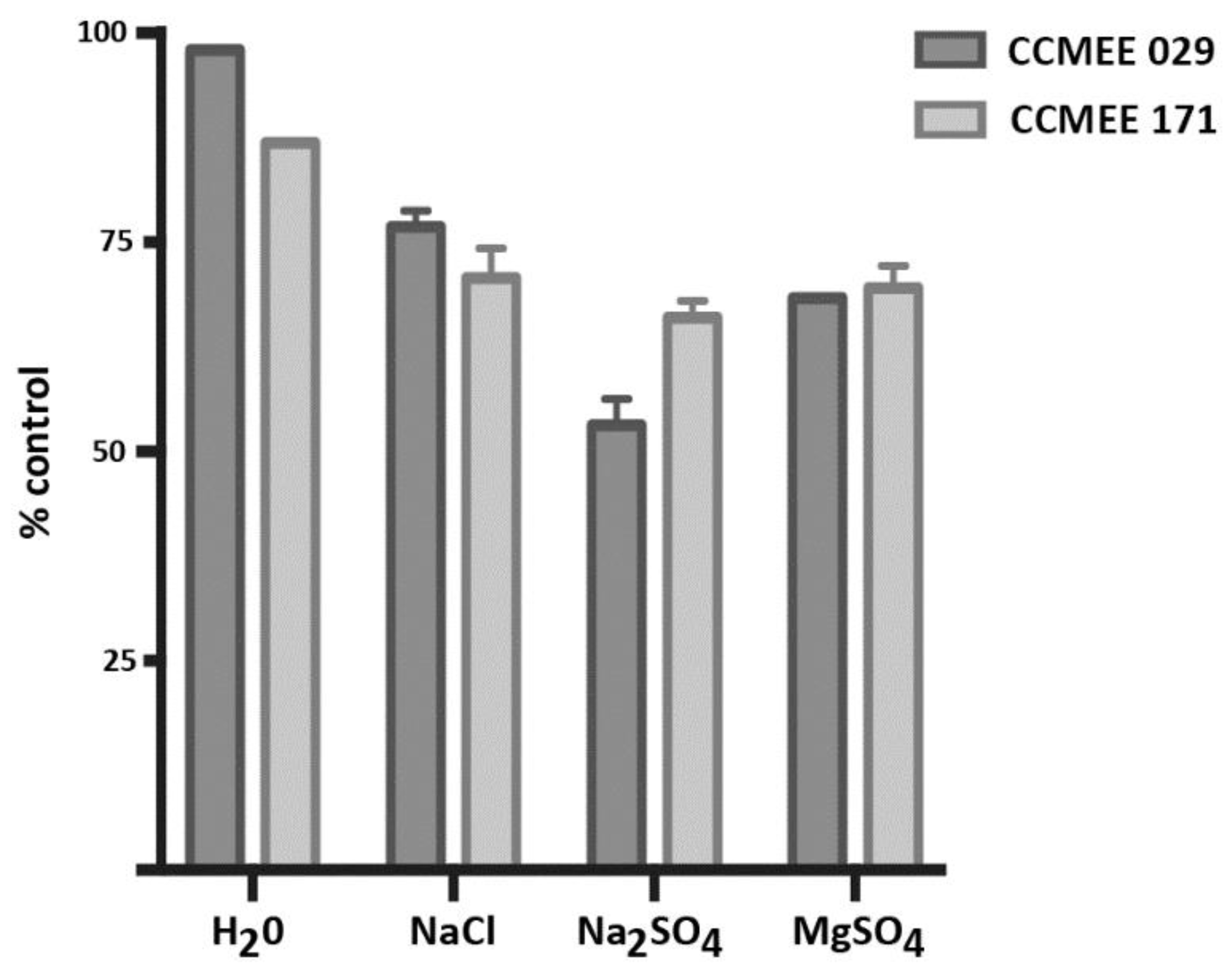
3.5. Incubation in ddH2O and Salt Solutions Reduced Cell Viability
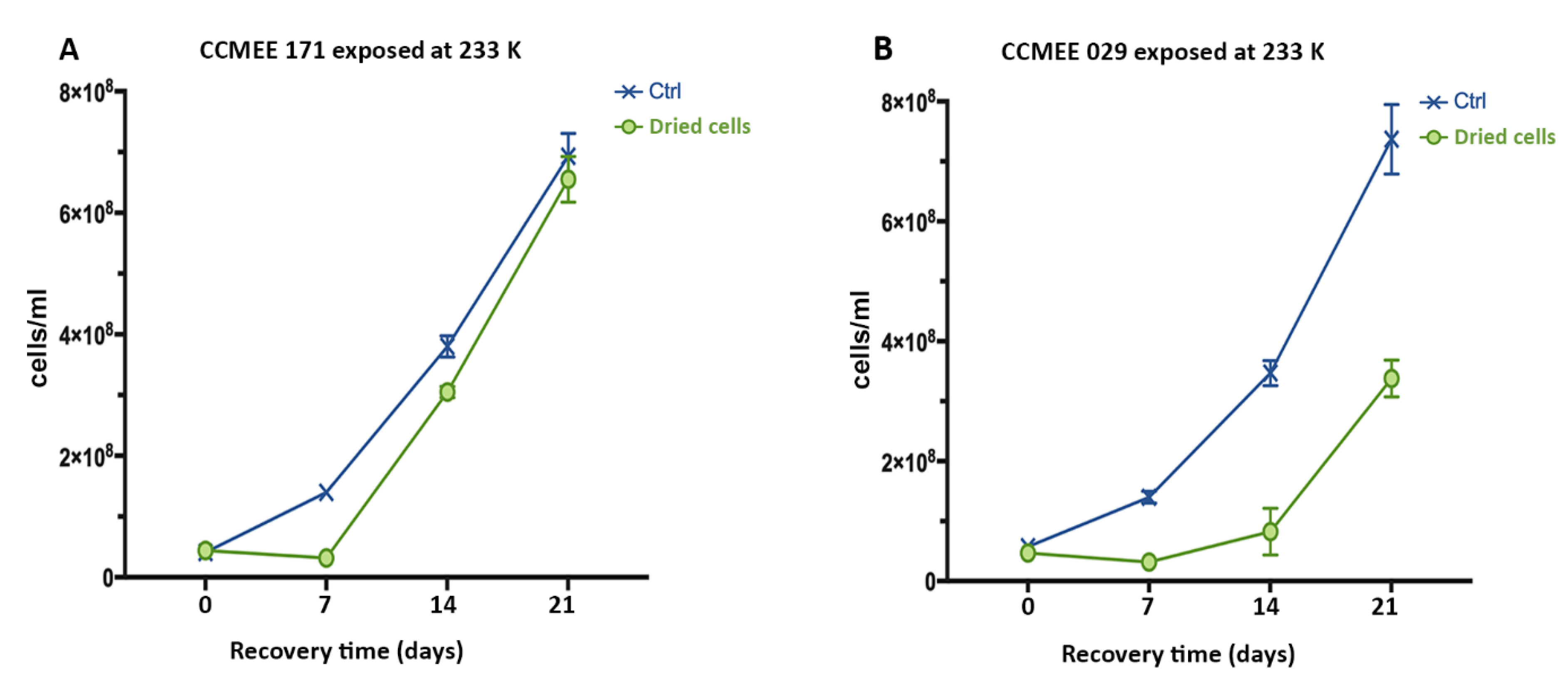
3.6. Air-dried Cells of Hot and Cold Desert Strains Survived Sub-Freezing Temperatures
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Cockell, C.S.; Bush, T.; Bryce, C.; Direito, S.; Fox-Powell, M.; Harrison, J.P.; Lammer, H.; Landenmark, H.; Martin-Torres, J.; Nicholson, N.; et al. Habitability: A review. Astrobiology 2016, 16, 89–117. [Google Scholar] [CrossRef] [PubMed]
- Chyba, C.F.; Phillips, C.B. Europa as an abode of life. Orig. Life Evol. Biosph. 2002, 32, 47–68. [Google Scholar] [CrossRef] [PubMed]
- Waite, J.H.; Glein, C.R.; Perryman, R.S.; Teolis, B.D.; Magee, B.A.; Miller, G.; Grimes, J.; Perry, M.E.; Miller, K.E.; Bouquet, A.; et al. Cassini finds molecular hydrogen in the Enceladus plume: Evidence for hydrothermal processes. Science 2017, 356, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Lowell, R.P.; DuBose, M. Hydrothermal systems on Europa. Geophys. Res. Lett. 2005, 32, L05202. [Google Scholar] [CrossRef]
- Deamer, D.; Damer, B. Can life begin on Enceladus? A perspective from hydrothermal chemistry. Astrobiology 2017, 17, 834–839. [Google Scholar] [CrossRef]
- Roth, L.; Saur, J.; Retherford, K.D.; Strobel, D.F.; Feldman, P.D.; McGrath, M.A.; Nimmo, F. Transient water vapor at Europa’s south pole. Science 2014, 343, 171–174. [Google Scholar] [CrossRef]
- Pappalardo, R.T.; Head, J.W.; Greeley, R.; Sullivan, R.J.; Pilcher, C.; Schubert, G.; Moore, W.B.; Carr, M.H.; Moore, J.M.; Belton, M.J.; et al. Geological evidence for solid-state convection in Europa’s ice shell. Nature 1998, 391, 365–368. [Google Scholar] [CrossRef]
- Laughlin, G.; Adams, F.C. The frozen Earth: Binary scattering events and the fate of the solar system. Icarus 2000, 145, 614–627. [Google Scholar] [CrossRef]
- Abbot, D.S.; Switzer, E.R. The Steppenwolf: A proposal for a habitable planet in interstellar space. Astrophys. J. Lett. 2011, 735, L27. [Google Scholar] [CrossRef]
- Lingam, M.; Loeb, A. Subsurface exolife. Int. J. Astrobiol. 2019, 18, 112–141. [Google Scholar] [CrossRef]
- Garcia-Lopez, E.; Cid, C. Glaciers and ice sheets as analog environments of potentially habitable icy worlds. Front. Microbiol. 2017, 8, 1407. [Google Scholar] [CrossRef] [PubMed]
- Mader, H.M.; Pettitt, M.E.; Wadham, J.L.; Wolff, E.W.; Parkes, R.J. Subsurface ice as a microbial habitat. Geology 2006, 34, 169–172. [Google Scholar] [CrossRef]
- Price, P.B. A habitat for psychrophiles in deep Antarctic ice. Proc. Natl. Acad. Sci. USA 2000, 97, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- Tung, H.C.; Price, P.B.; Bramall, N.E.; Vrdoljak, G. Microorganisms metabolizing on clay grains in 3-km-deep Greenland basal ice. Astrobiology 2006, 6, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Rohde, R.A.; Price, P.B. Diffusion-controlled metabolism for long-term survival of single isolated microorganisms trapped within ice crystals. Proc. Natl. Acad. Sci. USA 2007, 104, 16592–16597. [Google Scholar] [CrossRef]
- Boetius, A.; Anesio, A.M.; Deming, J.W.; Mikucki, J.A.; Rapp, J.Z. Microbial ecology of the cryosphere: Sea ice and glacial habitats. Nat. Rev. Microbiol. 2015, 13, 677–690. [Google Scholar] [CrossRef]
- Clarke, A.; Morris, G.J.; Fonseca, F.; Murray, B.J.; Acton, E.; Price, H.C. A low temperature limit for life on Earth. PLoS ONE 2013, 8, e66207. [Google Scholar] [CrossRef]
- Price, P.B.; Sowers, T. Temperature dependence of metabolic rates for microbial growth, maintenance, and survival. Proc. Natl. Acad. Sci. USA 2004, 101, 4631–4636. [Google Scholar] [CrossRef]
- McCarthy, C.; Cooper, R.F.; Kirby, S.H.; Rieck, K.D.; Stern, L.A. Solidification and microstructures of binary ice-I/hydrate eutectic aggregates. Am. Mineral. 2007, 92, 1550–1560. [Google Scholar] [CrossRef]
- Barletta, R.E.; Priscu, J.C.; Mader, H.M.; Jones, W.L.; Roe, C.H. Chemical analysis of ice vein microenvironments: II. Analysis of glacial samples from Greenland and Antarctica. J. Glaciol. 2012, 58, 1109–1118. [Google Scholar] [CrossRef]
- Dalton, J.B.; Shirley, J.H.; Prockter, L.M. Surface geology of Europa: A window to subsurface composition and habitability. Geophys. Res. Abstr. 2010, 12, 2270–2271. [Google Scholar]
- Hand, K.P.; Carlson, R.W. Europa’s surface color suggests an ocean rich with sodium chloride. Geophys. Res. Lett. 2015, 42, 3174–3178. [Google Scholar] [CrossRef]
- Dickson, J.L.; Head, J.W.; Levy, J.S.; Marchant, D.R. Don Juan Pond, Antarctica: Near-surface CaCl2—Brine feeding Earth’s most saline lake and implications for Mars. Sci. Rep. 2013, 3, 1166. [Google Scholar] [CrossRef] [PubMed]
- Pontefract, A.; Zhu, T.F.; Walker, V.K.; Hepburn, H.; Lui, C.; Zuber, M.T.; Ruvkun, G.; Carr, C.E. Microbial diversity in a hypersaline sulfate lake: A terrestrial analog of ancient Mars. Front. Microbiol. 2017, 8, 1819. [Google Scholar] [CrossRef]
- Sharma, A.; Scott, J.H.; Cody, G.D.; Fogel, M.L.; Hazen, R.M.; Hemley, R.J.; Huntress, W.T. Microbial activity at gigapascal pressures. Science 2002, 295, 1514–1516. [Google Scholar] [CrossRef]
- Billi, D. Anhydrobiotic Rock-Inhabiting Cyanobacteria: Potential for astrobiology and biotechnology. In Adaptation of Microbial Life to Environmental Extremes: Novel Research Results and Application; Stan-Lotter, H., Fendrihan, F., Eds.; Springer: Vienna, Austria, 2012; pp. 119–132. [Google Scholar]
- Billi, D.; Baqué, M.; Verseux, C.; Rothschild, L.J.; de Vera, J.-P. Desert cyanobacteria—Potential for space and Earth applications. In Adaption of Microbial Life to Environmental Extremes, 2nd ed.; Stan-Lotter, H., Fendrihan, F., Eds.; Springer International Publishing: New York, NY, USA, 2017; pp. 133–146. [Google Scholar]
- Goordial, J.; Davila, A.; Lacelle, D.; Pollard, W.; Marinova, M.M.; Greer, C.W.; DiRuggiero, J.; McKay, C.P.; Whyte, L.G. Nearing the cold-arid limits of microbial life in permafrost of an upper dry valley, Antarctica. ISME J. 2016, 10, 1613–1624. [Google Scholar] [CrossRef]
- Friedmann, E.I.; Kappen, L.; Meyer, M.A.; Nienow, J.A. Long-term productivity in the cryptoendolithic communities of the Ross desert, Antarctica. Microb. Ecol. 1992, 25, 51–69. [Google Scholar] [CrossRef]
- Baqué, M.; de Vera, J.P.; Rettberg, P.; Billi, D. The BOSS and BIOMEX space experiments on the EXPOSE-R2 mission: Endurance of the desert cyanobacterium Chroococcidiopsis under simulated space vacuum, Martian atmosphere, UVC radiation and temperature extremes. Acta Astronaut. 2013, 91, 180–186. [Google Scholar] [CrossRef]
- Ocampo-Friedmann, R.; Meyer, M.A.; Chen, M.; Friedmann, E.I. Temperature response of Antarctic cryptoendolithic photosynthetic microorganisms. Polarforschung 1988, 58, 121–124. [Google Scholar]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Billi, D. Desert cyanobacteria under space and planetary simulations: A tool for searching for life beyond Earth and supporting human space exploration. Int. J. Astrobiol. 2018, 18, 483–489. [Google Scholar] [CrossRef]
- Singh, S.M.; Elster, J. Cyanobacteria in Antarctic Lake Environments. In Algae and Cyanobacteria in Extreme Environments. Cellular Origin, Life in Extreme Habitats and Astrobiology; Seckbach, J., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 303–320. [Google Scholar]
- Chrismas, N.A.M.; Anesio, A.M.; Sánchez-Baracaldo, P. The future of genomics in polar and alpine cyanobacteria. FEMS Microbiol. Ecol. 2018, 94(4), fiy032. [Google Scholar] [CrossRef] [PubMed]
- Price, P.B. Microbial life in glacial ice and implications for a cold origin of life. FEMS Microbiol. Ecol. 2007, 59, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Rippka, R.; Waterbury, J.B.; Herdman, M.; Castenholz, R.W. The Cyanobacteria: Subsection 2 (formerly Pleurocapsales Geitler 1925, emend. Waterbury and Stanier 1978). In Bergeys Manual of Systematic Bacteriology; Boone, D.R., Castenholz, R.W., Eds.; Springer-Verlag: New York, NY, USA, 2001; pp. 1746–1770. [Google Scholar]
- Billi, D.; Potts, M. Life and death of dried prokaryotes. Res. Microbiol. 2002, 153, 7–12. [Google Scholar] [CrossRef]
- Stal, L.J.; Moezelaar, R. Fermentation in cyanobacteria. FEMS Microbiol. Rev. 1997, 21, 179–211. [Google Scholar] [CrossRef]
- Puente-Sánchez, F.; Arce-Rodríguez, A.; Oggerin, M.; García-Villadangos, M.; Moreno-Paz, M.; Blanco, Y.; Rodríguez, N.; Bird, L.; Lincoln, S.A.; Tornos, F.; et al. Viable cyanobacteria in the deep continental subsurface. PNAS USA 2018, 115, 10702–10707. [Google Scholar] [CrossRef]
- Heinz, J.; Schirmack, J.; Airo, A.; Kounaves, S.P.; Schulze-Makuch, D. Enhanced microbial survivability in subzero brines. Astrobiology 2018, 18, 1171–1180. [Google Scholar] [CrossRef]
- Heinz, J.; Waajen, A.C.; Airo, A.; Alibrandi, A.; Schirmack, J.; Schulze-Makuch, D. Bacterial growth in chloride and perchlorate brines: Halotolerances and salt stress responses of Planococcus halocryophilus. Astrobiology 2019, 19, 1377–1387. [Google Scholar] [CrossRef]
- Toner, J.D.; Catling, D.C.; Light, B. Soluble salts at the Phoenix Lander site, Mars: A reanalysis of the wet chemistry laboratory data. Geochim. Cosmochim Acta 2014, 136, 142–168. [Google Scholar] [CrossRef]
- Ewert, M.; Deming, J.W. Sea ice microorganisms: Environmental constraints and extracellular responses. Biology 2013, 2, 603–628. [Google Scholar] [CrossRef]
- Hershkovitz, N.; Oren, A.; Cohen, Y. Accumulation of trehalose and sucrose in cyanobacteria exposed to matric water stress. Appl. Environ. Microbiol. 1991, 57, 645–648. [Google Scholar] [PubMed]
- Klähn, S.; Hagemann, M. Compatible solute biosynthesis in cyanobacteria. Environ. Microbiol. 2011, 13, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.H.; Carpenter, J.F.; Crowe, L.M. The role of vitrification in anhydrobiosis. Annu. Rev. Physiol. 1998, 60, 73–103. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.F.; Prestrelski, S.J.; Arakawa, T. Separation of freezing- and drying-induced denaturation of lyophilized proteins using stress-specific stabilization. I. Enzyme activity and calorimetric studies. Arch. Biochem. Biophys. 1993, 303, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Seki, S.; Kleinhans, F.W.; Mazur, P. Intracellular ice formation in yeast cells vs. cooling rate: Predictions from modeling vs. experimental observations by differential scanning calorimetry. Cryobiology 2008, 58, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.H.; Childers, D.; Fox-Powell, M.; Nicholson, N.; Jhoti, E.; Cockell, C.S. Growth, viability, and death of planktonic and biofilm Sphingomonas desiccabilis in simulated Martian brines. Astrobiology 2019, 19, 87–98. [Google Scholar] [CrossRef]
- Schulze-Makuch, D.; Airo, A.; Schirmack, J. The Adaptability of Life on Earth and the Diversity of Planetary Habitats. Front. Microbiol. 2017, 8, 2011. [Google Scholar] [CrossRef]
- Martin, A.; McMinn, A. Sea ice, extremophiles and life on extra-terrestrial ocean worlds. Int. J. Astrobiol. 2017, 17, 1–16. [Google Scholar] [CrossRef]

| Liquid Solution | Hydrated Form of Salt at Eutectic Concentration | Initial Salt Concentration (mol/L) | Eutectic Temperature/Concentration (K/wt%) | Sample Temperature (K) |
|---|---|---|---|---|
| NaCl-H2O | NaCl·2H2O | 10−4 | 252.35/23.3 | 258 |
| 233 | ||||
| 203 | ||||
| Na2SO4-H2O | Na2SO4·10 H2O | 10−4 | 271.99/3.8 | 258 |
| 233 | ||||
| 203 | ||||
| MgSO4-H2O | MgSO4·11 H2O | 10−4 | 269.55/17.55 | 258 |
| 233 | ||||
| 203 | ||||
| ddH2O | Pure ice | - | - | 258 |
| 233 | ||||
| 203 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosciotti, B.; Balbi, A.; Ceccarelli, A.; Fagliarone, C.; Mattei, E.; Lauro, S.E.; Di Paolo, F.; Pettinelli, E.; Billi, D. Survivability of Anhydrobiotic Cyanobacteria in Salty Ice: Implications for the Habitability of Icy Worlds. Life 2019, 9, 86. https://doi.org/10.3390/life9040086
Cosciotti B, Balbi A, Ceccarelli A, Fagliarone C, Mattei E, Lauro SE, Di Paolo F, Pettinelli E, Billi D. Survivability of Anhydrobiotic Cyanobacteria in Salty Ice: Implications for the Habitability of Icy Worlds. Life. 2019; 9(4):86. https://doi.org/10.3390/life9040086
Chicago/Turabian StyleCosciotti, Barbara, Amedeo Balbi, Alessandra Ceccarelli, Claudia Fagliarone, Elisabetta Mattei, Sebastian Emanuel Lauro, Federico Di Paolo, Elena Pettinelli, and Daniela Billi. 2019. "Survivability of Anhydrobiotic Cyanobacteria in Salty Ice: Implications for the Habitability of Icy Worlds" Life 9, no. 4: 86. https://doi.org/10.3390/life9040086
APA StyleCosciotti, B., Balbi, A., Ceccarelli, A., Fagliarone, C., Mattei, E., Lauro, S. E., Di Paolo, F., Pettinelli, E., & Billi, D. (2019). Survivability of Anhydrobiotic Cyanobacteria in Salty Ice: Implications for the Habitability of Icy Worlds. Life, 9(4), 86. https://doi.org/10.3390/life9040086







