Importance of Using Sunscreen After Light or Laser Facial Treatment: A Literature Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Ablative Laser Treatment and Sunscreen Outcomes
3.1.1. Sunscreen After Ablative Laser Treatment
3.1.2. Hydroxyapatite-Based Sunscreen After Laser Ablation
3.2. Non-Ablative Laser Treatment and Sunscreen Outcomes
3.3. Intense Pulsed Light (IPL) Treatment and Sunscreen Outcomes
3.4. Light-Based Treatments for Specific Purposes
3.4.1. Sunscreen to Prevent Adverse Effect of Light Treatment
3.4.2. Sunscreen After Using Light Treatment to Treat Melasma
3.5. Sunscreen Formulation
4. Discussion
4.1. Importance of Sunscreen Use Post-Laser Treatment
4.2. Types of Sunscreens and Their Efficacy
4.3. Patient Education and Compliance
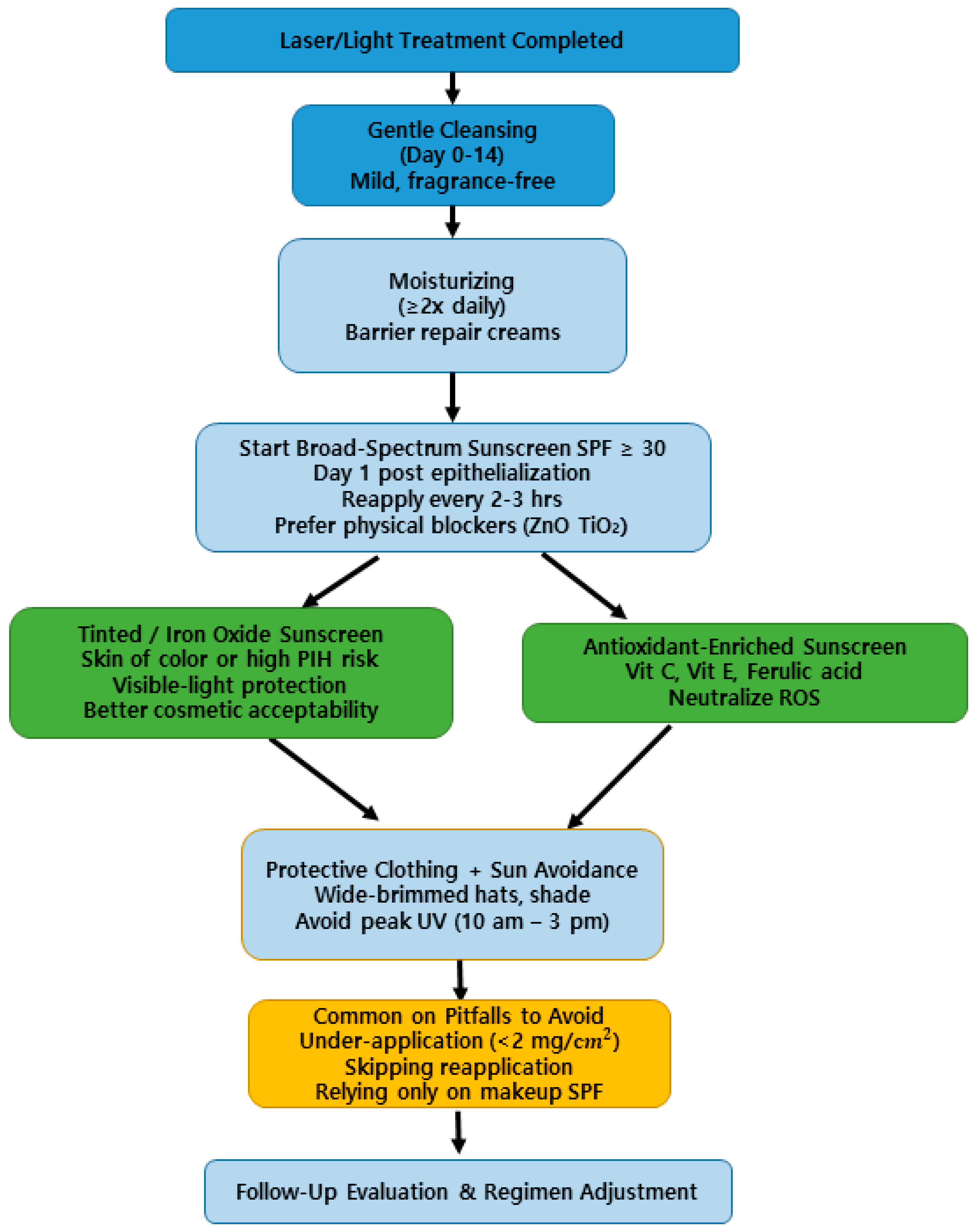
4.4. Practical Clinical Recommendations for Post-Laser Photoprotection
4.5. Future Research Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haykal, D.; Cartier, H.; Goldberg, D.; Gold, M. Advancements in laser technologies for skin rejuvenation: A comprehensive review of efficacy and safety. J. Cosmet. Dermatol. 2024, 23, 3078–3089. [Google Scholar] [CrossRef]
- Papadavid, E.; Katsambas, A. Lasers for facial rejuvenation: A review. Int. J. Dermatol. 2003, 42, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Britt, C.J.; Marcus, B. Energy-based facial rejuvenation: Advances in diagnosis and treatment. JAMA Facial Plast. Surg. 2017, 19, 64–71. [Google Scholar] [CrossRef] [PubMed]
- El-Domyati, M.; Medhat, W. Minimally invasive facial rejuvenation: Current concepts and future expectations. Expert Rev. Dermatol. 2013, 8, 565–580. [Google Scholar] [CrossRef]
- Ge, S.; Beasley, K.L.; Halvorson, C.R.; Weiss, R.A. Nonablative laser rejuvenation. In Textbook of Cosmetic Dermatology; CRC Press: Boca Raton, FL, USA, 2024; pp. 425–443. [Google Scholar]
- Clementoni, M.T.; Pedrelli, V.; Zaccaria, G.; Pontini, P.; Motta, L.R.; Azzopardi, E.A. New developments for fractional CO2 resurfacing for skin rejuvenation and scar reduction. Facial Plast. Surg. Clin. N. Am. 2020, 28, 17–28. [Google Scholar] [CrossRef]
- Gozali, M.V.; Zhou, B. Effective treatments of atrophic acne scars. J. Clin. Aesthet. Dermatol. 2015, 8, 33–40. [Google Scholar] [PubMed]
- Tam, C.; Khong, J.; Tam, K.; Vasilev, R.; Wu, W.; Hazany, S. A comprehensive review of non-energy-based treatments for atrophic acne scarring. Clin. Cosmet. Investig. Dermatol. 2022, 15, 455–469. [Google Scholar] [CrossRef]
- Jia, X.; Feng, Y. Energy-based skin rejuvenation: A review of mechanisms and thermal effects. J. Cosmet. Dermatol. 2025, 24, e16657. [Google Scholar] [CrossRef]
- Malone, C.H.; Walters, N.; Stroh, R.; Munavalli, G. New technologies in skin tightening. Curr. Otorhinolaryngol. Rep. 2021, 9, 422–435. [Google Scholar] [CrossRef]
- Dupont, E.; Gomez, J.; Bilodeau, D. Beyond UV radiation: A skin under challenge. Int. J. Cosmet. Sci. 2013, 35, 224–232. [Google Scholar] [CrossRef]
- Seago, M.; Shumaker, P.R.; Spring, L.K.; Alam, M.; Al-Niaimi, F.; Anderson, R.R.; Waibel, J. Laser treatment of traumatic scars and contractures: 2020 international consensus recommendations. Lasers Surg. Med. 2020, 52, 96–116. [Google Scholar]
- Cios, A.; Ciepielak, M.; Szymański, Ł.; Lewicka, A.; Cierniak, S.; Stankiewicz, W.; Lewicki, S. Effect of different wavelengths of laser irradiation on the skin cells. Int. J. Mol. Sci. 2021, 22, 2437. [Google Scholar] [CrossRef]
- Alster, T.S.; Li, M.K. Dermatologic laser side effects and complications: Prevention and management. Am. J. Clin. Dermatol. 2020, 21, 711–723. [Google Scholar] [CrossRef]
- Mahmoud, B.H.; Hexsel, C.L.; Hamzavi, I.H.; Lim, H.W. Effects of visible light on the skin. Photochem. Photobiol. 2008, 84, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Amici, J.M.; Cogrel, O.; Jourdan, M.; Raimbault, C.; Canchy, L.; Kerob, D.; Araviiskaia, E. Expert recommendations on supportive skin care for non-surgical and surgical procedures. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Madnani, N.; Vedmurthy, M.; Parthasarthi, A.; Dhawan, S.; Kandhari, R.; Pillai, R.; Nischal, K.C. Expert opinion on pre and post procedure care in aesthetic dermatology. Int. J. Res. Med. Sci. 2022, 10, 991. [Google Scholar]
- Ishaq, A.R.; Younis, T.; Akbar, T.; Mangat, M.A.; Fatima, M.; Cai, D. How to protect your skin from harmful radiation. In Natural Products for Skin Diseases: A Treasure Trove for Dermatologic Therapy; Bentham Science Publishers: Sharjah, United Arab Emirates, 2023; pp. 1–59. [Google Scholar]
- Carniol, P.J.; Hamilton, M.M.; Carniol, E.T. Current status of fractional laser resurfacing. JAMA Facial Plast. Surg. 2015, 17, 360–366. [Google Scholar] [CrossRef]
- Metelitsa, A.I.; Alster, T.S. Fractionated laser skin resurfacing treatment complications: A review. Dermatol. Surg. 2010, 36, 299–306. [Google Scholar] [CrossRef]
- Magdum, A.; Leonforte, F.; McNaughton, E.; Kim, J.; Patel, T.; Haywood, R. Sun protection—Do we know enough? J. Plast. Reconstr. Aesthet. Surg. 2012, 65, 1384–1389. [Google Scholar] [CrossRef]
- Wesson, K.M.; Silverberg, N.B. Sun protection education in the United States: What we know and what needs to be taught. Cutis 2003, 71, 71–74. [Google Scholar]
- Centre for Evidence-Based Medicine. Oxford Centre for Evidence-Based Medicine: Levels of Evidence (March 2009). Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009 (accessed on 5 February 2023).
- Fulton, J.E., Jr. Complications of laser resurfacing: Methods of prevention and management. Dermatol. Surg. 1998, 24, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, C. Carbon dioxide laser resurfacing: Long-term follow-up in 2123 patients. Clin. Plast. Surg. 1998, 25, 109–130. [Google Scholar] [CrossRef]
- Levy, T.; Lerman, I.; Waibel, J.; Gauglitz, G.G.; Clementoni, M.T.; Friedmann, D.P.; Artzi, O. Expert consensus on clinical recommendations for fractional ablative CO2 laser, in facial skin rejuvenation treatment. Lasers Surg. Med. 2025, 57, 15–26. [Google Scholar] [CrossRef]
- Alster, T.S.; Lupton, J.R. Prevention and treatment of side effects and complications of cutaneous laser resurfacing. Plast. Reconstr. Surg. 2002, 109, 308–316. [Google Scholar] [CrossRef]
- Chen, W.Y.; Fang, C.L.; Al-Suwayeh, S.A.; Yang, H.H.; Li, Y.C.; Fang, J.Y. Risk assessment of excess drug and sunscreen absorption via skin with ablative fractional laser resurfacing: Optimization of the applied dose for postoperative care. Lasers Med. Sci. 2013, 28, 1363–1374. [Google Scholar] [CrossRef]
- Lee, W.R.; Shen, S.C.; Al-Suwayeh, S.A.; Li, Y.C.; Fang, J.Y. Erbium: YAG laser resurfacing increases skin permeability and the risk of excessive absorption of antibiotics and sunscreens: The influence of skin recovery on drug absorption. Toxicol. Lett. 2012, 211, 150–158. [Google Scholar] [CrossRef]
- Wanitphakdeedecha, R.; Phuardchantuk, R.; Manuskiatti, W. The use of sunscreen starting on the first day after ablative fractional skin resurfacing. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Puaratanaarunkon, T.; Asawanonda, P. A randomized, double blinded, split-face study of the efficacy of using a broad spectrum sunscreen with anti-inflammatory agent to reduce post inflammatory hyperpigmentation after picosecond laser. Clin. Cosmet. Investig. Dermatol. 2022, 15, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Callender, V.D.; Barbosa, V.; Burgess, C.M.; Heath, C.; McMichael, A.J.; Ogunleye, T.; Taylor, S.C. Approach to treatment of medical and cosmetic facial concerns in skin of color patients. Cutis 2017, 100, 375–380. [Google Scholar]
- Zelickson, B.D.; Kilmer, S.L.; Bernstein, E.; Chotzen, V.A.; Dock, J.; Mehregan, D.; Coles, C. Pulsed dye laser therapy for sun damaged skin. Lasers Surg. Med. 1999, 25, 229–236. [Google Scholar] [CrossRef]
- Tempark, T.; Lueangarun, S.; Chatproedprai, S.; Panchaprateep, R.; Pongprutthipan, M.; Wananukul, S. Sun protection behavior and knowledge of patients attending laser clinic to prevent adverse events of laser: A cross-sectional, single-center, tertiary care study. Photodermatol. Photoimmunol. Photomed. 2018, 34, 374–386. [Google Scholar] [CrossRef]
- Halachmi, S.; Haedersdal, M.; Lapidoth, M. Melasma and laser treatment: An evidenced-based analysis. Lasers Med. Sci. 2014, 29, 589–598. [Google Scholar] [CrossRef]
- Piccirillo, C.; Fernández-Arias, M.; Boutinguiza, M.; Tobaldi, D.M.; Del Val, J.; Pintado, M.M.; Pou, J. Increased UV absorption properties of natural hydroxyapatite-based sunscreen through laser ablation modification in liquid. J. Am. Ceram. Soc. 2019, 102, 3163–3174. [Google Scholar] [CrossRef]
- Regazzetti, C.; Sormani, L.; Debayle, D.; Bernerd, F.; Tulic, M.K.; De Donatis, G.M.; Chignon-Sicard, B.; Rocchi, S.; Passeron, T. Melanocytes Sense Blue Light and Regulate Pigmentation through Opsin-3. J. Investig. Dermatol. 2018, 138, 171–178. [Google Scholar] [CrossRef]
- Wicks, N.L.; Chan, J.W.; Najera, J.A.; Ciriello, J.M.; Oancea, E. Blue Light Induces a Distinct Slow Kinetic Profile of Calcium Influx in Human Melanocytes. Photochem. Photobiol. 2011, 87, 993–1003. [Google Scholar] [CrossRef]
- Marques, C.; Hadjab, F.; Porcello, A.; Lourenço, K.; Scaletta, C.; Abdel-Sayed, P.; Hirt-Burri, N.; Applegate, L.A.; Laurent, A. Mechanistic insights into the multiple functions of niacinamide: Therapeutic implications and cosmeceutical applications in functional skincare products. Int. J. Mol. Sci. 2024, 13, 425. [Google Scholar] [CrossRef]
- Boo, Y.C. Mechanistic basis and clinical evidence for the role of niacinamide in skin health: A comprehensive review. Antioxidants 2021, 10, 1315. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Kim, H.O.; Woo, S.M.; Lee, D.H. Use of Dexpanthenol for Atopic Dermatitis—Benefits and Recommendations Based on Current Evidence. J. Clin. Med. 2022, 11, 3943. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Ceramella, J.; Iacopetta, D.; Marra, M.; Conforti, F.; Lupi, F.R.; Gabriele, D.; Borges, F.; Sinicropi, M.S. Aloe vera―An Extensive Review Focused on Recent Studies. Foods 2024, 13, 2155. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, A.; Kalinowska-Lis, U. 18β-Glycyrrhetinic acid: Its core biological properties and dermatological applications. Int. J. Cosmet. Sci. 2019, 41, 325–331. [Google Scholar] [CrossRef]
- Ruvolo, E.; Aeschliman, L.; Cole, C. Evaluation of sunscreen efficacy over time and re-application using hybrid diffuse reflectance spectroscopy. Photodermatol. Photoimmunol. Photomed. 2020, 36, 192–199. [Google Scholar] [CrossRef]
- Passeron, T.; Genedy, R.; Salah, L.; Fusade, T.; Kositratna, G.; Laubach, H.J.; Badawi, A. Laser treatment of hyperpigmented lesions: Position statement of the European Society of Laser in Dermatology. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 987–1005. [Google Scholar] [CrossRef]
- Jones, I.T.; Guiha, I.; Fabi, S.G. Open-label study assessing the efficacy and tolerability of topical skincare and sun protection products following intense pulsed light treatment. J. Cosmet. Dermatol. 2018, 17, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Krutmann, J.; Tian, Y.; Granger, C.; Piquero-Casals, J.; Trullàs, C.; Lai, W. Commentary: Facial aesthetic dermatological procedures and photoprotection in Chinese populations. Dermatol. Ther. 2023, 13, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, C.; Ramirez, O.M.; Pozner, J.N. Postoperative care following CO2 laser resurfacing: Avoiding pitfalls. Plast. Reconstr. Surg. 1997, 100, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.; Nguyen, T.; Patel, R. Immediate versus delayed sunscreen use after ablative laser therapy: A randomized controlled trial. Lasers Surg. Med. 2021, 53, 1032–1040. [Google Scholar]
- Xu, H.; Zhang, Y.; Wang, L. Efficacy of sunscreen use in preventing post-procedural complications: A systematic review and meta-analysis. J. Dermatol. Treat. 2022, 33, 3624–3632. [Google Scholar]
- López, S.; Miranda, A.; Taylor, C.R. Sunscreens and prevention of photoaging: Evidence in patients with skin of color. J. Am. Acad. Dermatol. 2020, 83, 1125–1133. [Google Scholar]
- Tuchinda, C.; Srivannaboon, S.; Lim, H.W. Visible light and pigmentary disorders: Update and implications for photoprotection. Photodermatol. Photoimmunol. Photomed. 2021, 37, 99–107. [Google Scholar]
- Boonchai, W.; Sathaworawong, A.; Wongpraparut, C.; Wanitphakdeedecha, R. The sensitization potential of sunscreen after ablative fractional skin resurfacing using modified human repeated insult patch test. J. Dermatolog. Treat. 2015, 26, 485–488. [Google Scholar] [CrossRef]
| Active Ingredients | Mechanism of Action | Clinical Benefit | References |
|---|---|---|---|
| Niacinamide (Vitamin B3) | Inhibits inflammatory mediators, reduces transepidermal water loss, enhances skin barrier function | Reduces erythema, improves barrier recovery post-laser treatment | Marques et al., 2024 [39]; Boo et al., 2024 [40] |
| Panthenol (Provitamin B5) | Converted to pantothenic acid; promotes epithelialization, has humectant and anti-inflammatory effects | Accelerates wound healing, soothes irritation | Cho et al., 2022 [41] |
| Aloe vera extract | Contains polysaccharides and glycoproteins that modulate inflammatory pathways | Reduces erythema, promotes healing | Catalano et al., 2024 [42] |
| Glycyrrhetinic acid (from licorice root) | Inhibits cyclooxygenase activity and prostaglandin E2 formation | Decreases redness, calms sensitive skin | Kowalska et al., 2019 [43] |
| Study (Year) | Laser/Light Type | Patient Skin Phototype | Sunscreen Type/Composition | Key Outcomes | Level of Evidence |
|---|---|---|---|---|---|
| Fulton et al. (1998) [24] | CO2 Laser Resurfacing | II–IV | Broad-spectrum physical SPF ≥ 30 | Reduced hyperpigmentation, erythema prevention | IV |
| Wanitphakdeedecha et al. (2014) [30] | Ablative Fractional Laser | III–V | Physical SPF 50 (zinc oxide + titanium dioxide) | Faster recovery, reduced inflammation | IIb |
| Puaratanaarunkon & Asawanonda (2022) [31] | Picosecond Laser | III–IV | Broad-spectrum + anti-inflammatory (niacinamide) | Lower PIH incidence | Ib |
| Passeron et al. (2019) [45] | Fractional/Q-switched Lasers | III–V | Tinted physical sunscreen + iron oxide | Reduced visible light-induced pigmentation | IIIa |
| Jones et al. (2018) [46] | IPL | II–III | Broad-spectrum SPF 50 | Reduced erythema, improved skin hydration | IV |
| Tran et al. (2021) [49] | Ablative Resurfacing | II–IV | Broad-spectrum physical SPF ≥ 30 | Immediate initiation reduced PIH | Ib |
| Xu et al. (2022) [50] | Multiple modalities (systematic review) | Mixed | Broad-spectrum photoprotection | Consistent sunscreen use improved recovery & patient satisfaction | Ia |
| López et al. (2020) [51] | Observational (Skin of color, multiple procedures) | IV–VI | Tinted sunscreen with iron oxides | Improved visible-light protection, better adherence in darker phototypes | IIIa |
| Tuchinda et al. (2021) [52] | Mechanistic & clinical studies | III–V | Iron oxide–based tinted formulations | Reduced blue light-induced pigmentation (opsin-3 pathway) | IIa |
| Do | Don’t |
|---|---|
| Use broad-spectrum SPF ≥ 30 from Day 1 [24,30,49] | Delay sunscreen application until redness subsides |
| Prefer physical blockers (zinc oxide, titanium dioxide) in early healing phase [14,53] | Use fragranced or alcohol-based sunscreens immediately post-procedure |
| Reapply every 2–3 h when outdoors [14,44] | Assume one morning application lasts all day |
| Consider tinted/iron oxide sunscreens for skin of color [45,51,52] | Ignore visible light as a pigmentation trigger |
| Combine with anti-inflammatory ingredients (niacinamide, panthenol) [31,32] | Overuse chemical filters that may cause irritation in compromised skin |
| Use antioxidant-enriched sunscreens (Vit C, E, Ferulic acid) [30,44,50] | Rely solely on sunscreen without other photoprotection measures (hats, shade) |
| Educate patients on PIH prevention [24,30,50] | Delay sunscreen application until redness subsides |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.W.A.; Chan, L.K.W.; Song, J.K.; Lee, C.H.; Kim, J.-H.; Yi, K.-H. Importance of Using Sunscreen After Light or Laser Facial Treatment: A Literature Review. Life 2025, 15, 1484. https://doi.org/10.3390/life15091484
Lee KWA, Chan LKW, Song JK, Lee CH, Kim J-H, Yi K-H. Importance of Using Sunscreen After Light or Laser Facial Treatment: A Literature Review. Life. 2025; 15(9):1484. https://doi.org/10.3390/life15091484
Chicago/Turabian StyleLee, Kar Wai Alvin, Lisa Kwin Wah Chan, Jong Keun Song, Cheuk Hung Lee, Jin-Hyun Kim, and Kyu-Ho Yi. 2025. "Importance of Using Sunscreen After Light or Laser Facial Treatment: A Literature Review" Life 15, no. 9: 1484. https://doi.org/10.3390/life15091484
APA StyleLee, K. W. A., Chan, L. K. W., Song, J. K., Lee, C. H., Kim, J.-H., & Yi, K.-H. (2025). Importance of Using Sunscreen After Light or Laser Facial Treatment: A Literature Review. Life, 15(9), 1484. https://doi.org/10.3390/life15091484






