The Prognostic Role of the Left Atrium in Hypertensive Patients with HFpEF: Does Function Matter More than Structure?
Abstract
1. Introduction
2. Materials and Methods
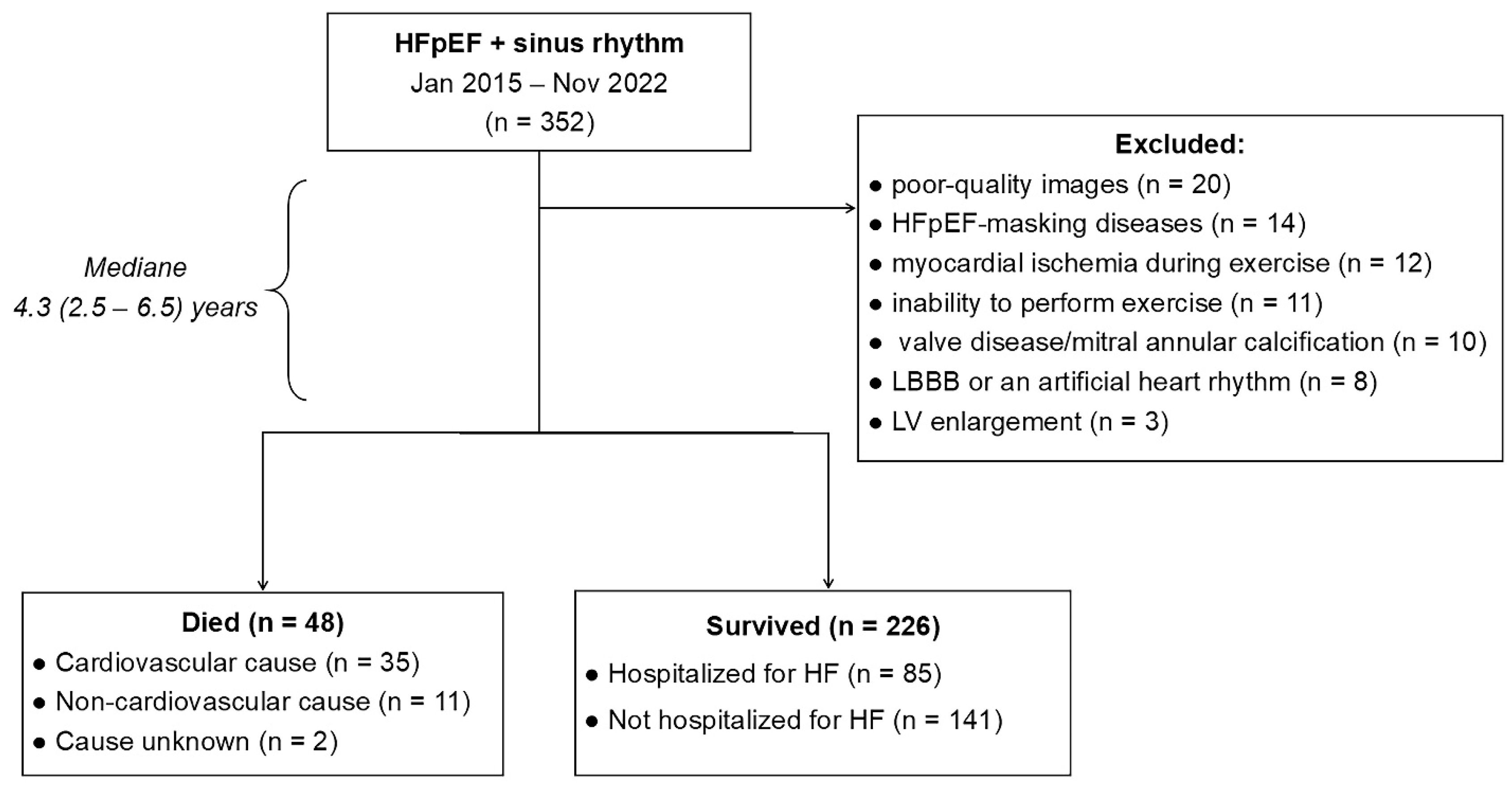
2.1. Study Population
2.2. Study Design
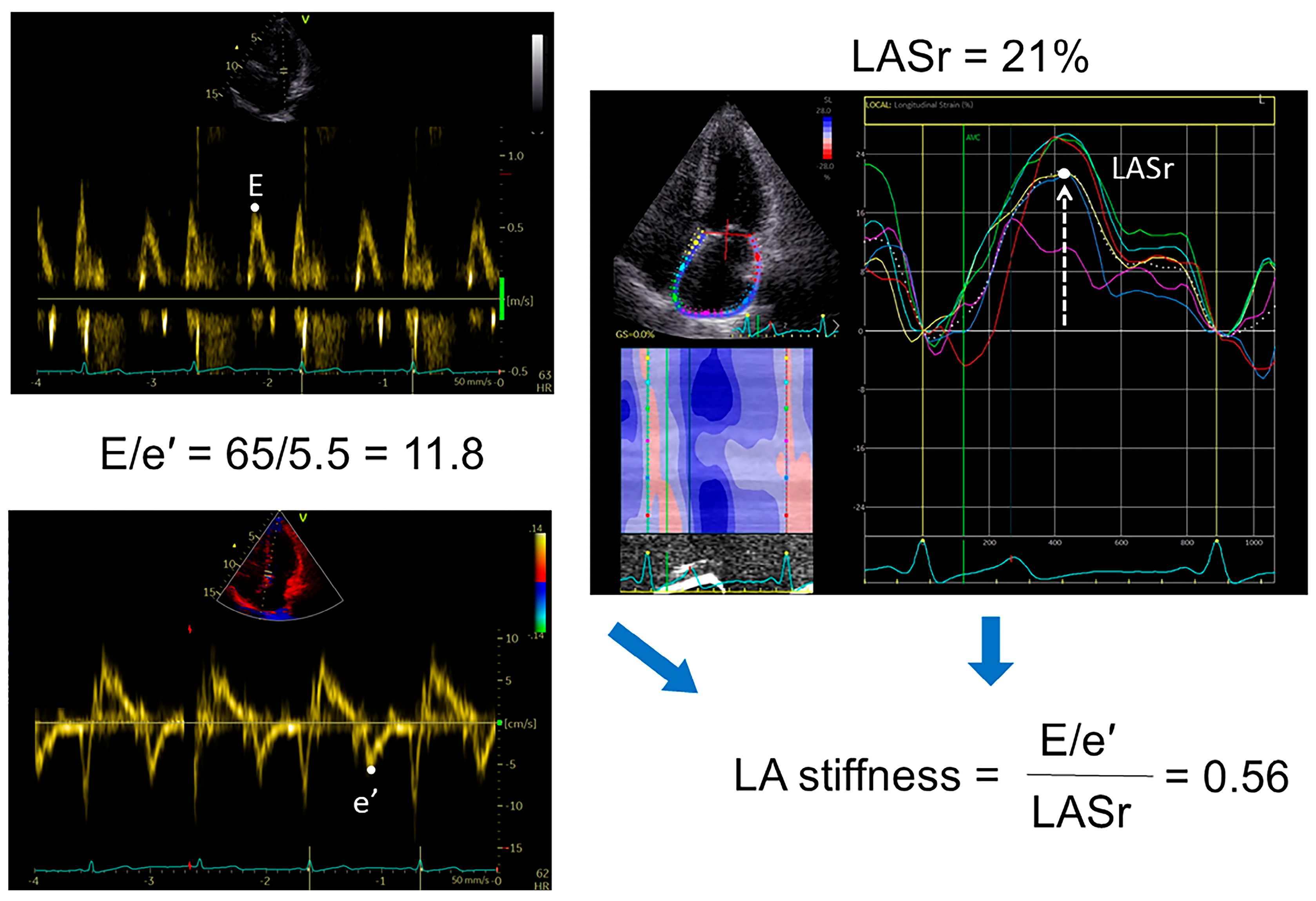
2.3. Echocardiography
2.4. Speckle Tracking Analysis
2.5. Diastolic Stress Test (DST)
2.6. NT-proBNP
2.7. Statistical Analysis
3. Results
3.1. Patient Baseline Characteristics
3.2. Comparison of Patients Who Died or Were Hospitalized Due to HF Exacerbation with Event-Free Survival Patients
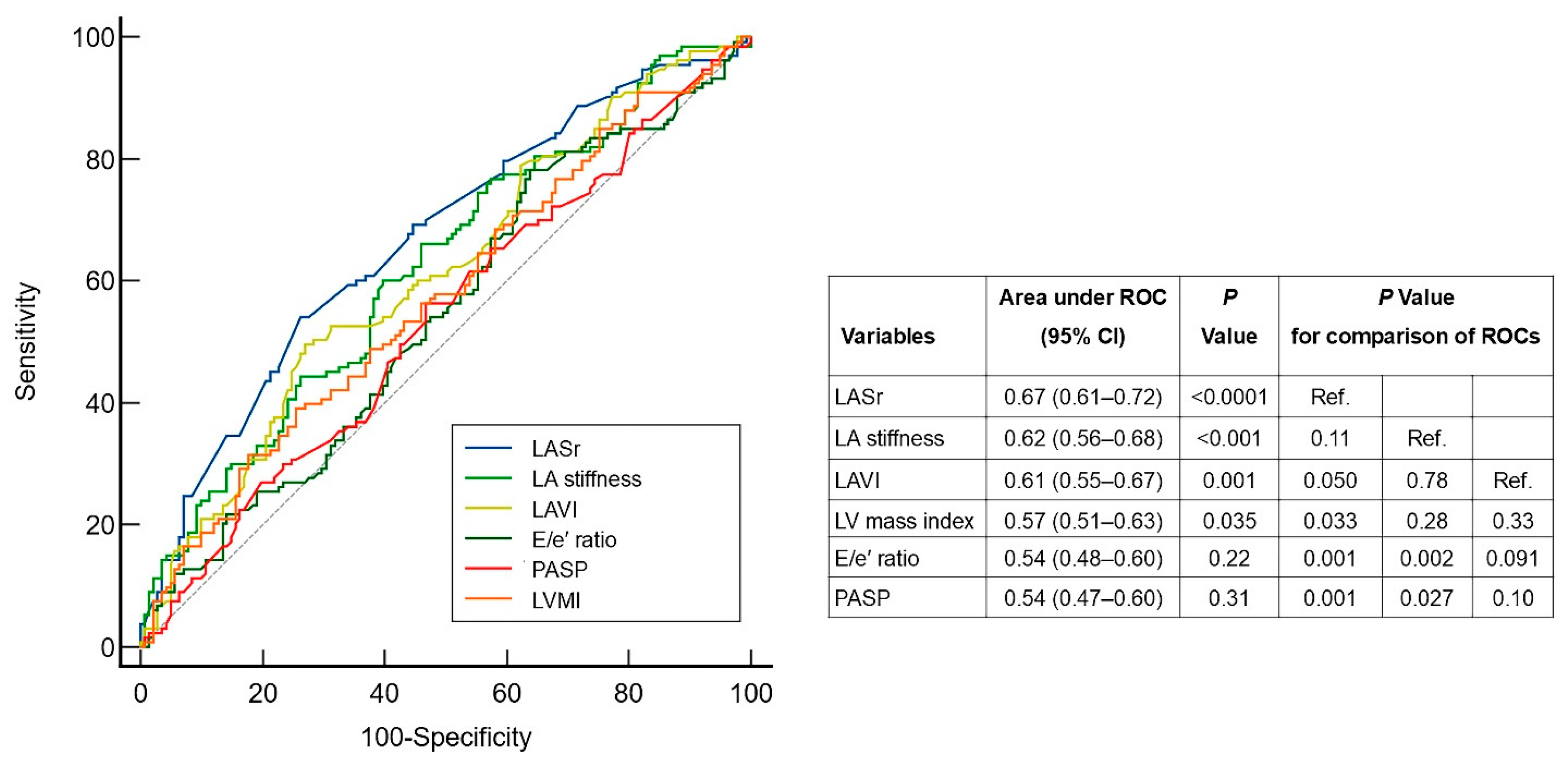
3.3. The Predictive Performance of Echocardiographic Parameters of LA Structure/Function and Parameters of LV Diastolic Dysfunction
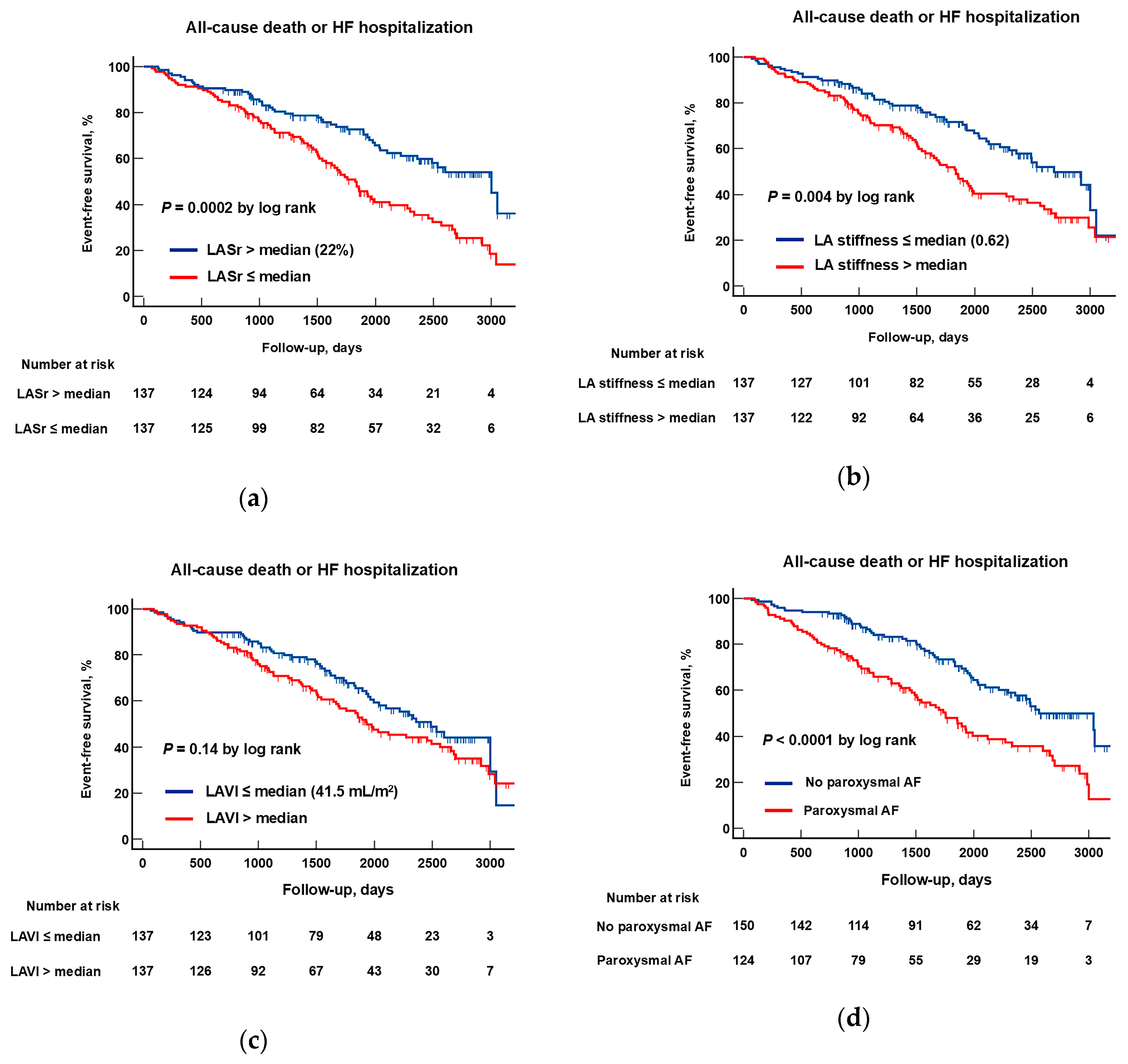
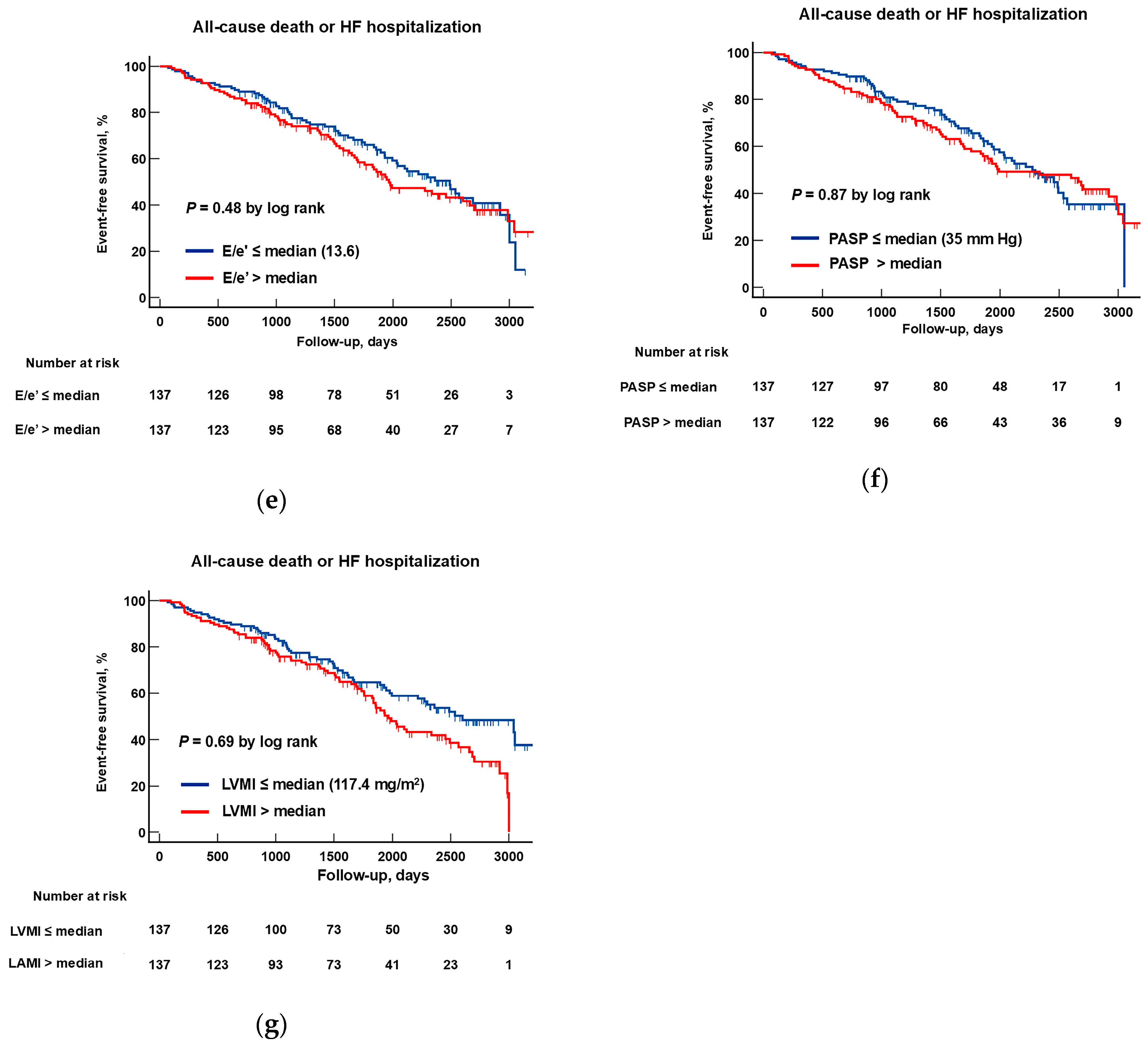
3.4. Identification of Prognostic Markers for HFpEF
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | Atrial fibrillation |
| AH | Arterial hypertension |
| CKD | Chronic kidney disease |
| DST | Diastolic stress test |
| E | Early inflow velocity |
| e′ | Annulus relaxation velocity |
| EDP | End-diastolic pressure |
| EDV | End-diastolic volume |
| EF | Ejection fraction |
| GLS | Global longitudinal strain |
| HFpEF | Heart failure with preserved ejection fraction |
| LA | Left atrial |
| LASr | Left atrial strain during the reservoir phase |
| LAVI | Left atrial volume index |
| LV | Left ventricular |
| LVDD | Left ventricular diastolic dysfunction |
| LVMI | Left ventricular mass index |
| NT-proBNP | N-terminal pro–brain natriuretic peptide |
| NYHA | New York Heart Association |
| PASP | Pulmonary artery systolic pressure |
| RA | Right atrial |
| RV | Right ventricular |
| RWT | Relative wall thickness |
| TAPSE | Tricuspid annular plane systolic excursion |
| T2DM | Type 2 diabetes mellitus |
| TR | Tricuspid regurgitation |
| 6-MWTD | 6 min walk test distance |
References
- Lam, C.S.; Roger, V.L.; Rodeheffer, R.J.; Bursi, F.; Borlaug, B.A.; Ommen, S.R.; Kass, D.A.; Redfield, M.M. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation 2007, 115, 1982–1990. [Google Scholar] [CrossRef]
- Messerli, F.H.; Rimoldi, S.F.; Bangalore, S. The Transition From Hypertension to Heart Failure: Contemporary Update. JACC Heart Fail. 2017, 5, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Nemtsova, V.; Burkard, T.; Vischer, A.S. Hypertensive Heart Disease: A Narrative Review Series-Part 2: Macrostructural and Functional Abnormalities. J. Clin. Med. 2023, 12, 5723. [Google Scholar] [CrossRef]
- Kockskämper, J.; Pluteanu, F. Left atrial myocardium in arterial hypertension. Cells 2022, 11, 3157. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikov, A.G.; Potekhina, A.; Belyavskiy, E.; Gvozdeva, A.; Ageev, F. Left atrial dysfunction as the major driver of heart failure with preserved ejection fraction syndrome. J. Clin. Ultrasound. 2022, 50, 1073–1083. [Google Scholar] [CrossRef]
- Melenovsky, V.; Hwang, S.J.; Redfield, M.M.; Zakeri, R.; Lin, G.; Borlaug, B.A. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ. Heart Fail. 2015, 8, 295–303. [Google Scholar] [CrossRef]
- Reddy, Y.N.V.; Obokata, M.; Wiley, B.; Koepp, K.E.; Jorgenson, C.C.; Egbe, A.; Melenovsky, V.; Carter, R.E.; Borlaug, B.A. The haemodynamic basis of lung congestion during exercise in heart failure with preserved ejection fraction. Eur. Heart J. 2019, 40, 3721–3730. [Google Scholar] [CrossRef]
- Obokata, M.; Olson, T.P.; Reddy, Y.N.V.; Melenovsky, V.; Kane, G.C.; Borlaug, B.A. Haemodynamics, dyspnoea, and pulmonary reserve in heart failure with preserved ejection fraction. Eur. Heart J. 2018, 39, 2810–2821. [Google Scholar] [CrossRef]
- Freed, B.H.; Daruwalla, V.; Cheng, J.Y.; Aguilar, F.G.; Beussink, L.; Choi, A.; Klein, D.A.; Dixon, D.; Baldridge, A.; Rasmussen-Torvik, L.J.; et al. Prognostic Utility and Clinical Significance of Cardiac Mechanics in Heart Failure With Preserved Ejection Fraction: Importance of Left Atrial Strain. Circ. Cardiovasc. Imaging 2016, 9, e003754. [Google Scholar] [CrossRef]
- Santos, A.B.; Roca, G.Q.; Claggett, B.; Sweitzer, N.K.; Shah, S.J.; Anand, I.S.; Fang, J.C.; Zile, M.R.; Pitt, B.; Solomon, S.D.; et al. Prognostic Relevance of Left Atrial Dysfunction in Heart Failure With Preserved Ejection Fraction. Circ. Heart Fail. 2016, 9, e002763. [Google Scholar] [CrossRef] [PubMed]
- Saheera, S.; Krishnamurthy, P. Cardiovascular Changes Associated with Hypertensive Heart Disease and Aging. Cell Transplant. 2020, 29, 963689720920830. [Google Scholar] [CrossRef]
- Hoit, B.D. Left atrial size and function: Role in prognosis. J. Am. Coll. Cardiol. 2014, 63, 493–505. [Google Scholar] [CrossRef]
- Thomas, L.; Marwick, T.H.; Popescu, B.A.; Donal, E.; Badano, L.P. Left Atrial Structure and Function, and Left Ventricular Diastolic Dysfunction: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1961–1977. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Memon, M.M.; Murad, M.H.; Vaduganathan, M.; Greene, S.J.; Hall, M.; Triposkiadis, F.; Lam, C.S.P.; Shah, A.M.; Butler, J.; et al. Left atrial function in heart failure with preserved ejection fraction: A systematic review and meta-analysis. Eur. J. Heart Fail. 2020, 22, 472–485. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Sanborn, D.Y.; Oh, J.K.; Anderson, B.; Billick, K.; Derumeaux, G.; Klein, A.; Koulogiannis, K.; Mitchell, C.; Shah, A.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography and for Heart Failure With Preserved Ejection Fraction Diagnosis: An Update From the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2025, 38, 537–569. [Google Scholar] [CrossRef]
- Ovchinnikov, A.G.; Potekhina, A.V.; Borisov, A.A.; Ibragimova, N.M.; Yushchyuk, E.N.; Masenko, V.P.; Ageev, F.T. The contribution of left atrial dysfunction to exercise intolerance in early heart failure with preserved left ventricular ejection fraction. Eur. Heart J. Cardiovasc. Imaging 2019, 21 (Suppl. S1), 1743. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.A.; Takeuchi, M.; Krisper, M.; Köhncke, C.; Bekfani, T.; Carstensen, T.; Hassfeld, S.; Dorenkamp, M.; Otani, K.; Takigiku, K.; et al. Normal values and clinical relevance of left atrial myocardial function analysed by speckle-tracking echocardiography: Multicentre study. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 364–372. [Google Scholar] [CrossRef]
- Morris, D.A.; Otani, K.; Bekfani, T.; Takigiku, K.; Izumi, C.; Yuda, S.; Sakata, K.; Ohte, N.; Tanabe, K.; Friedrich, K.; et al. Multidirectional global left ventricular systolic function in normal subjects and patients with hypertension: Multicenter evaluation. J. Am. Soc. Echocardiogr. 2014, 27, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Seo, J.H.; Choi, K.H.; Lee, S.H.; Choi, J.O.; Jeon, E.S.; Yang, J.H. Prognostic Implications of Left Atrial Stiffness Index in Heart Failure Patients With Preserved Ejection Fraction. JACC Cardiovasc. Imaging 2023, 16, 435–445. [Google Scholar] [CrossRef]
- Inoue, K.; Khan, F.H.; Remme, E.W.; Ohte, N.; García-Izquierdo, E.; Chetrit, M.; Moñivas-Palomero, V.; Mingo-Santos, S.; Andersen, Ø.S.; Gude, E.; et al. Determinants of left atrial reservoir and pump strain and use of atrial strain for evaluation of left ventricular filling pressure. Eur. Heart J. Cardiovasc. Imaging 2021, 23, 61–70. [Google Scholar] [CrossRef]
- Russo, C.; Jin, Z.; Homma, S.; Rundek, T.; Elkind, M.S.; Sacco, R.L.; Di Tullio, M.R. Left atrial minimum volume and reservoir function as correlates of left ventricular diastolic function: Impact of left ventricular systolic function. Heart 2012, 98, 813–820. [Google Scholar] [CrossRef]
- Ye, Z.; Miranda, W.R.; Yeung, D.F.; Kane, G.C.; Oh, J.K. Left Atrial Strain in Evaluation of Heart Failure with Preserved Ejection Fraction. J. Am. Soc. Echocardiogr. 2020, 33, 1490–1499. [Google Scholar] [CrossRef]
- O’Neal, W.T.; Sandesara, P.; Patel, N.; Venkatesh, S.; Samman-Tahhan, A.; Hammadah, M.; Kelli, H.M.; Soliman, E.Z. Echocardiographic predictors of atrial fibrillation in patients with heart failure with preserved ejection fraction. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 725–729. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Khan, S.U. Left Atrial Strain for Assessment of Left Ventricular Diastolic Function: Focus on Populations With Normal LVEF. JACC Cardiovasc. Imaging 2023, 16, 691–707. [Google Scholar] [CrossRef] [PubMed]
- Barbier, P.; Solomon, S.B.; Schiller, N.B.; Glantz, S.A. Left atrial relaxation and left ventricular systolic function determine left atrial reservoir function. Circulation 1999, 100, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Lisi, M.; Mondillo, S.; Padeletti, M.; Ballo, P.; Tsioulpas, C.; Bernazzali, S.; Maccherini, M. Left atrial longitudinal strain by speckle tracking echocardiography correlates well with left ventricular filling pressures in patients with heart failure. Cardiovasc. Ultrasound 2010, 8, 14. [Google Scholar] [CrossRef]
- Reddy, Y.N.V.; Obokata, M.; Egbe, A.; Yang, J.H.; Pislaru, S.; Lin, G.; Carter, R.; Borlaug, B.A. Left atrial strain and compliance in the diagnostic evaluation of heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2019, 21, 891–900. [Google Scholar] [CrossRef]
- Reddy, Y.N.V.; El Sabbagh, A.; Packer, D.; Nishimura, R.A. Evaluation of shortness of breath after atrial fibrillation ablation-Is there a stiff left atrium? Heart Rhythm 2018, 15, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.B.; Lam, C.S.P.; Svedlund, S.; Saraste, A.; Hage, C.; Tan, R.S.; Beussink-Nelson, L.; Tromp, J.; Sanchez, C.; Njoroge, J.; et al. Disproportionate left atrial myopathy in heart failure with preserved ejection fraction among participants of the PROMIS-HFpEF study. Sci. Rep. 2021, 11, 4885. [Google Scholar] [CrossRef]
- Ovchinnikov, A.; Belyavskiy, E.; Potekhina, A.; Ageev, F. Asymptomatic Left Ventricular Hypertrophy Is a Potent Risk Factor for the Development of HFpEF but Not HFrEF: Results of a Retrospective Cohort Study. J. Clin. Med. 2022, 11, 3885. [Google Scholar] [CrossRef]
- Kittipibul, V.; Lam, C.S.P. Heart failure with preserved ejection fraction and atrial fibrillation: Epidemiology, pathophysiology, and diagnosis interplay. Heart Fail. Rev. 2025, 30, 697–705. [Google Scholar] [CrossRef]
- Reddy, Y.N.V.; Obokata, M.; Verbrugge, F.H.; Lin, G.; Borlaug, B.A. Atrial Dysfunction in Patients With Heart Failure With Preserved Ejection Fraction and Atrial Fibrillation. J. Am. Coll. Cardiol. 2020, 76, 1051–1064. [Google Scholar] [CrossRef] [PubMed]
- Olsson, L.G.; Swedberg, K.; Ducharme, A.; Granger, C.B.; Michelson, E.L.; McMurray, J.J.; Puu, M.; Yusuf, S.; Pfeffer, M.A.; CHARM Investigators. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: Results from the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) program. J. Am. Coll. Cardiol. 2006, 47, 1997–2004. [Google Scholar] [CrossRef] [PubMed]
- Eapen, Z.J.; Greiner, M.A.; Fonarow, G.C.; Yuan, Z.; Mills, R.M.; Hernandez, A.F.; Curtis, L.H. Associations between atrial fibrillation and early outcomes of patients with heart failure and reduced or preserved ejection fraction. Am. Heart J. 2014, 167, 369–375.e2. [Google Scholar] [CrossRef]
- Mohammed, S.F.; Hussain, I.; AbouEzzeddine, O.F.; Takahama, H.; Kwon, S.H.; Forfia, P.; Roger, V.L.; Redfield, M.M. Right ventricular function in heart failure with preserved ejection fraction: A community-based study. Circulation 2014, 130, 2310–2320. [Google Scholar] [CrossRef]
- Hussain, I.; Mohammed, S.F.; Forfia, P.R.; Lewis, G.D.; Borlaug, B.A.; Gallup, D.S.; Redfield, M.M. Impaired Right Ventricular-Pulmonary Arterial Coupling and Effect of Sildenafil in Heart Failure With Preserved Ejection Fraction: An Ancillary Analysis From the Phosphodiesterase-5 Inhibition to Improve Clinical Status And Exercise Capacity in Diastolic Heart Failure (RELAX) Trial. Circ. Heart Fail. 2016, 9, e002729. [Google Scholar]
- Borlaug, B.A.; Blair, J.; Bergmann, M.W.; Bugger, H.; Burkhoff, D.; Bruch, L.; Celermajer, D.S.; Claggett, B.; Cleland, J.G.F.; Cutlip, D.E.; et al. Latent Pulmonary Vascular Disease May Alter the Response to Therapeutic Atrial Shunt Device in Heart Failure. Circulation 2022, 145, 1592–1604. [Google Scholar] [CrossRef]
- Ovchinnikov, A.; Potekhina, A.; Filatova, A.; Svirida, O.; Zherebchikova, K.; Ageev, F.; Belyavskiy, E. Effects of empagliflozin on functional capacity, LV filling pressure, and cardiac reserves in patients with type 2 diabetes mellitus and heart failure with preserved ejection fraction: A randomized controlled open-label trial. Cardiovasc. Diabetol. 2025, 24, 196. [Google Scholar] [CrossRef]
- Anker, S.D.; Usman, M.S.; Anker, M.S.; Butler, J.; Böhm, M.; Abraham, W.T.; Adamo, M.; Chopra, V.K.; Cicoira, M.; Cosentino, F.; et al. Patient phenotype profiling in heart failure with preserved ejection fraction to guide therapeutic decision making. A scientific statement of the Heart Failure Association, the European Heart Rhythm Association of the European Society of Cardiology, and the European Society of Hypertension. Eur. J. Heart Fail. 2023, 25, 936–955. [Google Scholar]
- Andersen, O.S.; Smiseth, O.A.; Dokainish, H.; Abudiab, M.M.; Schutt, R.C.; Kumar, A.; Sato, K.; Harb, S.; Gude, E.; Remme, E.W.; et al. Estimating Left Ventricular Filling Pressure by Echocardiography. J. Am. Coll. Cardiol. 2017, 69, 1937–1948. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, F.H.; Omote, K.; Reddy, Y.N.V.; Sorimachi, H.; Obokata, M.; Borlaug, B.A. Heart failure with preserved ejection fraction in patients with normal natriuretic peptide levels is associated with increased morbidity and mortality. Eur. Heart J. 2022, 43, 1941–1951. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef] [PubMed]
| Variables | All Patients (n = 274) | Died or Hospitalized for HF (n = 133) | Survived Without HF Hospitalization (n = 141) | p Value |
|---|---|---|---|---|
| Clinical variables | ||||
| Age, y | 68.7 (63.6–77.0) | 69.7 (64.1–76.8) | 67.9 (61.7–74.0) | 0.031 |
| Male gender, n (%) | 121 (44) | 58 (44) | 63 (45) | 0.86 |
| Body mass index, kg/m2 | 32.0 ± 5.4 | 31.3 ± 5.1 | 32.7 ± 5.6 | 0.037 |
| Systolic BP, mm Hg | 140 (130–150) | 140 (130–150) | 138 (130–145) | 0.13 |
| Diastolic BP, mm Hg | 85 (80–90) | 86 (80–93) | 85 (80–90) | 0.73 |
| Heart rate, min−1 | 65 (60–71) | 65 (60–71) | 65 (61–71) | 0.95 |
| 6-MWTD, m | 329 ± 77 | 322 ± 76 | 335 ± 78 | 0.16 |
| NYHA functional class: | 0.10 | |||
| I, n (%) | 41 (15) | 14 (11) | 27 (19) | 0.046 |
| II, n (%) | 160 (58) | 79 (59) | 81 (57) | 0.74 |
| III, n (%) | 73 (27) | 40 (30) | 33 (23) | 0.21 |
| NT-proBNP, pg/mL | 283 (193–449) | 312 (223–630) | 253 (158–365) | <0.0001 |
| eGFR, mL/min/1.73 m2 | 64 (46–72) | 63 (44–72) | 65 (50–74) | 0.10 |
| Comorbidities and associated conditions | ||||
| Overweight/obesity, 1 n (%) | 250 (91) | 121 (91) | 129 (92) | 0.88 |
| Hypertension, 2 n (%) | 274 (100) | 133 (100) | 141 (100) | 0.30 |
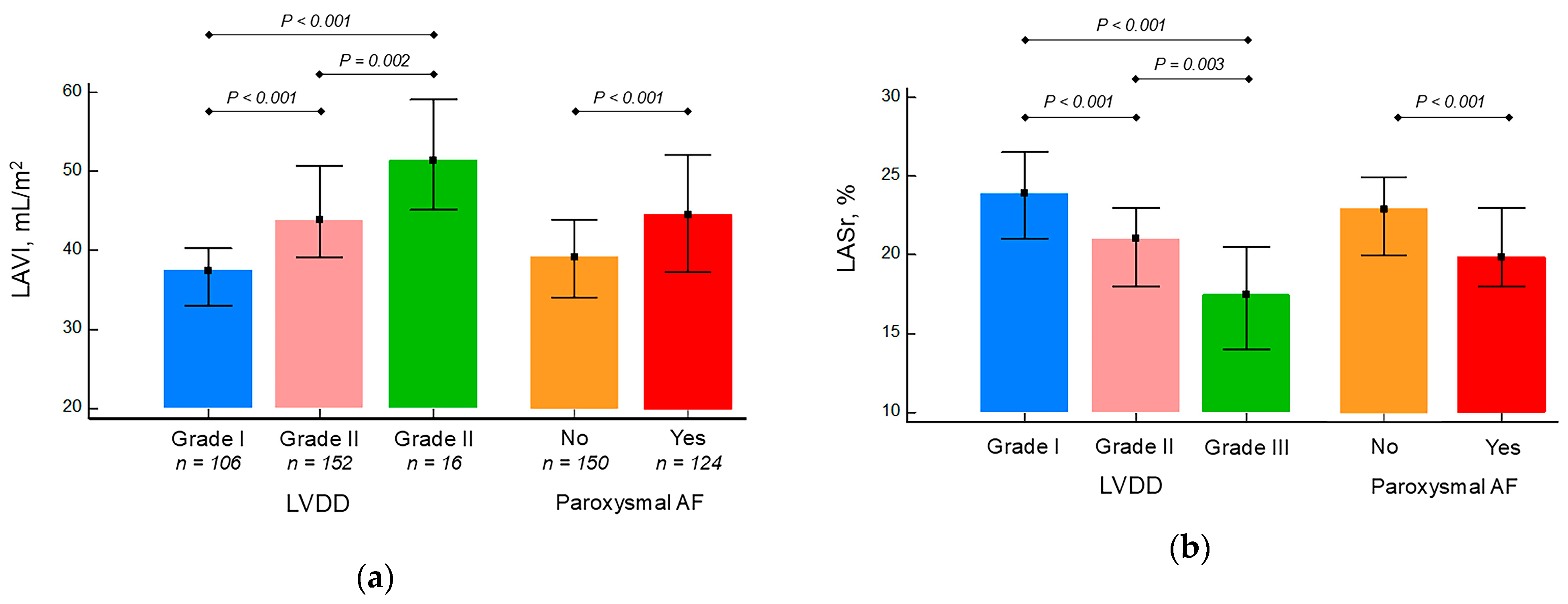
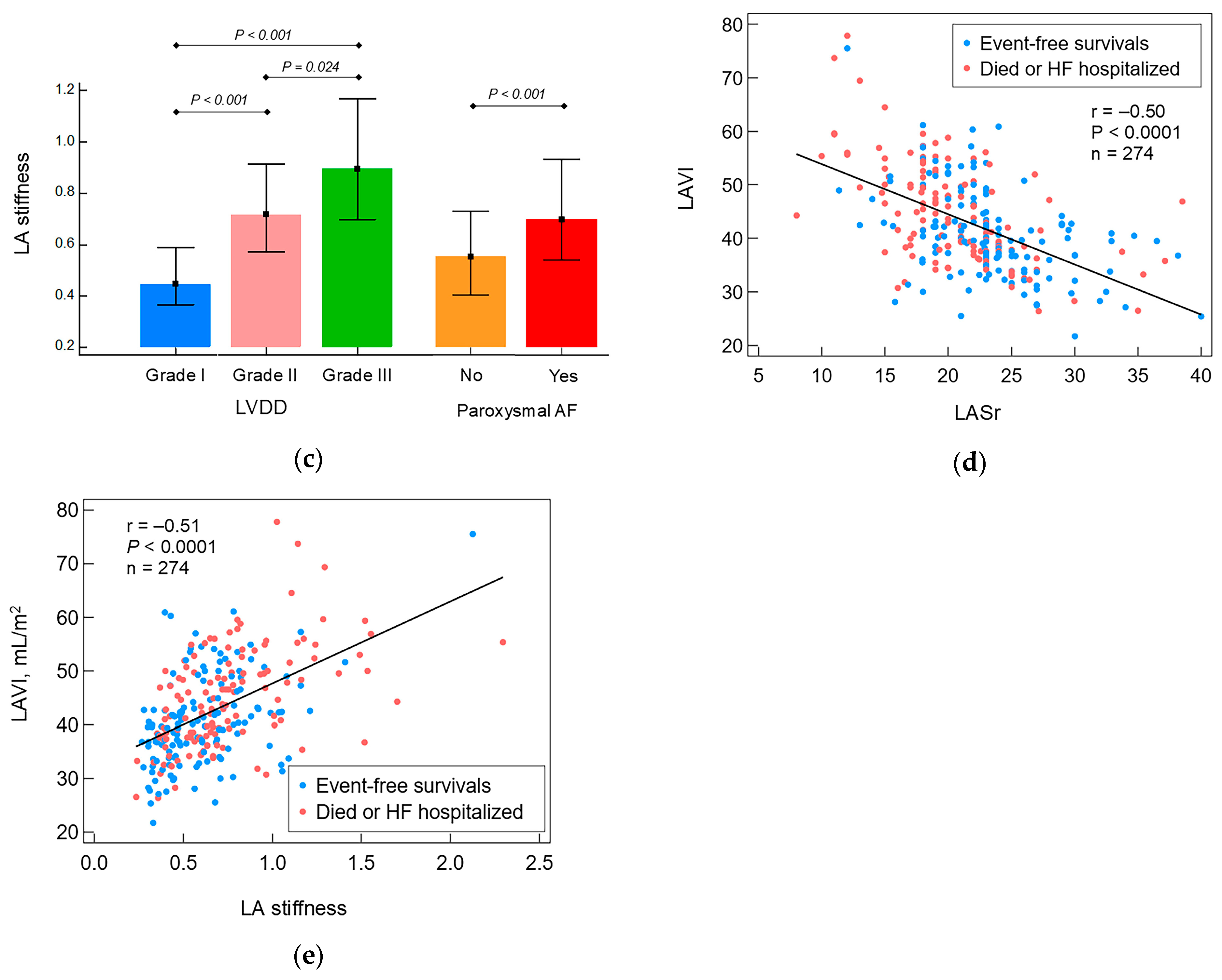
| Paroxysmal atrial fibrillation, n (%) | 124 (45) | 76 (57) | 48 (34) | 0.0001 |
| Ischemic heart disease, n (%) | 96 (35) | 48 (36) | 48 (34) | 0.72 |
| Previous myocardial infarction, n (%) | 40 (15) | 20 (15) | 20 (14) | 0.84 |
| Myocardial revascularization, n (%) | 74 (27) | 36 (27) | 28 (20) | 0.16 |
| Type 2 diabetes mellitus, n (%) | 110 (40) | 54 (41) | 56 (40) | 0.88 |
| Chronic kidney disease, 3 n (%) | 112 (41) | 60 (45) | 52 (37) | 0.17 |
| Stroke, n (%) | 15 (5) | 10 (8) | 5 (4) | 0.15 |
| Anemia, n (%) | 36 (13) | 21 (16) | 15 (11) | 0.21 |
| COPD, n (%) | 45 (16) | 23 (17) | 22 (16) | 0.51 |
| Cardiovascular medications | ||||
| ACEI/ARB, n (%) | 220 (80) | 112 (84) | 108 (77) | 0.11 |
| ARNI, n (%) | 49 (18) | 20 (15) | 29 (21) | 0.23 |
| SGLT2 inhibitors, n (%) | 39 (14) | 19 (14) | 20 (14) | 0.98 |
| MRA, n (%) | 36 (13) | 13 (10) | 23 (16) | 0.11 |
| Calcium channel blocker, n (%) | 125 (46) | 58 (44) | 67 (48) | 0.52 |
| β-Blockers, n (%) | 186 (68) | 90 (68) | 96 (68) | 0.94 |
| Loop diuretics, n (%) | 203 (74) | 108 (81) | 95 (67) | 0.009 |
| Statins, n (%) | 200 (73) | 97 (73) | 103 (73) | 0.98 |
| Variables | All Patients (n = 274) | Died or Hospitalized for HF (n = 133) | Survived Without HF Hospitalization (n = 141) | p Value |
|---|---|---|---|---|
| LV ejection fraction, % | 59.8 (55.8–65.5) | 59.4 (56.3–66.7) | 59.8 (55.4–65.2) | 0.45 |
| LV GLS, % | 18.5 ± 3.2 | 18.7 ± 2.9 | 18.2 ± 3.4 | 0.42 |
| LV EDV, mL | 93 (78–110) | 92 (80–108) | 94 (75–111) | 0.98 |
| LV mass index, g/m2 | 110.6 (101.1–127.7) | 112.9 (102.4–131.7) | 109.0 (99.1–122.5) | 0.037 |
| LV hypertrophy, 1 n (%) | 188 (69) | 97 (73) | 91 (65) | 0.14 |
| Relative wall thickness | 0.50 ± 0.09 | 0.50 ± 0.08 | 0.50 ± 0.09 | 0.98 |
| Types of LV geometry | 0.35 | |||
| Normal geometry | 21 (8) | 10 (8) | 11 (8) | 0.93 |
| Concentric remodeling | 65 (24) | 26 (19) | 39 (28) | 0.12 |
| Concentric hypertrophy | 165 (60) | 87 (65) | 78 (55) | 0.089 |
| Eccentric hypertrophy | 23 (8) | 10 (8) | 13 (9) | 0.61 |
| LAVI, mL/m2 | 41.5 (36.7–49.0) | 43.4 (37.6–50.0) | 40.0 (35.5–44.9) | 0.002 |
| LA dilatation, 2 n (%) | 230 (84) | 120 (90) | 110 (78) | 0.006 |
| LASr, % | 22 (19–24) | 20 (18–23) | 23 (20–26) | <0.0001 |
| LA dysfunction, 3 n (%) | 159 (58) | 93 (70) | 66 (47) | 0.0001 |
| LA stiffness | 0.62 (0.45–0.80) | 0.67 (0.51–0.90) | 0.57 (0.43–0.74) | 0.0006 |
| E/A ratio | 0.88 (0.70–1.16) | 0.83 (0.68–1.25) | 0.90 (0.76–1.13) | 0.27 |
| E/e′ ratio | 13.6 (10.8–15.8) | 13.7 (11.3–16.2) | 13.2 (10.3–15.8) | 0.22 |
| E/e′ > 14, n (%) | 123 (46) | 63 (47) | 60 (43) | 0.42 |
| LV diastolic dysfunction: | 0.014 | |||
| I, n (%) | 106 (39) | 42 (32) | 64 (45) | 0.019 |
| II, n (%) | 152 (55) | 79 (59) | 73 (52) | 0.21 |
| III, n (%) | 16 (6) | 12 (9) | 4 (3) | 0.029 |
| PASP, mm Hg | 35.0 (28.0–43.1) | 36.9 (28.8–46.9) | 33.3 (27.0–40.9) | 0.025 |
| Pulmonary hypertension, 4 n (%) | 138 (50) | 72 (54) | 66 (47) | 0.23 |
| TAPSE, cm | 2.1 ± 0.4 | 2.1 ± 0.4 | 2.2 ± 0.4 | 0.11 |
| RV dysfunction, 5 n (%) | 43 (16) | 23 (17) | 20 (14) | 0.48 |
| Increased RA pressure, 6 n (%) | 129 (47) | 64 (48) | 65 (46) | 0.74 |
| Parameters | Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) | p Value | Unadjusted HR (95% CI)—Model 1 | p Value | Adjusted HR (95% CI) 1—Model 2 | p Value | Adjusted HR (95% CI) 2—Model 3 | p Value | |
| LASr | 0.93 (0.90–0.97) | 0.0001 | 0.95 (0.90–0.99) | 0.025 | 0.95 (0.90–0.99) | 0.023 | 0.94 (0.90–0.99) | 0.023 |
| Paroxysmal AF | 2.03 (1.43–2.87) | 0.0001 | 1.71 (1.17–2.51) | 0.006 | 1.71 (1.16–2.50) | 0.006 | 1.77 (1.19–2.64) | 0.005 |
| LA stiffness | 1.75 (1.13–2.71) | 0.012 | – | – | – | – | – | – |
| No loop diuretics | 0.57 (0.37–0.89) | 0.012 | 0.79 (0.47–1.31) | 0.36 | 0.81 (0.48–1.37) | 0.43 | 0.86 (0.51–1.45) | 0.58 |
| LAVI | 1.02 (1.01–1.04) | 0.013 | 0.99 (0.97–1.02) | 0.56 | 0.99 (0.97–1.02) | 0.71 | 1.00 (0.97–1.03) | 0.83 |
| NYHA fc | 1.35 (1.05–1.75) | 0.020 | 1.15 (0.86–1.53) | 0.36 | 1.06 (0.81–1.47) | 0.58 | 1.10 (0.80–1.52) | 0.55 |
| LVDD | 1.35 (1.01–1.79) | 0.042 | 0.91 (0.63–1.32) | 0.63 | 0.94 (0.64–1.36) | 0.73 | 0.97 (0.66–1.42) | 0.87 |
| Systolic BP | 1.01 (1.00–1.02) | 0.073 | – | – | – | – | 1.01 (1.00–1.03) | 0.058 |
| Age | 1.02 (1.00–1.04) | 0.097 | – | – | 1.01 (0.99–1.03) | 0.48 | 1.01 (0.99–1.03) | 0.42 |
| Male gender | 0.78 (0.55–1.10) | 0.15 | – | – | 0.91 (0.63–1.33) | 0.63 | 0.90 (0.61–1.33) | 0.60 |
| NT-proBNP | 1.01 (0.99–1.09) | 0.18 | – | – | – | – | 1.00 (0.99–1.01) | 0.27 |
| CKD | 1.23 (0.87–1.73) | 0.25 | – | – | – | – | 1.03 (0.70–1.51) | 0.88 |
| T2DM | 1.16 (0.82–1.66) | 0.40 | – | – | – | – | 1.03 (0.69–1.53) | 0.89 |
| LVMI | 1.00 (0.99–1.01) | 0.41 | – | – | – | – | 1.00 (0.99–1.01) | 0.56 |
| PASP | 1.00 (0.98–1.01) | 0.51 | – | – | – | – | – | – |
| E/e′ ratio | 1.01 (0.97–1.06) | 0.52 | – | – | – | – | – | – |
| BMI | 1.00 (0.97–1.04) | 0.88 | – | – | 1.02 (0.98–1.06) | 0.34 | 1.02 (0.98–1.06) | 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ovchinnikov, A.; Potekhina, A.; Filatova, A.; Svirida, O.; Sobolevskaya, M.; Safiullina, A.; Ageev, F. The Prognostic Role of the Left Atrium in Hypertensive Patients with HFpEF: Does Function Matter More than Structure? Life 2025, 15, 1483. https://doi.org/10.3390/life15091483
Ovchinnikov A, Potekhina A, Filatova A, Svirida O, Sobolevskaya M, Safiullina A, Ageev F. The Prognostic Role of the Left Atrium in Hypertensive Patients with HFpEF: Does Function Matter More than Structure? Life. 2025; 15(9):1483. https://doi.org/10.3390/life15091483
Chicago/Turabian StyleOvchinnikov, Artem, Alexandra Potekhina, Anastasiia Filatova, Olga Svirida, Maria Sobolevskaya, Alfiya Safiullina, and Fail Ageev. 2025. "The Prognostic Role of the Left Atrium in Hypertensive Patients with HFpEF: Does Function Matter More than Structure?" Life 15, no. 9: 1483. https://doi.org/10.3390/life15091483
APA StyleOvchinnikov, A., Potekhina, A., Filatova, A., Svirida, O., Sobolevskaya, M., Safiullina, A., & Ageev, F. (2025). The Prognostic Role of the Left Atrium in Hypertensive Patients with HFpEF: Does Function Matter More than Structure? Life, 15(9), 1483. https://doi.org/10.3390/life15091483






