Multifactorial Refractory Acne in Women: Insights from a Case Series Involving Hormonal-, Metabolic-, and Corticosteroid-Related Triggers
Abstract
1. Introduction
2. Materials and Methods
3. Case Presentation
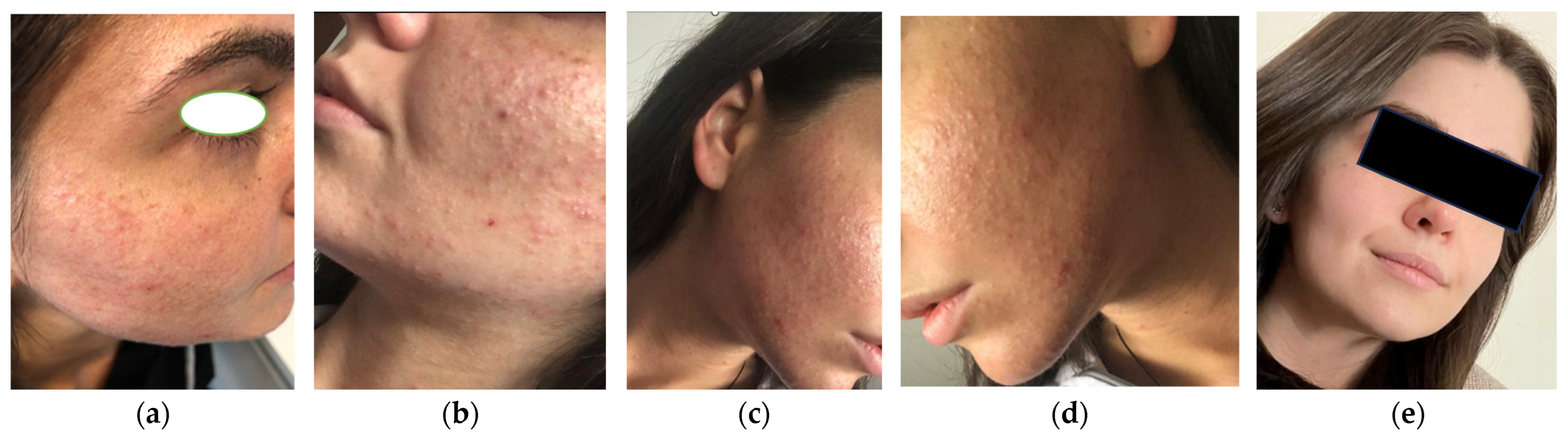
3.1. Case 1
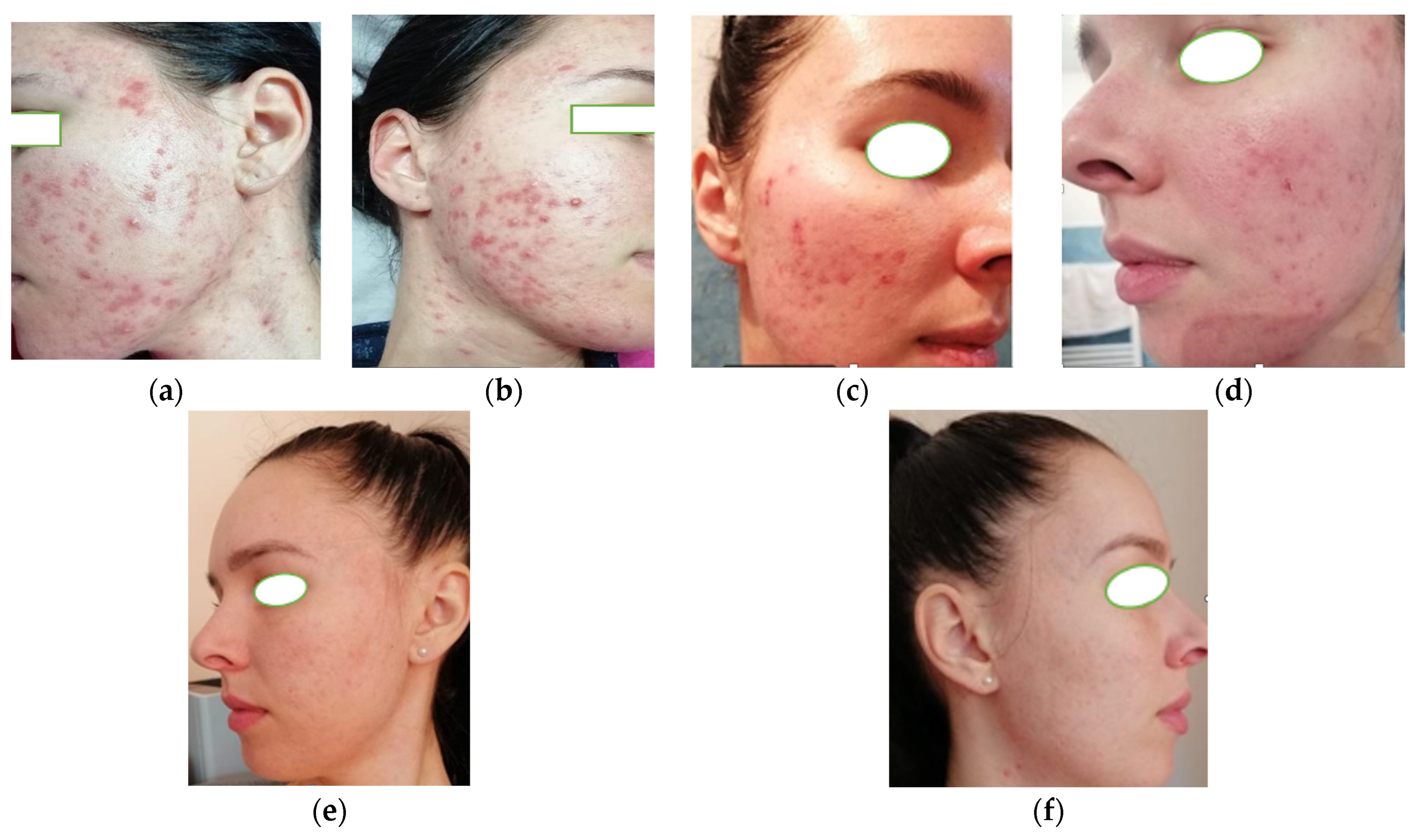
3.2. Case 2
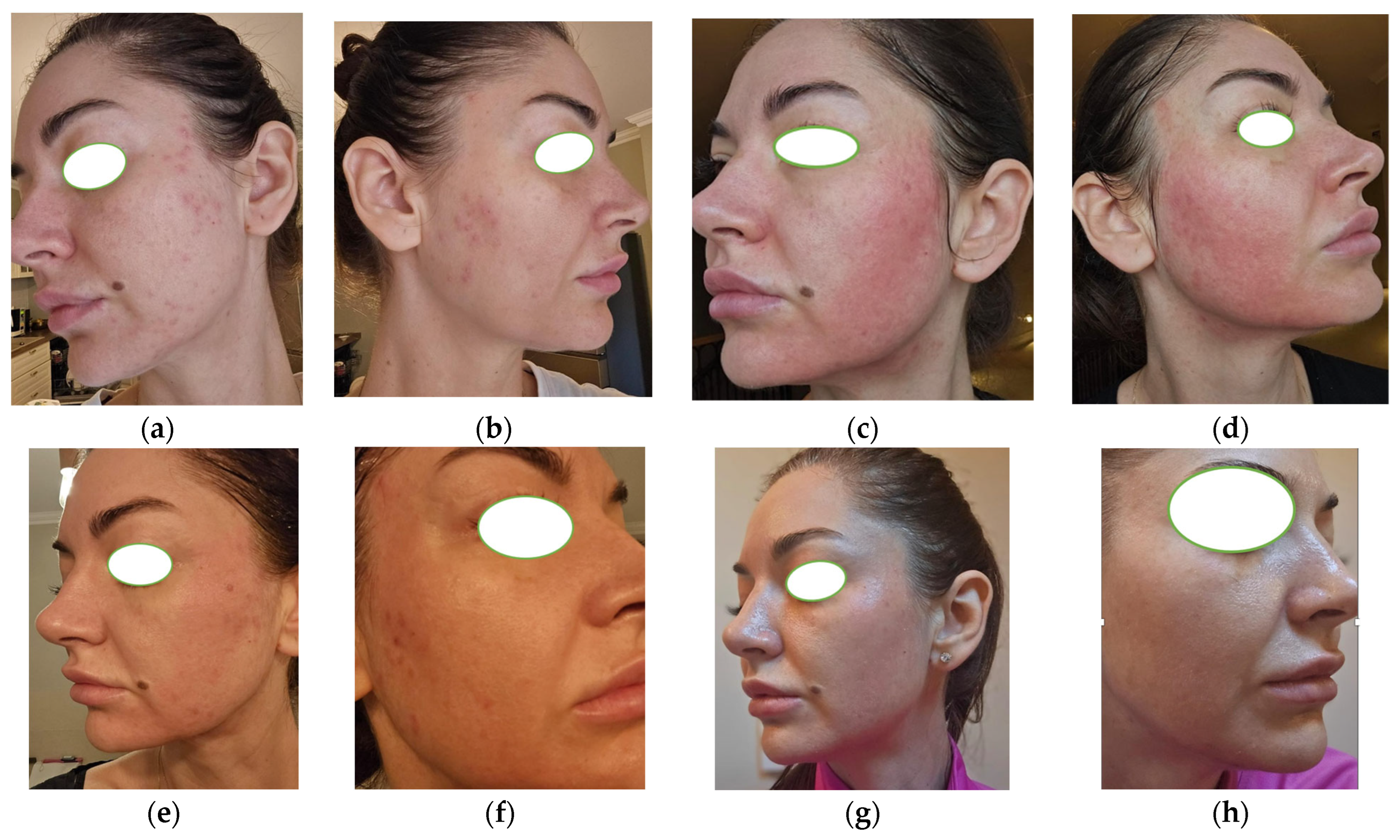
3.3. Case 3
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| C. acnes | Cutibacterium acnes |
| COC | Combined oral contraceptive |
| GEA | Global Evaluation Acne |
| IGF-1 | Insulin-like growth factor 1 |
| IL | Interleukin |
| LDL | Low-density lipoprotein |
| LTB4 | Leukotriene B4 |
| mg | Milligram |
| NF-κB | Nuclear factor kappa B |
| PCOS | Polycystic ovary syndrome |
| TLR | Toll-like receptors |
References
- Liu, L.; Xue, Y.; Chen, Y.; Chen, T.; Zhong, J.; Shao, X.; Chen, J. Prevalence and Risk Factors of Acne Scars in Patients with Acne Vulgaris. Ski. Res. Technol. 2023, 29, e13386. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, T.C.; Yin, X.L.; Man, J.Y.; Yang, X.R.; Lu, M. Magnitude and Temporal Trend of Acne Vulgaris Burden in 204 Countries and Territories from 1990 to 2019: An Analysis from the Global Burden of Disease Study 2019*. Br. J. Dermatol. 2022, 186, 673–683. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhong, X.; Luo, Z.; Liu, M.; Zhang, H.; Zheng, H.; Li, J. Global, Regional and National Burdens of Acne Vulgaris in Adolescents and Young Adults Aged 10–24 Years from 1990 to 2021: A Trend Analysis. Br. J. Dermatol. 2025, 192, 228–237. [Google Scholar] [CrossRef]
- Hagenström, K.; Müller, K.; Klinger, T.; Stephan, B.; Augustin, M. Epidemiology and Healthcare of Juvenile and Late-Onset Acne: Claims Data Analysis. Dermatol. Ther. 2024, 14, 3017–3031. [Google Scholar] [CrossRef]
- Samuels, D.V.; Rosenthal, R.; Lin, R.; Chaudhari, S.; Natsuaki, M.N. Acne Vulgaris and Risk of Depression and Anxiety: A Meta-Analytic Review. J. Am. Acad. Dermatol. 2020, 83, 532–541. [Google Scholar] [CrossRef]
- Morshed, A.S.M.; Noor, T.; Uddin Ahmed, M.A.; Mili, F.S.; Ikram, S.; Rahman, M.; Ahmed, S.; Uddin, M.B. Understanding the Impact of Acne Vulgaris and Associated Psychological Distress on Self-Esteem and Quality of Life via Regression Modeling with CADI, DLQI, and WHOQoL. Sci. Rep. 2023, 13, 21084. [Google Scholar] [CrossRef]
- Leyden, J.J. New Understandings of the Pathogenesis of Acne. J. Am. Acad. Dermatol. 1995, 32, S15–S25. [Google Scholar] [CrossRef]
- Melnik, B.C. Acne Vulgaris: The Metabolic Syndrome of the Pilosebaceous Follicle. Clin. Dermatol. 2018, 36, 29–40. [Google Scholar] [CrossRef]
- Perkins, A.C.; Maglione, J.; Hillebrand, G.G.; Miyamoto, K.; Kimball, A.B. Acne Vulgaris in Women: Prevalence across the Life Span. J. Womens Health 2012, 21, 223–230. [Google Scholar] [CrossRef]
- Bagatin, E.; Freitas, T.H.P.D.; Rivitti Machado, M.C.; Ribeiro, B.M.; Nunes, S.; Rocha, M.A.D. Da Adult Female Acne: A Guide to Clinical Practice. Bras. Dermatol. 2019, 94, 62–75. [Google Scholar] [CrossRef]
- Okoro, O.E.; Camera, E.; Flori, E.; Ottaviani, M. Insulin and the Sebaceous Gland Function. Front. Physiol. 2023, 14, 1252972. [Google Scholar] [CrossRef]
- Fatima, F.; Das, A.; Kumar, P.; Datta, D. Skin and Metabolic Syndrome: An Evidence Based Comprehensive Review. Indian J. Dermatol. 2021, 66, 302. [Google Scholar] [CrossRef]
- Xia, J.; Ding, L.; Liu, G. Metabolic Syndrome and Dermatological Diseases: Association and Treatment. Nutr. Metab. 2025, 22, 36. [Google Scholar] [CrossRef]
- Bertolani, M.; Rodighiero, E.; Saleri, R.; Pedrazzi, G.; Bertoli, S.; Leone, A.; Feliciani, C.; Lotti, T.; Satolli, F. The Influence of Mediterranean Diet in Acne Pathogenesis and the Correlation with Insulin-like Growth Factor-1 Serum Levels: Implications and Results. Dermatol. Rep. 2022, 14, 9143. [Google Scholar] [CrossRef]
- Chandak, S.; Singh, A.; Madke, B.; Jawade, S.; Khandelwal, R. Acne Vulgaris and Metabolic Syndrome: A Possible Association. Cureus 2022, 14, e24750. [Google Scholar] [CrossRef]
- Bungau, A.F.; Radu, A.F.; Bungau, S.G.; Vesa, C.M.; Tit, D.M.; Endres, L.M. Oxidative Stress and Metabolic Syndrome in Acne Vulgaris: Pathogenetic Connections and Potential Role of Dietary Supplements and Phytochemicals. Biomed. Pharmacother. 2023, 164, 115003. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, Y.H.; Wertz, P.W.; Kim, H.J.; Kim, Y.H. Exploring Acne Treatments: From Pathophysiological Mechanisms to Emerging Therapies. Int. J. Mol. Sci. 2024, 25, 5302. [Google Scholar] [CrossRef]
- ElAttar, Y.; Mourad, B.; Alngomy, H.A.; Shams El Deen, A.; Ismail, M. Study of Interleukin-1 Beta Expression in Acne Vulgaris and Acne Scars. J. Cosmet. Dermatol. 2022, 21, 4864–4870. [Google Scholar] [CrossRef]
- Bergler-Czop, B.; Brzezińska-Wcisło, L. Pro-Inflammatory Cytokines in Patients with Various Kinds of Acne Treated with Isotretinoin. Postep. Dermatol. Alergol. 2014, 31, 21–28. [Google Scholar] [CrossRef]
- Bungau, S.G.; Tit, D.M.; Vesa, C.M.; Abid, A.; Szilagyi, D.-V.; Radu, A.-F.; Bungau, A.F.; Tarce, A.G.; Behl, T.; Stoicescu, M.; et al. Non-Conventional Therapeutical Approaches to Acne Vulgaris Related to Its Association with Metabolic Disorders. Eur. J. Pharmacol. 2022, 923, 174936. [Google Scholar] [CrossRef] [PubMed]
- Dréno, B.; Poli, F.; Pawin, H.; Beylot, C.; Faure, M.; Chivot, M.; Auffret, N.; Moyse, D.; Ballanger, F.; Revuz, J. Development and Evaluation of a Global Acne Severity Scale (GEA Scale) Suitable for France and Europe. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 43–48. [Google Scholar] [CrossRef]
- Bungau, A.F.; Tit, D.M.; Bungau, S.G.; Vesa, C.M.; Radu, A.F.; Marin, R.C.; Endres, L.M.; Moleriu, L.C. Exploring the Metabolic and Endocrine Preconditioning Associated with Thyroid Disorders: Risk Assessment and Association with Acne Severity. Int. J. Mol. Sci. 2024, 25, 721. [Google Scholar] [CrossRef]
- Nast, A.; Dréno, B.; Bettoli, V.; Bukvic Mokos, Z.; Degitz, K.; Dressler, C.; Finlay, A.Y.; Haedersdal, M.; Lambert, J.; Layton, A.; et al. European Evidence-Based (S3) Guideline for the Treatment of Acne—Update 2016—Short Version. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1261–1268. [Google Scholar] [CrossRef]
- Thiboutot, D.; Gollnick, H.; Bettoli, V.; Dréno, B.; Kang, S.; Leyden, J.J.; Shalita, A.R.; Lozada, V.T.; Berson, D.; Finlay, A.; et al. New Insights into the Management of Acne: An Update from the Global Alliance to Improve Outcomes in Acne Group. J. Am. Acad. Dermatol. 2009, 60, S1–S50. [Google Scholar] [CrossRef]
- Reynolds, R.V.; Yeung, H.; Cheng, C.E.; Cook-Bolden, F.; Desai, S.R.; Druby, K.M.; Freeman, E.E.; Keri, J.E.; Stein Gold, L.F.; Tan, J.K.L.; et al. Guidelines of Care for the Management of Acne Vulgaris. J. Am. Acad. Dermatol. 2024, 90, 1006.e1–1006.e30. [Google Scholar] [CrossRef] [PubMed]
- Rademaker, M. Isotretinoin: Dose, Duration and Relapse. What Does 30 Years of Usage Tell Us? Australas. J. Dermatol. 2013, 54, 157–162. [Google Scholar] [CrossRef] [PubMed]
- IPLEDGE Risk Evaluation and Mitigation Strategy (REMS)|FDA. Available online: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/ipledge-risk-evaluation-and-mitigation-strategy-rems (accessed on 14 July 2025).
- Gruszczyńska, M.; Sadowska-Przytocka, A.; Szybiak, W.; Więckowska, B.; Lacka, K. Insulin Resistance in Patients with Acne Vulgaris. Biomedicines 2023, 11, 2294. [Google Scholar] [CrossRef] [PubMed]
- Saraswat, A.; Lahiri, K.; Chatterjee, M.; Barua, S.; Coondoo, A.; Mittal, A.; Panda, S.; Rajagopalan, M.; Sharma, R.; Abraham, A.; et al. Topical Corticosteroid Abuse on the Face: A Prospective, Multicenter Study of Dermatology Outpatients. Indian J. Dermatol. Venereol. Leprol. 2011, 77, 160–166. [Google Scholar] [CrossRef]
- Maskey, A.R.; Sasaki, A.; Sargen, M.; Kennedy, M.; Tiwari, R.K.; Geliebter, J.; Safai, B.; Li, X.M. Breaking the Cycle: A Comprehensive Exploration of Topical Steroid Addiction and Withdrawal. Front. Allergy 2025, 6, 1547923. [Google Scholar] [CrossRef]
- Harlan, S.L. Steroid Acne and Rebound Phenomenon. J. Drugs Dermatol. 2008, 7, 547–550. [Google Scholar]
- Sharma, R.; Abrol, S.; Wani, M. Misuse of Topical Corticosteroids on Facial Skin. A Study of 200 Patients. J. Dermatol. Case Rep. 2017, 11, 5–8. [Google Scholar] [CrossRef]
- Mahar, S.; Mahajan, K.; Agarwal, S.; Kar, H.K.; Bhattacharya, S.K. Topical Corticosteroid Misuse: The Scenario in Patients Attending a Tertiary Care Hospital in New Delhi. J. Clin. Diagn. Res. 2016, 10, FC16. [Google Scholar] [CrossRef]
- Jain, S.; Mohapatra, L.; Mohanty, P.; Jena, S.; Behera, B. Study of Clinical Profile of Patients Presenting with Topical Steroid-Induced Facial Dermatosis to a Tertiary Care Hospital. Indian Dermatol. Online J. 2020, 11, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.A.; Saleh, H.M.; Salazar, F.J. Acneiform Eruptions; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Daniele, S.G.; Kim, S.R.; Grada, A.; Moore, A.Y.; Suozzi, K.C.; Bunick, C.G. Truncal Acne and Scarring: A Comprehensive Review of Current Medical and Cosmetic Approaches to Treatment and Patient Management. Am. J. Clin. Dermatol. 2023, 24, 199–223. [Google Scholar] [CrossRef]
- Ghannoum, M.; Gamal, A.; Kadry, A.; Del Rosso, J.Q.; Stein Gold, L.; Kircik, L.H.; Harper, J.C. Criticality of Benzoyl Peroxide and Antibiotic Fixed Combinations in Combating Rising Resistance in Cutibacterium Acnes. Clin. Cosmet. Investig. Dermatol. 2025, 18, 755–766. [Google Scholar] [CrossRef]
- Li, Y.; Hu, X.; Dong, G.; Wang, X.; Liu, T. Acne Treatment: Research Progress and New Perspectives. Front. Med. 2024, 11, 1425675. [Google Scholar] [CrossRef]
- Thiboutot, D.M.; Dréno, B.; Abanmi, A.; Alexis, A.F.; Araviiskaia, E.; Barona Cabal, M.I.; Bettoli, V.; Casintahan, F.; Chow, S.; da Costa, A.; et al. Practical Management of Acne for Clinicians: An International Consensus from the Global Alliance to Improve Outcomes in Acne. J. Am. Acad. Dermatol. 2018, 78, S1–S23.e1. [Google Scholar] [CrossRef]
- Kurokawa, I.; Kobayashi, M.; Nomura, Y.; Abe, M.; Kerob, D.; Dreno, B. The Role and Benefits of Dermocosmetics in Acne Management in Japan. Dermatol. Ther. 2023, 13, 1423–1433. [Google Scholar] [CrossRef]
- Tempark, T.; Shem, A.; Lueangarun, S. Efficacy of Ceramides and Niacinamide-Containing Moisturizer versus Hydrophilic Cream in Combination with Topical Anti-Acne Treatment in Mild to Moderate Acne Vulgaris: A Split Face, Double-Blinded, Randomized Controlled Trial. J. Cosmet. Dermatol. 2024, 23, 1758–1765. [Google Scholar] [CrossRef]
- Shibata, M.; Katsuyama, M.; Onodera, T.; Ehama, R.; Hosoi, J.; Tagami, H. Glucocorticoids Enhance Toll-like Receptor 2 Expression in Human Keratinocytes Stimulated with Propionibacterium Acnes or Proinflammatory Cytokines. J. Investig. Dermatol. 2009, 129, 375–382. [Google Scholar] [CrossRef]
- Zaenglein, A.L.; Pathy, A.L.; Schlosser, B.J.; Alikhan, A.; Baldwin, H.E.; Berson, D.S.; Bowe, W.P.; Graber, E.M.; Harper, J.C.; Kang, S.; et al. Guidelines of Care for the Management of Acne Vulgaris. J. Am. Acad. Dermatol. 2016, 74, 945–973.e33. [Google Scholar] [CrossRef]
- Grandi, G.; Guariglia, G.; Facchinetti, F. The Role of Combined Oral Contraceptives Containing Norgestimate for Acne Vulgaris Treatment: A Review. Eur. J. Contracept. Reprod. Health Care 2023, 28, 184–191. [Google Scholar] [CrossRef]
- Arowojolu, A.O.; Gallo, M.F.; Lopez, L.M.; Grimes, D.A. Combined Oral Contraceptive Pills for Treatment of Acne. Cochrane Database Syst. Rev. 2012, 2012, CD004425. [Google Scholar] [CrossRef]
- Ebede, T.L.; Arch, E.L.; Berson, D. Hormonal Treatment of Acne in Women. J. Clin. Aesthet. Dermatol. 2009, 2, 16–22. [Google Scholar] [PubMed]
- Mosorin, M.E.; Piltonen, T.; Rantala, A.S.; Kangasniemi, M.; Korhonen, E.; Bloigu, R.; Tapanainen, J.S.; Morin-Papunen, L. Oral and Vaginal Hormonal Contraceptives Induce Similar Unfavorable Metabolic Effects in Women with PCOS: A Randomized Controlled Trial. J. Clin. Med. 2023, 12, 2827. [Google Scholar] [CrossRef]
- Goodman, N.F.; Cobin, R.H.; Futterweit, W.; Glueck, J.S.; Legro, R.S.; Carmina, E. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and Pcos Society Disease State Clinical Review: Guide to the Best Practices in the Evaluation and Treatment of Polycystic Ovary Syndrome—Part 1. Endocr. Pract. 2015, 21, 1291–1300. [Google Scholar] [CrossRef]
- Cappel, M.; Mauger, D.; Thiboutot, D. Correlation between Serum Levels of Insulin-like Growth Factor 1, Dehydroepiandrosterone Sulfate, and Dihydrotestosterone and Acne Lesion Counts in Adult Women. Arch. Dermatol. 2005, 141, 333–338. [Google Scholar] [CrossRef]
- Carmina, E.; Dreno, B.; Lucky, W.A.; Agak, W.G.; Dokras, A.; Kim, J.J.; Lobo, R.A.; Ramezani Tehrani, F.; Dumesic, D. Female Adult Acne and Androgen Excess: A Report From the Multidisciplinary Androgen Excess and PCOS Committee. J. Endocr. Soc. 2022, 6, bvac003. [Google Scholar] [CrossRef]
- Christ, J.P.; Cedars, M.I. Current Guidelines for Diagnosing PCOS. Diagnostics 2023, 13, 1113. [Google Scholar] [CrossRef] [PubMed]
- Kartal, D.; Yildiz, H.; Ertas, R.; Borlu, M.; Utas, S. Association between Isolated Female Acne and Insulin Resistance: A Prospective Study. G. Ital. Dermatol. Venereol. 2016, 151, 353–357. [Google Scholar] [PubMed]
- Thielitz, A.; Gollnick, H. Topical Retinoids in Acne Vulgaris: Update on Efficacy and Safety. Am. J. Clin. Dermatol. 2008, 9, 369–381. [Google Scholar] [CrossRef] [PubMed]
- El-Domyati, M.; Barakat, M.; Awad, S.; Medhat, W.; El-Fakahany, H.; Farag, H. Multiple Microneedling Sessions for Minimally Invasive Facial Rejuvenation: An Objective Assessment. Int. J. Dermatol. 2015, 54, 1361–1369. [Google Scholar] [CrossRef]
- Asif, M.; Kanodia, S.; Singh, K. Combined Autologous Platelet-Rich Plasma with Microneedling Verses Microneedling with Distilled Water in the Treatment of Atrophic Acne Scars: A Concurrent Split-Face Study. J. Cosmet. Dermatol. 2016, 15, 434–443. [Google Scholar] [CrossRef]
- Combined Use of Skin Needling and Platelet-Rich Plasma in Acne Scarring Treatment|Request PDF. Available online: https://www.researchgate.net/publication/283158408_Combined_use_of_skin_needling_and_platelet-rich_plasma_in_acne_scarring_treatment (accessed on 14 July 2025).
- Kang, C.; Lu, D. Combined Effect of Microneedling and Platelet-Rich Plasma for the Treatment of Acne Scars: A Meta-Analysis. Front. Med. 2022, 8, 788754. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.S.; Bagatin, E.; Yang, Z.; Pacheco, R.L.; Magin, P.; de Sá Urtiga Santos, L.; Pereira, T.; Riera, R. Systemic Pharmacological Treatments for Acne: An Overview of Systematic Reviews. Cochrane Database Syst. Rev. 2021, 2021, CD014917. [Google Scholar] [CrossRef]
- Branisteanu, D.; Toader, M.; Porumb, E.; Serban, I.; Pinzariu, A.; Branisteanu, C.; Vicovan, A.; Dimitriu, A.; Fartusnic, I.-A.; Boda, D.; et al. Adult Female Acne: Clinical and Therapeutic Particularities (Review). Exp. Ther. Med. 2022, 23, 151. [Google Scholar] [CrossRef] [PubMed]
- Dréno, B.; Layton, A.; Zouboulis, C.C.; López-Estebaranz, J.L.; Zalewska-Janowska, A.; Bagatin, E.; Zampeli, V.A.; Yutskovskaya, Y.; Harper, J.C. Adult Female Acne: A New Paradigm. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 1063–1070. [Google Scholar] [CrossRef]
- Albalat, W.; Darwish, H.; Abd-Elaal, W.H.; AbouHadeed, M.H.; Essam, R. The Potential Role of Insulin-like Growth Factor 1 in Acne Vulgaris and Its Correlation with the Clinical Response before and after Treatment with Metformin. J. Cosmet. Dermatol. 2022, 21, 6209–6214. [Google Scholar] [CrossRef]
- Kamboj, P.; Kaushik, A.; Handa, S.; Dutta, P.; Saikia, U.N.; Pal, A.; De, D. Effects of Metformin on Clinical, Hormonal and Relevant Gene Expression Parameters in Patients with Acne: An Observational Study. Clin. Exp. Dermatol. 2023, 48, 617–622. [Google Scholar] [CrossRef]
- Nguyen, S.; Nguyen, M.-L.; Roberts, W.S.; Wu, M.; Smith, B.; Rahaman, T.; Nguyen, H. The Efficacy of Metformin as a Therapeutic Agent in the Treatment of Acne Vulgaris: A Systematic Review. Cureus 2024, 16, e56246. [Google Scholar] [CrossRef]
- Yen, H.; Chang, Y.T.; Yee, F.J.; Huang, Y.C. Metformin Therapy for Acne in Patients with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Am. J. Clin. Dermatol. 2021, 22, 11–23. [Google Scholar] [CrossRef]
- Pawelczyk, L.; Spaczynski, R.Z.; Banaszewska, B.; Duleba, A.J. Metformin Therapy Increases Insulin-like Growth Factor Binding Protein-1 in Hyperinsulinemic Women with Polycystic Ovary Syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 113, 209–213. [Google Scholar] [CrossRef]
- Andreadi, A.; Muscoli, S.; Tajmir, R.; Meloni, M.; Minasi, A.; Muscoli, C.; Ilari, S.; Mollace, V.; Della Morte, D.; Bellia, A.; et al. Insulin Resistance and Acne: The Role of Metformin as Alternative Therapy in Men. Pharmaceuticals 2023, 16, 27. [Google Scholar] [CrossRef]
- Shamim, H.; Jean, M.; Umair, M.; Muddaloor, P.; Farinango, M.; Ansary, A.; Dakka, A.; Nazir, Z.; White, C.T.; Habbal, A.B.; et al. Role of Metformin in the Management of Polycystic Ovarian Syndrome-Associated Acne: A Systematic Review. Cureus 2022, 14, e28462. [Google Scholar] [CrossRef]
- Szefler, L.; Szybiak-Skora, W.; Sadowska-Przytocka, A.; Zaba, R.; Wieckowska, B.; Lacka, K. Metformin Therapy for Acne Vulgaris: A Meta-Analysis. Pharmaceuticals 2024, 17, 728. [Google Scholar] [CrossRef]
- Sadati, M.S.; Yazdanpanah, N.; Shahriarirad, R.; Javaheri, R.; Parvizi, M.M. Efficacy of Metformin vs. Doxycycline in Treating Acne Vulgaris: An Assessor-Blinded, Add-on, Randomized, Controlled Clinical Trial. J. Cosmet. Dermatol. 2023, 22, 2816–2823. [Google Scholar] [CrossRef]
- Thiboutot, D.M.; Weiss, J.; Bucko, A.; Eichenfield, L.; Jones, T.; Clark, S.; Liu, Y.; Graeber, M.; Kang, S. Adapalene-Benzoyl Peroxide, a Fixed-Dose Combination for the Treatment of Acne Vulgaris: Results of a Multicenter, Randomized Double-Blind, Controlled Study. J. Am. Acad. Dermatol. 2007, 57, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Versatility of Azelaic Acid (AzA) 15% Gel in Treatment of Acne Vulgaris: A Review of Clinical Literature. J. Am. Acad. Dermatol. 2007, 56, AB18. [CrossRef]
- Addor, F.A.S.; Schalka, S. Acne in Adult Women: Epidemiological, Diagnostic and Therapeutic Aspects. Bras. Dermatol. 2010, 85, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Wise, E.M.; Graber, E.M. Clinical Pearl: Comedone Extraction for Persistent Macrocomedones While on Isotretinoin Therapy. J. Clin. Aesthet. Dermatol. 2011, 4, 20. [Google Scholar]
- Baldwin, H. Oral Antibiotic Treatment Options for Acne Vulgaris. J. Clin. Aesthet. Dermatol. 2020, 13, 26. [Google Scholar] [PubMed]
- Dessinioti, C.; Katsambas, A. Antibiotics and Antimicrobial Resistance in Acne: Epidemiological Trends and Clinical Practice Considerations. Yale J. Biol. Med. 2022, 95, 429. [Google Scholar]
| Therapeutic Component | Product/Approach |
|---|---|
| Systemic antibiotics | Doxycycline 100 mg/day, for 3 months |
| Antihistamine | Desloratadine 5 mg/day, for 3 weeks |
| Corticosteroid withdrawal | Abrupt discontinuation of methylprednisolone aceponate 0.1% |
| Cleanser | Soap-free gel with zinc gluconate and niacinamide, supplemented by micellar water |
| Barrier-repair moisturizer | Ceramide-based emollient with panthenol, applied twice daily |
| Topical anti-acne therapy | Benzoyl peroxide 5% (morning), azelaic acid 15–20% (evening), clindamycin/erythromycin (alternated) |
| Photoprotection | SPF 50+, non-comedogenic fluid, applied daily |
| Cosmetic procedures | Monthly manual comedone extraction under sterile conditions |
| Maintenance phase | Cleansing + retinoid- or fruit acid-based creams 2–3×/week for long-term prevention |
| Therapeutic Component | Product/Approach |
|---|---|
| Systemic antibiotics | Doxycycline 100 mg/day, for 3 months |
| Hormonal therapy | Combined oral contraceptive (ethinylestradiol + cyproterone acetate), for 6 months |
| Cleanser | Gentle gel cleanser, soap-free, for acne-prone skin (twice daily) |
| Morning topical therapy | Benzoyl peroxide 5% (alternating with azelaic acid 20%) |
| Evening topical therapy | Adapalene 0.1% (first choice) or clindamycin/erythromycin on inflamed lesions |
| Moisturizer | Non-comedogenic cream with hyaluronic acid, used after actives |
| Photoprotection | SPF 50+, non-comedogenic, broad spectrum, applied daily |
| Procedures | Monthly comedone extraction and microneedling (dermapen) for atrophic scarring |
| Maintenance strategy | Extended retinoid-based evening routine; monitoring of metabolic profile |
| Therapeutic Component | Product/Approach |
|---|---|
| Systemic antibiotics | Doxycycline 100 mg/day, for 3–4 months |
| Topical regimen | AM: benzoyl peroxide 5% or azelaic acid 20%; PM: adapalene 0.1% or clindamycin/erythromycin |
| Cleanser | Soap-free, non-irritating cleanser for acne-prone skin (2×/day) |
| Moisturizer | Non-comedogenic cream with niacinamide, as needed |
| Photoprotection | Broad-spectrum SPF 50+, non-comedogenic formulation |
| Lifestyle changes | Hypocaloric Mediterranean-style diet + aerobic exercise (150 min/week) |
| Metabolic therapy | Metformin 500–1000 mg/day initiated by diabetologist |
| Cosmetic procedures | Monthly comedone extraction to assist lesion clearance |
| Maintenance phase | Ongoing topical retinoids and cosmetic follow-up, adapted to response and tolerance |
| Parameter | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Age (years) | 21 | 26 | 39 |
| Acne type | Steroid-induced acne | Moderate-to-severe acne (PCOS-related) | Adult-onset acne (metabolic syndrome) |
| Comedones | Present | Numerous | Present |
| Inflammatory lesions | Papules, pustules | Papules, pustules, nodules | Moderate papules |
| Atrophic scarring | Punctate atrophic scars | Present | Absent |
| Seborrhea | Marked | Marked | Moderate |
| Baseline GEA grade | 3 | 4 | 3 |
| Systemic treatment | Doxycycline 100 mg/day, antihistamines | Doxycycline 100 mg/day+ combined oral contraceptives | Doxycycline + metformin |
| Topical treatment | Benzoyl peroxide, azelaic acid, topical antibiotics | Adapalene, benzoyl peroxide, azelaic acid | Tretinoin, benzoyl peroxide, niacinamide |
| Cosmetic procedures | Manual comedone extraction | Comedone extraction, microneedling | Monthly comedone extraction |
| Response at intermediary evaluation | Gradual improvement/GEA grade 3 | Significant improvement/GEA grade 2 | Modest reduction GEA grade 3 |
| Response at 6 months | Near-complete remission/GEA grade 1 | Near remission/GEA grade 1 | Partial remission/GEA grade 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bungau, A.F.; Marin, R.C.; Tit, D.M.; Bungau, G.; Radu, A.; Branisteanu, D.E.; Endres, L.M. Multifactorial Refractory Acne in Women: Insights from a Case Series Involving Hormonal-, Metabolic-, and Corticosteroid-Related Triggers. Life 2025, 15, 1196. https://doi.org/10.3390/life15081196
Bungau AF, Marin RC, Tit DM, Bungau G, Radu A, Branisteanu DE, Endres LM. Multifactorial Refractory Acne in Women: Insights from a Case Series Involving Hormonal-, Metabolic-, and Corticosteroid-Related Triggers. Life. 2025; 15(8):1196. https://doi.org/10.3390/life15081196
Chicago/Turabian StyleBungau, Alexa Florina, Ruxandra Cristina Marin, Delia Mirela Tit, Gabriela Bungau, Ada Radu, Daciana Elena Branisteanu, and Laura Maria Endres. 2025. "Multifactorial Refractory Acne in Women: Insights from a Case Series Involving Hormonal-, Metabolic-, and Corticosteroid-Related Triggers" Life 15, no. 8: 1196. https://doi.org/10.3390/life15081196
APA StyleBungau, A. F., Marin, R. C., Tit, D. M., Bungau, G., Radu, A., Branisteanu, D. E., & Endres, L. M. (2025). Multifactorial Refractory Acne in Women: Insights from a Case Series Involving Hormonal-, Metabolic-, and Corticosteroid-Related Triggers. Life, 15(8), 1196. https://doi.org/10.3390/life15081196







