Coexistence of Acute Appendicitis and Mesenteric Cystic Lymphatic Malformation in an Adult: A Case Report and Narrative Review of Intraoperative Management Strategies
Abstract
1. Introduction
2. Case Presentation
2.1. Patient Information
2.2. Clinical Findings
2.3. Timeline
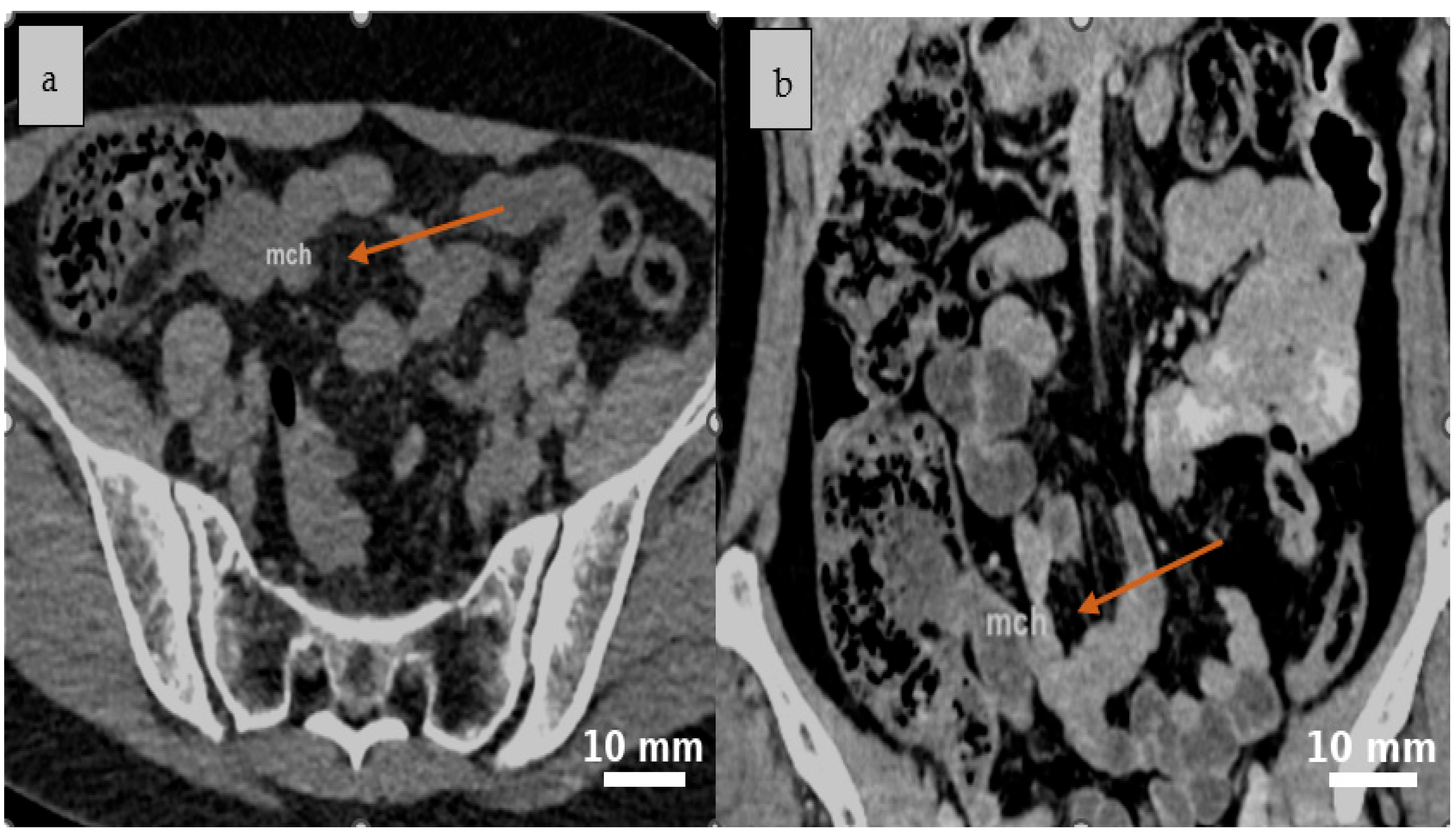
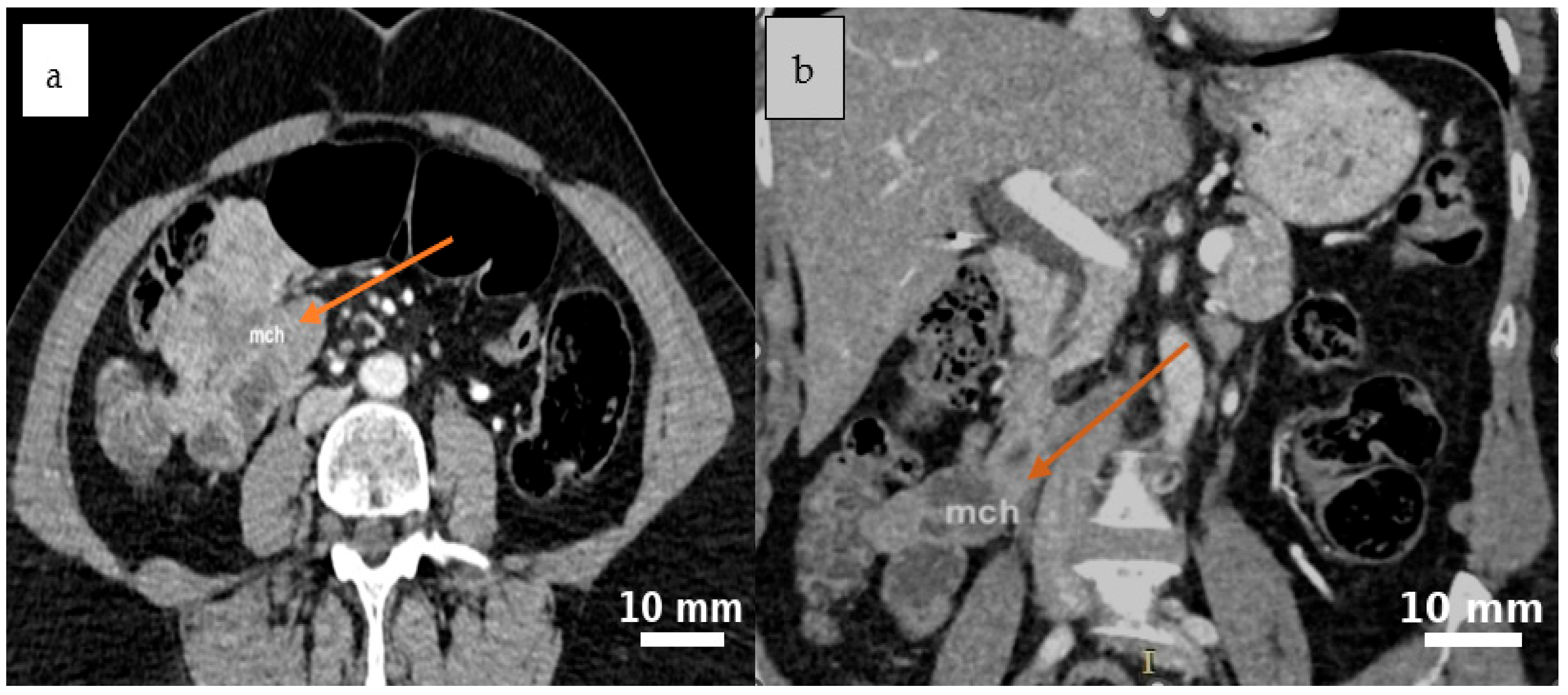
2.4. Diagnostic Assessment
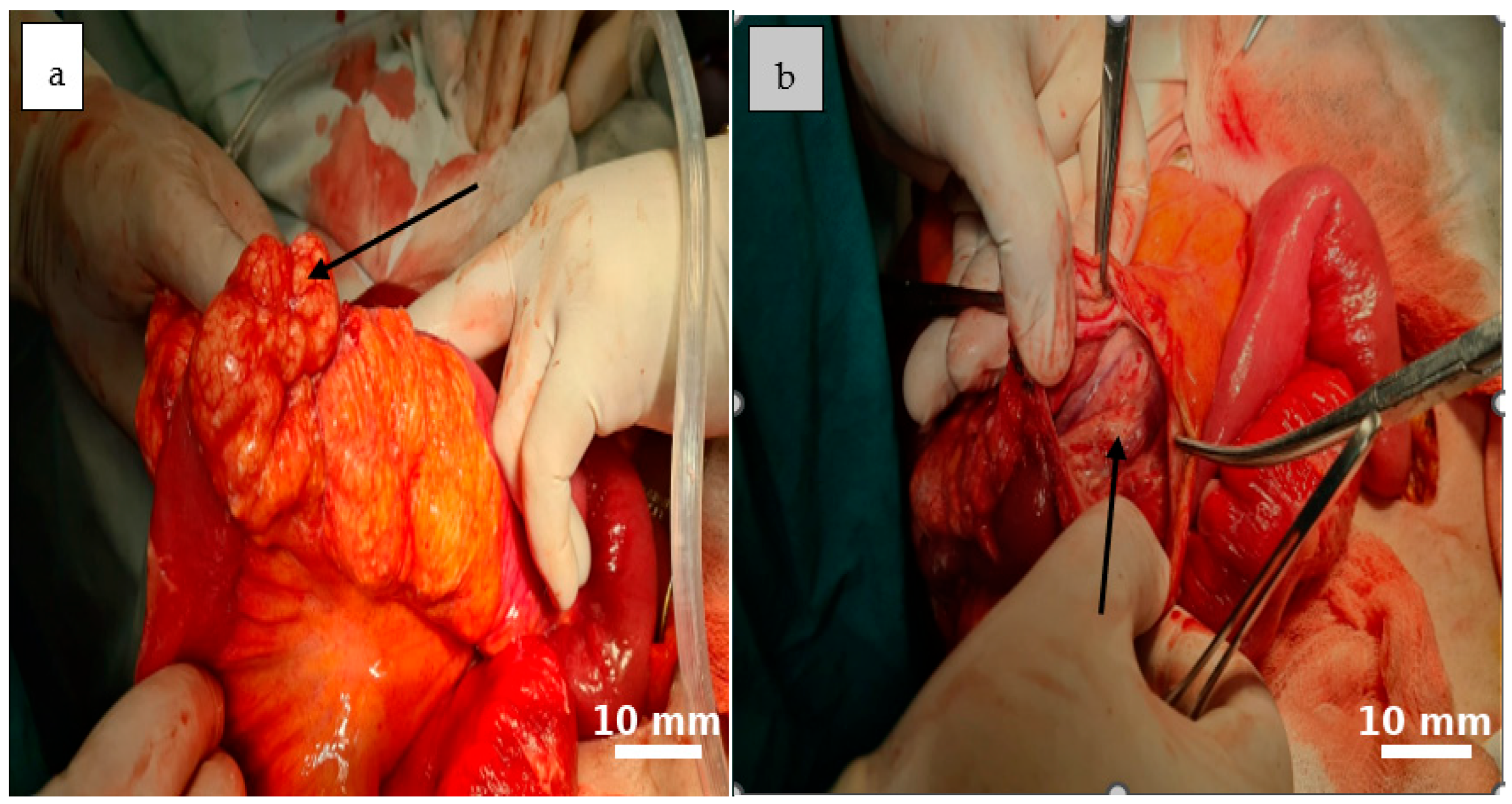
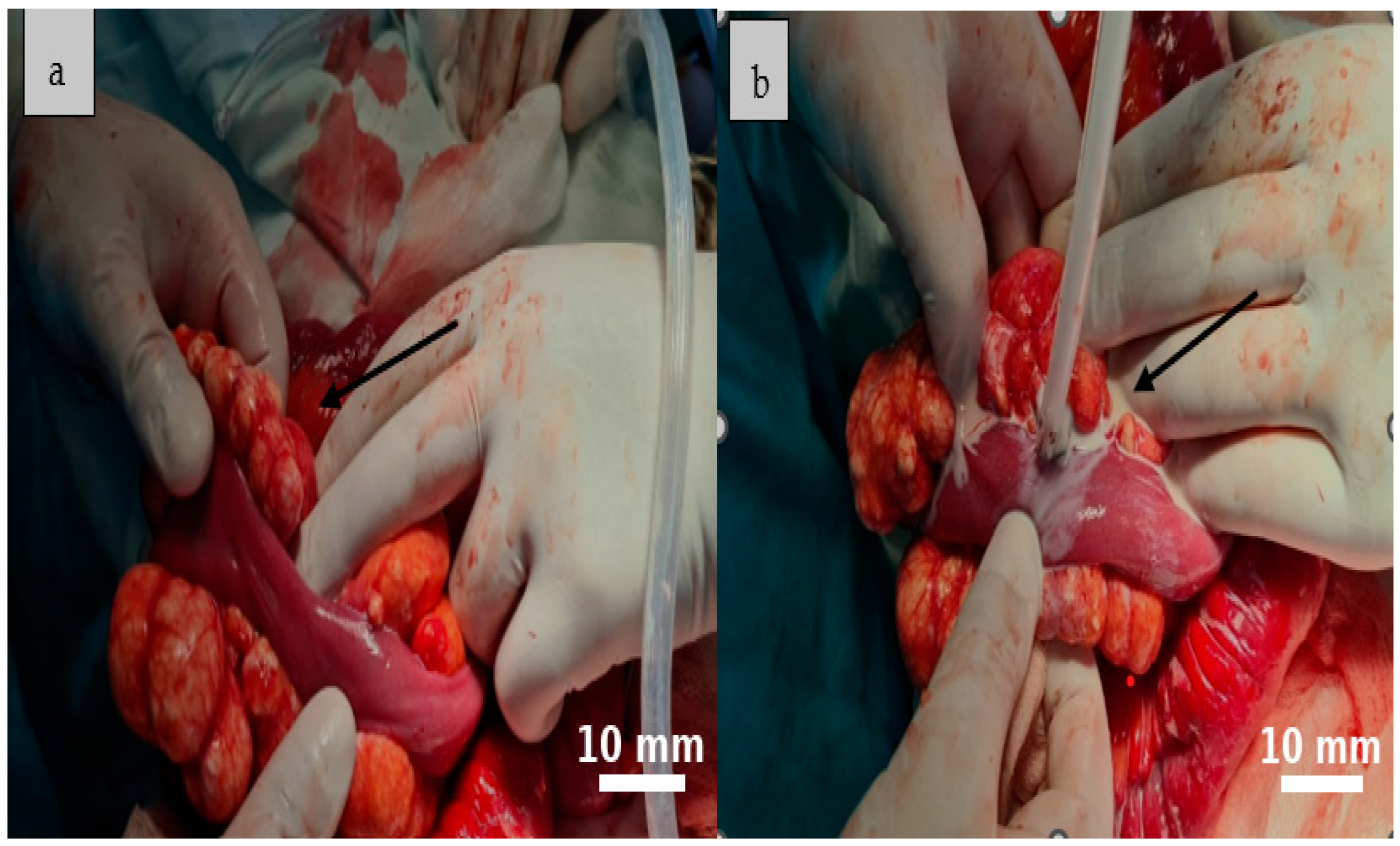
2.5. Therapeutic Intervention
2.6. Follow-Up and Outcomes
2.7. Histopathological Findings
3. Discussion
3.1. Epidemiology and Etiopathogenesis of Mesenteric Cystic Lymphatic Malformations
3.1.1. Rarity and Clinical Significance
3.1.2. Pediatric and Adult Presentations
3.1.3. Pathogenesis and Recurrence Risk
3.1.4. Intraoperative Considerations
3.1.5. Possible Interaction with Acute Inflammation
3.2. Clinical and Imaging Features
3.2.1. Incidental Discovery in Emergency Settings
3.2.2. Differential Diagnosis
3.2.3. Role and Limitations of Imaging
3.3. Incidental Detection and Diagnostic Pitfalls
3.3.1. Incidental Discovery of Mesenteric Lesions
3.3.2. Mesenteric Cystic Lymphatic Malformations in Context
3.3.3. Implications for Emergency Surgery
3.4. Surgical Management and Operative Strategies
3.4.1. Surgical Classification of Intra-Abdominal Lymphatic Malformations
3.4.2. Role of Surgical Exploration in Emergency Settings
3.4.3. Extent of Resection and Patient-Specific Considerations
3.4.4. Surgical Indications and Non-Surgical Alternatives
3.4.5. Prognosis
3.4.6. Minimally Invasive Versus Open Surgery
3.4.7. Recent Literature and Technique Selection
3.4.8. When Complete Excision Is Not Feasible
3.4.9. Intraoperative Decision-Making Principles
3.4.10. Histologic Classification and Diagnosis
3.4.11. Clinical Presentation and Complications
3.4.12. Summary of Reported Cases and Case Relevance
3.4.13. Pathology, Genetic Basis, and Multidisciplinary Management
3.5. Case Contribution and Surgical Challenges
3.6. Limitation of Follow-Up
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saxena, A.; Kakodkar, P.; Zhang, D.; Poulin, A.; Shekari, N.; Kanthan, S.C.; Kanthan, R. Intra-Abdominal Multi Cystic Lymphangiomas: A Case Series with Adult and Pediatric Literature Review. Arch. Clin. Gastroenterol. 2024, 10, 027–039. [Google Scholar] [CrossRef]
- Khunger, N. Lymphatic malformations: Current status. J. Cutan. Aesthet. Surg. 2010, 3, 137–138. [Google Scholar] [CrossRef]
- Yan, J.; Wang, L.; Xie, C.; Peng, C.; Pang, W.; Chen, Y. Preoperative complications in children with mesenteric lymphatic malformations: Incidence, risk factors and outcomes. Front. Pediatr. 2022, 10, 1033897. [Google Scholar] [CrossRef]
- Alqurashi, H.E.; Alaryni, A.A.; Alsairafi, R.A.; Alharbi, A.M.; Alaqla, A.A. Mesenteric Cyst: A Case Report. Cureus 2023, 15, e34325. [Google Scholar] [CrossRef]
- Li, Y.Y.; Wang, Q.; Zhu, J. Mesenteric cystic lymphatic malformation: A rare case report and review of the literature. AME Case Rep. 2023, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Shewaye, A.B.; Berhane, K.A.; Gebresilassie, A.G.; Daniel Fanta, A.; Regassa, M.; Ayalew, F.; Fekede, E.; Ayalew, B.D. Giant Mesenteric Cyst in a Young Adult Mimicking Refractory Ascites: A Diagnostic and Surgical Challenge-A Case Report. Case Rep. Gastrointest. Med. 2025, 2025, 7405161. [Google Scholar] [CrossRef]
- Talarico, F.; Iusco, D.; Negri, L.; Valieri, L. Mesenteric cystic lymphangioma treated with laparoscopic excision: Case report and review of the literature. G. Chir. 2009, 30, 362–364. [Google Scholar] [PubMed]
- Varela, J.E.; Wilson, S.E.; Nguyen, N.T. Laparoscopic surgery significantly reduces surgical-site infections compared with open surgery. Surg. Endosc. 2010, 24, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.; Connelly, M.; Lee, S.; Wang, L.; Nguyen, N. Mesenteric lymphatic malformation associated with acute appendicitis: A case report. J. Med. Case Rep. 2009, 3, 9030. [Google Scholar] [CrossRef]
- Cupido, B.D.; Low, G. Incidental Cystic Lymphangioma of the Small Bowel Mesentery. J. Clin. Imaging Sci. 2015, 5, 55. [Google Scholar] [CrossRef]
- Hancock, B.J.; St-Vil, D.; Luks, F.I.; Di Lorenzo, M.; Blanchard, H. Complications of lymphangiomas in children. J. Pediatr. Surg. 1992, 27, 220–224; discussion 224–226. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.C.; Song, O.P. Cystic lymphangioma of the jejunal mesentery presenting with acute abdomen in an adult. Can. J. Surg. 2009, 52, E286–E288. [Google Scholar] [PubMed]
- Luks, V.L.; Kamitaki, N.; Vivero, M.P.; Uller, W.; Rab, R.; Bovée, J.V.; Rialon, K.L.; Guevara, C.J.; Alomari, A.I.; Greene, A.K.; et al. Lymphatic and other vascular malformative/overgrowth disorders are caused by somatic mutations in PIK3CA. J. Pediatr. 2015, 166, 1048–1054.e5. [Google Scholar] [CrossRef]
- Kurtz, R.J.; Heimann, T.M.; Holt, J.; Beck, A.R. Mesenteric and retroperitoneal cysts. Ann. Surg. 1986, 203, 109–112. [Google Scholar] [CrossRef]
- Barbu, L.A.; Mărgăritescu, N.-D.; Cercelaru, L.; Vîlcea, I.-D.; Șurlin, V.; Mogoantă, S.-S.; Mogoș, G.F.R.; Țenea Cojan, T.S.; Vasile, L. Mesenteric Cysts as Rare Causes of Acute Abdominal Masses: Diagnostic Challenges and Surgical Insights from a Literature Review. J. Clin. Med. 2025, 14, 4888. [Google Scholar] [CrossRef]
- Barbu, L.A.; Mărgăritescu, N.D.; Ghiluşi, M.C.; Belivacă, D.; Georgescu, E.F.; Ghelase, Ş.M.; Marinescu, D. Severe upper gastrointestinal bleeding from gastrointestinal stromal tumor of the stomach. Rom. J. Morphol. Embryol. 2016, 57, 1397–1401. [Google Scholar]
- Dufay, C.; Abdelli, A.; Le Pennec, V.; Chiche, L. Mesenteric tumors: Diagnosis and treatment. J. Visc. Surg. 2012, 149, e239–e251. [Google Scholar] [CrossRef]
- Barbu, L.A.; Vasile, L.; Țenea-Cojan, T.Ș.; Mogoș, G.F.R.; Șurlin, V.; Vîlcea, I.D.; Cercelaru, L.; Mogoantă, S.Ș.; Mărgăritescu, N.D. Endometrioid adenofibroma of ovary—A literature review. Rom. J. Morphol. Embryol. 2025, 66, 39–49. [Google Scholar] [CrossRef]
- Rao, P.M.; Rhea, J.T.; Novelline, R.A.; Mostafavi, A.A.; McCabe, C.J. Effect of computed tomography of the appendix on treatment of patients and use of hospital resources. N. Engl. J. Med. 1998, 338, 141–146. [Google Scholar] [CrossRef]
- Losanoff, J.E.; Richman, B.W.; El-Sherif, A.; Rider, K.D.; Jones, J.W. Mesenteric cystic lymphangioma. J. Am. Coll. Surg. 2003, 196, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Siddique, K.; Bhandari, S.; Basu, S. Giant mesenteric lymphangioma: A rare cause of a life-threatening complication in an adult. BMJ Case Rep. 2010, 2010, bcr0420102896. [Google Scholar] [CrossRef] [PubMed]
- Iwabuchi, A.; Otaka, M.; Okuyama, A.; Jin, M.; Otani, S.; Itoh, S.; Sasahara, H.; Odashima, M.; Kotanagi, H.; Satoh, M.; et al. Disseminated intra-abdominal cystic lymphangiomatosis with severe intestinal bleeding. A case report. J. Clin. Gastroenterol. 1997, 25, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Rieker, R.J.; Quentmeier, A.; Weiss, C.; Kretzschmar, U.; Amann, K.; Mechtersheimer, G.; Bläker, H.; Herwart, O.F. Cystic lymphangioma of the small-bowel mesentery: Case report and a review of the literature. Pathol. Oncol. Res. 2000, 6, 146–148. [Google Scholar] [CrossRef]
- van Randen, A.; Laméris, W.; van Es, H.W.; van Heesewijk, H.P.; van Ramshorst, B.; Ten Hove, W.; Bouma, W.H.; van Leeuwen, M.S.; van Keulen, E.M.; Bossuyt, P.M.; et al. OPTIMA Study Group. A comparison of the accuracy of ultrasound and computed tomography in common diagnoses causing acute abdominal pain. Eur. Radiol. 2011, 21, 1535–1545. [Google Scholar] [CrossRef]
- Nepal, P.; Wells, M.; Ojili, V.; Khandelwal, K.; Lalwani, N.; Khandelwal, A. Problem-solving with MRI in acute abdominopelvic conditions, part 1: Gastrointestinal, hepatobiliary, and pancreatic diseases. Emerg. Radiol. 2021, 28, 1161–1172. [Google Scholar] [CrossRef]
- Humes, D.J.; Simpson, J. Acute appendicitis. BMJ 2006, 333, 530–534. [Google Scholar] [CrossRef]
- Sauerland, S.; Jaschinski, T.; Neugebauer, E.A. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst. Rev. 2010, CD001546. [Google Scholar] [CrossRef]
- Udwadia, T.E. Diagnostic laparoscopy. Surg. Endosc. 2004, 18, 6–10. [Google Scholar] [CrossRef]
- Hebra, A.; Brown, M.F.; McGeehin, K.M.; Ross, A.J., 3rd. Mesenteric, omental, and retroperitoneal cysts in children: A clinical study of 22 cases. South. Med. J. 1993, 86, 173–176. [Google Scholar] [CrossRef]
- de Perrot, M.; Bründler, M.; Tötsch, M.; Mentha, G.; Morel, P. Mesenteric cysts. Toward less confusion? Dig. Surg. 2000, 17, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Mărgăritescu, N.D.; Ciobanu, M.O.; Nemeş, R.N.; Ghelase, Ş.M.; Pleşea, R.M.; Georgescu, I.; Voinea, B.; Pleşea, I.E.; Chiuțu, L.C. The morphological profile of small bowel tumors—Our experience. Rom. J. Morphol. Embryol. 2016, 57, 1241–1252. [Google Scholar] [PubMed]
- Yin, W.; Yu, R.; Xia, D. A gaint mesenteric cystic lymphangioma of small intestinal in an adult: A case report and literature review. Medicine 2025, 104, e42394. [Google Scholar] [CrossRef]
- Watanabe, A.; Suzuki, H.; Kubo, N.; Kobayashi, T.; Araki, K.; Sasaki, S.; Shimura, T.; Oyama, T.; Kuwano, H. A case of mesenteric cystic lymphangioma in an adult which caused duodenal stenosis after resection. Int. J. Surg. Case Rep. 2013, 4, 212–215. [Google Scholar] [CrossRef]
- Bellamlih, H.; El Farouki, A.; Mssrouri, R.; Derqaoui, S.; Jahid, A.; Moatassim Billah, N.; Nassar, I. Cystic mass of the right iliac fossa: Don’t forget about lymphatic malformation. BJR Case Rep. 2020, 7, 20200165. [Google Scholar] [CrossRef]
- Zhuo, C.H.; Shi, D.B.; Ying, M.G.; Cheng, Y.F.; Wang, Y.W.; Zhang, W.M.; Cai, S.J.; Li, X.X. Laparoscopic segmental colectomy for colonic lymphangiomas: A definitive, minimally invasive surgical option. World J. Gastroenterol. 2014, 20, 8745–8750. [Google Scholar] [CrossRef]
- Bezzola, T.; Bühler, L.; Chardot, C.; Morel, P. Le traitement chirurgical du lymphangiome kystique abdominal chez l’adulte et chez l’enfant [Surgical therapy of abdominal cystic lymphangioma in adults and children]. J. Chir. (In French). 2008, 145, 238–243. [Google Scholar] [CrossRef]
- Amrutha, T.; Kuladeep, B.; Mendon, A.S.; Parikshith, H. Cyst in the Mist: A Surgical Perspective on Mesenteric Cysts. Cureus 2025, 17, e381178. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.T.M.; Chok, A.Y.; Farah, B.L.; Yan, Y.Y.; Toh, E.L. Spontaneous partial regression of a microcystic jejunal mesenteric lymphangioma and a proposed management algorithm. BMJ Case Rep. 2019, 12, e231037. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.N.; Chen, C.P.; Lin, C.J.; Chen, S.W. Prenatal Ultrasound Evaluation and Outcome of Pregnancy with Fetal Cystic Hygromas and Lymphangiomas. J. Med. Ultrasound. 2017, 25, 12–15. [Google Scholar] [CrossRef]
- Prior-Rosas, J.E.; Mejía-Ruíz, B.; Magdaleno-Becerra, B.A.; Nava-Tenorio, C.G.; Alonso-Domínguez, S.M.; Botello-Ortiz, G.E. Giant benign mesenteric cysts (mesothelioma and lymphangioma): A report of two cases. Int. J. Surg. Case Rep. 2024, 125, 110587. [Google Scholar] [CrossRef]
- Aprea, G.; Guida, F.; Canfora, A.; Ferronetti, A.; Giugliano, A.; Ciciriello, M.B.; Savanelli, A.; Amato, B. Mesenteric cystic lymphangioma in adult: A case series and review of the literature. BMC Surg. 2013, 13 (Suppl. 1), A4. [Google Scholar] [CrossRef]
- Chen, C.W.; Hsu, S.D.; Lin, C.H.; Cheng, M.F.; Yu, J.C. Cystic lymphangioma of the jejunal mesentery in an adult: A case report. World J. Gastroenterol. 2005, 11, 5084–5086. [Google Scholar] [CrossRef]
- Chin, S.; Kikuyama, S.; Hashimoto, T.; Tomita, T.; Hasegawa, T.; Ohno, Y. Lymphangioma of the jejunal mesentery in an adult: A case report and a review of the Japanese literature. Keio J. Med. 1993, 42, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Fundarò, S.; Medici, L.; Perrone, S.; Natalini, G. Cystic lymphangioma of the mesentery. A case of intestinal obstruction and a brief review of the literature. Minerva Chir. 1998, 53, 939–942. (In Italian) [Google Scholar] [PubMed]
- Yoo, E.; Kim, M.J.; Kim, K.W.; Chung, J.J.; Kim, S.H.; Choi, J.Y. A case of mesenteric cystic lymphangioma: Fat saturation and chemical shift MR imaging. J. Magn. Reson. Imaging 2006, 23, 77–80. [Google Scholar] [CrossRef]
- Tsukada, H.; Takaori, K.; Ishiguro, S.; Tsuda, T.; Ota, S.; Yamamoto, T. Giant cystic lymphangioma of the small bowel mesentery: Report of a case. Surg. Today 2002, 32, 734–737. [Google Scholar] [CrossRef]
- Nagano, H.; Kimura, T.; Iida, A.; Togawa, T.; Goi, T.; Sato, Y. Cystic lymphangioma in the peripheral jejunal mesentery in an adult and excision with laparoscopic-assisted surgery: A case report. World J. Surg. Oncol. 2019, 17, 170. [Google Scholar] [CrossRef]
- Jayasundara, J.; Perera, E.; Chandu de Silva, M.V.; Pathirana, A.A. Lymphangioma of the jejunal mesentery and jejunal polyps presenting as an acute abdomen in a teenager. Ann. R. Coll. Surg. Engl. 2017, 99, e108–e109. [Google Scholar] [CrossRef]
- Khattala, K.; Rami, M.; Elmadi, A.; Mahmoudi, A.; Bouabdallah, Y. Giant cystic lymphangioma of the small bowel mesentery: Case report. Pan Afr. Med. J. 2011, 9, 46. [Google Scholar] [CrossRef][Green Version]
- Ramírez-Ortega, M.A.; Villegas-Romero, J.; Márquez-Díaz, A.; Gómez-Díaz, A. A cystic mesenteric lymphangioma presented at the colon sigmoid. Case report. Rev. Med. Inst. Mex. Seguro Soc. 2010, 48, 557–562. [Google Scholar]
- Mahfoud, H.; Flissate, F.; Tligui, S.; Benammi, S.; Etber, A.; Baidada, A. Mesenteric cystic lymphangioma misdiagnosed as ovarian cyst in a 63-year-old female: A case report and review of literature. Int. J. Surg. Case Rep. 2024, 120, 109846. [Google Scholar] [CrossRef] [PubMed]
- Wilting, J.; Papoutsi, M.; Christ, B.; Nicolaides, K.H.; von Kaisenberg, C.S.; Borges, J.; Stark, G.B.; Alitalo, K.; Tomarev, S.I.; Niemeyer, C.; et al. The transcription factor Prox1 is a marker for lymphatic endothelial cells in normal and diseased human tissues. Faseb J. 2002, 16, 1271–1273. [Google Scholar] [CrossRef]
- Lui, S.A.; Nyo, Y.L.; Mali, V.P. Ileal Cystic Lymphangioma presenting with Acute Appendicitis. J. Indian. Assoc. Pediatr. Surg. 2018, 23, 36–38. [Google Scholar] [CrossRef] [PubMed]
- Barbu, L.A.; Mărgăritescu, N.D.; Cercelaru, L.; Caragea, D.C.; Vîlcea, I.D.; Șurlin, V.; Mogoantă, S.Ș.; Mogoș, G.F.R.; Vasile, L.; Țenea Cojan, T.Ș. Can Thrombosed Abdominal Aortic Dissecting Aneurysm Cause Mesenteric Artery Thrombosis and Ischemic Colitis?- A Case Report and a Review of Literature. J. Clin. Med. 2025, 14, 3092. [Google Scholar] [CrossRef] [PubMed]
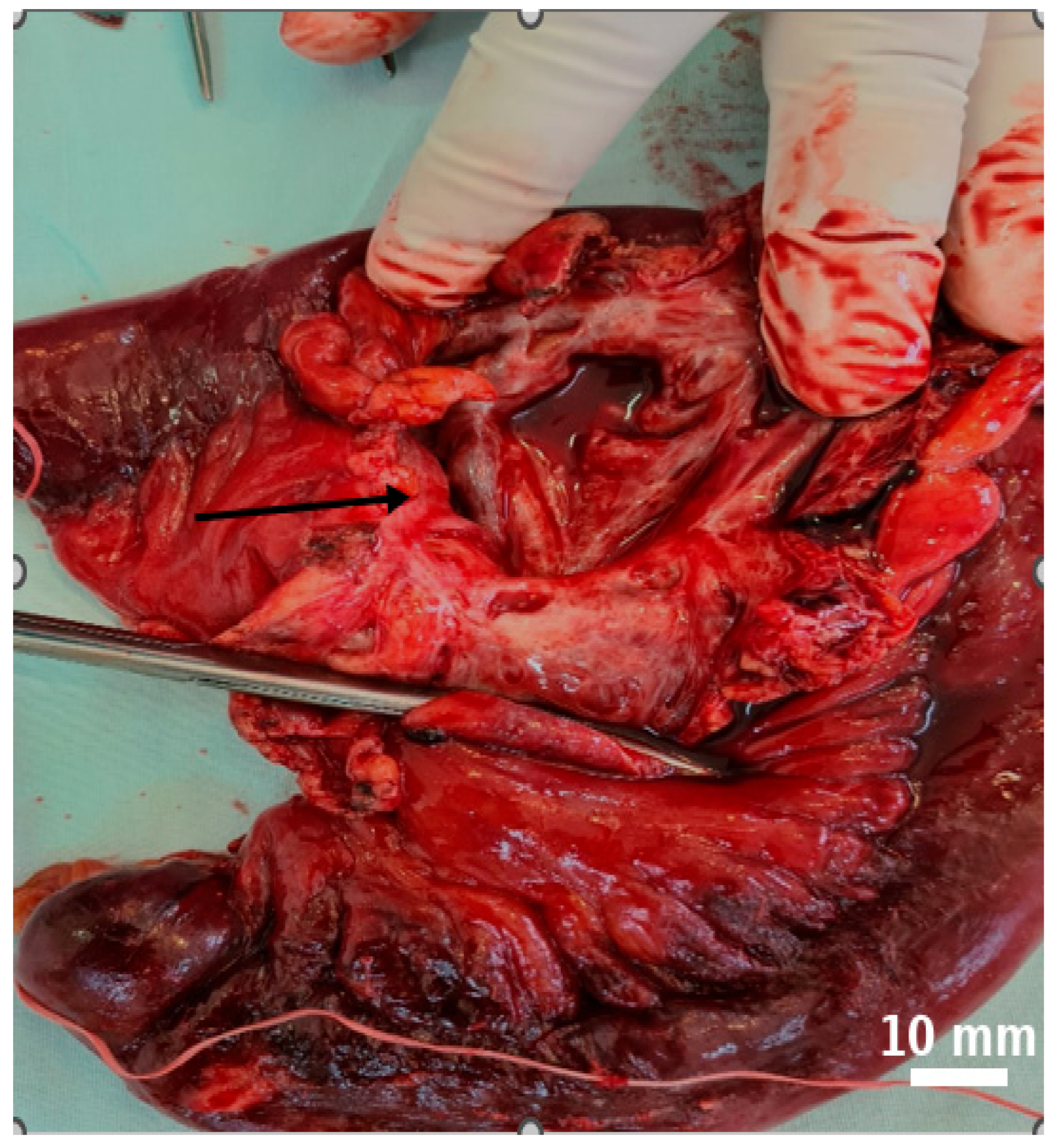
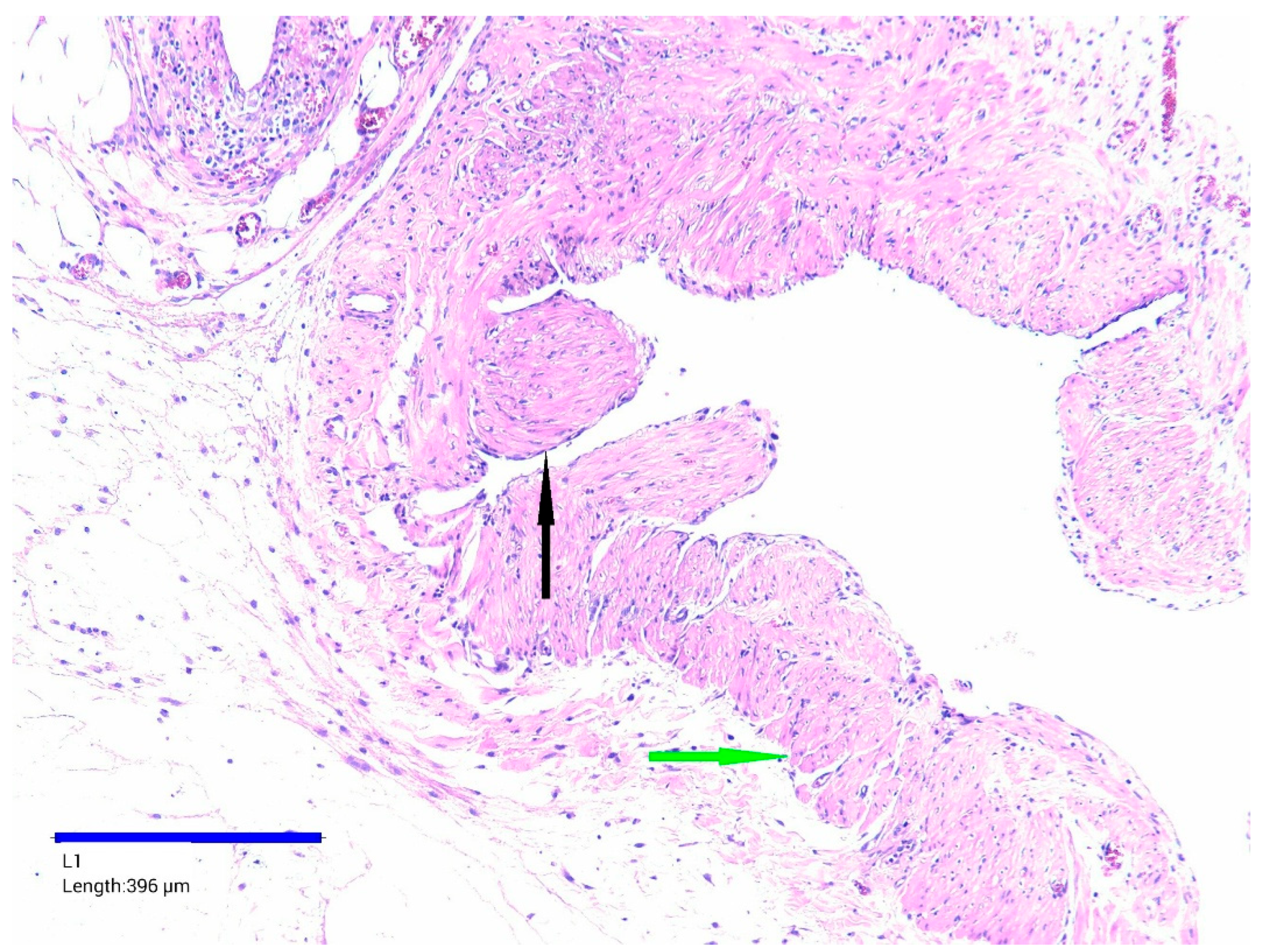
| Day | Clinical Event |
|---|---|
| Day 0 | Onset of right lower quadrant pain, associated with nausea and vomiting. |
| Day 1 (Morning) | Worsening abdominal pain; presentation to the Emergency Department. |
| Physical examination, laboratory investigations, and contrast-enhanced CT imaging performed. | |
| Day 1 (Afternoon) | Decision for emergency surgical intervention; midline laparotomy performed. |
| Postoperative Day 1–2 | Intravenous antibiotics administered; patient tolerated oral intake by Day 2. |
| Postoperative Day 3–5 | Continued clinical improvement with no complications. |
| Postoperative Day 5 | Discharged home in stable condition. |
| Parameter | Laborator Results | Normal Range |
|---|---|---|
| White blood cell | 13 × 103 cells/µL | 4–10 × 103 cells/µL |
| Hemoglobin | 14 g/dl | 13.5–17.5 g/d |
| Platelet count | 340 × 103 cells/µL | 150–450 × 103 cells/µL |
| Neutrophilia | 85.14% | 40–70% |
| Creatinine | 1 mg/dL | 0.6–1.3 mg/dL |
| Urea | 100 mg/dL | 15–45 mg/dL |
| INR | 1.3 | 0.8–1.2 |
| C-reactive protein | 25 mg/dL | <0.5 mg/dL |
| Fibrinogen | 450 mg/dL | 200–400 mg/dL |
| Parameter | Laparoscopic Approach | Open Surgery |
|---|---|---|
| Access to retroperitoneal cysts | Limited exposure; may require conversion to open in complex or extensive cases | Excellent exposure for large, deep, or complex lesions |
| Hemorrhage control | Technically more challenging due to limited working space | More straightforward control, especially for major vessels |
| Hospital stay duration | Typically shorter with faster recovery | Slightly longer due to larger incision and postoperative pain |
| Emergency suitability | Feasible in selected cases with experienced team; useful for diagnostic exploration and optimal incision planning | Generally appropriate for acute presentations or when extensive resection anticipated |
| Author | Patient Type | Surgical Intervention | Imaging/IHC | Follow-Up | Key Findings |
|---|---|---|---|---|---|
| Saxena [1] | Adult | Cystectomy ± bowel resection | D2-40, CD31 | ~50 months | Complete resection with no recurrence |
| Yin [32] | Adult | Complete resection | CT/MRI | 1 month | Large cyst (~15 cm) with chronic course over 20 years |
| Li [5] | Adult (sigmoid) | Laparoscopic fenestration and drainage | EUS, MRI, IHC | 3 months | Rare sigmoid location, successfully managed laparoscopically |
| Amrutha [33] | Adult | Elective laparotomy with complete excision | Ultrasound + CECT | Uneventful recovery | Large (12 cm × 15 cm) mesenteric cyst mimicking gynecologic pathology; successful complete resection |
| Yan [3] | Pediatric | Robotic laparoscopic management | Clinical + radiological evaluation | Not reported | Cysts ≥ 7.5 cm and jejunal location correlated with higher complication risk |
| Alqurashi [4] | Adult | Segmental colectomy (8 cm) + cyst excision | CT abdomen | Uneventful postoperative recovery | Rare localization (transverse mesocolon); complete resection with good outcome |
| Proposed Intraoperative Management Algorithm for Mesenteric Cystic Lymphatic Malformations |
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Author (Ref.) | Age at Diagnosis | Sex | Gastrointestinal Simptoms | Cystic Size | Surgical Treatment | Localization |
|---|---|---|---|---|---|---|
| Prior-Rosas [40] | 47 years | Male | Abdominal pain and distension, dyspepsia | 20.73 cm × 21 cm | Complete resection of the cyst | Ileum |
| Aprea [41] | 67 years | Female | Distension, hyper active bowel sounds and tenderness | 6 × 3.5 cm | Complete resection of the cyst and appendicectomy | Ileum |
| Aprea [41] | 68 years | Male | Abdominal pain and a mass in RIF and RH | 10 × 10 cm | Complete resection of the cyst and Choledochorrhaphy | Common hepatic duct |
| Aprea [41] | 80 years | Male | Abdominal pain and occlusive state | 8.6 × 5 cm | Complete resection | Jejunal loops and stomach |
| Aprea [41] | 65 years | Male | Asymptomatic | 18 × 12 cm | Complete resection | Stomach and pancreas |
| Chen [42] | 27 years | Female | Abdominal pain | 15 × 8 cm | Complete resection | Jejunum |
| Chin [43] | 34 years | Male | Tumor in the left lower abdomen. | 15 × 12 cm | Enterectomy | Jejunum |
| Fundarò [44] | 45 years | Male | Ileal volvulus | 3.5 × 3 cm | Complete resection of the cyst | Ileum |
| Yoo [45] | 56 years | Female | Epigastric pain | 8 × 8 cm | Complete resection of the cyst | Mesentery |
| Tsukada [46] | 48 years | Male | Abdominal distention and pain | 30 × 5 cm | Complete resection of the cyst | Small bowel mesentery |
| Nagano [47] | 40 years | Male | Tenderness in the lower left quadrant. | 4.3 × 4 cm | Enterectomy | Jejunum |
| Jayasundara [48] | 18 years | Female | Abdominal pain and vomiting | 10 × 10 cm | Enterectomy | Jejunal mesentery |
| Yin [32] | 66 years | Male | Abdominal pain | 20 × 10 cm | Enterectomy | Jejunal mesentery |
| Khattala [49] | 12 years | Female | Palpable abdominopelvic mass | 20 × 5 cm | Enterectomy | Jejunal mesentery |
| Ramirez-Ortega [50] | 5 years | Male | Abdominal distension | 18 × 11 cm | Complete resection of the cyst and sigmoidectomy | Sigmoid colon |
| Mahfoud [51] | 63 years | Female | Pelvic Pain | 7 × 6 cm | Enterectomy | Jejunal mesentery |
| Siddique [21] | 24 years | Male | Tenderness over the left lower abdomen | 20 × 16 cm | Enterectomy | Jejuno-ileal junction. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbu, L.A.; Cercelaru, L.; Vîlcea, I.-D.; Șurlin, V.; Mogoantă, S.-S.; Țenea Cojan, T.S.; Mărgăritescu, N.-D.; Popescu, M.; Mogoș, G.F.R.; Vasile, L. Coexistence of Acute Appendicitis and Mesenteric Cystic Lymphatic Malformation in an Adult: A Case Report and Narrative Review of Intraoperative Management Strategies. Life 2025, 15, 1390. https://doi.org/10.3390/life15091390
Barbu LA, Cercelaru L, Vîlcea I-D, Șurlin V, Mogoantă S-S, Țenea Cojan TS, Mărgăritescu N-D, Popescu M, Mogoș GFR, Vasile L. Coexistence of Acute Appendicitis and Mesenteric Cystic Lymphatic Malformation in an Adult: A Case Report and Narrative Review of Intraoperative Management Strategies. Life. 2025; 15(9):1390. https://doi.org/10.3390/life15091390
Chicago/Turabian StyleBarbu, Laurențiu Augustus, Liliana Cercelaru, Ionică-Daniel Vîlcea, Valeriu Șurlin, Stelian-Stefaniță Mogoantă, Tiberiu Stefăniță Țenea Cojan, Nicolae-Dragoș Mărgăritescu, Mihai Popescu, Gabriel Florin Răzvan Mogoș, and Liviu Vasile. 2025. "Coexistence of Acute Appendicitis and Mesenteric Cystic Lymphatic Malformation in an Adult: A Case Report and Narrative Review of Intraoperative Management Strategies" Life 15, no. 9: 1390. https://doi.org/10.3390/life15091390
APA StyleBarbu, L. A., Cercelaru, L., Vîlcea, I.-D., Șurlin, V., Mogoantă, S.-S., Țenea Cojan, T. S., Mărgăritescu, N.-D., Popescu, M., Mogoș, G. F. R., & Vasile, L. (2025). Coexistence of Acute Appendicitis and Mesenteric Cystic Lymphatic Malformation in an Adult: A Case Report and Narrative Review of Intraoperative Management Strategies. Life, 15(9), 1390. https://doi.org/10.3390/life15091390






