Periprosthetic Joint Infection by Streptococcus bovis Reveals Hidden Colorectal Cancer: A Case Report
Abstract
1. Introduction
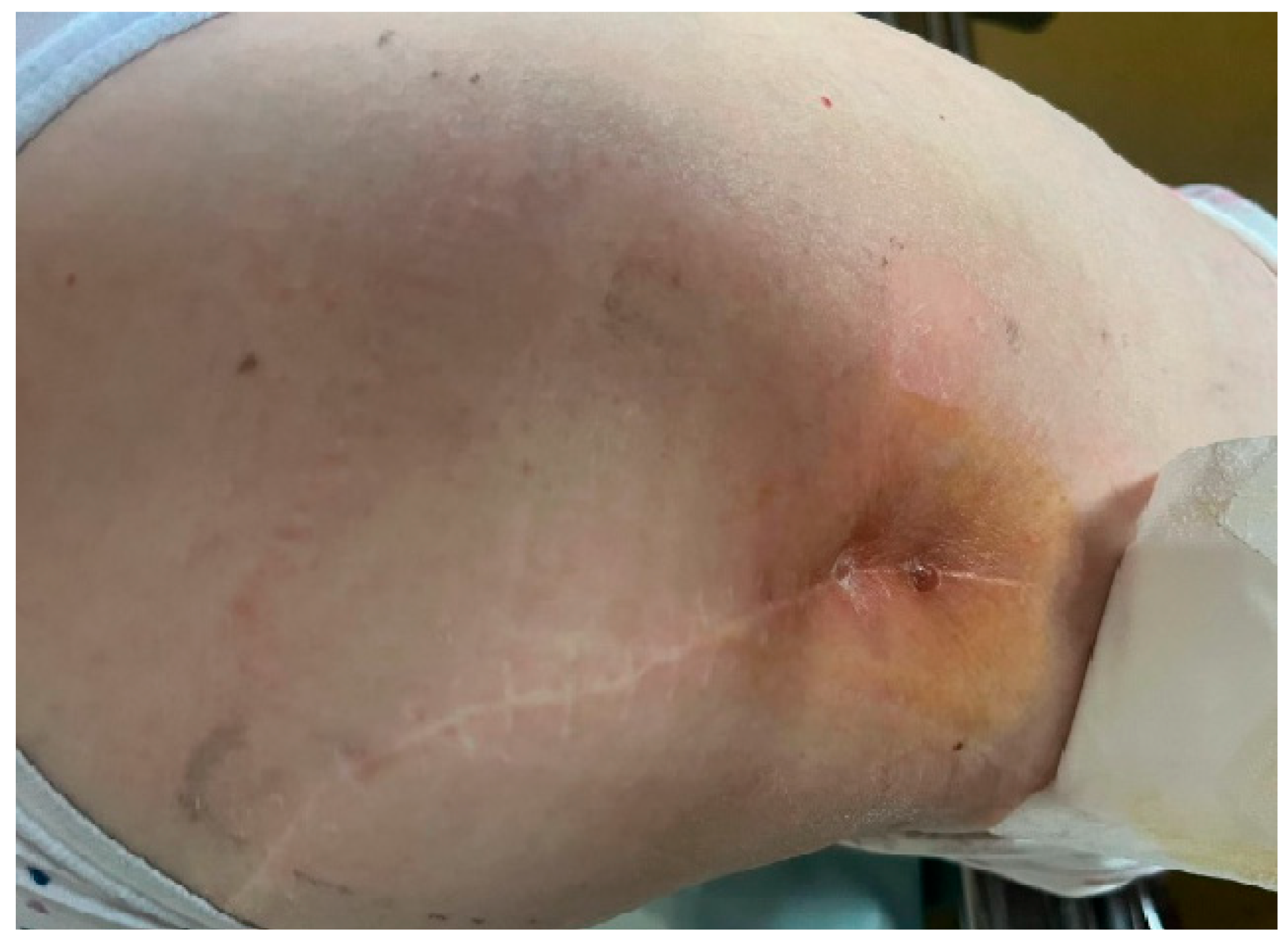
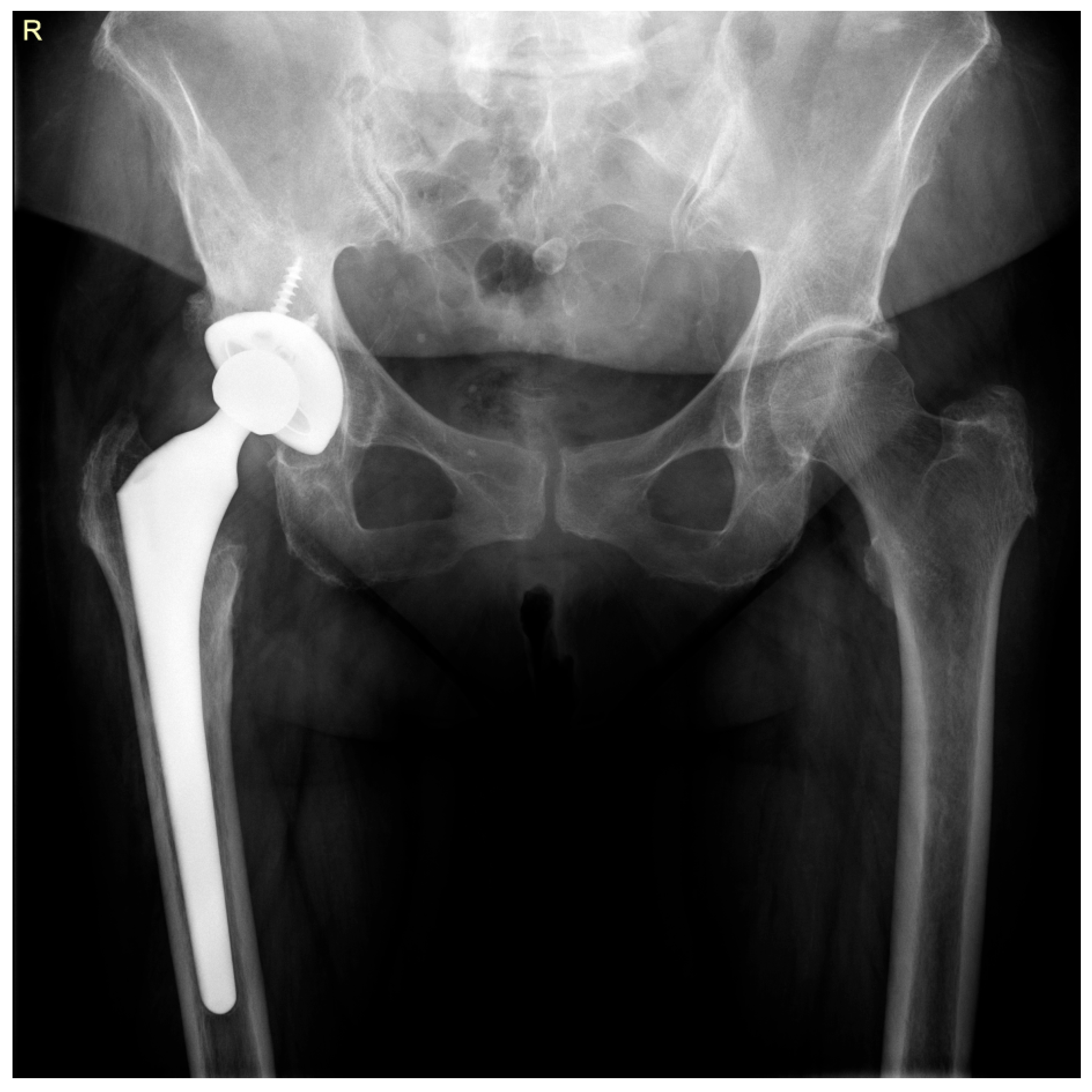
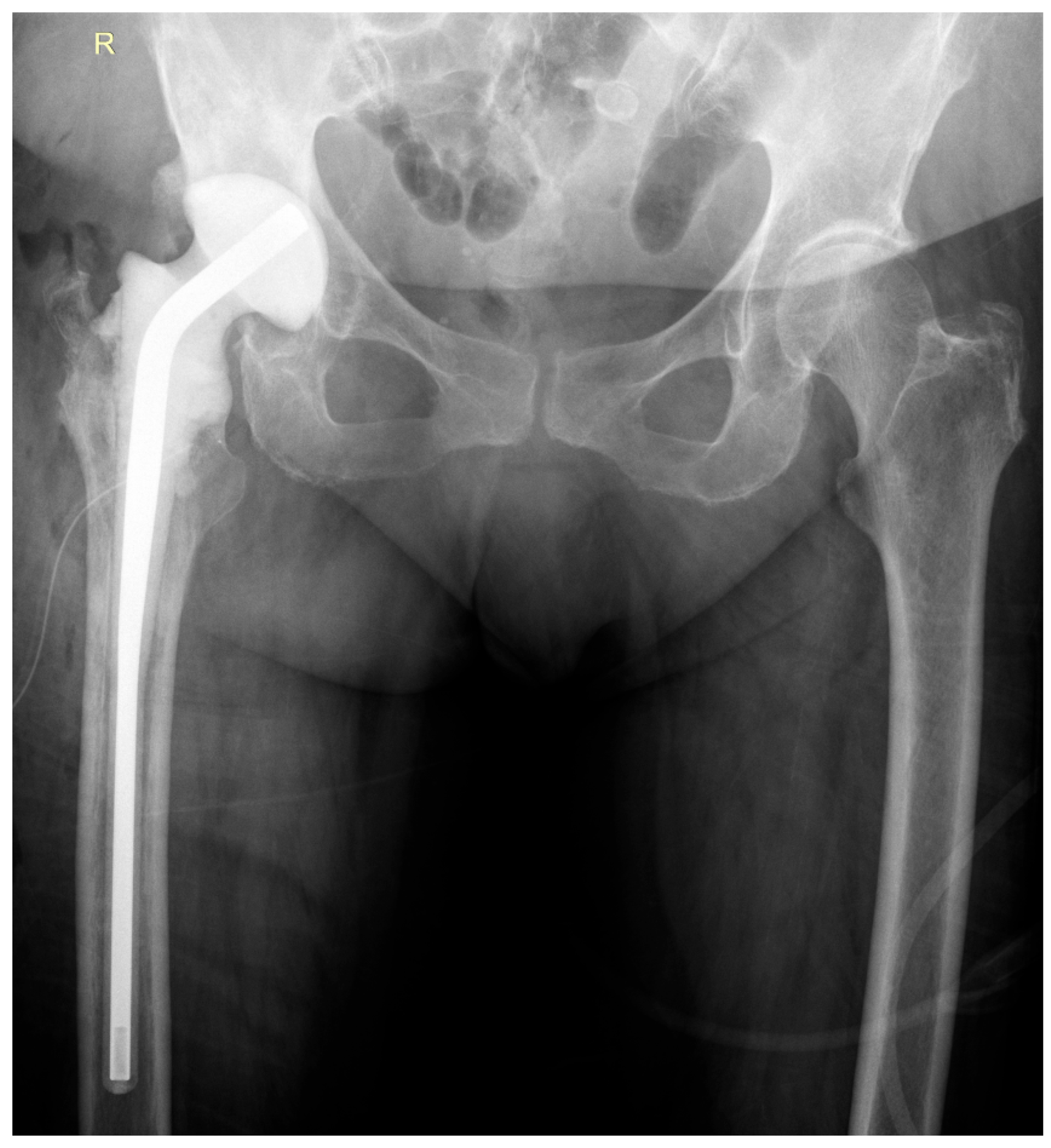
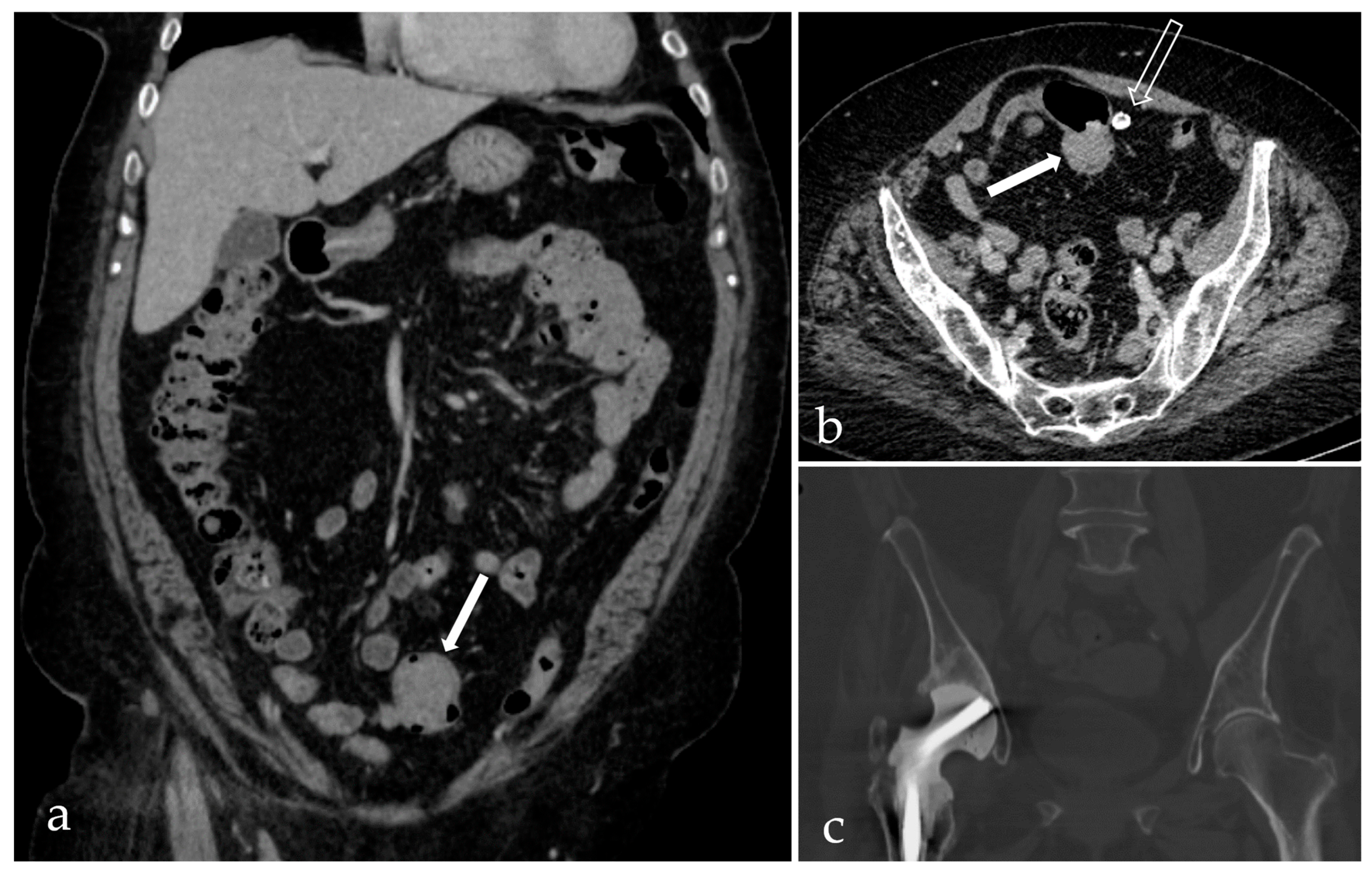
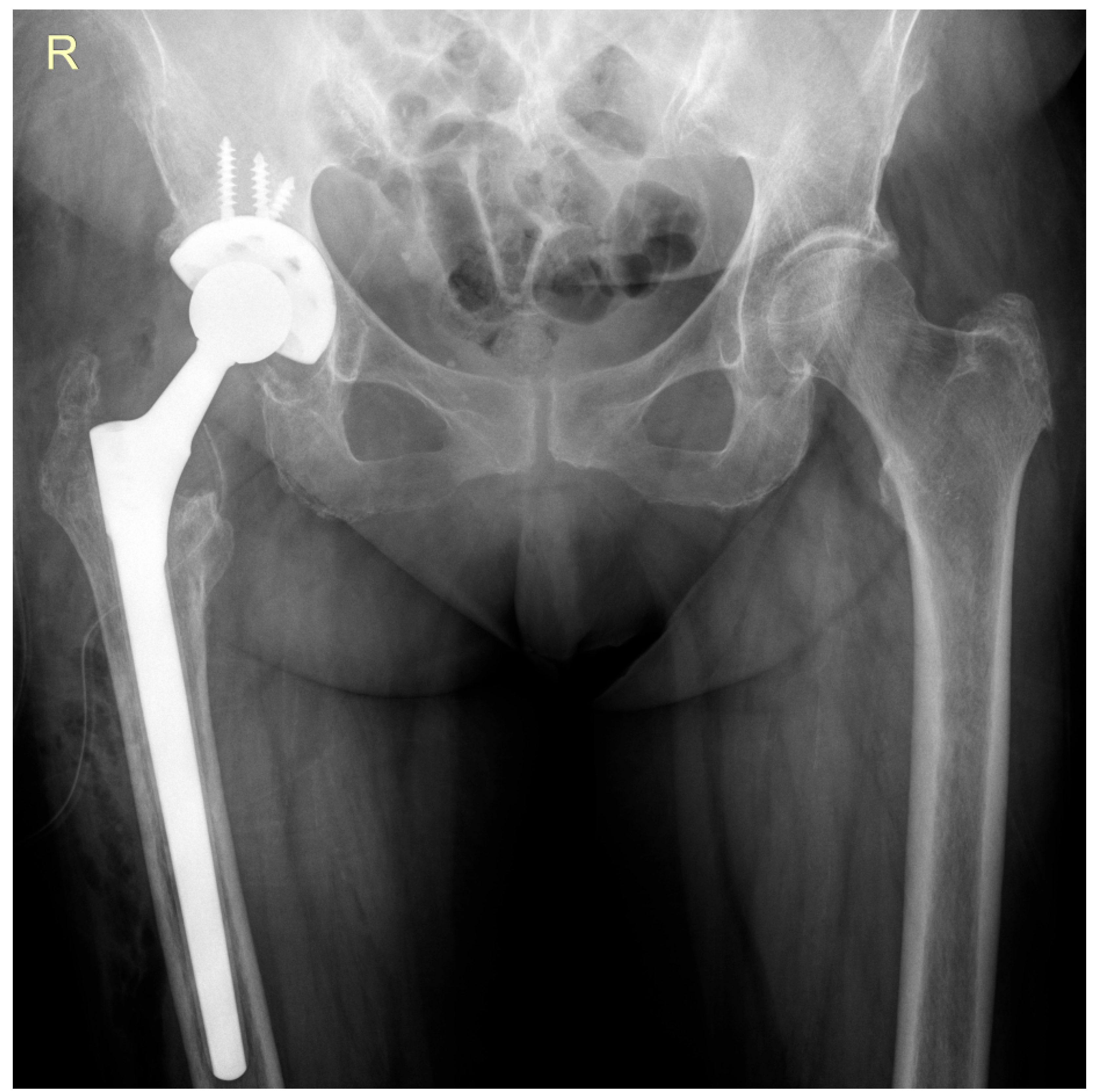
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRP | C-reactive protein |
| ESR | Erythrocyte sedimentation rate |
| Hb | Hemoglobin |
| PJI | Periprosthetic joint infection |
| S. bovis | Streptococcus bovis |
References
- Ayoade, F.; Li, D.; Mabrouk, A.; Todd, J.R. Periprosthetic Joint Infection. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Tarabichi, S.; Chen, A.F.; Higuera, C.A.; Parvizi, J.; Polkowski, G.G. 2022 American Association of Hip and Knee Surgeons Symposium: Periprosthetic Joint Infection. J. Arthroplast. 2023, 38, S45–S49. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.-J.; Yao, F.-M.; He, W.; Wei, Q.-S.; He, M.-C. Incidence of periprosthetic joint infection after primary total hip arthroplasty is underestimated: A synthesis of meta-analysis and bibliometric analysis. J. Orthop. Surg. Res. 2023, 18, 610. [Google Scholar] [CrossRef] [PubMed]
- McNally, M.; Sousa, R.; Wouthuyzen-Bakker, M.; Chen, A.F.; Soriano, A.; Vogely, H.C.; Clauss, M.; Higuera, C.A.; Trebše, R. The EBJIS definition of periprosthetic joint infection. Bone Jt. J. 2021, 103-B, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Kherabi, Y.; Zeller, V.; Kerroumi, Y.; Meyssonnier, V.; Heym, B.; Lidove, O.; Marmor, S. Streptococcal and Staphylococcus aureus prosthetic joint infections: Are they really different? BMC Infect. Dis. 2022, 22, 555. [Google Scholar] [CrossRef]
- Tsikopoulos, K.; Meroni, G. Periprosthetic Joint Infection Diagnosis: A Narrative Review. Antibiotics 2023, 12, 1485. [Google Scholar] [CrossRef]
- Martin, A.; Irrazábal, T. Microbiota and Colon Cancer: Orchestrating Neoplasia Through DNA Damage and Immune Dysregulation. In Encyclopedia of Cancer, 3rd ed.; Boffetta, P., Hainaut, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 458–471. [Google Scholar]
- Öberg, J.; Bo, N.; Patrik, G.; Magnus, R.; Inghammar, M. Bacteraemia and infective endocarditis with Streptococcus bovis-Streptococcus equinus-complex: A retrospective cohort study. Infect. Dis. 2022, 54, 760–765. [Google Scholar] [CrossRef]
- Aymeric, L.; Donnadieu, F.; Mulet, C.; du Merle, L.; Nigro, G.; Saffarian, A.; Bérard, M.; Poyart, C.; Robine, S.; Regnault, B.; et al. Colorectal cancer specific conditions promote Streptococcus gallolyticus gut colonization. Proc. Natl. Acad. Sci. USA 2018, 115, E283–E291. [Google Scholar] [CrossRef]
- Thompson, J.C.; Goldman, A.H.; Tande, A.J.; Osmon, D.R.; Sierra, R.J. Streptococcus bovis Hip and Knee Periprosthetic Joint Infections: A Series of 9 Cases. J. Bone Jt. Infect. 2020, 5, 1–6. [Google Scholar] [CrossRef]
- Posse, J.R.; Werly, T.D.A.B.; Souza, F.; Cavanellas, N.T.; Matos, J.A.; Barbato, K.B.G. When prosthetic joint infection may lead to premalignant colorectal lesion detection. Int. J. Res. Orthop. 2023, 9, 1063–1067. [Google Scholar] [CrossRef]
- Shanti, I.; Samardali, M.; Gebremedhen, A. Prosthetic Joint Infection by Streptococcus lutetiensis (Bovis) With Noncancerous Polyps and Negative Blood Cultures: A Case Report. Cureus 2024, 16, e74264. [Google Scholar] [CrossRef]
- Liddle, A.D.; Abram, S.; Iyer, S.; Andrade, A.J. Streptococcus gallolyticus prosthetic joint infection associated with undiagnosed colonic malignancy. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 1069–1070. [Google Scholar] [CrossRef] [PubMed]
- Gold, J.S.; Bayar, S.; Salem, R.R. Association of Streptococcus bovis bacteremia with colonic neoplasia and extracolonic malignancy. Arch. Surg. 2004, 139, 760–765. [Google Scholar] [CrossRef]
- Corredoira, J.C.; Alonso, M.P.; García-País, M.J.; Rabuñal, R.; García-Garrote, F.; López-Roses, L.; Lancho, A.; Coira, A.; Pita, J.; Velasco, D.; et al. Is colonoscopy necessary in cases of infection by Streptococcus bovis biotype II? Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 171–177. [Google Scholar] [CrossRef]
- Fernández-González, R.; Otero-Villar, J.; Estévez-Vilar, R.; Díaz-López, M.D. Streptococcus gallolyticus subsp. gallolyticus knee periprosthetic joint infection. Enfermedades Infecc. Microbiol. Clínica (Engl. Ed.) 2022, 40, 337–338. [Google Scholar] [CrossRef]
- Meseeha, M.; Attia, M. Streptococcus bovis Bactremia and Colon Cancer: A Well Established but Frequently Forgotten Association: 1456. Am. J. Gastroenterol. 2018, 113, S835–S836. [Google Scholar] [CrossRef]
- Deng, Q.; Wang, C.; Yu, K.; Wang, Y.; Yang, Q.; Zhang, J.; Xu, X. Streptococcus bovis Contributes to the Development of Colorectal Cancer via Recruiting CD11b+TLR-4+ Cells. Med. Sci. Monit. 2020, 26, e921886. [Google Scholar] [CrossRef] [PubMed]
- Abdulamir, A.S.; Hafidh, R.R.; Abu Bakar, F. The association of Streptococcus bovis/gallolyticus with colorectal tumors: The nature and the underlying mechanisms of its etiological role. J. Exp. Clin. Cancer Res. 2011, 30, 11. [Google Scholar] [CrossRef]
- Paritsky, M.; Pastukh, N.; Brodsky, D.; Isakovich, N.; Peretz, A. Association of Streptococcus bovis presence in colonic content with advanced colonic lesion. World J. Gastroenterol. 2015, 21, 5663–5667. [Google Scholar] [CrossRef]
- Brüggemann, A.; Hailer, N.P. Never mind the bug: No differences in infection-free survival after periprosthetic joint infections with Staphylococcus aureus, Coagulase-negative Staphylococcus, or Streptococcus. Front. Microbiol. 2024, 15, 1503928. [Google Scholar] [CrossRef]
- Thompson, O.; Påhlman, L.I. Frequency of haematogenous periprosthetic joint infection due to bacteraemia caused by gram-positive cocci. Infect. Dis. 2025, 57, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Pompilio, A.; Di Bonaventura, G.; Gherardi, G. An Overview on Streptococcus bovis/Streptococcus equinus Complex Isolates: Identification to the Species/Subspecies Level and Antibiotic Resistance. Int. J. Mol. Sci. 2019, 20, 480. [Google Scholar] [CrossRef]
- Dekker, J.P.; Lau, A.F. An Update on the Streptococcus bovis Group: Classification, Identification, and Disease Associations. J. Clin. Microbiol. 2016, 54, 1694–1699. [Google Scholar] [CrossRef] [PubMed]
- Biarc, J.; Nguyen, I.S.; Pini, A.; Gossé, F.; Richert, S.; Thiersé, D.; Van Dorsselaer, A.; Leize-Wagner, E.; Raul, F.; Klein, J.P.; et al. Carcinogenic properties of proteins with pro-inflammatory activity from Streptococcus infantarius (formerly S.bovis). Carcinogenesis 2004, 25, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Longo, U.G.; Lalli, A.; Bandini, B.; Angeletti, S.; Lustig, S.; Budhiparama, N.C. The influence of gut microbiome on periprosthetic joint infections: State-of-the art. J. ISAKOS 2024, 9, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Mahamud, E.; Gallart, X.; Soriano, A. One-stage revision arthroplasty for infected hip replacements. Open Orthop. J. 2013, 7, 184–189. [Google Scholar] [CrossRef]
- Ure, K.J.; Amstutz, H.C.; Nasser, S.; Schmalzried, T.P. Direct-exchange arthroplasty for the treatment of infection after total hip replacement. An average ten-year follow-up. J. Bone Jt. Surg. Am. 1998, 80, 961–968. [Google Scholar] [CrossRef]
- Chandler, C.C.; Clair, A.J.; Metcalf, R.W.; Hietpas, K.T.; Fehring, T.K.; Otero, J.E. Reinfection Patterns Following Two-Stage Exchange for Periprosthetic Joint Infection: A Retrospective Analysis. J. Arthroplast. 2025, 40, 1961–1966. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viscopoleanu, G.; Valeanu, M.-S.; Capitanu, B.-S.; Dragosloveanu, S.; Scheau, C. Periprosthetic Joint Infection by Streptococcus bovis Reveals Hidden Colorectal Cancer: A Case Report. Life 2025, 15, 1385. https://doi.org/10.3390/life15091385
Viscopoleanu G, Valeanu M-S, Capitanu B-S, Dragosloveanu S, Scheau C. Periprosthetic Joint Infection by Streptococcus bovis Reveals Hidden Colorectal Cancer: A Case Report. Life. 2025; 15(9):1385. https://doi.org/10.3390/life15091385
Chicago/Turabian StyleViscopoleanu, George, Mihai-Sebastian Valeanu, Bogdan-Sorin Capitanu, Serban Dragosloveanu, and Cristian Scheau. 2025. "Periprosthetic Joint Infection by Streptococcus bovis Reveals Hidden Colorectal Cancer: A Case Report" Life 15, no. 9: 1385. https://doi.org/10.3390/life15091385
APA StyleViscopoleanu, G., Valeanu, M.-S., Capitanu, B.-S., Dragosloveanu, S., & Scheau, C. (2025). Periprosthetic Joint Infection by Streptococcus bovis Reveals Hidden Colorectal Cancer: A Case Report. Life, 15(9), 1385. https://doi.org/10.3390/life15091385






