Blue-Light Stimulation for Myopia Prevention: Only Retinal but Not Optic Disc Stimulation Modulates the Pattern ERG
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Approval
2.2. Participants
2.3. General Experimental Procedure
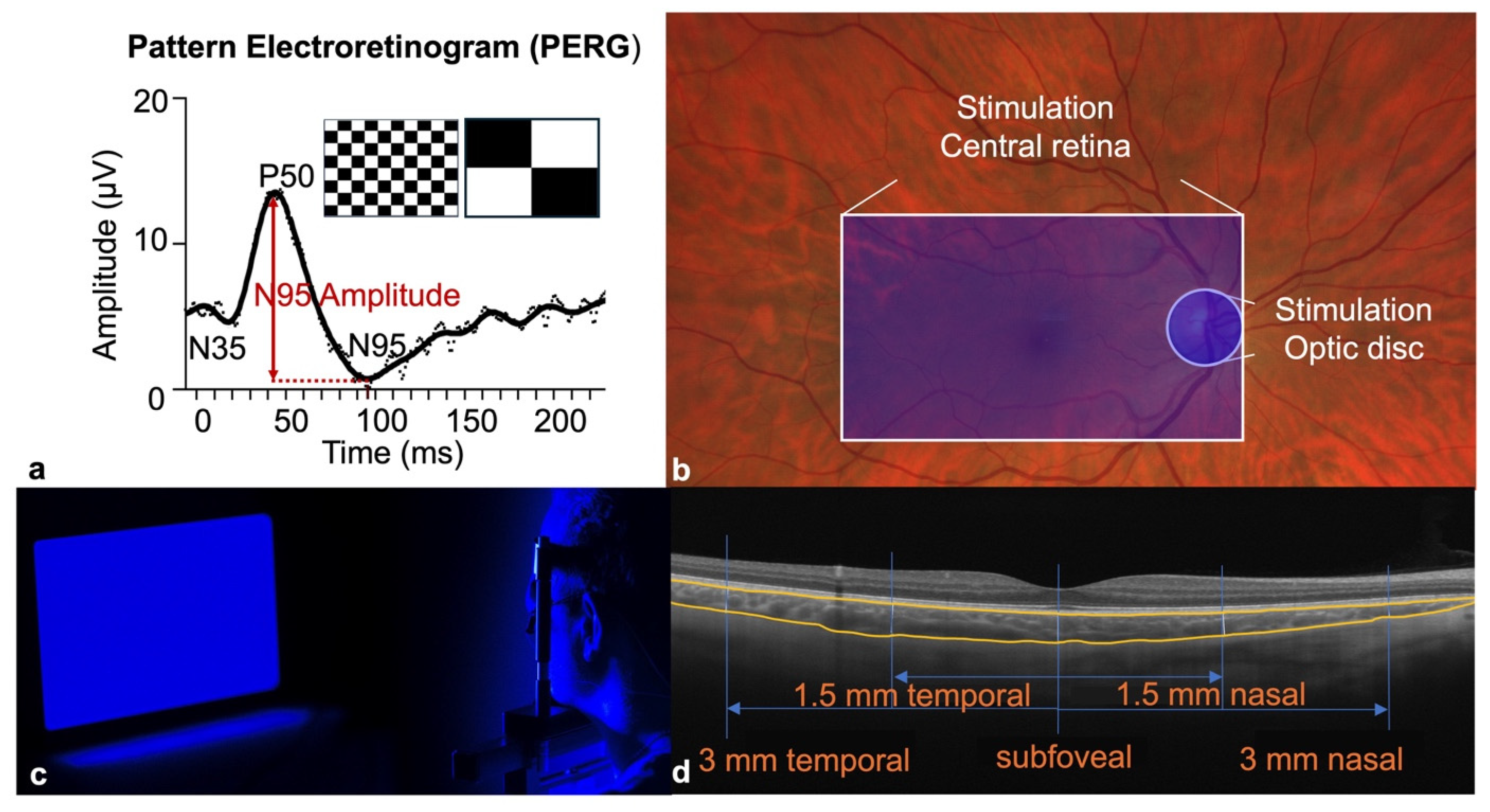
2.4. Blue-Light Stimulation
2.5. PERG Measurements
2.6. Choroidal Thickness and Axial Length Measurements
2.7. Statistical Analysis
3. Results
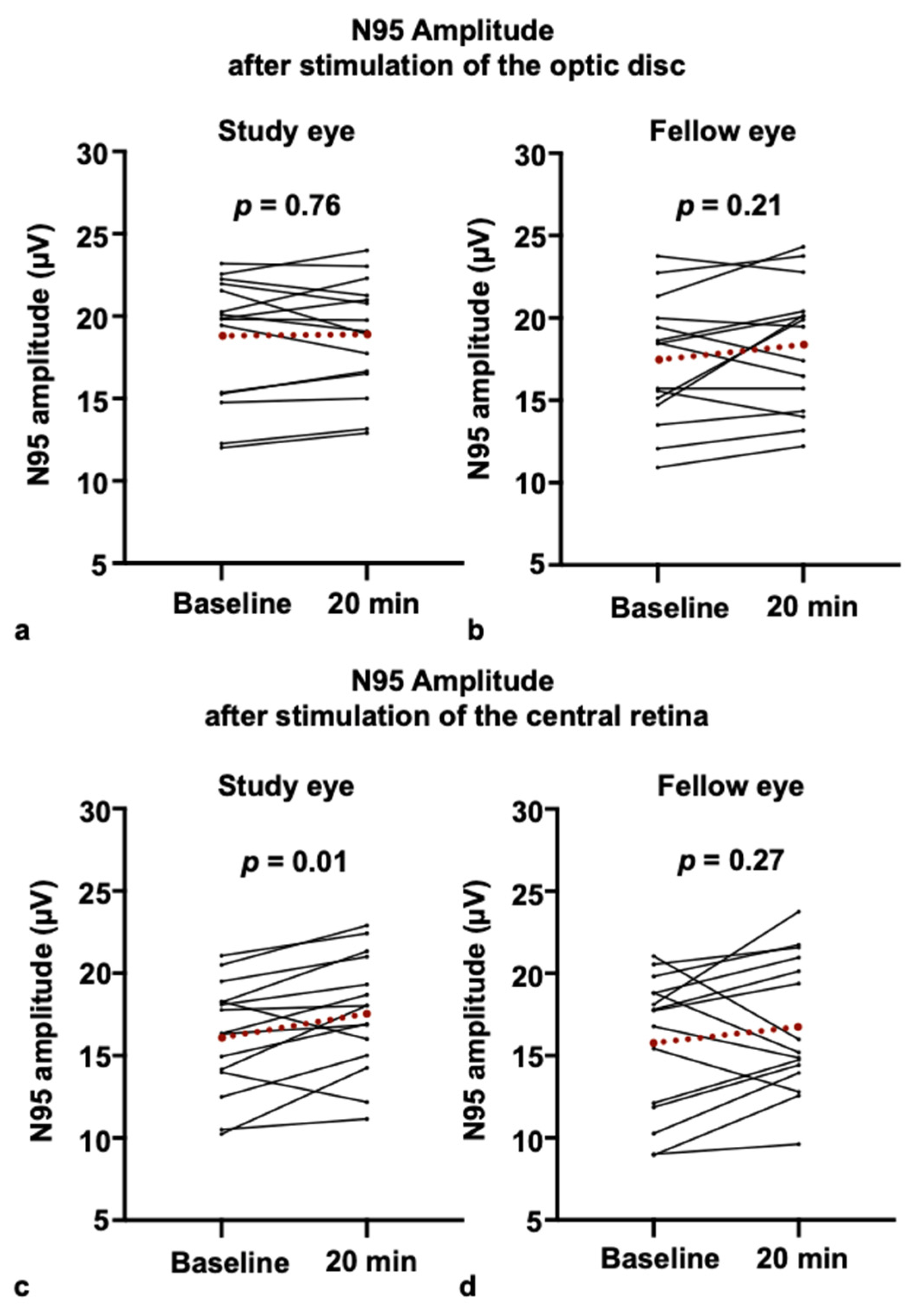
3.1. PERG Response After Blue-Light Stimulation of the Optic Disc
3.2. PERG Responses After Blue-Light Stimulation of the Retina
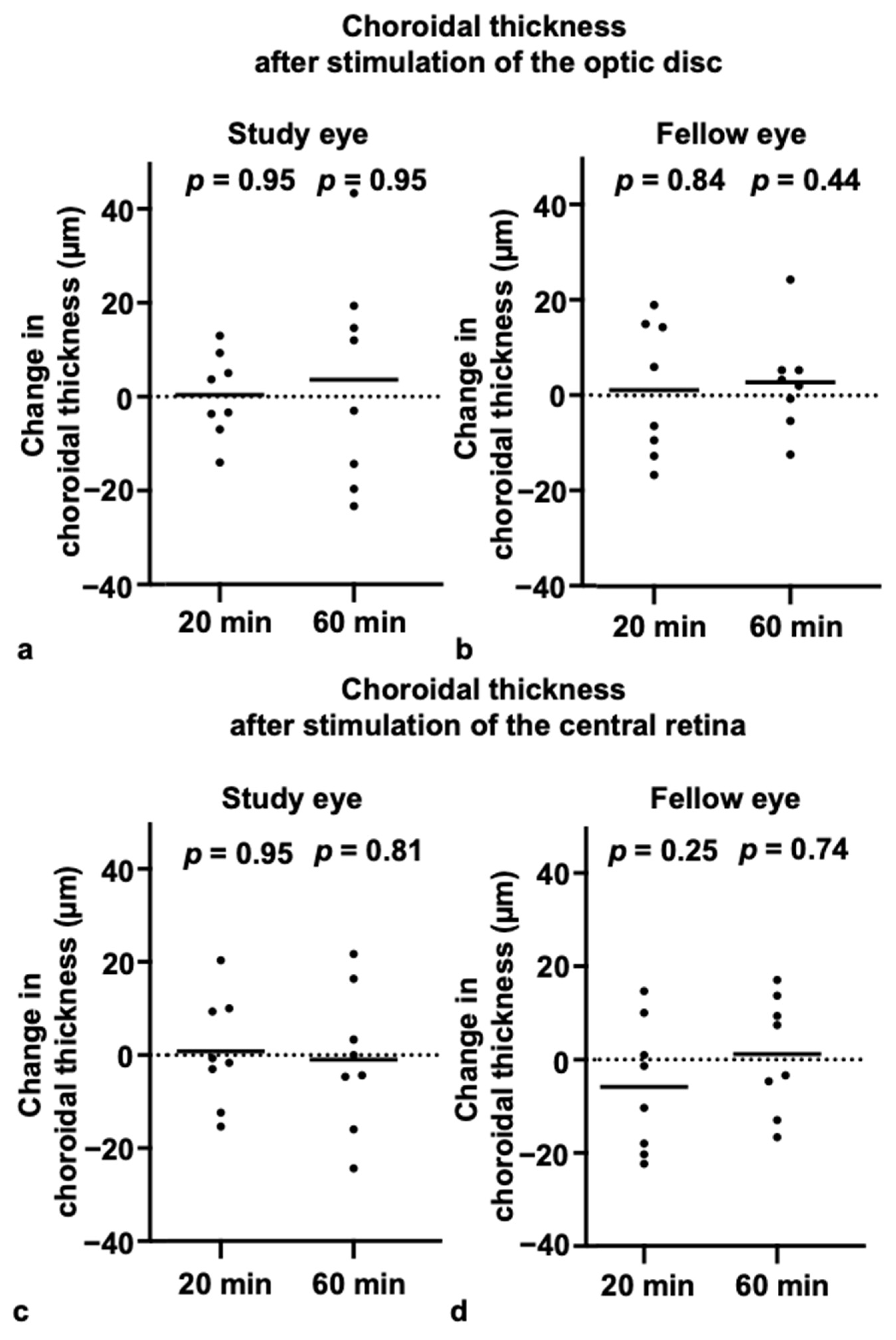
3.3. Choroidal Thickness and Axial Length After Blue-Light Stimulation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ipRGC | Intrinsically photosensitive retinal ganglion cells |
| DAC | Dopaminergic amacrine cells |
| PERG | Pattern electroretinogram |
| ChT | Choroidal thickness |
| AL | Axial length |
| OS | Left eye |
| OD | Right eye |
| FrACT | Freiburg Visual Acuity and Contrast Test |
| ICC | Intraclass correlation coefficient |
| VR | Virtual reality |
References
- Morgan, I.G.; French, A.N.; Ashby, R.S.; Guo, X.; Ding, X.; He, M.; Rose, K.A. The Epidemics of Myopia: Aetiology and Prevention. Prog. Retin. Eye Res. 2018, 62, 134–149. [Google Scholar] [CrossRef]
- Dirani, M.; Tong, L.; Gazzard, G.; Zhang, X.; Chia, A.; Young, T.L.; Rose, K.A.; Mitchell, P.; Saw, S.-M. Outdoor Activity and Myopia in Singapore Teenage Children. Br. J. Ophthalmol. 2009, 93, 997–1000. [Google Scholar] [CrossRef]
- Lingham, G.; Mackey, D.A.; Lucas, R.; Yazar, S. How Does Spending Time Outdoors Protect against Myopia? A Review. Br. J. Ophthalmol. 2020, 104, 593–599. [Google Scholar] [CrossRef]
- Amorim-de-Sousa, A.; Chakraborty, R.; Collins, M.J.; Fernandes, P.; González-Méijome, J.; Hannibal, J.; Hoseini-Yazdi, H.; Read, S.A.; Ellrich, J.; Schilling, T. Blue Light Stimulation of the Blind Spot in Human: From Melanopsin to Clinically Relevant Biomarkers of Myopia. Bioelectron. Med. 2024, 10, 26. [Google Scholar] [CrossRef]
- Xiong, R.; Zhu, Z.; Jiang, Y.; Wang, W.; Zhang, J.; Chen, Y.; Bulloch, G.; Yuan, Y.; Zhang, S.; Xuan, M.; et al. Longitudinal Changes and Predictive Value of Choroidal Thickness for Myopia Control after Repeated Low-Level Red-Light Therapy. Ophthalmology 2023, 130, 286–296. [Google Scholar] [CrossRef]
- Bailes, H.J.; Lucas, R.J. Human Melanopsin Forms a Pigment Maximally Sensitive to Blue Light (Λmax ≈ 479 Nm) Supporting Activation of G(q/11) and G(i/o) Signalling Cascades. Proc. Biol. Sci. 2013, 280, 20122987. [Google Scholar] [CrossRef]
- Chakraborty, R.; Landis, E.G.; Mazade, R.; Yang, V.; Strickland, R.; Hattar, S.; Stone, R.A.; Iuvone, P.M.; Pardue, M.T. Melanopsin Modulates Refractive Development and Myopia. Exp. Eye Res. 2022, 214, 108866. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-Q.; Wong, K.Y.; Sollars, P.J.; Berson, D.M.; Pickard, G.E.; McMahon, D.G. Intraretinal Signaling by Ganglion Cell Photoreceptors to Dopaminergic Amacrine Neurons. Proc. Natl. Acad. Sci. USA 2008, 105, 14181–14186. [Google Scholar] [CrossRef]
- Feldkaemper, M.; Schaeffel, F. An Updated View on the Role of Dopamine in Myopia. Exp. Eye Res. 2013, 114, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Amorim-de-Sousa, A.; Schilling, T.; Fernandes, P.; Seshadri, Y.; Bahmani, H.; González-Méijome, J.M. Blue Light Blind-Spot Stimulation Upregulates b-Wave and Pattern ERG Activity in Myopes. Sci. Rep. 2021, 11, 9273. [Google Scholar] [CrossRef]
- Thompson, D.A.; Bach, M.; McAnany, J.J.; Šuštar Habjan, M.; Viswanathan, S.; Robson, A.G. ISCEV Standard for Clinical Pattern Electroretinography (2024 Update). Doc. Ophthalmol. Adv. Ophthalmol. 2024, 148, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Hoseini-Yazdi, H.; Read, S.A.; Collins, M.J.; Bahmani, H.; Ellrich, J.; Schilling, T. Increase in Choroidal Thickness after Blue Light Stimulation of the Blind Spot in Young Adults. Bioelectron. Med. 2024, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Read, S.A.; Collins, M.J.; Vincent, S.J. Light Exposure and Eye Growth in Childhood. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6779–6787. [Google Scholar] [CrossRef]
- Yam, J.C.; Zhang, X.J.; Zhang, Y.; Yip, B.H.K.; Tang, F.; Wong, E.S.; Bui, C.H.T.; Kam, K.W.; Ng, M.P.H.; Ko, S.T.; et al. Effect of Low-Concentration Atropine Eyedrops vs Placebo on Myopia Incidence in Children: The LAMP2 Randomized Clinical Trial. JAMA 2023, 329, 472–481. [Google Scholar] [CrossRef]
- Gardner, D.J.; Walline, J.J.; Mutti, D.O. Choroidal Thickness and Peripheral Myopic Defocus during Orthokeratology. Optom. Vis. Sci. 2015, 92, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Farassat, N. Topical Atropine for Myopia Control: A Review. Klin. Monatsbl. Augenheilkd. 2024, 241, 1134–1139. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y.; Huang, D.; Yang, J.; Fan, C.; Chen, C.; Li, J.; Wang, Q.; Li, S.; Jiang, B.; et al. The Efficacy of Defocus Incorporated Multiple Segments Lenses in Slowing Myopia Progression: Results from Diverse Clinical Circumstances. Ophthalmology 2023, 130, 542–550. [Google Scholar] [CrossRef]
- Mavilio, A.; Scrimieri, F.; Errico, D. Can Variability of Pattern ERG Signal Help to Detect Retinal Ganglion Cells Dysfunction in Glaucomatous Eyes? BioMed Res. Int. 2015, 2015, 571314. [Google Scholar] [CrossRef]
- Holopigian, K.; Snow, J.; Seiple, W.; Siegel, I. Variability of the Pattern Electroretinogram. Doc. Ophthalmol. Adv. Ophthalmol. 1988, 70, 103–115. [Google Scholar] [CrossRef]
- Fredette, M.-J.; Anderson, D.R.; Porciatti, V.; Feuer, W. Reproducibility of Pattern Electroretinogram in Glaucoma Patients with a Range of Severity of Disease with the New Glaucoma Paradigm. Ophthalmology 2008, 115, 957–963. [Google Scholar] [CrossRef]
- Peirce, J.; Hirst, R.; MacAskill, M. Building Experiments in PsychoPy, 2nd ed.; Sage Publications: Thousand Oaks, CA, USA, 2022. [Google Scholar]
- Schilling, T.; Amorim-de-Sousa, A.; A Wong, N.; Bahmani, H.; González-Méijome, J.M.; Fernandes, P. Increase in B-Wave Amplitude after Light Stimulation of the Blind Spot Is Positively Correlated with the Axial Length of Myopic Individuals. Sci. Rep. 2022, 12, 4785. [Google Scholar] [CrossRef]
- Joo, H.R.; Peterson, B.B.; Dacey, D.M.; Hattar, S.; Chen, S.-K. Recurrent Axon Collaterals of Intrinsically Photosensitive Retinal Ganglion Cells. Vis. Neurosci. 2013, 30, 175–182. [Google Scholar] [CrossRef]
- Carpena-Torres, C.; Schilling, T.; Huete-Toral, F.; Bahmani, H.; Carracedo, G. Increased Ocular Dopamine Levels in Rabbits after Blue Light Stimulation of the Optic Nerve Head. Exp. Eye Res. 2023, 234, 109604. [Google Scholar] [CrossRef]
- Schilling, T.; Soltanlou, M.; Nuerk, H.-C.; Bahmani, H. Blue-Light Stimulation of the Blind-Spot Constricts the Pupil and Enhances Contrast Sensitivity. PLoS ONE 2023, 18, e0286503. [Google Scholar] [CrossRef]
- Newkirk, G.S.; Hoon, M.; Wong, R.O.; Detwiler, P.B. Inhibitory Inputs Tune the Light Response Properties of Dopaminergic Amacrine Cells in Mouse Retina. J. Neurophysiol. 2013, 110, 536–552. [Google Scholar] [CrossRef]
- Porciatti, V.; Ventura, L.M. Physiologic Significance of Steady-State Pattern Electroretinogram Losses in Glaucoma: Clues from Simulation of Abnormalities in Normal Subjects. J. Glaucoma 2009, 18, 535–542. [Google Scholar] [CrossRef]
- Mathis, U.; Feldkaemper, M.; Liu, H.; Schaeffel, F. Studies on the Interactions of Retinal Dopamine with Choroidal Thickness in the Chicken. Graefe’s Arch. Clin. Exp. Ophthalmol. 2023, 261, 409–425. [Google Scholar] [CrossRef]
- Lee, S.S.-Y.; Alonso-Caneiro, D.; Lingham, G.; Chen, F.K.; Sanfilippo, P.G.; Yazar, S.; Mackey, D.A. Choroidal Thickening During Young Adulthood and Baseline Choroidal Thickness Predicts Refractive Error Change. Investig. Ophthalmol. Vis. Sci. 2022, 63, 34. [Google Scholar] [CrossRef]
- Zhou, X.; Ye, C.; Wang, X.; Zhou, W.; Reinach, P.; Qu, J. Choroidal Blood Perfusion as a Potential “Rapid Predictive Index” for Myopia Development and Progression. Eye Vis. Lond. Engl. 2021, 8, 1. [Google Scholar] [CrossRef]
- Ostrin, L.A.; Harb, E.; Nickla, D.L.; Read, S.A.; Alonso-Caneiro, D.; Schroedl, F.; Kaser-Eichberger, A.; Zhou, X.; Wildsoet, C.F. IMI-The Dynamic Choroid: New Insights, Challenges, and Potential Significance for Human Myopia. Investig. Ophthalmol. Vis. Sci. 2023, 64, 4. [Google Scholar] [CrossRef]
- Read, S.A.; Pieterse, E.C.; Alonso-Caneiro, D.; Bormann, R.; Hong, S.; Lo, C.-H.; Richer, R.; Syed, A.; Tran, L. Daily Morning Light Therapy Is Associated with an Increase in Choroidal Thickness in Healthy Young Adults. Sci. Rep. 2018, 8, 8200. [Google Scholar] [CrossRef]
- Thakur, S.; Dhakal, R.; Verkicharla, P.K. Short-Term Exposure to Blue Light Shows an Inhibitory Effect on Axial Elongation in Human Eyes Independent of Defocus. Investig. Ophthalmol. Vis. Sci. 2021, 62, 22. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, Y.; Wang, K.; Yang, S.; Luan, C.; Wu, B.; Zhang, W.; Hao, R. Effects of Blue Light Exposure on Ocular Parameters and Choroidal Blood Perfusion in Guinea Pig. Exp. Eye Res. 2023, 235, 109619. [Google Scholar] [CrossRef] [PubMed]
- Swiatczak, B.; Schaeffel, F. Emmetropic, but Not Myopic Human Eyes Distinguish Positive Defocus from Calculated Defocus in Monochromatic Red Light. Vision Res. 2022, 192, 107974. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhu, Z.; Tan, X.; Kong, X.; Zhong, H.; Zhang, J.; Xiong, R.; Yuan, Y.; Zeng, J.; Morgan, I.G.; et al. Effect of Repeated Low-Level Red-Light Therapy for Myopia Control in Children: A Multicenter Randomized Controlled Trial. Ophthalmology 2022, 129, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, M.; Yan, X.; Li, M.; Xiang, Y. The Effect of Repeated Low-Level Red-Light Therapy on Myopia Control and Choroid. Transl. Vis. Sci. Technol. 2024, 13, 29. [Google Scholar] [CrossRef]

| Characteristics | General Study Population | PERG Protocol Only | ChT/AL Protocol Only | |||
|---|---|---|---|---|---|---|
| n = 46 | n = 30 | n = 16 | ||||
| Mean/N | SD/(%) | Mean/N | SD/(%) | Mean/N | SD/(%) | |
| Age (years) | 24.25 | 2.90 | 24.75 | 2.66 | 23.75 | 3.13 |
| Sex Female Male | ||||||
| 16 | 34.78 | 19 | 63.33 | 11 | 68.75 | |
| 30 | 65.22 | 11 | 36.67 | 5 | 31.25 | |
| Visual acuity (logMAR) | −0.23 | 0.10 | −0.23 | 0.09 | −0.24 | 0.11 |
| Axial length (mm) | 23.53 | 0.69 | 23.50 | 0.58 | 23.56 | 0.81 |
| Spherical equivalent (diopters) | −0.64 | 1.23 | −0.37 | 0.94 | −0.92 | 1.53 |
| Refraction Myopes Non-Myopes | ||||||
| 19 | 41.30 | 10 | 33.33 | 9 | 56.25 | |
| 27 | 58.70 | 20 | 66.67 | 7 | 43.75 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehler, I.S.E.; Heinrich, S.P.; Böhringer, D.; Simon, V.; Bleul, T.; Küchlin, S.; Lagrèze, W.A.; Farassat, N. Blue-Light Stimulation for Myopia Prevention: Only Retinal but Not Optic Disc Stimulation Modulates the Pattern ERG. Life 2025, 15, 1384. https://doi.org/10.3390/life15091384
Mehler ISE, Heinrich SP, Böhringer D, Simon V, Bleul T, Küchlin S, Lagrèze WA, Farassat N. Blue-Light Stimulation for Myopia Prevention: Only Retinal but Not Optic Disc Stimulation Modulates the Pattern ERG. Life. 2025; 15(9):1384. https://doi.org/10.3390/life15091384
Chicago/Turabian StyleMehler, Isabella Silke Elisabeth, Sven Pascal Heinrich, Daniel Böhringer, Valentin Simon, Tim Bleul, Sebastian Küchlin, Wolf Alexander Lagrèze, and Navid Farassat. 2025. "Blue-Light Stimulation for Myopia Prevention: Only Retinal but Not Optic Disc Stimulation Modulates the Pattern ERG" Life 15, no. 9: 1384. https://doi.org/10.3390/life15091384
APA StyleMehler, I. S. E., Heinrich, S. P., Böhringer, D., Simon, V., Bleul, T., Küchlin, S., Lagrèze, W. A., & Farassat, N. (2025). Blue-Light Stimulation for Myopia Prevention: Only Retinal but Not Optic Disc Stimulation Modulates the Pattern ERG. Life, 15(9), 1384. https://doi.org/10.3390/life15091384






