Comprehensive Evaluation of Leaf Structure, Photosynthetic Characteristics, and Drought Resistance in Six Jackfruit (Artocarpus heterophyllus) Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
2.2.1. Methods for Sampling Plant Leaves
2.2.2. Observation of Leaf Morphological Characteristics
2.2.3. Measurement of Leaf Anatomy
2.2.4. Determination of Leaf Chlorophyll Concentration
2.2.5. Determination of Leaf Photosynthetic Indexes
2.3. Statistics and Analysis of Data
3. Results and Analysis
3.1. Analysis of the Leaf Structure of Different Strains of Jackfruit
3.1.1. Morphological Characteristics of the Leaf Blades of Different Strains of Jackfruit
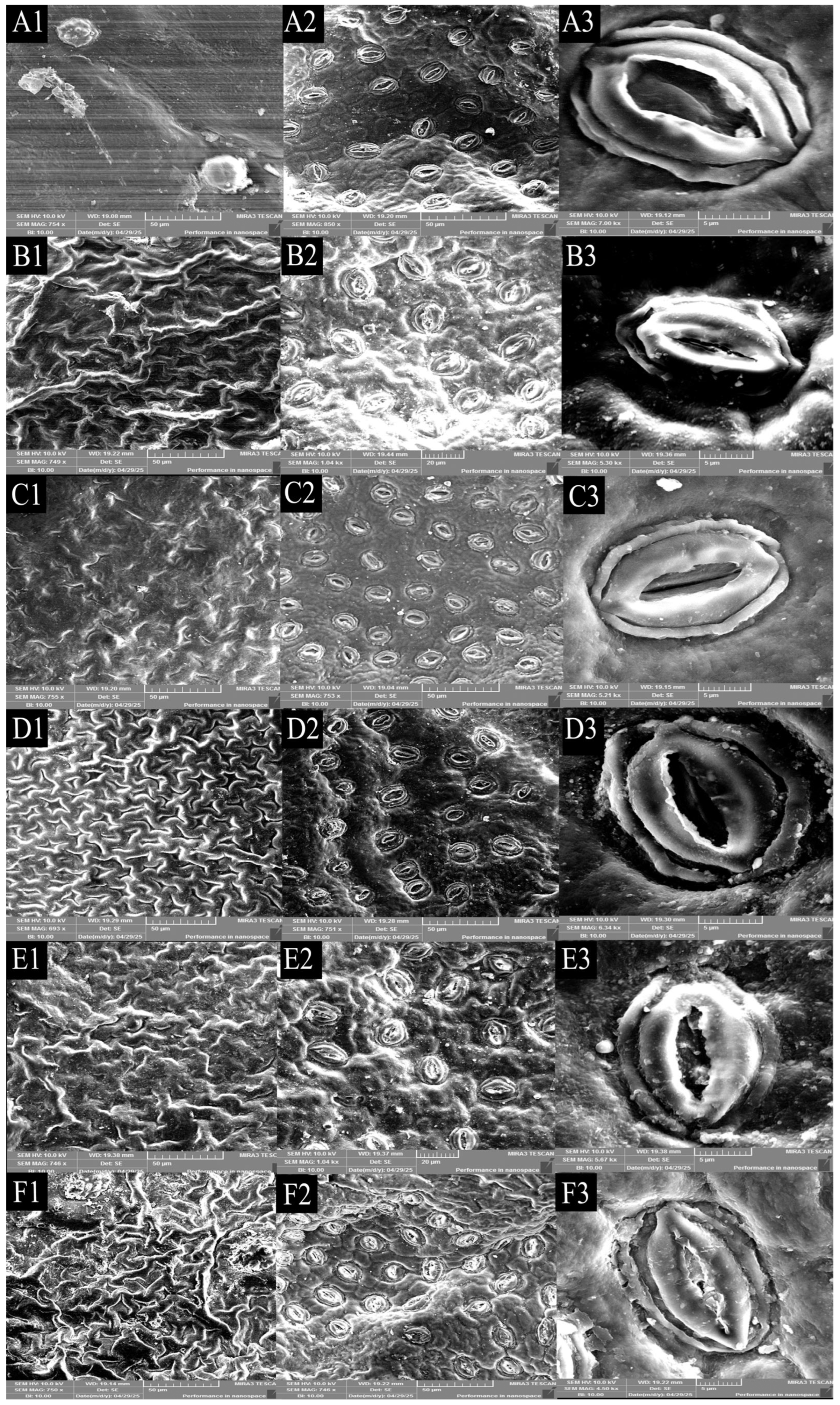
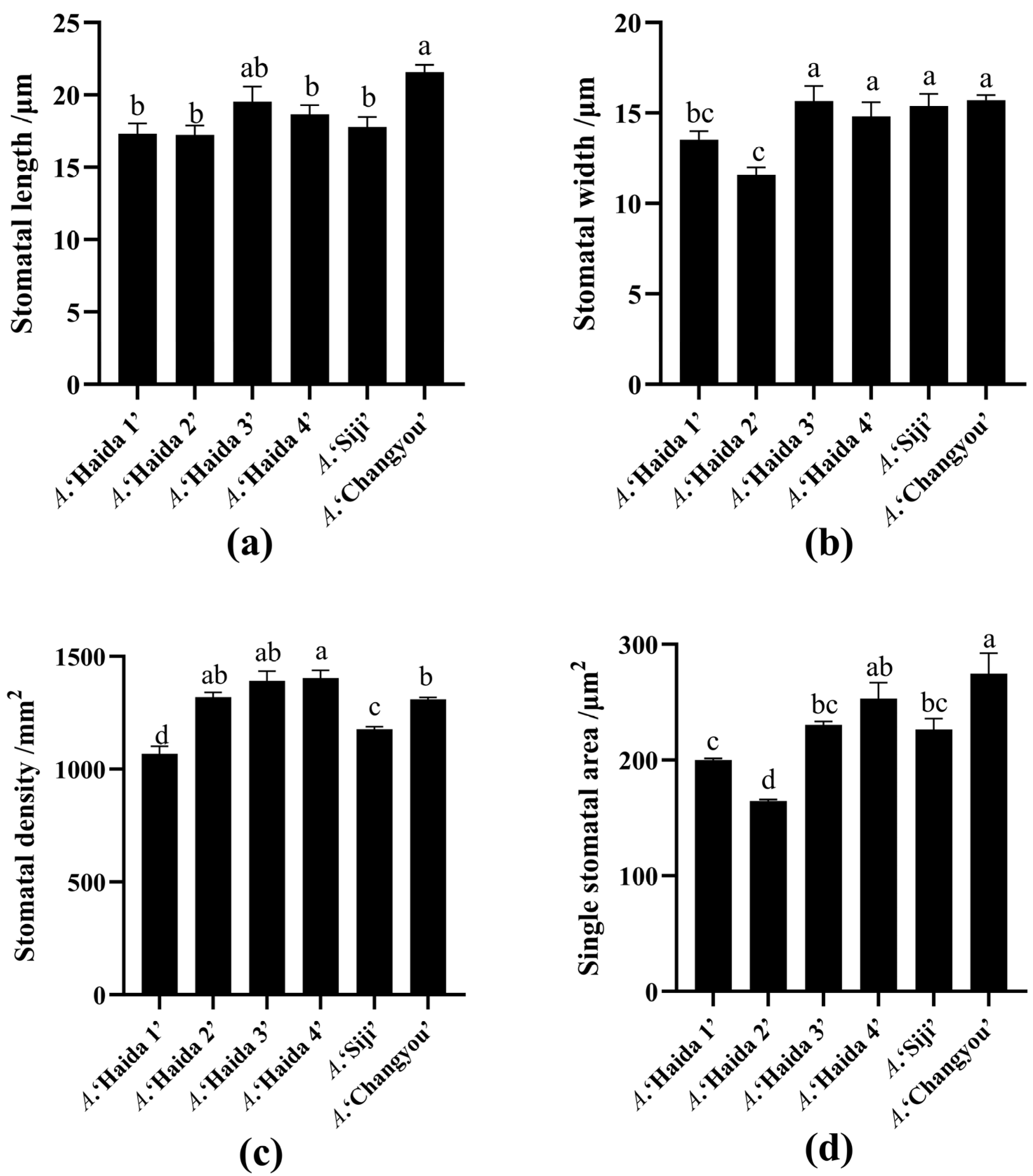
3.1.2. Stomatal Size of Leaves of Different Strains of Jackfruit
3.1.3. Anatomical Structure of the Leaf Blades of Different Strains of Jackfruit
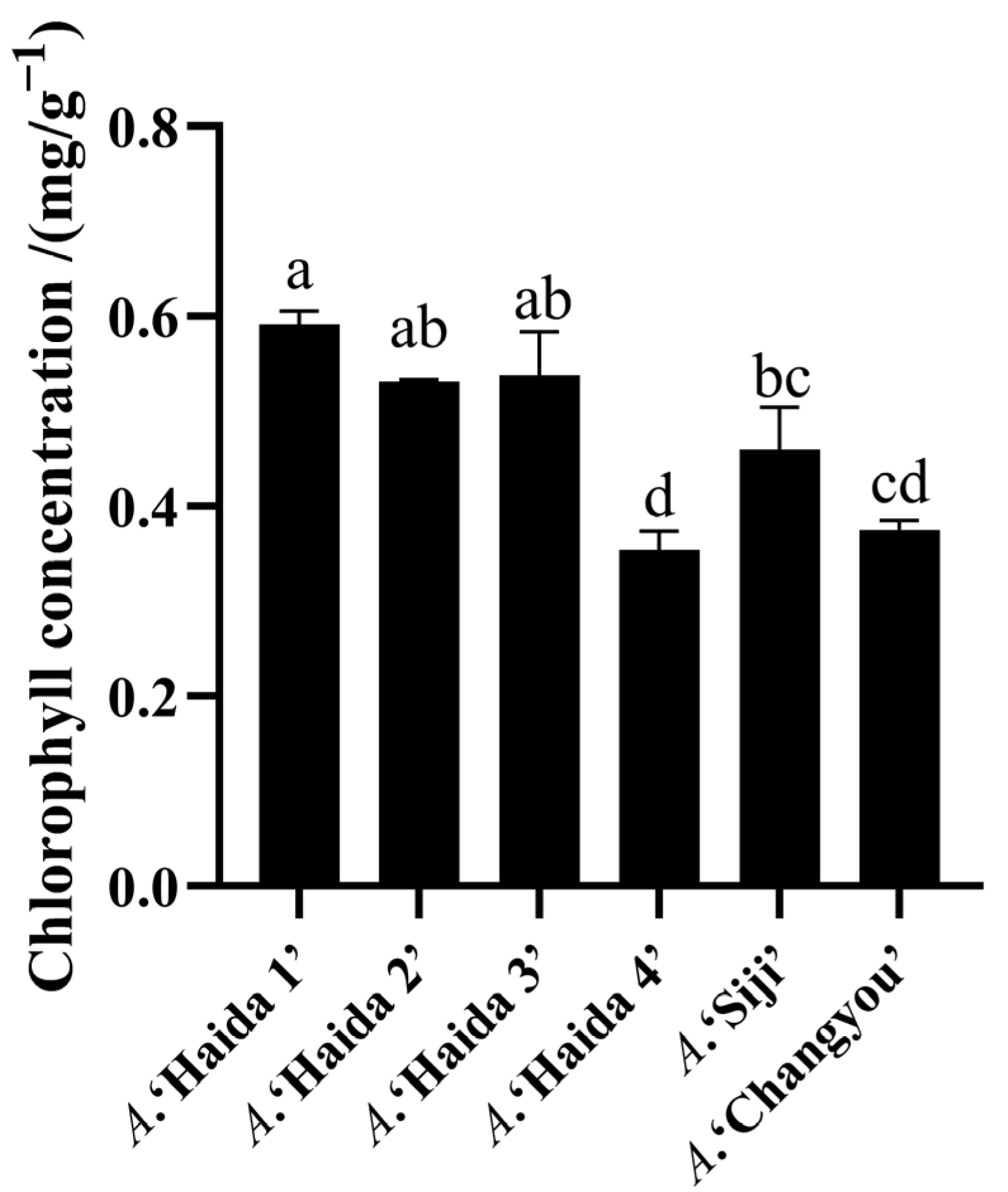
3.2. Comparison of Chlorophyll Concentration of Different Strains of Jackfruit
3.3. Comparison of Photosynthetic Characteristics of Different Strains of Jackfruit
3.3.1. Analysis of Daily Variation in Photosynthesis in Different Strains of Jackfruit
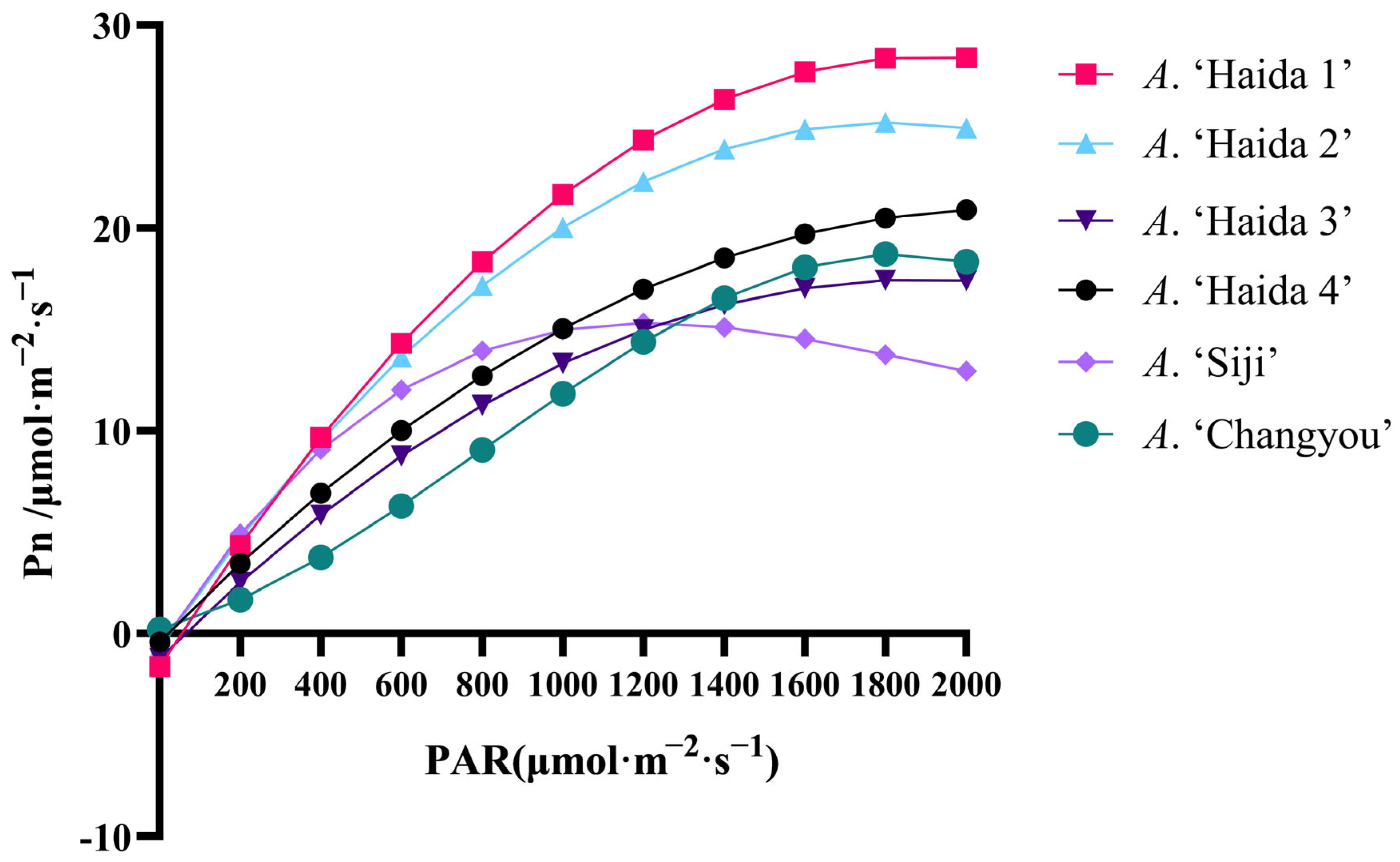
3.3.2. Analysis of Light Intensity Response of Leaves of Different Strains of Jackfruit
3.4. Evaluation of Drought Tolerance of Different Cultivars of Jackfruit
3.4.1. Correlation Analysis of Jackfruit Leaf Indicators
3.4.2. Comprehensive Evaluation of Drought Tolerance
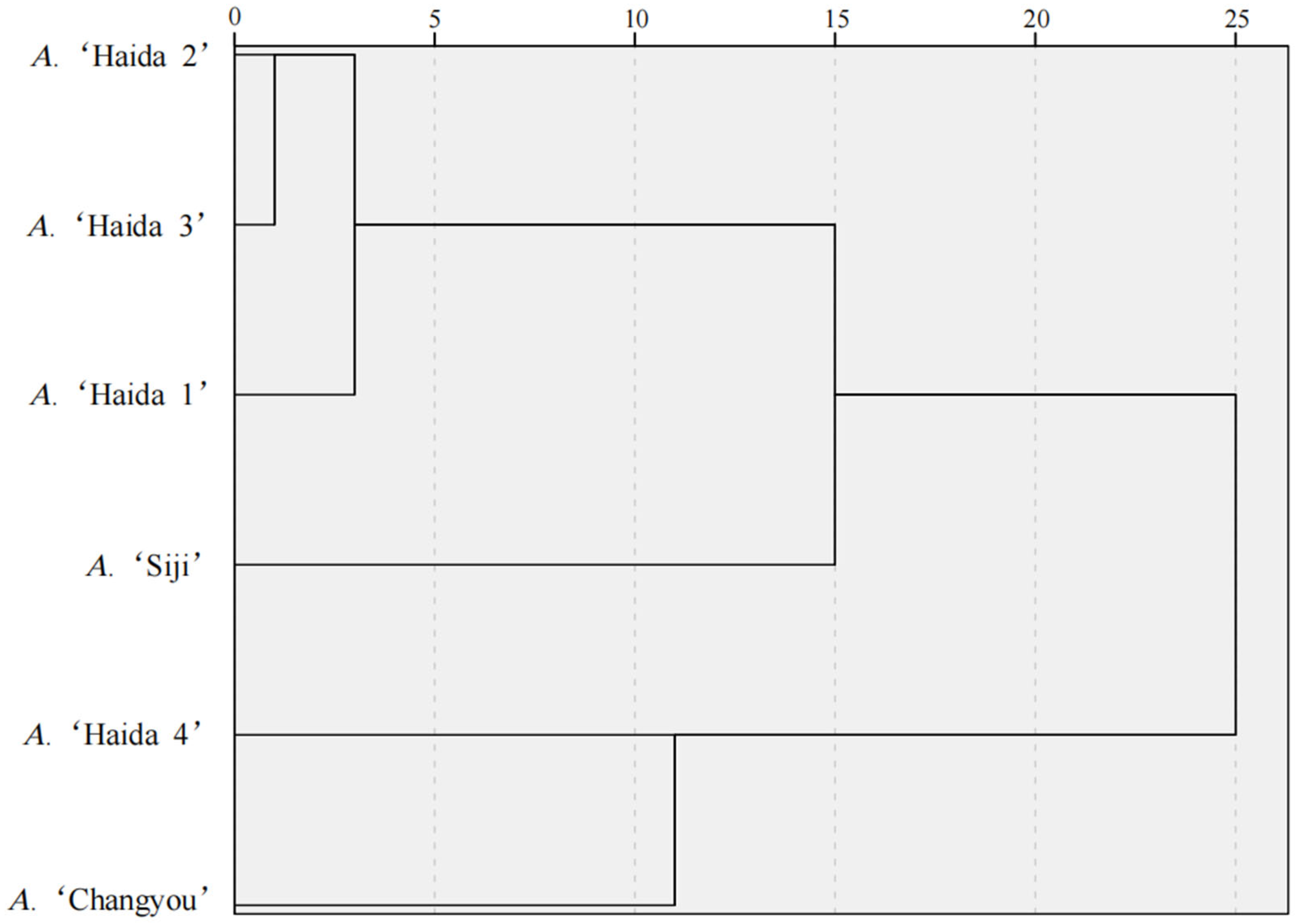
3.4.3. Cluster Analysis of Drought Tolerance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baliga, M.S.; Shivashankara, A.R.; Haniadka, R.; Dsouza, J.; Bhat, H.P. Phytochemistry, Nutritional and Pharmacological Properties of Artocarpus heterophyllus Lam (Jackfruit): A Review. Food Res. Int. 2011, 44, 1800–1811. [Google Scholar] [CrossRef]
- Palupi, D.; Daryono, B.S. Genetic Diversity of Jackfruit (Artocarpus heterophyllus Lam.) Provenances Based on Morphological Parameters. SABRAO J. Breed. Genet. 2021, 53, 479–498. [Google Scholar]
- Ranasinghe, R.A.S.N.; Maduwanthi, S.D.T.; Marapana, R.A.U.J. Nutritional and Health Benefits of Jackfruit (Artocarpus heterophyllus Lam.): A Review. Int. J. Food Sci. 2019, 2019, 4327183. [Google Scholar] [CrossRef]
- Wang, S.; Liang, D.; Li, C.; Hao, Y.; Ma, F.; Shu, H. Influence of Drought Stress on the Cellular Ultrastructure and Antioxidant System in Leaves of Drought-Tolerant and Drought-Sensitive Apple Rootstocks. Plant Physiol. Biochem. 2012, 51, 81–89. [Google Scholar] [CrossRef]
- Everingham, S.E.; Offord, C.A.; Sabot, M.E.B.; Moles, A.T. Leaf Morphological Traits Show Greater Responses to Changes in Climate than Leaf Physiological Traits and Gas Exchange Variables. Ecol. Evol. 2024, 14, e10941. [Google Scholar] [CrossRef] [PubMed]
- Injamum-Ul-Hoque, M.; Uddin, M.N.; Solaiman, M.; Fakir, A.; Rasel, M. Drought and Salinity Affect Leaf and Root Anatomical Structures in Three Maize Genotypes. J. Bangladesh Agric. Univ. 2018, 16, 47–55. [Google Scholar] [CrossRef]
- Li, J.L.; Luan, X.Y.; Tang, Z.H.; Han, A.Z.; Guo, L. Evaluation of Drought Resistance in Apricot Germplasm Resources Based on Leaf Microstructure. Acta Hortic. Sin. 2025. [Google Scholar] [CrossRef]
- Pamungkas, S.S.T.; Suwarto; Suprayogi; Farid, N. Drought Stress: Responses and Mechanism in Plants. Rev. Agric. Sci. 2022, 10, 168–185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, L.Q.; Zhao, Y.; Yu, D.; Li, L.L.; Zhou, L.; Ma, K. Comparison of Leaf Structure and Photosynthetic Characteristics of Two Main Walnut Cultivars in Xinjiang. Non-Wood For. Res. 2022, 40, 153–163+182. [Google Scholar] [CrossRef]
- Bao, W.W.; Chen, J.N.; Liu, Z.D. Evaluation of Leaf Morphology, Anatomical Structure and Drought Resistance of Different Kiwifruit Varieties. Acta Agric. Boreali-Occident. Sin. 2024, 33, 1329–1336. [Google Scholar]
- Lei, Y.; Lin, Y.; Gao, A.P.; Liu, F.Z.; Liu, Q.G.; Zhang, Z.X. Comprehensive Evaluation of Leaf Anatomical Structure Characteristics and Drought Resistance of 14 Mango Germplasm Resources. Southwest China J. Agric. Sci. 2025, 38, 250–258. [Google Scholar] [CrossRef]
- Ma, J.; He, X.Y.; Tao, L.; Wu, C.; Li, Z.Q.; Gong, L.D. Evaluation of Drought Resistance in Macadamia Germplasm Resources Based on Leaf Anatomical Structure. Chin. J. Trop. Crops 2023, 44, 1392–1399. [Google Scholar]
- Li, S.K.; Zhang, S.Q.; Yan, C.B.; Zhao, Y.; Xiao, M.; Fan, H.Y. Physiological Basis of Bark Splitting Disease in Jackfruit Induced by Drought Stress. China Trop. Agric. 2024, 2, 35–43. [Google Scholar]
- Abobatta, W.F. Fruit Orchards under Climate Change Conditions: Adaptation Strategies and Management. J. Appl. Biotechnol. Bioeng. 2021, 8, 99–102. [Google Scholar] [CrossRef]
- Matsuda, H. Jackfruit (Artocarpus heterophyllus Lam.) Grafting as Affected by Nitrogen Fertilizer Application to Irrigation Water. Sci. Hortic. 2024, 324, 112596. [Google Scholar] [CrossRef]
- Kaur, J.; Singh, Z.; Mazhar, M.S.; Afrifa-Yamoah, E.; Woodward, A. Variability in Fruit Quality Traits of Tropical Australian Jackfruit (Artocarpus heterophyllus Lam.) Genotypes. Sci. Hortic. 2024, 338, 113771. [Google Scholar] [CrossRef]
- Lan, Z.X.; Feng, F.; Huang, H.S.; Min, T.Y.; Ding, Y.; Ye, C.H. Comparative Test of Jackfruit Varieties (Lines) Based on Fuzzy Membership Function Method. South China Fruits 2023, 52, 61–65. [Google Scholar] [CrossRef]
- Huang, W.L.; Zhang, C.K.; Zhang, D.M.; Fu, X.X.; Chen, X.Y. Study on the Genetic Law of Leaf Traits in Guava Hybrid Progeny. Chin. J. Trop. Agric. 2025, 45, 17–25. [Google Scholar]
- Yang, P.D.; Zhao, Y.; Qu, F.R.; Cheng, Y.; Liu, Y.; Liu, Z. Identification of Tea Plant Triploid Resources and Study on Ploidy Differences in Bud and Leaf Traits. Chin. J. Trop. Crops 2025, 46, 924–936. [Google Scholar]
- Peng, L.H.; Chen, N.; Yang, Z.; Jang, H.D.; Wei, X.; Chai, S.F.; Zeng, D.J. Comparative Study on Photosynthetic Characteristics and Leaf Anatomical Structure of Three Rare and Endangered Dendrobium Species. J. Chin. Med. Mater. 2024, 12, 2966–2973. [Google Scholar]
- Miao, T.T.; Wu, Z.N.; Liu, J.L.; Wang, X.; Wang, J.C.; Cao, Z.H.; Sun, H. Physiological Response to Natural Drought Stress and Drought Resistance Evaluation of Different Pecan Varieties (Lines). Anhui For. Sci. Technol. 2025, 51, 14–22+34. [Google Scholar]
- Lv, Z.; Zhao, W.; Kong, S.; Li, L.; Lin, S. Overview of Molecular Mechanisms of Plant Leaf Development: A Systematic Review. Front. Plant Sci. 2023, 14, 1293424. [Google Scholar] [CrossRef]
- Kulkarni, M.; Deshpande, U. Anatomical Breeding for Altered Leaf Parameters in Tomato Genotypes Imparting Drought Resistance Using Leaf Strength Index. Asian J. Plant Sci. 2006, 5, 414–420. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General Mechanisms of Drought Response and Their Application in Drought Resistance Improvement in Plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Guo, S.J.; Wu, Y.Q. Characteristics of Leaf Anatomical Structure of Chestnut and Its Relationship with Drought Resistance. J. Northwest A F Univ. (Nat. Sci. Ed.) 2018, 46, 51–59. [Google Scholar] [CrossRef]
- Abdulraham, A.A.; Oladele, F.A. Response of Trichomes to Water Stress in Two Species of Jatropha. Insight Bot. 2011, 1, 15–21. [Google Scholar] [CrossRef]
- Pu, W.C.; Xu, Y.L.; Yu, Z.X.; Ma, H.C. Leaf Anatomical Structure Characteristics and Drought Resistance Analysis of Typical Drought-Tolerant Plants in Yuanjiang Dry-Hot Valley. J. Southwest. For. Univ. Nat. Sci. 2019, 39, 58–68. [Google Scholar]
- Hu, X.Y.; Yu, H.Y.; Cui, Y.F.; Fan, S.Q.; Bi, Q.X.; Li, Y.C.; Wang, L.B. Response of Stomatal Distribution Characteristics of Different Xanthoceras sorbifolium Provenances to Water Stress. For. Res. 2019, 32, 169–174. [Google Scholar] [CrossRef]
- Zheng, S.Z.; Kuang, B.C. Study on Drought Resistance Morphology and Anatomy of Tectona grandis Provenances. For. Res. 1993, 2, 124–130+235. [Google Scholar]
- Guha, A.; Sengupta, D.; Rasineni, G.K.; Reddy, A.R. An Integrated Diagnostic Approach to Understand Drought Tolerance in Mulberry (Morus indica L.). Flora 2010, 205, 144–151. [Google Scholar] [CrossRef]
- Ali, H.E.; Saad, Z.H.; Elsayed, H. The Effect of Drought on Chlorophyll, Proline and Chemical Composition of Three Varieties of Egyptian Rice. J. Biol. Chem. Environ. Sci. 2020, 15, 21–30. [Google Scholar]
- Sharifi, P.; Mohammadkhani, N. Effects of Drought Stress on Photosynthesis Factors in Wheat Genotypes during Anthesis. Cereal Res. Commun. 2016, 44, 229–239. [Google Scholar] [CrossRef]
- Yang, Y.; Nan, R.; Mi, T.; Song, Y.; Shi, F.; Liu, X.; Wang, Y.; Sun, F.; Xi, Y.; Zhang, C. Rapid and Nondestructive Evaluation of Wheat Chlorophyll under Drought Stress Using Hyperspectral Imaging. Int. J. Mol. Sci. 2023, 24, 5825. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors Influencing Leaf Chlorophyll Content in Natural Forests at the Biome Scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef]
- Hörtensteiner, S.; Kräutler, B. Chlorophyll Breakdown in Higher Plants. Biochim. Biophys. Acta Bioenerg. 2011, 1807, 977–988. [Google Scholar] [CrossRef]
- Nikolaeva, M.K.; Maevskaya, S.N.; Shugaev, A.G.; Bukhov, N.G. Effect of Drought on Chlorophyll Content and Antioxidant Enzyme Activities in Leaves of Three Wheat Cultivars Varying in Productivity. Russ. J. Plant Physiol. 2010, 57, 87–95. [Google Scholar] [CrossRef]
- Chowdhury, M.K.; Hasan, M.A.; Bahadur, M.M.; Islam, M.R.; Hakim, M.A.; Iqbal, M.A.; Javed, T.; Raza, A.; Shabbir, R.; Sorour, S.; et al. Evaluation of Drought Tolerance of Some Wheat (Triticum aestivum L.) Genotypes through Phenology, Growth, and Physiological Indices. Agronomy 2021, 11, 1792. [Google Scholar] [CrossRef]
- Chen, J.L.; Zhao, X.Y.; Zhang, Y.Q.; Li, Y.Q.; Luo, Y.Q.; Ning, Z.Y.; Wang, R.X.; Wang, P.Y.; Cong, A.Q. Effects of Drought and Rehydration on the Physiological Responses of Artemisia halodendron. Water 2019, 11, 793. [Google Scholar] [CrossRef]
- Xie, H.Y.; Li, M.R.; Chen, Y.J.; Zhou, Q.P.; Liu, W.H.; Liang, G.L.; Jia, Z.F. Important Physiological Changes Due to Drought Stress on Oat. Front. Ecol. Evol. 2021, 9, 644726. [Google Scholar] [CrossRef]
- Wasaya, A.; Manzoor, S.; Yasir, T.A.; Sarwar, N.; Mubeen, K.; Ismail, I.A.; Raza, A.; Rehman, A.; Hossain, A.; El Sabagh, A. Evaluation of Fourteen Bread Wheat (Triticum aestivum L.) Genotypes by Observing Gas Exchange Parameters, Relative Water and Chlorophyll Content, and Yield Attributes under Drought Stress. Sustainability 2021, 13, 4799. [Google Scholar] [CrossRef]
- Saha, S.; Begum, H.H.; Nasrin, S.; Samad, R. Effects of Drought Stress on Pigment and Protein Contents and Antioxidant Enzyme Activities in Five Varieties of Rice (Oryza sativa L.). Bangladesh J. Bot. 2020, 49, 997–1002. [Google Scholar] [CrossRef]
- Hinge, P.; Kale, A.; Pawar, B.; Jadhav, A.; Chimote, V.; Gadakh, S. Effect of PEG Induced Water Stress on Chlorophyll Content, Membrane Injury Index, Osmoprotectants and Antioxidant Enzymes Activities in Sorghum (Sorghum bicolor (L.) Moench). Maydica 2015, 60, 1–10. [Google Scholar]
- Epila, J.; Hubeau, M.; Steppe, K. Drought Effects on Photosynthesis and Implications of Photoassimilate Distribution in 11C-Labeled Leaves in the African Tropical Tree Species Maesopsis eminii Engl. Forests 2018, 9, 109. [Google Scholar] [CrossRef]
- Santos, T.d.O.; Amaral Junior, A.T.d.; Moulin, M.M. Maize Breeding for Low Nitrogen Inputs in Agriculture: Mechanisms Underlying the Tolerance to the Abiotic Stress. Stresses 2023, 3, 136–152. [Google Scholar] [CrossRef]
- Wang, K.; An, J.C.; Zhu, C.S.; Liang, X.J.; Li, K.X. Leaf Anatomical Structure Characteristics and Drought Resistance of Different Chemotypes of Oil Cinnamomum camphora. J. South. Agric. 2019, 50, 2525–2531. [Google Scholar]
- Wei, J.T.; Ma, X.Y.; Li, X.W.; Zhang, Z.J.; Li, C.; Ma, F.W. Drought Resistance Evaluation of 8 Apple Germplasm Resources. J. Fruit. Sci. 2024, 41, 569–578. [Google Scholar]
- Lin, X.; Feng, C.; Lin, T.; Harris, A.J.; Li, Y.; Kang, M. Jackfruit Genome and Population Genomics Provide Insights into Fruit Evolution and Domestication History in China. Hortic. Res. 2022, 9, uhac173. [Google Scholar] [CrossRef] [PubMed]
- Karunarathne, S.I.; Ranaweera, L.T.; Daundasekera, D.M.K.C.; Ananda, G.K.S.; Kannangara, S.K.; Ranathunga, A.P.D.T.; Madhukalpani, O.V.S.; Weebadde, C.K.; Sooriyapathirana, S.D.S.S. Assessment of the Applicability of Morphometric, Organoleptic and Phylogenetic Analyses to Differentiate Soft and Firm Flesh Bearing Trees of Jackfruit (Artocarpus heterophyllus Lam.) in Sri Lanka. J. Agric. Sci.-Sri Lanka 2018, 13, 200–218. [Google Scholar] [CrossRef]
- Yao, X.; Sun, H.; Zhou, S.; Li, L. Evaluating Photosynthetic Light Response Models for Leaf Photosynthetic Traits in Paddy Rice (Oryza sativa L.) Under Field Conditions. Plants 2025, 14, 23. [Google Scholar] [CrossRef]




| Number | Variety | Country of Origin | Variety Approval Number |
|---|---|---|---|
| 1 | A. ‘Haida 1’ | Donghai Island, Zhanjiang | Guangdong 2013004 |
| 2 | A. ‘Haida 2’ | Changshan Town, Lianjiang, Zhanjiang | Guangdong 2014009 |
| 3 | A. ‘Haida 3’ | Nanshan Town, Xuwen, Zhanjiang | Guangdong 2014003 |
| 4 | A. ‘Haida 4’ | Nanshan Town, Xuwen, Zhanjiang | Guangdong 2023006 |
| 5 | A. ‘Siji’ | Dongan Town, Gaozhou, Maoming | Guangdong 2009019 |
| 6 | A. ‘Changyou’ | Fruit Tree Research Institute, Maoming | Guangdong 2008001 |
| Indexes | Principal Component Coefficient | |||
|---|---|---|---|---|
| C1 | C2 | C3 | C4 | |
| X1 | 0.309 | −0.037 | 0.629 | 0.267 |
| X2 | 0.809 | 0.432 | 0.214 | 0.319 |
| X3 | 0.725 | 0.407 | 0.307 | 0.355 |
| X4 | −0.743 | −0.582 | 0.052 | −0.21 |
| X5 | 0.462 | 0.677 | 0.367 | −0.425 |
| X6 | −0.748 | −0.258 | 0.251 | 0.558 |
| X7 | 0.774 | −0.586 | 0.007 | 0.209 |
| X8 | 0.373 | 0.375 | −0.799 | 0.17 |
| X9 | 0.23 | −0.652 | 0.034 | 0.714 |
| X10 | 0.625 | 0.341 | −0.455 | −0.159 |
| X11 | 0.488 | 0.607 | −0.193 | 0.009 |
| X12 | −0.846 | 0.3 | 0.231 | −0.113 |
| X13 | 0.735 | −0.327 | 0.322 | 0.494 |
| X14 | 0.489 | −0.688 | 0.471 | 0.097 |
| X15 | 0.608 | −0.739 | −0.1 | 0.257 |
| X16 | 0.228 | 0.19 | −0.893 | 0.262 |
| X17 | 0.394 | −0.478 | 0.129 | −0.718 |
| X18 | 0.757 | −0.19 | 0.546 | −0.304 |
| X19 | −0.452 | 0.357 | 0.723 | 0.377 |
| X20 | 0.74 | −0.313 | 0.048 | −0.594 |
| X21 | 0.636 | 0.726 | 0.222 | 0.078 |
| X22 | 0.916 | 0.192 | 0.076 | −0.217 |
| X23 | 0.5 | 0.707 | −0.026 | 0.486 |
| X24 | −0.902 | 0.34 | −0.136 | 0.123 |
| X25 | 0.115 | −0.553 | −0.757 | 0.222 |
| X26 | −0.896 | 0.307 | 0.317 | 0.046 |
| Characteristic root | 10.539 | 5.934 | 4.393 | 3.302 |
| Contribution rate% | 40.533 | 22.824 | 16.898 | 12.7 |
| Cumulative contribution% | 40.533 | 63.357 | 80.255 | 92.955 |
| Cultivars | Principal Component Score | Value of the Affiliation Function | Consolidated Assessed Value | Rankings | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C1 | C2 | C3 | C4 | Comprehensive Evaluation Value (D) | ||
| A. ‘Haida 1’ | −12.62 | 4.55 | −0.16 | −2.87 | 0.00 | 1.00 | 0.57 | 0.00 | 0.32 | 5 |
| A. ‘Haida 2’ | −3.46 | 3.10 | 0.59 | 6.41 | 0.35 | 0.90 | 0.63 | 1.00 | 0.58 | 3 |
| A. ‘Haida 3’ | −2.38 | 2.36 | 4.42 | −1.98 | 0.39 | 0.86 | 0.96 | 0.10 | 0.53 | 4 |
| A. ‘Haida 4’ | 11.66 | 4.02 | −6.66 | −1.03 | 0.92 | 0.96 | 0.00 | 0.20 | 0.62 | 2 |
| A. ‘Siji’ | −7.07 | −10.58 | −3.01 | −0.03 | 0.21 | 0.00 | 0.32 | 0.31 | 0.18 | 6 |
| A. ‘Changyou’ | 13.87 | −3.45 | 4.82 | −0.50 | 1.00 | 0.47 | 1.00 | 0.26 | 0.71 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Yang, C.; Lin, S.; Li, W.; Ou, S.; Guo, J.; Huang, X.; Liu, X.; Feng, F. Comprehensive Evaluation of Leaf Structure, Photosynthetic Characteristics, and Drought Resistance in Six Jackfruit (Artocarpus heterophyllus) Cultivars. Life 2025, 15, 1346. https://doi.org/10.3390/life15091346
Wu W, Yang C, Lin S, Li W, Ou S, Guo J, Huang X, Liu X, Feng F. Comprehensive Evaluation of Leaf Structure, Photosynthetic Characteristics, and Drought Resistance in Six Jackfruit (Artocarpus heterophyllus) Cultivars. Life. 2025; 15(9):1346. https://doi.org/10.3390/life15091346
Chicago/Turabian StyleWu, Weihao, Chongcheng Yang, Shiting Lin, Wei Li, Suhui Ou, Jinson Guo, Xiaojia Huang, Xuemin Liu, and Feng Feng. 2025. "Comprehensive Evaluation of Leaf Structure, Photosynthetic Characteristics, and Drought Resistance in Six Jackfruit (Artocarpus heterophyllus) Cultivars" Life 15, no. 9: 1346. https://doi.org/10.3390/life15091346
APA StyleWu, W., Yang, C., Lin, S., Li, W., Ou, S., Guo, J., Huang, X., Liu, X., & Feng, F. (2025). Comprehensive Evaluation of Leaf Structure, Photosynthetic Characteristics, and Drought Resistance in Six Jackfruit (Artocarpus heterophyllus) Cultivars. Life, 15(9), 1346. https://doi.org/10.3390/life15091346





