FGF14 Peptide Derivative Differentially Regulates Nav1.2 and Nav1.6 Function
Abstract
1. Introduction
2. Methods and Materials
2.1. Chemicals
2.2. Cell Culture
2.3. Automated Planar Hardware and Software
2.3.1. Hardware
2.3.2. Software
2.4. Automated Planar Patch Recordings Using the Porta-Patch
2.4.1. Whole Cell Voltage-Clamp Solutions
2.4.2. Automated Planar Cell Viability and Density Assessment
2.5. Automated Patch-Clamp Recordings Using the Synchropatch 384i
2.6. Voltage-Clamp Data Analysis
2.7. Computational CA1 Pyramidal Neuron Modeling
2.8. Statistical Analysis of Data
3. Results
3.1. ZL0177 Modulates Na+ Currents in an Isoform-Specific Manner
3.2. AlphaFold Structure of Nav1.2 and Nav1.6 Channels and Predicted ZL0177 Binding Sites
3.3. Zl0177 Elicits Phenotypically Divergent Action Potentials in CA1 Pyramidal Neuron Computational Models
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Royeck, M.; Horstmann, M.-T.; Remy, S.; Reitze, M.; Yaari, Y.; Beck, H. Role of Axonal NaV1.6 Sodium Channels in Action Potential Initiation of CA1 Pyramidal Neurons. J. Neurophysiol. 2008, 100, 2361–2380. [Google Scholar] [CrossRef]
- Hu, W.; Tian, C.; Li, T.; Yang, M.; Hou, H.; Shu, Y. Distinct Contributions of Na(v)1.6 and Na(v)1.2 in Action Potential Initiation and Backpropagation. Nat. Neurosci. 2009, 12, 996–1002. [Google Scholar] [CrossRef]
- Shu, Y. Neuronal signaling in central nervous system. Sheng Li Xue Bao 2011, 63, 1–8. [Google Scholar] [PubMed]
- Noujaim, S.F.; Kaur, K.; Milstein, M.; Jones, J.M.; Furspan, P.; Jiang, D.; Auerbach, D.S.; Herron, T.; Meisler, M.H.; Jalife, J. A Null Mutation of the Neuronal Sodium Channel NaV1.6 Disrupts Action Potential Propagation and Excitation-Contraction Coupling in the Mouse Heart. FASEB J. 2012, 26, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Yang, J.; Tian, C.; Zhu, Q.; Yin, L.; Jiang, S.; Yang, M.; Shu, Y. Differential Roles of NaV1.2 and NaV1.6 in Regulating Neuronal Excitability at Febrile Temperature and Distinct Contributions to Febrile Seizures. Sci. Rep. 2018, 8, 753. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.D.; Wang, C.; Banks, E.; Fenton, T.; DeKeyser, J.-M.; Abramova, T.V.; Jr, A.L.G.; Ben-Shalom, R.; Hackos, D.H.; Bender, K.J. Differential Roles of NaV1.2 and NaV1.6 in Neocortical Pyramidal Cell Excitability. eLife 2025, 14, RP105696. [Google Scholar] [CrossRef]
- Liao, Y.; Deprez, L.; Maljevic, S.; Pitsch, J.; Claes, L.; Hristova, D.; Jordanova, A.; Ala-Mello, S.; Bellan-Koch, A.; Blazevic, D.; et al. Molecular Correlates of Age-Dependent Seizures in an Inherited Neonatal-Infantile Epilepsy. Brain 2010, 133, 1403–1414. [Google Scholar] [CrossRef]
- Van Wart, A.; Trimmer, J.S.; Matthews, G. Polarized Distribution of Ion Channels within Microdomains of the Axon Initial Segment. J. Comp. Neurol. 2007, 500, 339–352. [Google Scholar] [CrossRef]
- Gazina, E.V.; Leaw, B.T.W.; Richards, K.L.; Wimmer, V.C.; Kim, T.H.; Aumann, T.D.; Featherby, T.J.; Churilov, L.; Hammond, V.E.; Reid, C.A.; et al. ‘Neonatal’ Nav1.2 Reduces Neuronal Excitability and Affects Seizure Susceptibility and Behaviour. Hum. Mol. Genet. 2015, 24, 1457–1468. [Google Scholar] [CrossRef]
- Brackenbury, W.J.; Calhoun, J.D.; Chen, C.; Miyazaki, H.; Nukina, N.; Oyama, F.; Ranscht, B.; Isom, L.L. Functional Reciprocity between Na+ Channel Nav1.6 and Beta1 Subunits in the Coordinated Regulation of Excitability and Neurite Outgrowth. Proc. Natl. Acad. Sci. USA 2010, 107, 2283–2288. [Google Scholar] [CrossRef]
- Imbrici, P.; Camerino, D.C.; Tricarico, D. Major Channels Involved in Neuropsychiatric Disorders and Therapeutic Perspectives. Front. Genet. 2013, 4, 76. [Google Scholar] [CrossRef] [PubMed]
- Akin, E.J.; Solé, L.; Johnson, B.; el Beheiry, M.; Masson, J.-B.; Krapf, D.; Tamkun, M.M. Single-Molecule Imaging of Nav1.6 on the Surface of Hippocampal Neurons Reveals Somatic Nanoclusters. Biophys. J. 2016, 111, 1235–1247. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Li, X.; Tian, H.; Wang, L.; Guo, B.; Wang, Y.; Li, W.; Wang, F.; Sun, T. SCN1A Mutation—Beyond Dravet Syndrome: A Systematic Review and Narrative Synthesis. Front. Neurol. 2021, 12, 743726. [Google Scholar] [CrossRef] [PubMed]
- Akin, E.J.; Solé, L.; Dib-Hajj, S.D.; Waxman, S.G.; Tamkun, M.M. Preferential Targeting of Nav1.6 Voltage-Gated Na+ Channels to the Axon Initial Segment during Development. PLoS ONE 2015, 10, e0124397. [Google Scholar] [CrossRef]
- Dvorak, N.M.; Wadsworth, P.A.; Aquino-Miranda, G.; Wang, P.; Engelke, D.S.; Zhou, J.; Nguyen, N.; Singh, A.K.; Aceto, G.; Haghighijoo, Z.; et al. Enhanced Motivated Behavior Mediated by Pharmacological Targeting of the FGF14/Nav1.6 Complex in Nucleus Accumbens Neurons. Nat. Commun. 2025, 16, 110. [Google Scholar] [CrossRef]
- Katz, E.; Stoler, O.; Scheller, A.; Khrapunsky, Y.; Goebbels, S.; Kirchhoff, F.; Gutnick, M.J.; Wolf, F.; Fleidervish, I.A. Role of Sodium Channel Subtype in Action Potential Generation by Neocortical Pyramidal Neurons. Proc. Natl. Acad. Sci. USA 2018, 115, E7184–E7192. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Eaton, M.; Wu, J.; Ma, Z.; Lai, S.; Park, A.; Ahmad, T.S.; Que, Z.; Lee, J.H.; et al. Severe Deficiency of the Voltage-Gated Sodium Channel NaV1.2 Elevates Neuronal Excitability in Adult Mice. Cell Rep. 2021, 36, 109495. [Google Scholar] [CrossRef]
- Meisler, M.H.; Hill, S.F.; Yu, W. Sodium Channelopathies in Neurodevelopmental Disorders. Nat. Rev. Neurosci. 2021, 22, 152–166. [Google Scholar] [CrossRef]
- Berg, A.T.; Thompson, C.H.; Myers, L.S.; Anderson, E.; Evans, L.; Kaiser, A.J.E.; Paltell, K.; Nili, A.N.; DeKeyser, J.-M.L.; Abramova, T.V.; et al. Expanded Clinical Phenotype Spectrum Correlates with Variant Function in SCN2A-Related Disorders. Brain 2024, 147, 2761–2774. [Google Scholar] [CrossRef]
- Eltokhi, A.; Lundstrom, B.N.; Li, J.; Zweifel, L.S.; Catterall, W.A.; Gamal El-Din, T.M. Pathogenic Gating Pore Current Conducted by Autism-Related Mutations in the NaV1.2 Brain Sodium Channel. Proc. Natl. Acad. Sci. USA 2024, 121, e2317769121. [Google Scholar] [CrossRef]
- Jia, L.; Li, M.; Pachernegg, S.; Sedo, A.; Jancovski, N.; Burbano, L.E.; Dalby, K.; Nemiroff, A.; Reid, C.; Maljevic, S.; et al. Variant-Specific in Vitro Neuronal Network Phenotypes and Drug Sensitivity in SCN2A Developmental and Epileptic Encephalopathy. J. Neurochem. 2024, 168, 3950–3961. [Google Scholar] [CrossRef] [PubMed]
- Shabani, K.; Krupp, J.; Lemesre, E.; Lévy, N.; Tran, H. Voltage-Gated Ion Channel Compensatory Effect in DEE: Implications for Future Therapies. Cells 2024, 13, 1763. [Google Scholar] [CrossRef] [PubMed]
- Hack, J.B.; Watkins, J.C.; Schreiber, J.M.; Hammer, M.F. Patients Carrying Pathogenic SCN8A Variants with Loss- and Gain-of-Function Effects Can Be Classified into Five Subgroups Exhibiting Varying Developmental and Epileptic Components of Encephalopathy. Epilepsia 2024, 65, 3324–3334. [Google Scholar] [CrossRef]
- Quinn, S.; Zhang, N.; Fenton, T.A.; Brusel, M.; Muruganandam, P.; Peleg, Y.; Giladi, M.; Haitin, Y.; Lerche, H.; Bassan, H.; et al. Complex Biophysical Changes and Reduced Neuronal Firing in an SCN8A Variant Associated with Developmental Delay and Epilepsy. Biochim. Et Biophys. Acta (BBA)—Mol. Basis Dis. 2024, 1870, 167127. [Google Scholar] [CrossRef] [PubMed]
- Vanoye, C.G.; Abramova, T.V.; DeKeyser, J.-M.; Ghabra, N.F.; Oudin, M.J.; Burge, C.B.; Helbig, I.; Thompson, C.H.; George, A.L. Molecular and Cellular Context Influences SCN8A Variant Function. JCI Insight 2024, 9, e177530. [Google Scholar] [CrossRef]
- Xiao, M.; Bosch, M.K.; Nerbonne, J.M.; Ornitz, D.M. FGF14 localization and organization of the axon initial segment. Mol. Cell Neurosci. 2013, 56, 393–403. [Google Scholar] [CrossRef]
- Ali, S.R.; Liu, Z.; Nenov, M.N.; Folorunso, O.; Singh, A.; Scala, F.; Chen, H.; James, T.F.; Alshammari, M.; Panova-Elektronova, N.I.; et al. Functional Modulation of Voltage-Gated Sodium Channels by a FGF14-Based Peptidomimetic. ACS Chem. Neurosci. 2018, 9, 976–987. [Google Scholar] [CrossRef]
- Hsu, W.-C.J.; Scala, F.; Nenov, M.N.; Wildburger, N.C.; Elferink, H.; Singh, A.K.; Chesson, C.B.; Buzhdygan, T.; Sohail, M.; Shavkunov, A.S.; et al. CK2 Activity Is Required for the Interaction of FGF14 with Voltage-Gated Sodium Channels and Neuronal Excitability. FASEB J. 2016, 30, 2171–2186. [Google Scholar] [CrossRef]
- Liu, Z.; Wadsworth, P.; Singh, A.K.; Chen, H.; Wang, P.; Folorunso, O.; Scaduto, P.; Ali, S.R.; Laezza, F.; Zhou, J. Identification of Peptidomimetics as Novel Chemical Probes Modulating Fibroblast Growth Factor 14 (FGF14) and Voltage-Gated Sodium Channel 1.6 (Nav1.6) Protein-Protein Interactions. Bioorg Med. Chem. Lett. 2019, 29, 413–419. [Google Scholar] [CrossRef]
- Dvorak, N.M.; Wadsworth, P.A.; Wang, P.; Chen, H.; Zhou, J.; Laezza, F. Bidirectional Modulation of the Voltage-Gated Sodium (Nav1.6) Channel by Rationally Designed Peptidomimetics. Molecules 2020, 25, 3365. [Google Scholar] [CrossRef]
- Singh, A.K.; Wadsworth, P.A.; Tapia, C.M.; Aceto, G.; Ali, S.R.; Chen, H.; D’Ascenzo, M.; Zhou, J.; Laezza, F. Mapping of the FGF14:Nav1.6 Complex Interface Reveals FLPK as a Functionally Active Peptide Modulating Excitability. Physiol. Rep. 2020, 8, e14505. [Google Scholar] [CrossRef] [PubMed]
- Wadsworth, P.A.; Singh, A.K.; Nguyen, N.; Dvorak, N.M.; Tapia, C.M.; Russell, W.K.; Stephan, C.; Laezza, F. JAK2 Regulates Nav1.6 Channel Function via FGF14Y158 Phosphorylation. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118786. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wadsworth, P.A.; Dvorak, N.M.; Singh, A.K.; Chen, H.; Liu, Z.; Zhou, R.; Holthauzen, L.M.F.; Zhou, J.; Laezza, F. Design, Synthesis, and Pharmacological Evaluation of Analogs Derived from the PLEV Tetrapeptide as Protein-Protein Interaction Modulators of Voltage-Gated Sodium Channel 1.6. J. Med. Chem. 2020, 63, 11522–11547. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, N.M.; Tapia, C.M.; Singh, A.K.; Baumgartner, T.J.; Wang, P.; Chen, H.; Wadsworth, P.A.; Zhou, J.; Laezza, F. Pharmacologically Targeting the Fibroblast Growth Factor 14 Interaction Site on the Voltage-Gated Na+ Channel 1.6 Enables Isoform-Selective Modulation. Int. J. Mol. Sci. 2021, 22, 13541. [Google Scholar] [CrossRef]
- Cummins, T.R.; Rush, A.M.; Estacion, M.; Dib-Hajj, S.D.; Waxman, S.G. Voltage-Clamp and Current-Clamp Recordings from Mammalian DRG Neurons. Nat. Protoc. 2009, 4, 1103–1112. [Google Scholar] [CrossRef]
- Hines, M.; Carnevale, N. The NEURON simulation environment. Neural Comput. 1997, 9, 1179–1209. [Google Scholar] [CrossRef]
- Migliore, R.; Lupascu, C.A.; Bologna, L.L.; Romani, A.; Courcol, J.-D.; Antonel, S. The Physiological Variability of Channel Density in Hippocampal CA1 Pyramidal Cells and Interneurons Explored Using a Unified Data-Driven Modeling Workflow. PLoS Comput. Biol. 2018, 14, 1006423. [Google Scholar] [CrossRef]
- Migliore, M.; Ascoli, G.A.; Jaffe, D.B. CA3 Cells: Detailed and Simplified Pyramidal Cell Models. In Hippocampal Microcircuits; Springer Series in Computational Neuroscience; Cutsuridis, V., Graham, B., Cobb, S., Vida, I., Eds.; Springer: New York, NY, USA, 2010; Volume 5. [Google Scholar]
- Kruth, K.A.; Grisolano, T.M.; Ahern, C.A.; Williams, A.J. SCN2A channelopathies in the autism spectrum of neuropsychiatric disorders: A role for pluripotent stem cells? Mol Autism. 2020, 11, 23. [Google Scholar] [CrossRef]
- Ben-Shalom, R.; Keeshen, C.M.; Berrios, K.N.; An, J.Y.; Sanders, S.J.; Bender, K.J. Opposing Effects on NaV1.2 Function Underlie Differences between SCN2A Variants Observed in Individuals with Autism Spectrum Disorder or Infantile Seizures. Biol. Psychiatry 2017, 82, 224–232. [Google Scholar] [CrossRef]
- Berecki, G.; Tao, E.; Howell, K.B.; Coorg, R.K.; Andersen, E.; Kahlig, K.; Wolff, M.; Corry, B.; Petrou, S. Nav1.2 channel mutations preventing fast inactivation lead to SCN2A encephalopathy. Brain 2025, 148, 212–226. [Google Scholar] [CrossRef]
- Tian, C.; Wang, K.; Ke, W.; Guo, H.; Shu, Y. Molecular Identity of Axonal Sodium Channels in Human Cortical Pyramidal Cells. Front. Cell. Neurosci. 2014, 8, 297. [Google Scholar] [CrossRef] [PubMed]
- Akin, E.J.; Weigel, A.V.; Krapf, D.; Tamkun, M.M. Single-Particle Tracking of Nav1.6 Demonstrates Different Mechanisms for Sodium Channel Anchoring within the AIS versus the Soma of Hippocampal Neurons. Biophys. J. 2013, 104, 138a. [Google Scholar] [CrossRef]
- Kaczmarek, L.K. Loss of NaV1.2-Dependent Backpropagating Action Potentials in Dendrites Contributes to Autism and Intellectual Disability. Neuron 2019, 103, 551–553. [Google Scholar] [CrossRef] [PubMed]
- Spratt, P.W.E.; Ben-Shalom, R.; Sahagun, A.; Keeshen, C.M.; Sanders, S.J.; Bender, K.J. Paradoxical Hyperexcitability from NaV1.2 Sodium Channel Loss in Neocortical Pyramidal Cells. bioRxiv 2021, 36, 109483. [Google Scholar] [CrossRef]
- Blumenfeld, H.; Lampert, A.; Klein, J.P.; Mission, J.; Chen, M.C.; Rivera, M.; Dib-Hajj, S.; Brennan, A.R.; Hains, B.C.; Waxman, S.G. Role of Hippocampal Sodium Channel Nav1.6 in Kindling Epileptogenesis. Epilepsia 2009, 50, 44–55. [Google Scholar] [CrossRef]
- Patel, R.R.; Barbosa, C.; Brustovetsky, T.; Brustovetsky, N.; Cummins, T.R. Aberrant Epilepsy-Associated Mutant Nav1.6 Sodium Channel Activity Can Be Targeted with Cannabidiol. Brain 2016, 139, 2164–2181. [Google Scholar] [CrossRef]
- Blanchard, M.G.; Willemsen, M.H.; Walker, J.B.; Dib-Hajj, S.D.; Waxman, S.G.; Jongmans, M.C.J.; Kleefstra, T.; van de Warrenburg, B.P.; Praamstra, P.; Nicolai, J.; et al. De Novo Gain-of-Function and Loss-of-Function Mutations of SCN8A in Patients with Intellectual Disabilities and Epilepsy. J. Med. Genet. 2015, 52, 330–337. [Google Scholar] [CrossRef]
- O’Brien, J.E.; Meisler, M.H. Sodium Channel SCN8A (Nav1.6): Properties and de Novo Mutations in Epileptic Encephalopathy and Intellectual Disability. Front. Genet. 2013, 4, 213. [Google Scholar] [CrossRef]
- Liu, Y.; Schubert, J.; Sonnenberg, L.; Helbig, K.L.; Hoei-Hansen, C.E.; Koko, M.; Rannap, M.; Lauxmann, S.; Huq, M.; Schneider, M.C.; et al. Neuronal Mechanisms of Mutations in SCN8A Causing Epilepsy or Intellectual Disability. Brain 2019, 142, 376–390. [Google Scholar] [CrossRef]
- Wagnon, J.L.; Barker, B.S.; Ottolini, M.; Park, Y.; Volkheimer, A.; Valdez, P.; Swinkels, M.E.M.; Patel, M.K.; Meisler, M.H. Loss-of-Function Variants of SCN8A in Intellectual Disability without Seizures. Neurol. Genet. 2017, 3, e170. [Google Scholar] [CrossRef]
- de Lera Ruiz, M.; Kraus, R.L. Voltage-Gated Sodium Channels: Structure, Function, Pharmacology, and Clinical Indications. J. Med. Chem. 2015, 58, 7093–7118. [Google Scholar] [CrossRef]
- Dvorak, N.M.; Wadsworth, P.A.; Wang, P.; Zhou, J.; Laezza, F. Development of Allosteric Modulators of Voltage-Gated Na+ Channels: A Novel Approach for an Old Target. Curr. Top. Med. Chem. 2021, 21, 841–848. [Google Scholar] [CrossRef]
- Ali, S.R.; Singh, A.K.; Laezza, F. Identification of Amino Acid Residues in Fibroblast Growth Factor 14 (FGF14) Required for Structure-Function Interactions with Voltage-Gated Sodium Channel Nav1.6. J. Biol. Chem. 2016, 291, 11268–11284. [Google Scholar] [CrossRef]
- Laezza, F.; Lampert, A.; Kozel, M.A.; Gerber, B.R.; Rush, A.M.; Nerbonne, J.M.; Waxman, S.G.; Dib-Hajj, S.D.; Ornitz, D.M. FGF14 N-Terminal Splice Variants Differentially Modulate Nav1.2 and Nav1.6-Encoded Sodium Channels. Mol. Cell. Neurosci. 2009, 42, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Shavkunov, A.S.; Wildburger, N.C.; Nenov, M.N.; James, T.F.; Buzhdygan, T.P.; Panova-Elektronova, N.I.; Green, T.A.; Veselenak, R.L.; Bourne, N.; Laezza, F. The Fibroblast Growth Factor 14·voltage-Gated Sodium Channel Complex Is a New Target of Glycogen Synthase Kinase 3 (GSK3). J. Biol. Chem. 2013, 288, 19370–19385. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.-Y.; Laezza, F.; Gerber, B.R.; Xiao, M.; Yamada, K.A.; Hartmann, H.; Craig, A.M.; Nerbonne, J.M.; Ornitz, D.M. Fibroblast Growth Factor 14 Is an Intracellular Modulator of Voltage-Gated Sodium Channels. J. Physiol. 2005, 569, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Effraim, P.R.; Huang, J.; Lampert, A.; Stamboulian, S.; Zhao, P.; Black, J.A.; Dib-Hajj, S.D.; Waxman, S.G. Fibroblast Growth Factor Homologous Factor 2 (FGF-13) Associates with Nav1.7 in DRG Neurons and Alters Its Current Properties in an Isoform-Dependent Manner. Neurobiol. Pain. 2019, 6, 100029. [Google Scholar] [CrossRef]
- Goldfarb, M.; Schoorlemmer, J.; Williams, A.; Diwakar, S.; Wang, Q.; Huang, X.; Giza, J.; Tchetchik, D.; Kelley, K.; Vega, A.; et al. Fibroblast Growth Factor Homologous Factors Control Neuronal Excitability through Modulation of Voltage-Gated Sodium Channels. Neuron 2007, 55, 449–463. [Google Scholar] [CrossRef]
- Carbone, E. Fast Inactivation of Nav1.3 Channels by FGF14 Proteins: An Unconventional Way to Regulate the Slow Firing of Adrenal Chromaffin Cells. J. Gen. Physiol. 2021, 153, e202112879. [Google Scholar] [CrossRef]
- O’Leary, M.E.; Chahine, M. Mechanisms of Drug Binding to Voltage-Gated Sodium Channels. In Voltage-Gated Sodium Channels: Structure, Function and Channelopathies; Springer: Cham, Switzerland, 2017; pp. 209–231. ISBN 978-3-319-90284-5. [Google Scholar]
- Cha, A.; Ruben, P.C.; George, A.L., Jr. Voltage Sensors in Domains III and IV, but Not I and II, Are Immobilized by Na+ Channel Fast Inactivation. Neuron 1999, 22, 73–87. [Google Scholar] [CrossRef]
- Goldin, A.L. Mechanisms of Sodium Channel Inactivation. Curr. Opin. Neurobiol. 2003, 13, 284–290. [Google Scholar] [CrossRef]
- Groome, J.; Lehmann-Horn, F.; Holzherr, B. Open- and Closed-State Fast Inactivation in Sodium Channels: Differential Effects of a Site-3 Anemone Toxin. Channels 2011, 5, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Li, Z.; Zhou, Q. Structure of the Human Voltage-Gated Sodium Channel Nav1.4 in Complex with Beta1. Science 2018, 362, eaau2486. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Kaneko, Y.; Dharmawan, T.; Kurabayashi, M. Role of the Voltage Sensor Module in Nav Domain IV on Fast Inactivation in Sodium Channelopathies: The Implication of Closed-State Inactivation. Channels 2019, 13, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Spratt, P.W.E.; Ben-Shalom, R.; Keeshen, C.M.; Burke, K.J.; Clarkson, R.L.; Sanders, S.J.; Bender, K.J. The Autism-Associated Gene Scn2a Contributes to Dendritic Excitability and Synaptic Function in the Prefrontal Cortex. Neuron 2019, 103, 673–685.e5. [Google Scholar] [CrossRef]
- Goodchild, S.J.; Shuart, N.G.; Williams, A.D.; Ye, W.; Parrish, R.R.; Soriano, M.; Thouta, S.; Mezeyova, J.; Waldbrook, M.; Dean, R.; et al. Molecular Pharmacology of Selective NaV1.6 and Dual NaV1.6/NaV1.2 Channel Inhibitors That Suppress Excitatory Neuronal Activity Ex Vivo. ACS Chem. Neurosci. 2024, 15, 1169–1184. [Google Scholar] [CrossRef]
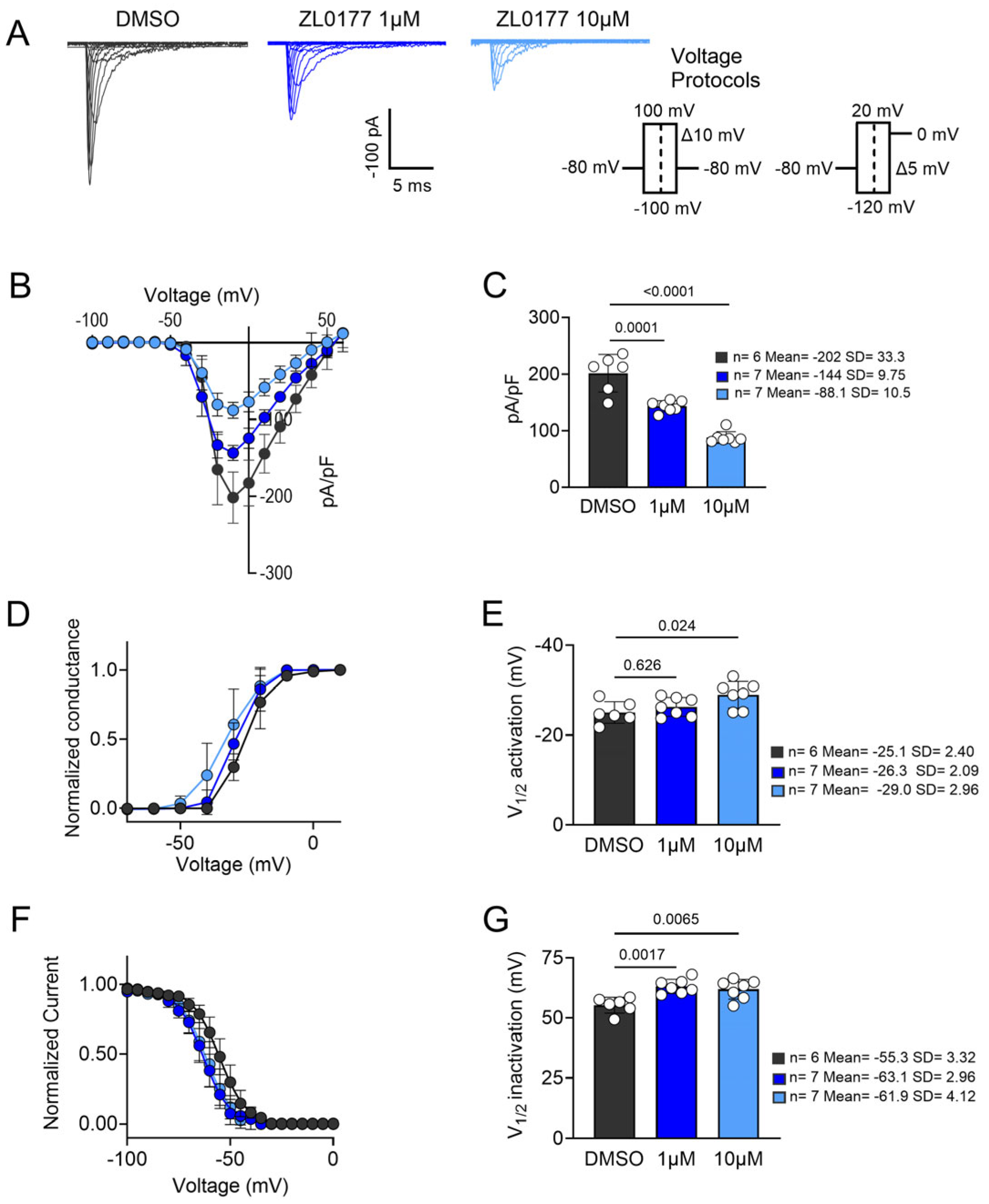

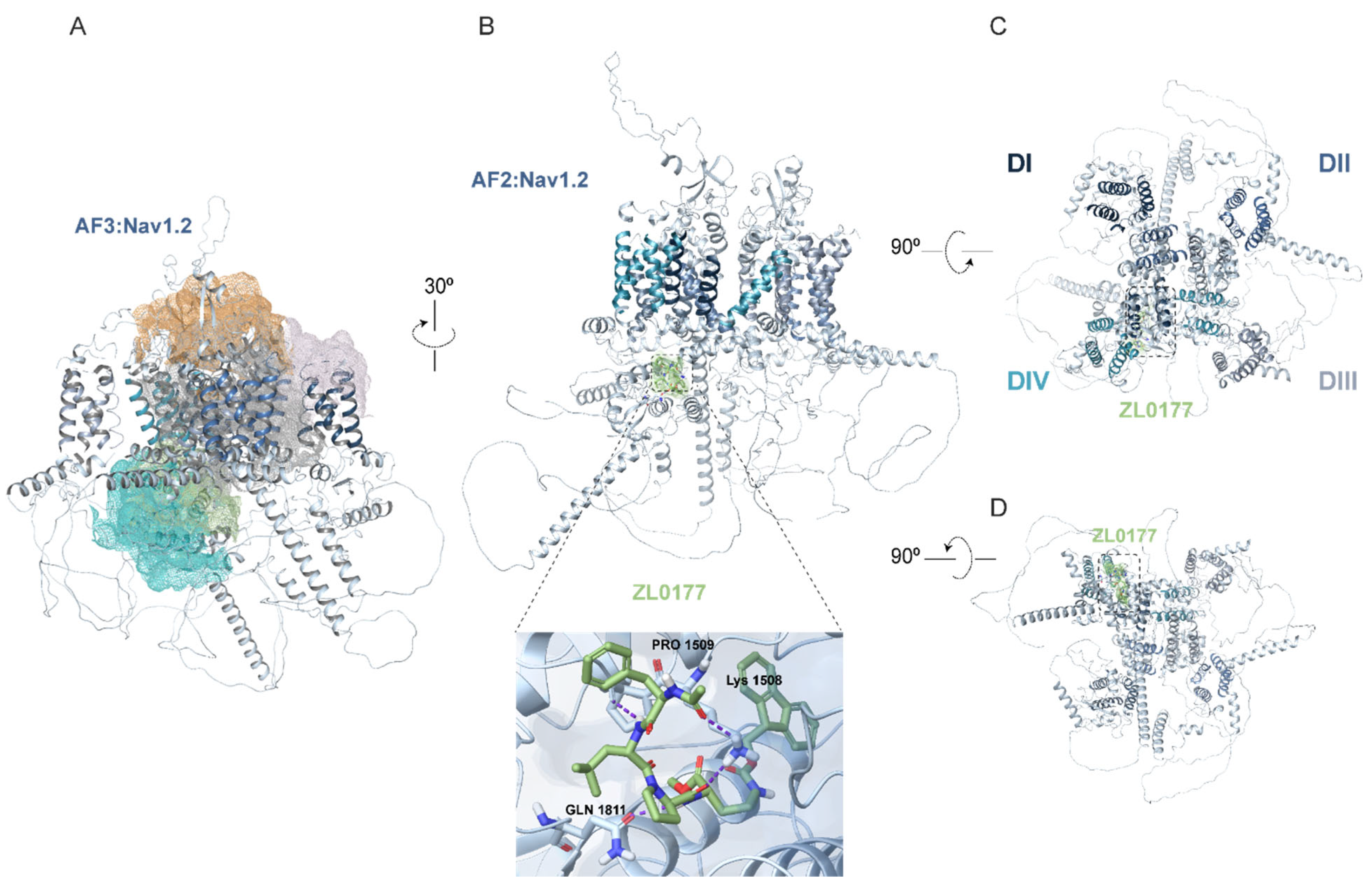
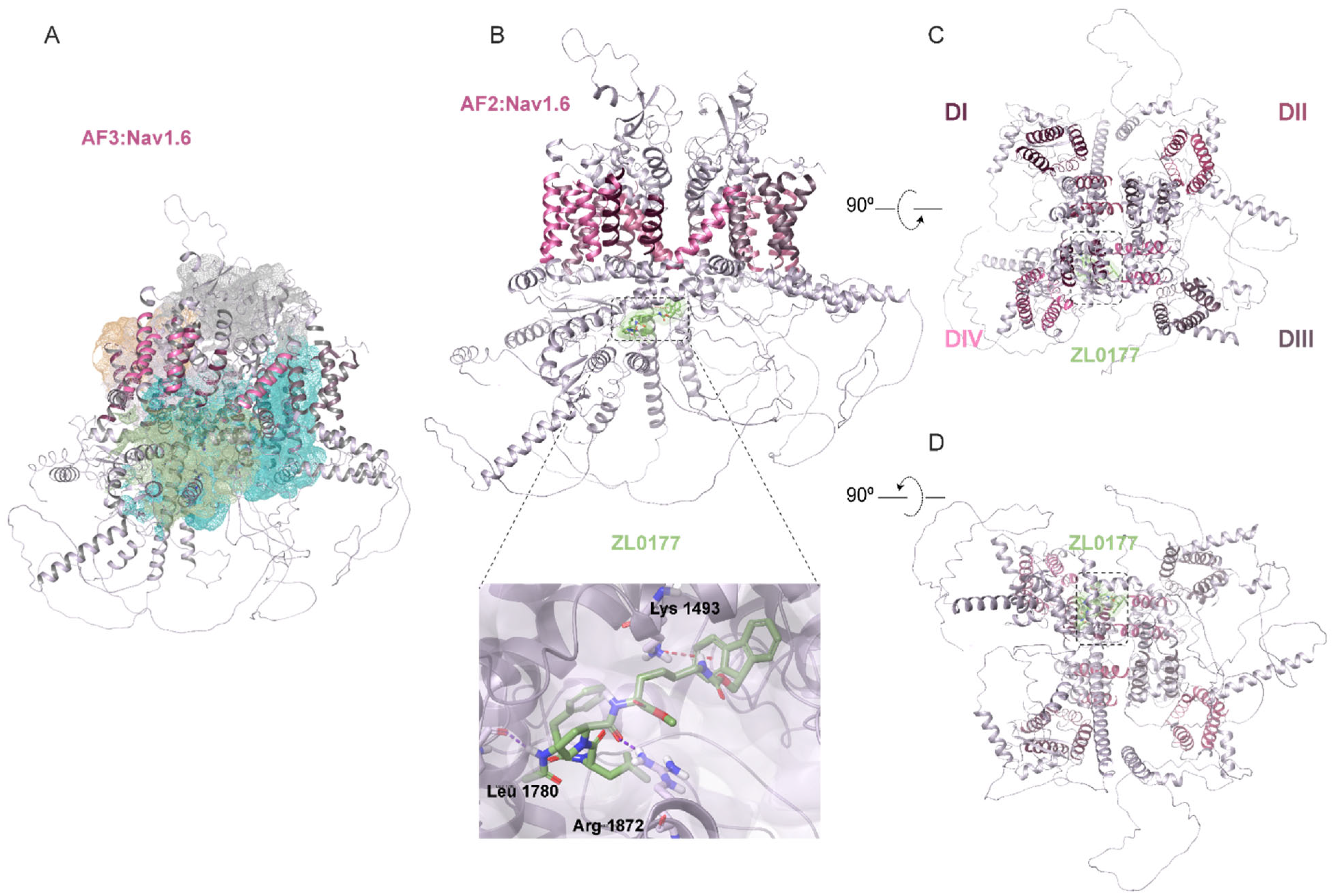
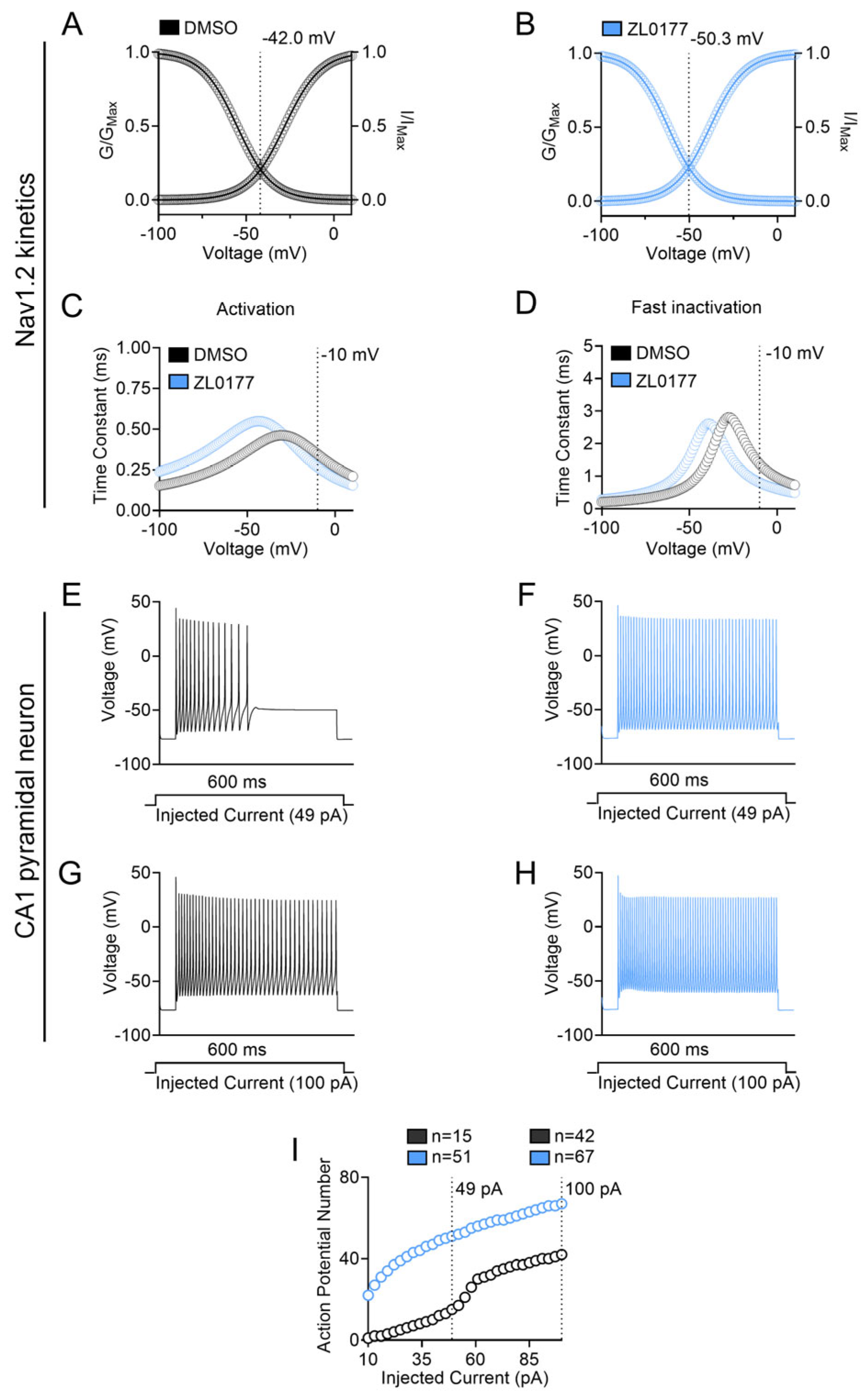

| Nav Isoform | Condition | Peak Current Density (pA/pF) |
|---|---|---|
| Nav1.2 | DMSO | −228.3 ± 133.3 (22) |
| ZL177 | −130.7 ± 92.8 (17) a | |
| Nav1.6 | DMSO | −51.2 ± 28.2 (17) |
| ZL177 | −21.6 ± 7.7 (20) b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arman, P.; Haghighijoo, Z.; Lupascu, C.A.; Singh, A.K.; Goode, N.A.; Baumgartner, T.J.; Singh, J.; Xue, Y.; Wang, P.; Chen, H.; et al. FGF14 Peptide Derivative Differentially Regulates Nav1.2 and Nav1.6 Function. Life 2025, 15, 1345. https://doi.org/10.3390/life15091345
Arman P, Haghighijoo Z, Lupascu CA, Singh AK, Goode NA, Baumgartner TJ, Singh J, Xue Y, Wang P, Chen H, et al. FGF14 Peptide Derivative Differentially Regulates Nav1.2 and Nav1.6 Function. Life. 2025; 15(9):1345. https://doi.org/10.3390/life15091345
Chicago/Turabian StyleArman, Parsa, Zahra Haghighijoo, Carmen A. Lupascu, Aditya K. Singh, Nana A. Goode, Timothy J. Baumgartner, Jully Singh, Yu Xue, Pingyuan Wang, Haiying Chen, and et al. 2025. "FGF14 Peptide Derivative Differentially Regulates Nav1.2 and Nav1.6 Function" Life 15, no. 9: 1345. https://doi.org/10.3390/life15091345
APA StyleArman, P., Haghighijoo, Z., Lupascu, C. A., Singh, A. K., Goode, N. A., Baumgartner, T. J., Singh, J., Xue, Y., Wang, P., Chen, H., Antunes, D. A., Lijffijt, M., Zhou, J., Migliore, M., & Laezza, F. (2025). FGF14 Peptide Derivative Differentially Regulates Nav1.2 and Nav1.6 Function. Life, 15(9), 1345. https://doi.org/10.3390/life15091345









