Non-Variceal Upper Gastrointestinal Bleeding: A Retrospective Cohort of 364 Cases, Historical Comparison, and Updated Management Algorithm
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Inclusion and Exclusion Criteria
2.3. Data Collection
- demographics: age, sex;
- bleeding etiology and comorbidities: cardiovascular, pulmonary, renal, and hepatic diseases;
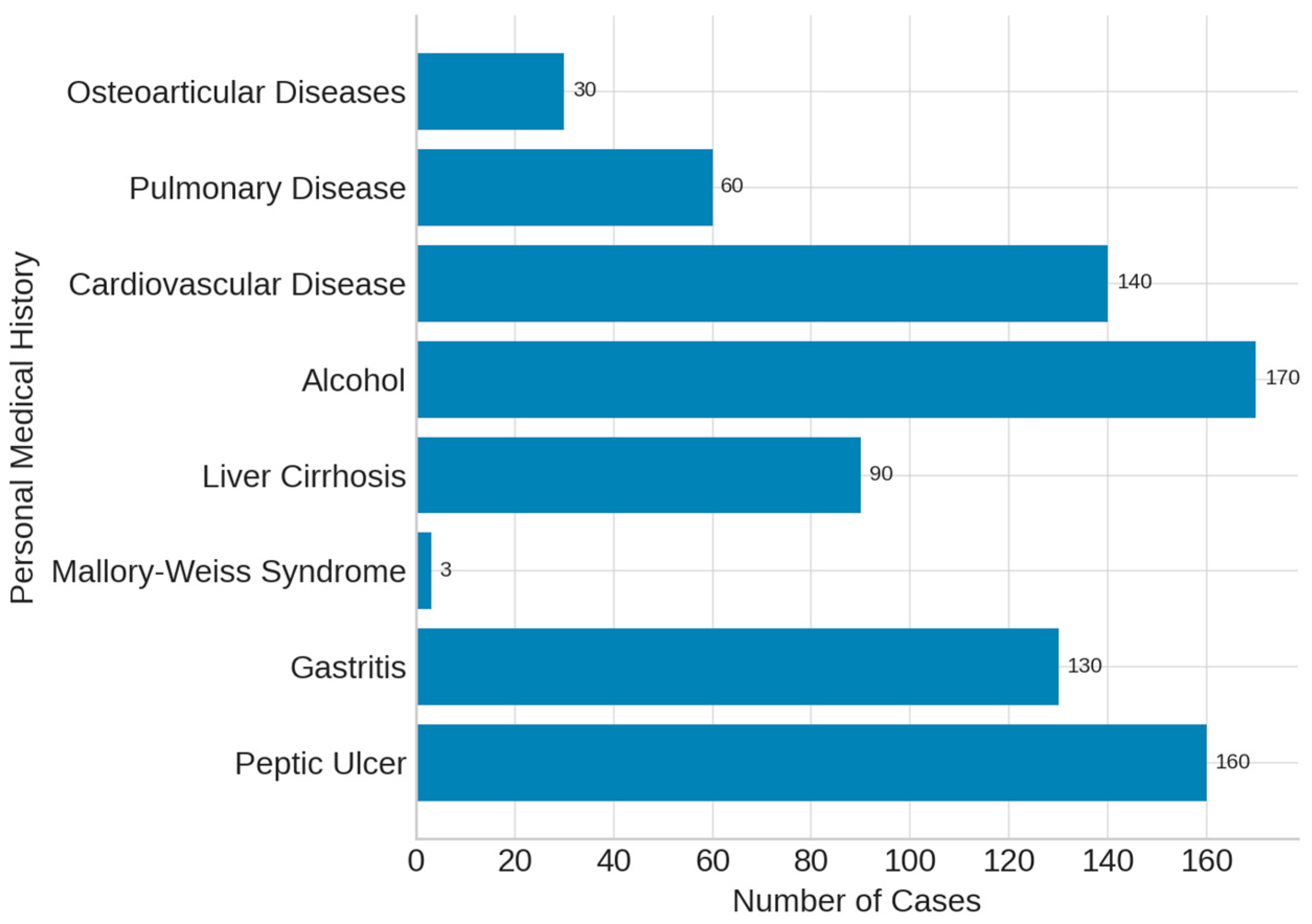
- risk factors: prior peptic ulcer, gastritis, Mallory–Weiss syndrome, liver cirrhosis, chronic alcohol use, NSAIDs, acetylsalicylic acid, paracetamol, oral anticoagulants.
- Baseline laboratory parameters: hemoglobin, hematocrit, platelet count, creatinine, urea;
- timing variables: onset-to-admission, admission-to-endoscopy, and onset-to-surgery intervals;
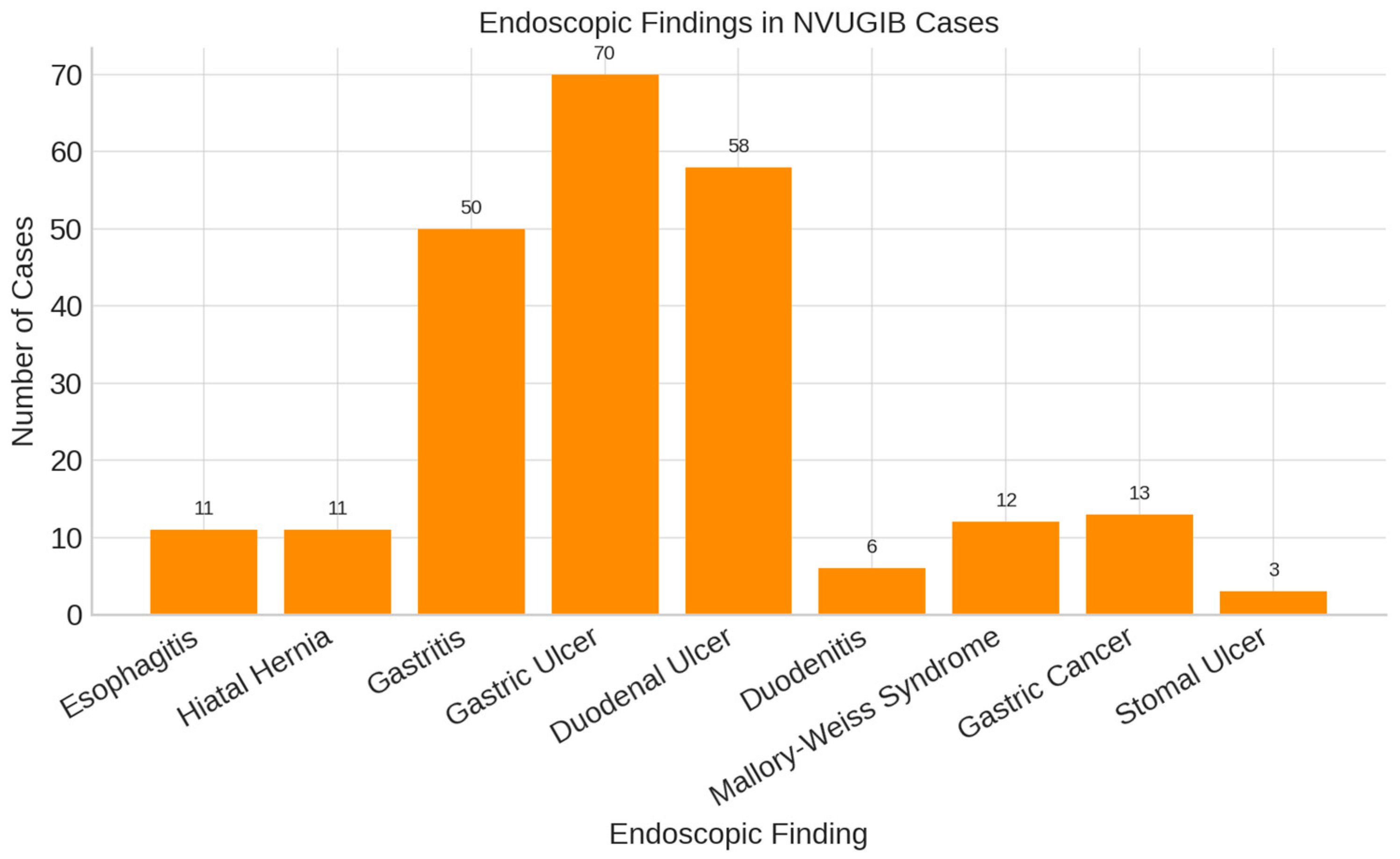
- endoscopic findings, lesion types and classification;
- therapeutic interventions: medical, endoscopic, and surgical;
- clinical outcomes: mortality, rebleeding, and drug-related bleeding.
2.4. Endoscopic and Surgical Management
2.5. Statistical Analysis
2.6. Ethics Statement
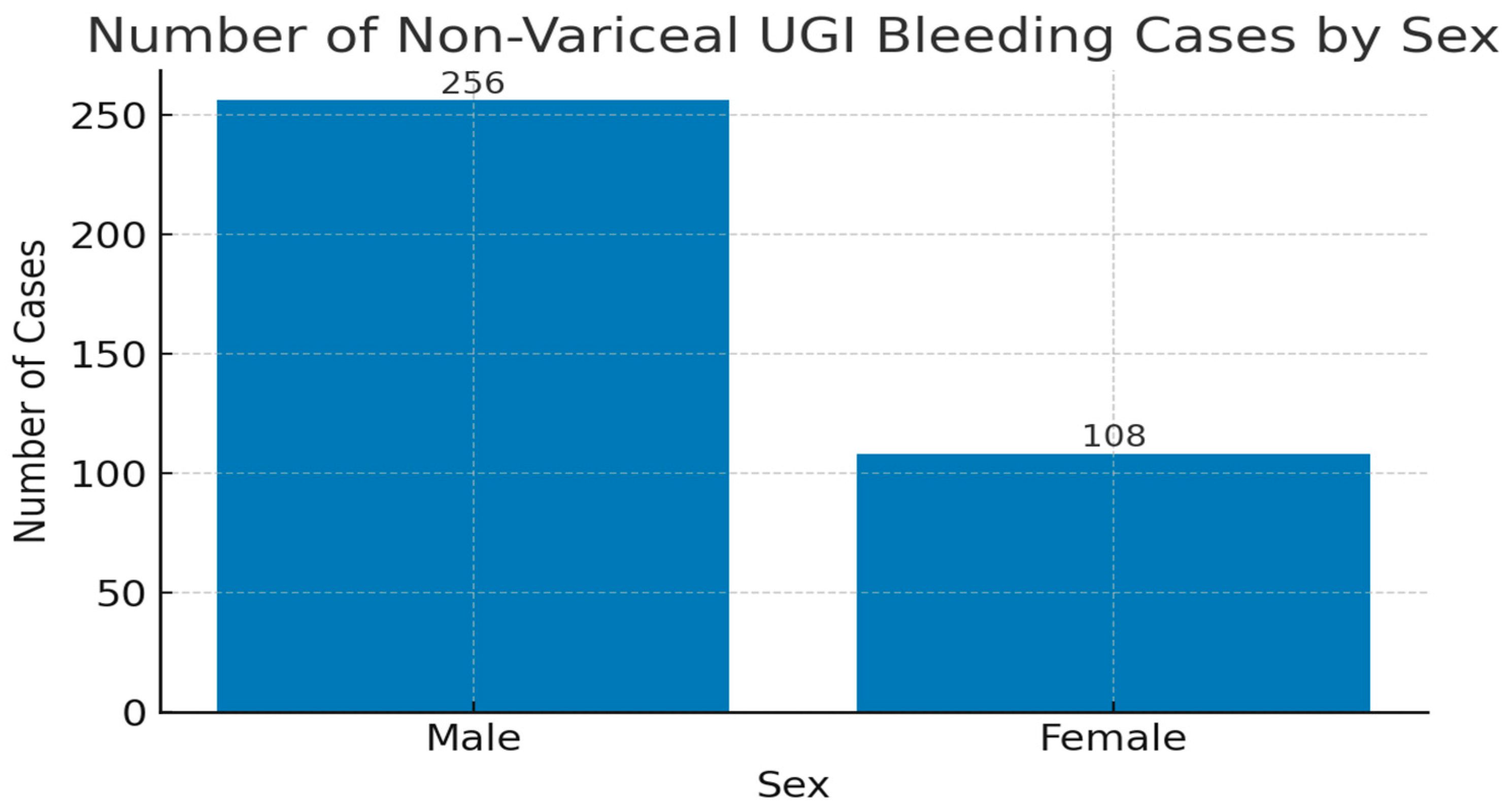
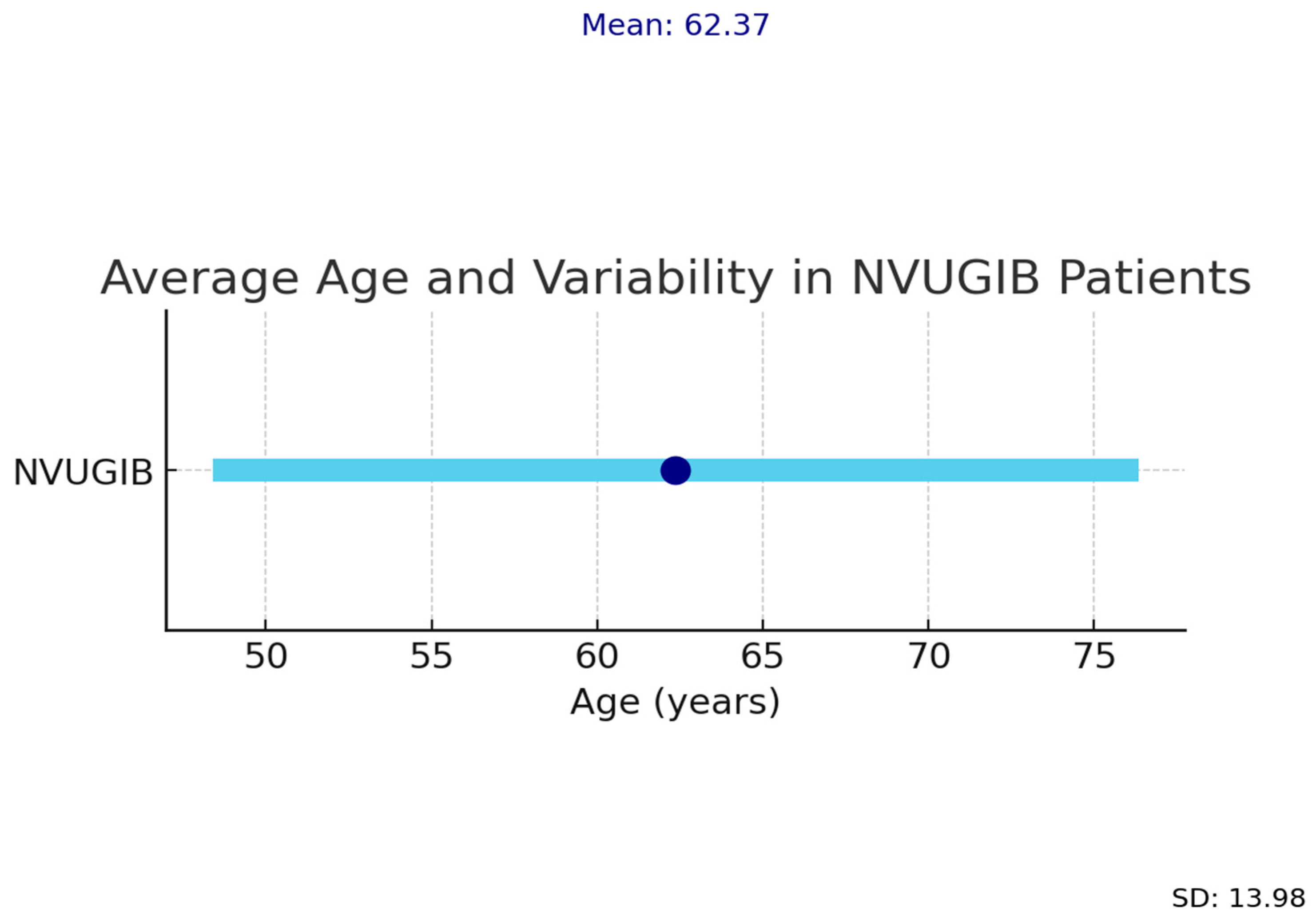
3. Results
4. Discussion
4.1. Anticoagulation and Endoscopic Timing
Resumption Strategies After Hemostasis
4.2. Pharmacological Therapy
- inhibition of platelet aggregation;
- clot lysis via pepsin activation at low pH;
- enhanced fibrinolysis in acidic environments;
- optimal clot stability at pH 6–6.5, as shown in experimental models [46].
4.3. Endoscopic Therapy
4.4. Limitations of Endoscopic Therapy in Severe Gastrointestinal Bleeding
4.5. Post-Endoscopic Management
4.6. Predictive Factors for Endoscopic Therapy Failure in Upper Gastrointestinal Bleeding
4.7. The Role and Timing of Surgical Intervention in Upper Gastrointestinal Bleeding
4.8. Type of Surgical Intervention in Peptic Ulcer Bleeding
4.9. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laine, L.; Shah, A. Randomized trial of urgent vs. elective colonoscopy in patients hospitalized with lower GI bleeding. Am. J. Gastroenterol. 2010, 105, 2636–2641; quiz 2642. [Google Scholar] [CrossRef]
- Targownik, L.E.; Nabalamba, A. Trends in management and outcomes of acute nonvariceal upper gastrointestinal bleeding: 1993–2003. Clin. Gastroenterol. Hepatol. 2006, 4, 1459–1466. [Google Scholar] [CrossRef]
- Lanas, A.; García-Rodríguez, L.A.; Polo-Tomás, M.; Ponce, M.; Alonso-Abreu, I.; Perez-Aisa, M.A.; Perez-Gisbert, J.; Bujanda, L.; Castro, M.; Muñoz, M.; et al. Time trends and impact of upper and lower gastrointestinal bleeding and perforation in clinical practice. Am. J. Gastroenterol. 2009, 104, 1633–1641. [Google Scholar] [CrossRef]
- Wuerth, B.A.; Rockey, D.C. Changing Epidemiology of Upper Gastrointestinal Hemorrhage in the Last Decade: A Nationwide Analysis. Dig. Dis. Sci. 2018, 63, 1286–1293. [Google Scholar] [CrossRef]
- Wollenman, C.S.; Chason, R.; Reisch, J.S.; Rockey, D.C. Impact of ethnicity in upper gastrointestinal hemorrhage. J. Clin. Gastroenterol. 2014, 48, 343–350. [Google Scholar] [CrossRef]
- Sung, J.J.; Tsoi, K.K.; Ma, T.K.; Yung, M.Y.; Lau, J.Y.; Chiu, P.W. Causes of mortality in patients with peptic ulcer bleeding: A prospective cohort study of 10,428 cases. Am. J. Gastroenterol. 2010, 105, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Barbu, L.A.; Mărgăritescu, N.D.; Şurlin, M.V. Diagnosis and Treatment Algorithms of Acute Variceal Bleeding. Curr. Health Sci. J. 2017, 43, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Marinescu, D.; Lazar, M.; Zaharie, S.; Comanescu, C.; Tudoran, C.; Bica, M.; Barbu, L. Upper Gastrointestinal Bleeding in Chronic Kidney Disease Patients. Curr. Health Sci. J. 2016, 42, 226–230. [Google Scholar] [CrossRef]
- Mărgăritescu, N.D.; Ciobanu, M.O.; Nemeş, R.N.; Ghelase, Ş.M.; Pleşea, R.M.; Georgescu, I.; Voinea, B.; Pleşea, I.E.; ChiuŢu, L.C. The morphological profile of small bowel tumors—Our experience. Rom. J. Morphol. Embryol. 2016, 57, 1241–1252. [Google Scholar] [PubMed]
- Laine, L.; McQuaid, K.R. Endoscopic therapy for bleeding ulcers: An evidence-based approach based on meta-analyses of randomized controlled trials. Clin. Gastroenterol. Hepatol. 2009, 7, 33–47; quiz 1–2. [Google Scholar] [CrossRef]
- Mujtaba, S.; Chawla, S.; Massaad, J.F. Diagnosis and Management of Non-Variceal Gastrointestinal Hemorrhage: A Review of Current Guidelines and Future Perspectives. J. Clin. Med. 2020, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Holster, I.L.; van Beusekom, H.M.; Kuipers, E.J.; Leebeek, F.W.; de Maat, M.P.; Tjwa, E.T. Effects of a hemostatic powder hemospray on coagulation and clot formation. Endoscopy 2015, 47, 638–645. [Google Scholar] [CrossRef]
- Changela, K.; Papafragkakis, H.; Ofori, E.; Ona, M.A.; Krishnaiah, M.; Duddempudi, S.; Anand, S. Hemostatic powder spray: A new method for managing gastrointestinal bleeding. Therap. Adv. Gastroenterol. 2015, 8, 125–135. [Google Scholar] [CrossRef]
- Chen, Y.I.; Barkun, A.; Nolan, S. Hemostatic powder TC-325 in the management of upper and lower gastrointestinal bleeding: A two-year experience at a single institution. Endoscopy 2015, 47, 167–171. [Google Scholar] [CrossRef]
- Pittayanon, R.; Rerknimitr, R.; Barkun, A. Prognostic factors affecting outcomes in patients with malignant GI bleeding treated with a novel endoscopically delivered hemostatic powder. Gastrointest. Endosc. 2018, 87, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, A.; Almadi, M.; Barkun, A.; Martel, M. REASON Investigators. Predictors of a variceal source among patients presenting with upper gastrointestinal bleeding. Can. J. Gastroenterol. 2012, 26, 187–192. [Google Scholar] [CrossRef]
- Romcea, A.A.; Tanţău, M.; Seicean, A.; Pascu, O. The etiology of upper gastrointestinal bleeding in cirrhotic patients. Clujul Med. 2013, 86, 21–23. [Google Scholar] [PubMed]
- Laine, L.; Barkun, A.N.; Saltzman, J.R.; Martel, M.; Leontiadis, G.I. ACG Clinical Guideline: Upper Gastrointestinal and Ulcer Bleeding. Am. J. Gastroenterol. 2021, 116, 899–917. [Google Scholar] [CrossRef]
- Pongprasobchai, S.; Nimitvilai, S.; Chasawat, J.; Manatsathit, S. Upper gastrointestinal bleeding etiology score for predicting variceal and non-variceal bleeding. World J. Gastroenterol. 2009, 15, 1099–1104. [Google Scholar] [CrossRef]
- Matei, D.; Groza, I.; Furnea, B.; Puie, L.; Levi, C.; Chiru, A.; Cruciat, C.; Mester, G.; Vesa, S.C.; Tantau, M. Predictors of variceal or nonvariceal source of upper gastrointestinal bleeding. An etiology predictive score established and validated in a tertiary referral center. J. Gastrointest. Liver Dis. 2013, 22, 379–384. [Google Scholar]
- Chai-Adisaksopha, C.; Crowther, M.; Isayama, T.; Lim, W. The impact of bleeding complications in patients receiving target-specific oral anticoagulants: A systematic review and meta-analysis. Blood 2014, 124, 2450–2458. [Google Scholar] [CrossRef]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef]
- Ansell, J.E.; Bakhru, S.H.; Laulicht, B.E.; Steiner, S.S.; Grosso, M.; Brown, K.; Dishy, V.; Noveck, R.J.; Costin, J.C. Use of PER977 to reverse the anticoagulant effect of edoxaban. N. Engl. J. Med. 2014, 371, 2141–2142. [Google Scholar] [CrossRef]
- Abraham, N.S.; Castillo, D.L. Novel anticoagulants: Bleeding risk and management strategies. Curr. Opin. Gastroenterol. 2013, 29, 676–683. [Google Scholar] [CrossRef]
- Holbrook, A.; Schulman, S.; Witt, D.M.; Vandvik, P.O.; Fish, J.; Kovacs, M.J.; Svensson, P.J.; Veenstra, D.L.; Crowther, M.; Guyatt, G.H. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141 (Suppl. S2), e152S–e184S. [Google Scholar] [CrossRef]
- Baron, T.H.; Kamath, P.S.; McBane, R.D. New anticoagulant and antiplatelet agents: A primer for the gastroenterologist. Clin. Gastroenterol. Hepatol. 2014, 12, 187–195. [Google Scholar] [CrossRef]
- Karaca, M.A.; Erbil, B.; Ozmen, M.M. Use and effectiveness of prothrombin complex concentrates vs fresh frozen plasma in gastrointestinal hemorrhage due to warfarin usage in the ED. Am. J. Emerg. Med. 2014, 32, 660–664. [Google Scholar] [CrossRef]
- Barkun, A.N.; Almadi, M.; Kuipers, E.J.; Laine, L.; Sung, J.; Tse, F.; Leontiadis, G.I.; Abraham, N.S.; Calvet, X.; Chan, F.K.L.; et al. Management of Nonvariceal Upper Gastrointestinal Bleeding: Guideline Recommendations From the International Consensus Group. Ann. Intern. Med. 2019, 171, 805–822. [Google Scholar] [CrossRef]
- Kang, S.J.; Tae, C.H.; Bang, C.S.; Shin, C.M.; Jeong, Y.H.; Choi, M.; Hwang, J.H.; Saito, Y.; Chiu, P.W.Y.; Rerknimitr, R.; et al. International Digestive Endoscopy Network Consensus on the Management of Antithrombotic Agents in Patients Undergoing Gastrointestinal Endoscopy. Gut Liver 2024, 18, 764–780. [Google Scholar] [CrossRef]
- Gralnek, I.M.; Dumonceau, J.M.; Kuipers, E.J.; Lanas, A.; Sanders, D.S.; Kurien, M.; Rotondano, G.; Hucl, T.; Dinis-Ribeiro, M.; Marmo, R.; et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015, 47, a1–a46. [Google Scholar] [CrossRef]
- Lau, J.Y.; Sung, J.J.; Lee, K.K.; Yung, M.Y.; Wong, S.K.; Wu, J.C.; Chan, F.K.; Ng, E.K.; You, J.H.; Lee, C.W.; et al. Effect of intravenous omeprazole on recurrent bleeding after endoscopic treatment of bleeding peptic ulcers. N. Engl. J. Med. 2000, 343, 310–316. [Google Scholar] [CrossRef]
- Sachar, H.; Vaidya, K.; Laine, L. Intermittent vs continuous proton pump inhibitor therapy for high-risk bleeding ulcers: A systematic review and meta-analysis. JAMA Intern. Med. 2014, 174, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Leontiadis, G.I.; Sharma, V.K.; Howden, C.W. Proton pump inhibitor therapy for peptic ulcer bleeding: Cochrane collaboration meta-analysis of randomized controlled trials. Mayo Clin. Proc. 2007, 82, 286–296. [Google Scholar] [CrossRef]
- Andriulli, A.; Loperfido, S.; Focareta, R.; Leo, P.; Fornari, F.; Garripoli, A.; Tonti, P.; Peyre, S.; Spadaccini, A.; Marmo, R.; et al. High- versus low-dose proton pump inhibitors after endoscopic hemostasis in patients with peptic ulcer bleeding: A multicentre, randomized study. Am. J. Gastroenterol. 2008, 103, 3011–3018. [Google Scholar] [CrossRef]
- Wang, C.H.; Ma, M.H.; Chou, H.C.; Yen, Z.S.; Yang, C.W.; Fang, C.C.; Chen, S.C. High-dose vs non-high-dose proton pump inhibitors after endoscopic treatment in patients with bleeding peptic ulcer: A systematic review and meta-analysis of randomized controlled trials. Arch. Intern. Med. 2010, 170, 751–758. [Google Scholar] [CrossRef]
- Gluud, L.L.; Klingenberg, S.L.; Langholz, E. Tranexamic acid for upper gastrointestinal bleeding. Cochrane Database Syst. Rev. 2012, 1, CD006640. [Google Scholar] [CrossRef]
- Theivanayagam, S.; Lim, R.G.; Cobell, W.J.; Gowda, J.T.; Matteson, M.L.; Choudhary, A.; Bechtold, M.L. Administration of erythromycin before endoscopy in upper gastrointestinal bleeding: A meta-analysis of randomized controlled trials. Saudi J. Gastroenterol. 2013, 19, 205–210. [Google Scholar] [CrossRef]
- Graham, D.Y. Limited value of early endoscopy in the management of acute upper gastrointestinal bleeding. Prospective controlled trial. Am. J. Surg. 1980, 140, 284–290. [Google Scholar] [CrossRef]
- Peterson, W.L.; Barnett, C.C.; Smith, H.J.; Allen, M.H.; Corbett, D.B. Routine early endoscopy in upper-gastrointestinal-tract bleeding: A randomized, controlled trial. N. Engl. J. Med. 1981, 304, 925–929. [Google Scholar] [CrossRef]
- Sacks, H.S.; Chalmers, T.C.; Blum, A.L.; Berrier, J.; Pagano, D. Endoscopic hemostasis. An effective therapy for bleeding peptic ulcers. JAMA 1990, 264, 494–499. [Google Scholar] [CrossRef]
- Yen, D.; Hu, S.C.; Chen, L.S.; Liu, K.; Kao, W.F.; Tsai, J.; Chern, C.H.; Lee, C.H. Arterial oxygen desaturation during emergent nonsedated upper gastrointestinal endoscopy in the emergency department. Am. J. Emerg. Med. 1997, 15, 644–647. [Google Scholar] [CrossRef]
- Hill, D.B.; Stokes, B.; Gilinsky, N.H. Arterial oxygen saturation during emergency esophagogastroduodenoscopy. The effects of nasal oxygen. J. Clin. Gastroenterol. 1994, 18, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.W. Endoscopic Management of Peptic Ulcer Bleeding: Recent Advances. Clin. Endosc. 2019, 52, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Cipolletta, L.; Bianco, M.A.; Rotondano, G.; Marmo, R.; Piscopo, R. Outpatient management for low-risk nonvariceal upper GI bleeding: A randomized controlled trial. Gastrointest. Endosc. 2002, 55, 1–5. [Google Scholar] [CrossRef]
- Vreeburg, E.M.; Levi, M.; Rauws, E.A.; Deventer, S.J.; Snel, P.; Bartelsman, J.W.; Ten Cate, J.W.; Tytgat, G.N. Enhanced mucosal fibrinolytic activity in gastroduodenal ulcer haemorrhage and the beneficial effect of acid suppression. Aliment. Pharmacol. Ther. 2001, 15, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.J.; Hernández-Díaz, S.; García Rodríguez, L.A. Acid suppressants reduce risk of gastrointestinal bleeding in patients on antithrombotic or anti-inflammatory therapy. Gastroenterology 2011, 141, 71–79. [Google Scholar] [CrossRef]
- Cardoso, R.N.; Benjo, A.M.; DiNicolantonio, J.J.; Garcia, D.C.; Macedo, F.Y.; El-Hayek, G.; Nadkarni, G.N.; Gili, S.; Iannaccone, M.; Konstantinidis, I.; et al. Incidence of cardiovascular events and gastrointestinal bleeding in patients receiving clopidogrel with and without proton pump inhibitors: An updated meta-analysis. Open Heart 2015, 2, e000248. [Google Scholar] [CrossRef]
- Sung, J.J.; Chiu, P.W.; Chan, F.K.L.; Lau, J.Y.; Goh, K.L.; Ho, L.H.; Jung, H.Y.; Sollano, J.D.; Gotoda, T.; Reddy, N.; et al. Asia-Pacific working group consensus on non-variceal upper gastrointestinal bleeding: An update 2018. Gut 2018, 67, 1757–1768. [Google Scholar] [CrossRef]
- Botianu, A.; Matei, D.; Tantau, M.; Acalovschi, M. Mortality and need of surgical treatment in acute upper gastrointestinal bleeding: A one year study in a tertiary center with a 24 hours/day-7 days/week endoscopy call. Has anything changed? Chirurgia 2013, 108, 312–318. [Google Scholar]
- Cook, D.J.; Guyatt, G.H.; Salena, B.J.; Laine, L.A. Endoscopic therapy for acute nonvariceal upper gastrointestinal hemorrhage: A meta-analysis. Gastroenterology 1992, 102, 139–148. [Google Scholar] [CrossRef]
- Kahi, C.J.; Jensen, D.M.; Sung, J.J.; Bleau, B.L.; Jung, H.K.; Eckert, G.; Imperiale, T.F. Endoscopic therapy versus medical therapy for bleeding peptic ulcer with adherent clot: A meta-analysis. Gastroenterology 2005, 129, 855–862. [Google Scholar] [CrossRef]
- Lin, H.J.; Perng, C.L.; Sun, I.C.; Tseng, G.Y. Endoscopic haemoclip versus heater probe thermocoagulation plus hypertonic saline-epinephrine injection for peptic ulcer bleeding. Dig. Liver Dis. 2003, 35, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Swain, C.P.; Storey, D.W.; Bown, S.G.; Heath, J.; Mills, T.N.; Salmon, P.R.; Northfield, T.C.; Kirkham, J.S.; O’Sullivan, J.P. Nature of the bleeding vessel in recurrently bleeding gastric ulcers. Gastroenterology 1986, 90, 595–608. [Google Scholar] [CrossRef]
- Raju, G.S.; Gajula, L. Endoclips for GI endoscopy. Gastrointest. Endosc. 2004, 59, 267–279. [Google Scholar] [CrossRef]
- Alali, A.A.; Moosavi, S.; Martel, M.; Almadi, M.; Barkun, A.N. Topical hemostatic agents in the management of upper gastrointestinal bleeding: A meta-analysis. Endosc. Int. Open 2023, 11, E368–E385. [Google Scholar] [CrossRef] [PubMed]
- Gralnek, I.M.; Stanley, A.J.; Morris, A.J.; Camus, M.; Lau, J.; Lanas, A.; Laursen, S.B.; Radaelli, F.; Papanikolaou, I.S.; Cúrdia Gonçalves, T.; et al. Endoscopic diagnosis and management of nonvariceal upper gastrointestinal hemorrhage (NVUGIH): European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2021. Endoscopy 2021, 53, 300–332. [Google Scholar] [CrossRef] [PubMed]
- Grottke, O.; Afshari, A.; Ahmed, A.; Arnaoutoglou, E.; Bolliger, D.; Fenger-Eriksen, C.; von Heymann, C. Clinical guideline on reversal of direct oral anticoagulants in patients with life threatening bleeding. Eur. J. Anaesthesiol. 2024, 41, 327–350. [Google Scholar] [CrossRef]
- Javid, G.; Zargar, S.A.; U-Saif, R.; Khan, B.A.; Yatoo, G.N.; Shah, A.H.; Gulzar, G.M.; Sodhi, J.S.; Khan, M.A. Comparison of p.o. or i.v. proton pump inhibitors on 72-h intragastric pH in bleeding peptic ulcer. J. Gastroenterol. Hepatol. 2009, 24, 1236–1243. [Google Scholar] [CrossRef]
- Douketis, J.D.; Spyropoulos, A.C.; Spencer, F.A.; Mayr, M.; Jaffer, A.K.; Eckman, M.H.; Dunn, A.S.; Kunz, R. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141 (Suppl. S2), e326S–e350S. [Google Scholar] [CrossRef]
- Elmunzer, B.J.; Young, S.D.; Inadomi, J.M.; Schoenfeld, P.; Laine, L. Systematic review of the predictors of recurrent hemorrhage after endoscopic hemostatic therapy for bleeding peptic ulcers. Am. J. Gastroenterol. 2008, 103, 2625–2632; quiz 2633. [Google Scholar] [CrossRef]
- Barbu, L.A.; Mărgăritescu, N.-D.; Cercelaru, L.; Caragea, D.-C.; Vîlcea, I.-D.; Șurlin, V.; Mogoantă, S.-Ș.; Mogoș, G.F.R.; Vasile, L.; Țenea Cojan, T.Ș. Can Thrombosed Abdominal Aortic Dissecting Aneurysm Cause Mesenteric Artery Thrombosis and Ischemic Colitis?—A Case Report and a Review of Literature. J. Clin. Med. 2025, 14, 3092. [Google Scholar] [CrossRef] [PubMed]
- Robson, A.J.; Richards, J.M.; Ohly, N.; Nixon, S.J.; Paterson-Brown, S. The effect of surgical subspecialization on outcomes in peptic ulcer disease complicated by perforation and bleeding. World J. Surg. 2008, 32, 1456–1461. [Google Scholar] [CrossRef]
- Paspatis, G.A.; Matrella, E.; Kapsoritakis, A.; Leontithis, C.; Papanikolaou, N.; Chlouverakis, G.J.; Kouroumalis, E. An epidemiological study of acute upper gastrointestinal bleeding in Crete, Greece. Eur. J. Gastroenterol. Hepatol. 2000, 12, 1215–1220. [Google Scholar] [CrossRef]
- van Leerdam, M.E.; Vreeburg, E.M.; Rauws, E.A.; Geraedts, A.A.; Tijssen, J.G.; Reitsma, J.B.; Tytgat, G.N. Acute upper GI bleeding: Did anything change? Time trend analysis of incidence and outcome of acute upper GI bleeding between 1993/1994 and 2000. Am. J. Gastroenterol. 2003, 98, 1494–1499. [Google Scholar] [CrossRef]
- Dicu, D.; Pop, F.; Ionescu, D.; Dicu, T. Comparison of risk scoring systems in predicting clinical outcome at upper gastrointestinal bleeding patients in an emergency unit. Am. J. Emerg. Med. 2013, 31, 94–99. [Google Scholar] [CrossRef]
- Cheung, F.K.; Lau, J.Y. Management of massive peptic ulcer bleeding. Gastroenterol. Clin. N. Am. 2009, 38, 231–243. [Google Scholar] [CrossRef]
- Saperas, E.; Piqué, J.M.; Pérez Ayuso, R.; Bordas, J.M.; Terés, J.; Pera, C. Conservative management of bleeding duodenal ulcer without a visible vessel: Prospective randomized trial. Br. J. Surg. 1987, 74, 784–786. [Google Scholar] [CrossRef]
- Imhof, M.; Ohmann, C.; Röher, H.D.; Glutig, H.; DUESUC Study Group. Endoscopic versus operative treatment in high-risk ulcer bleeding patients—Results of a randomised study. Langenbecks Arch. Surg. 2003, 387, 327–336. [Google Scholar] [CrossRef]
- Bender, J.S.; Bouwman, D.L.; Weaver, D.W. Bleeding gastroduodenal ulcers: Improved outcome from a unified surgical approach. Am. Surg. 1994, 60, 313–315. [Google Scholar] [PubMed]
- Mueller, X.; Rothenbuehler, J.M.; Amery, A.; Meyer, B.; Harder, F. Outcome of peptic ulcer hemorrhage treated according to a defined approach. World J. Surg. 1994, 18, 406–409; discussion 409–410. [Google Scholar] [CrossRef] [PubMed]
- Poxon, V.A.; Keighley, M.R.; Dykes, P.W.; Heppinstall, K.; Jaderberg, M. Comparison of minimal and conventional surgery in patients with bleeding peptic ulcer: A multicentre trial. Br. J. Surg. 1991, 78, 1344–1345. [Google Scholar] [CrossRef]
- Caragher, S.; Wang, D.; Moonsamy, P.; Fagenholz, P. Hepatic arterial haemorrhage caused by duodenal ulcer. BMJ Case Rep. 2022, 15, e249523. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.G.; Sundbom, M.; Gustavsson, S.; Nyman, R. Endoscopic marking with a metallic clip facilitates transcatheter arterial embolization in upper peptic ulcer bleeding. J. Vasc. Interv. Radiol. 2006, 17, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Fukamatsu, F.; Yamada, K.; Takekoshi, D.; Aonuma, T.; Oyama, K.; Yanagisawa, S.; Yamada, A.; Shimizu, A.; Fujinaga, Y. Embolization using both n-butyl cyanoacrylate and gelatin sponges in a patient with a posterior superior pancreaticoduodenal artery pseudoaneurysm that ruptured and bled into the drain tube. Radiol. Case Rep. 2023, 19, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, C.; Bañares, R.; Beceiro, I.; Menchén, P.; Catalina, M.V.; Echenagusia, A.; Turegano, F. Comparison of transcatheter arterial embolization and surgery for treatment of bleeding peptic ulcer after endoscopic treatment failure. J. Vasc. Interv. Radiol. 2004, 15, 447–450. [Google Scholar] [CrossRef]

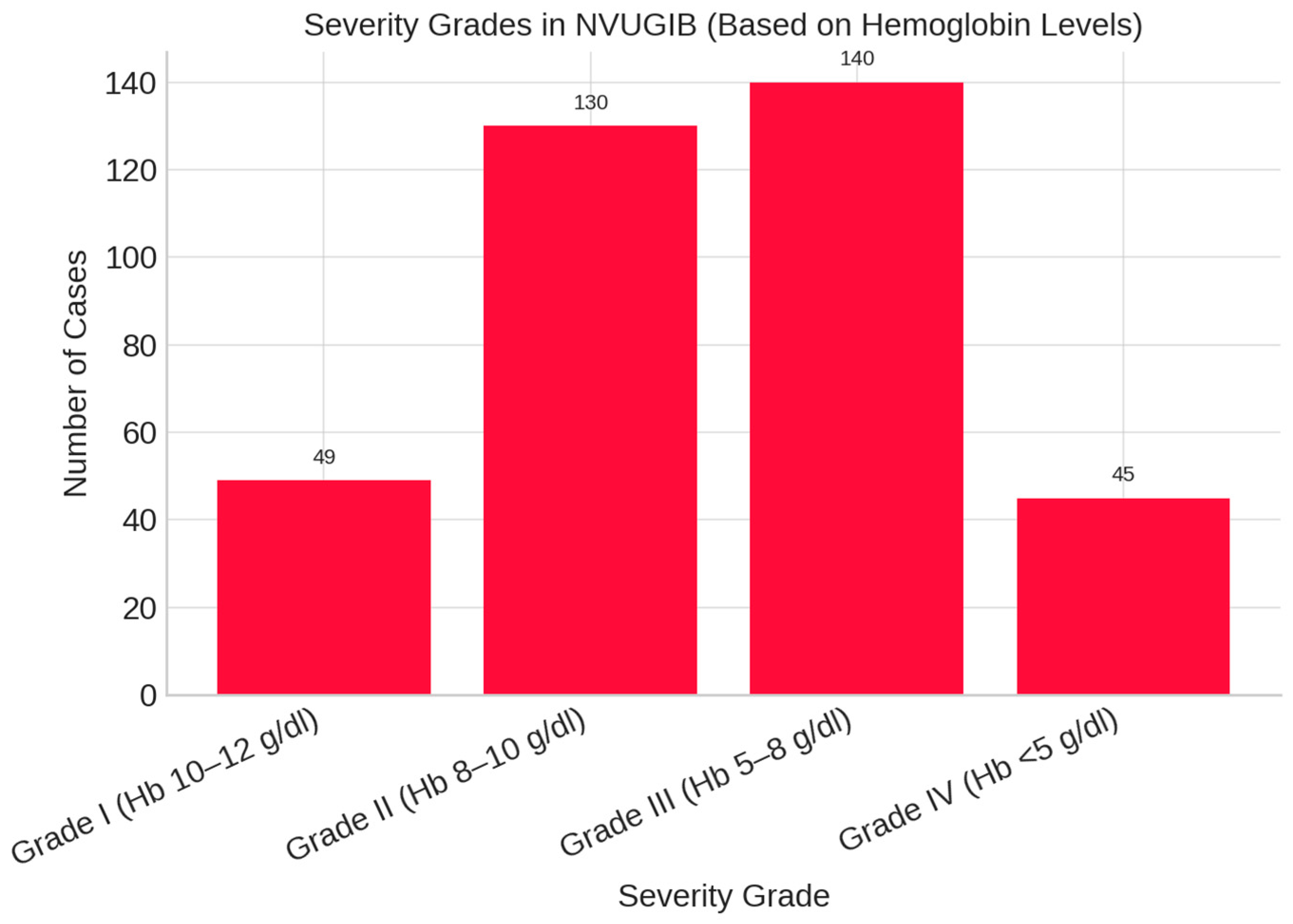
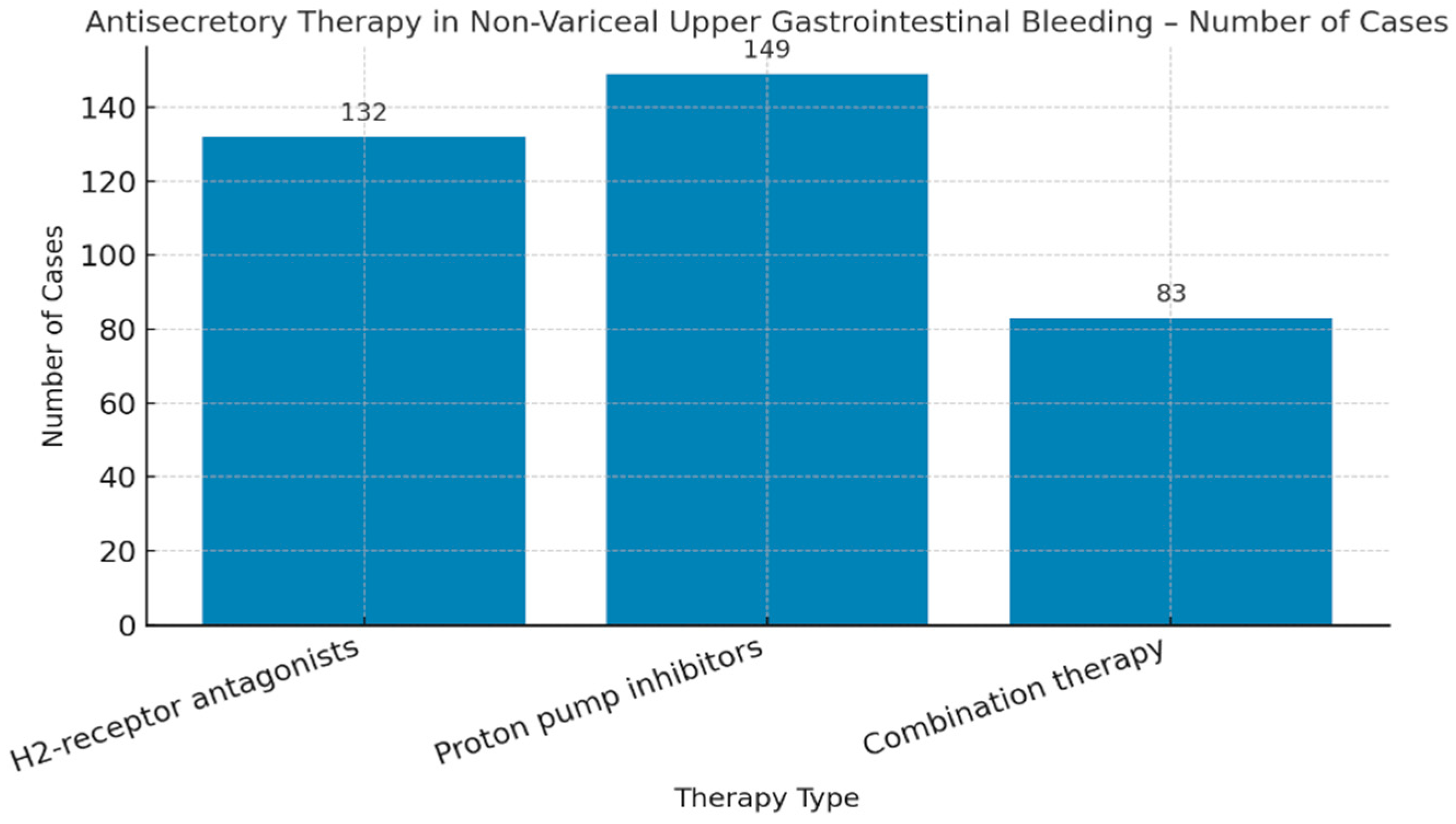
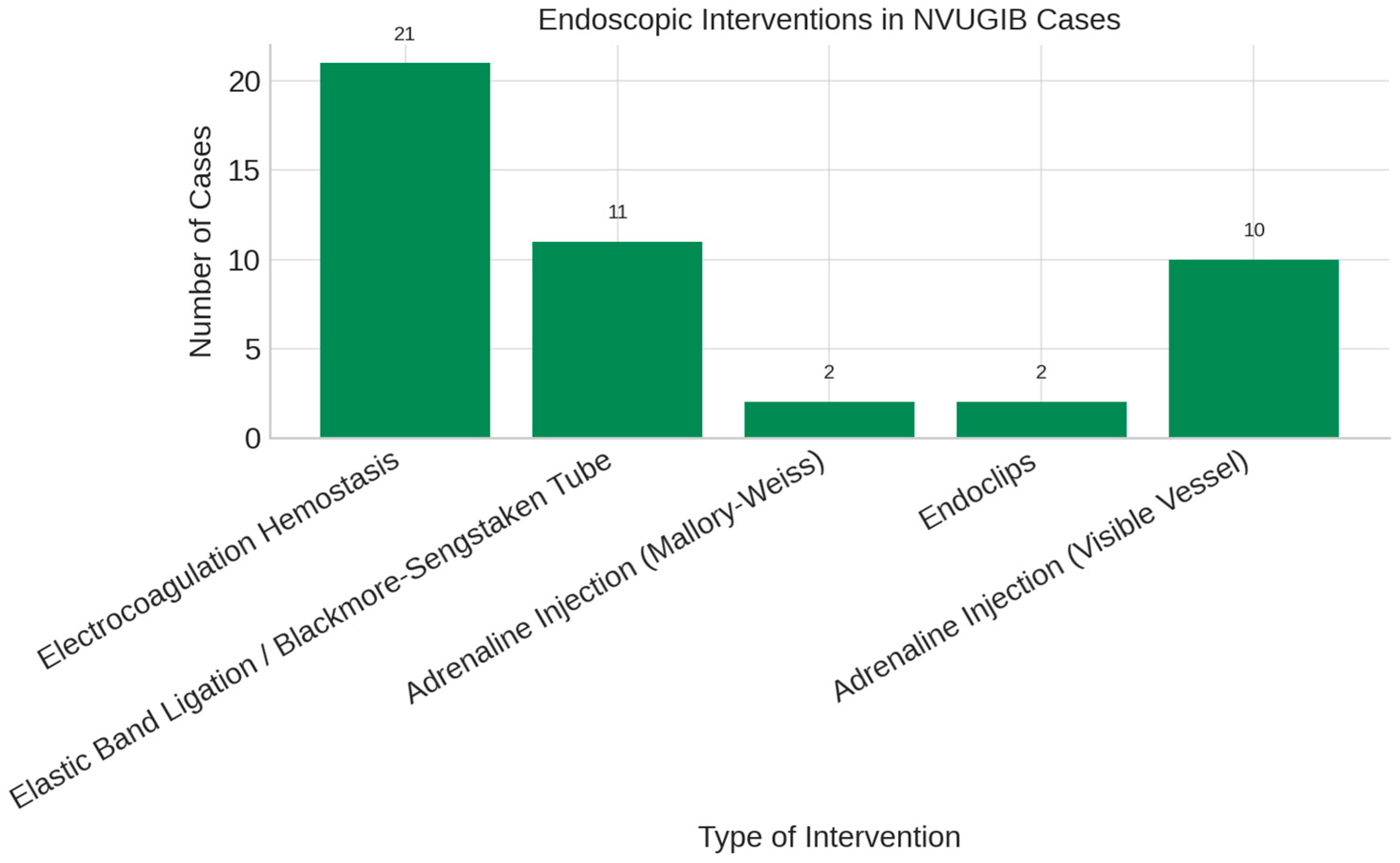


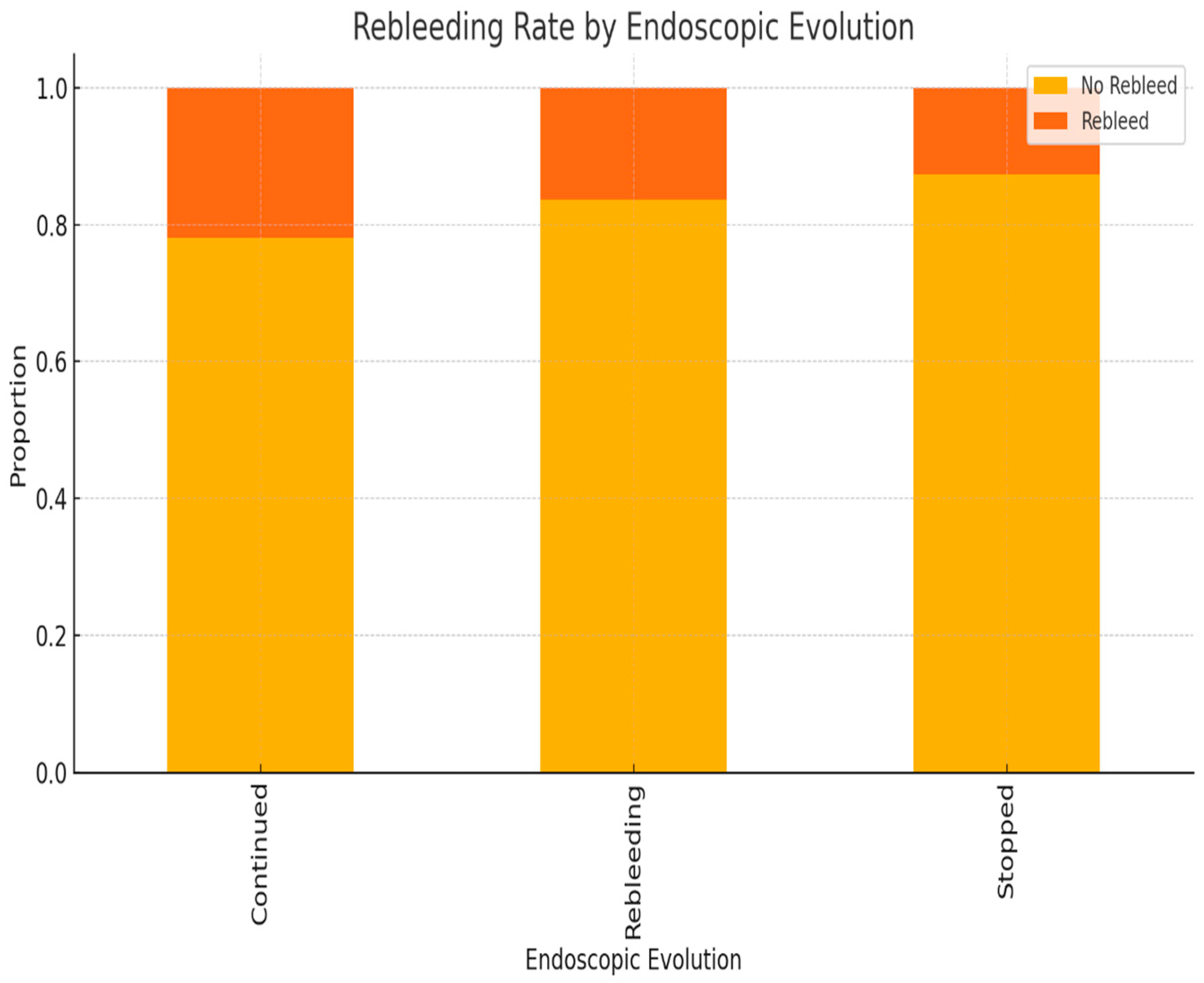
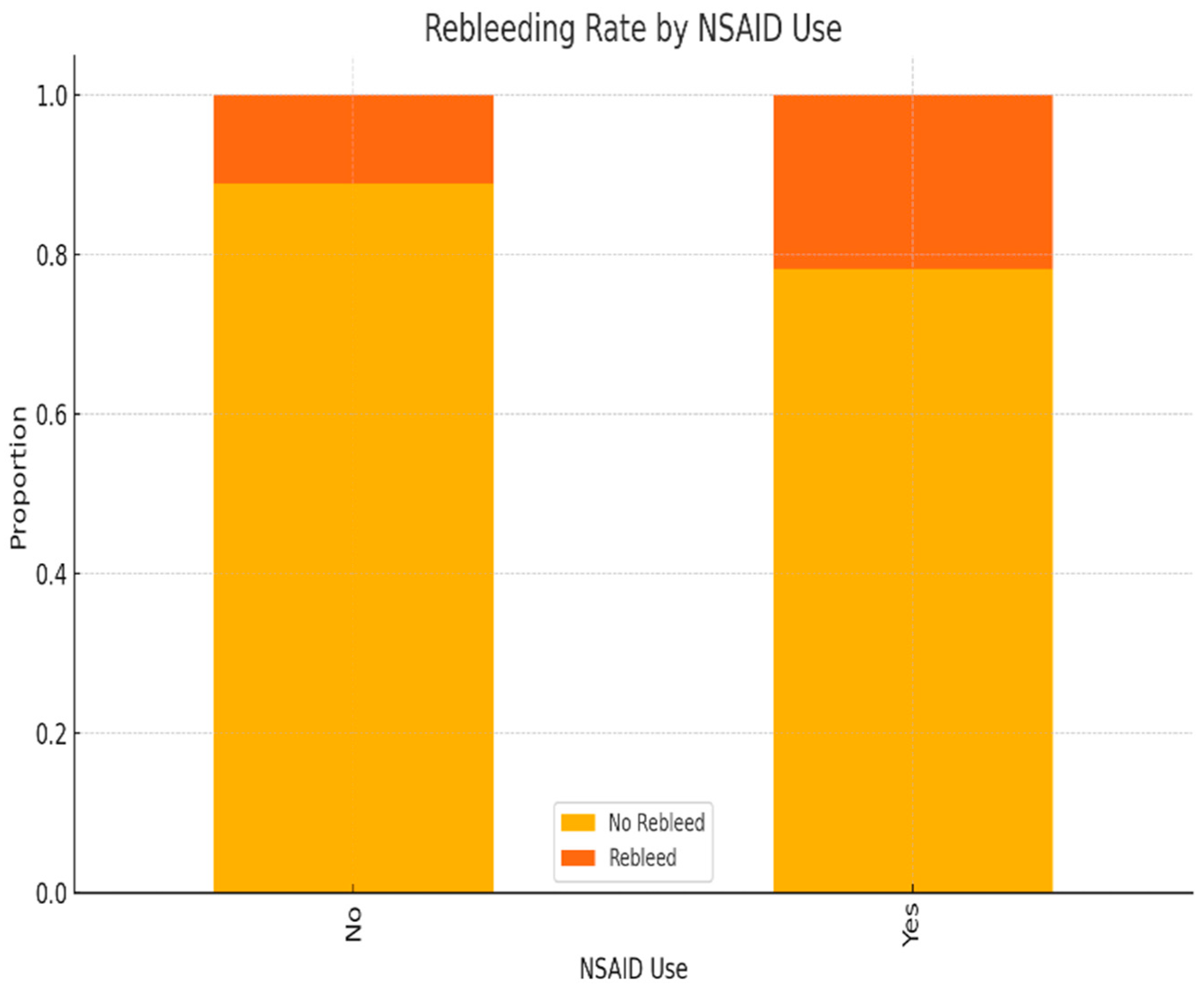

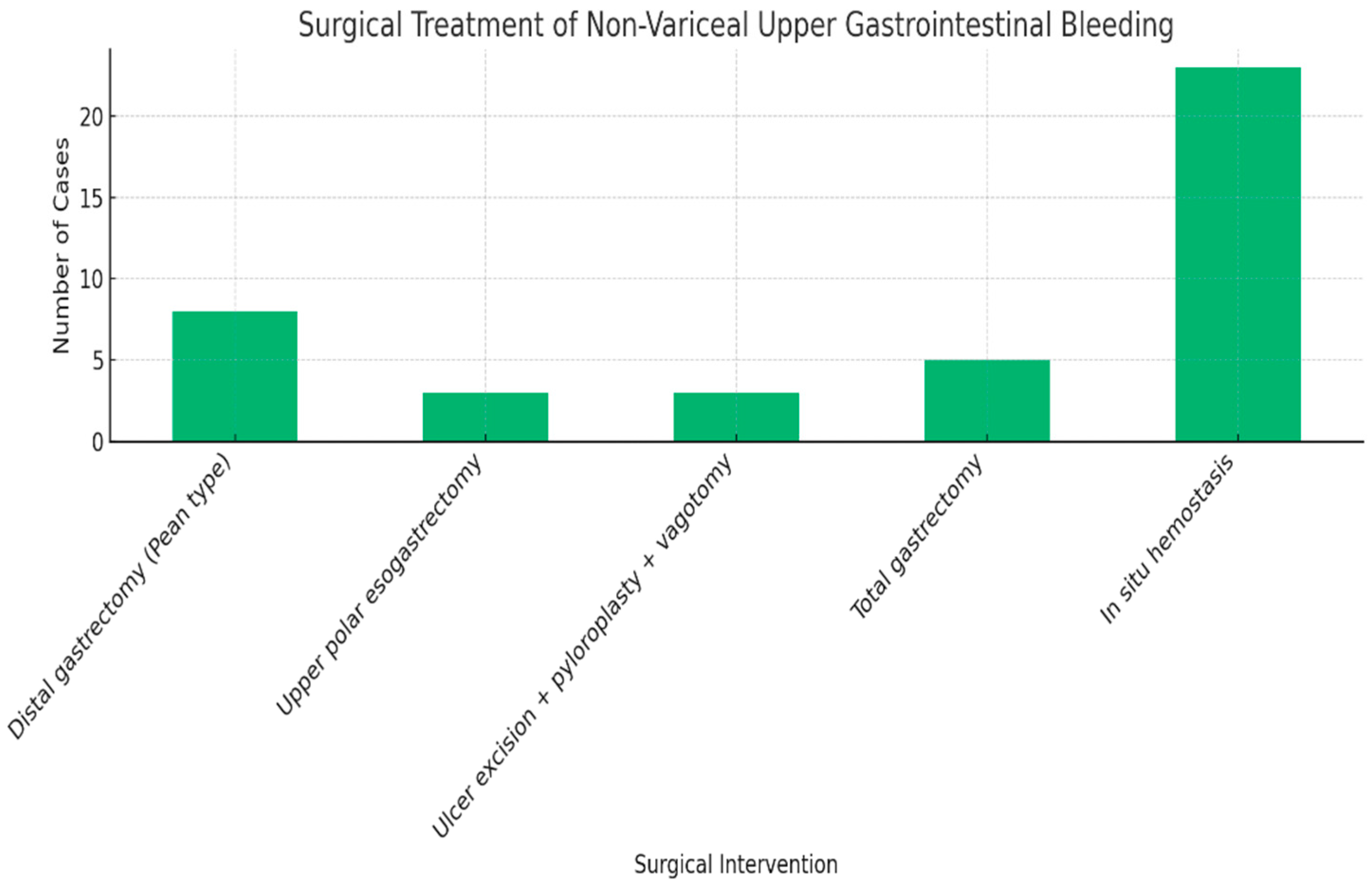
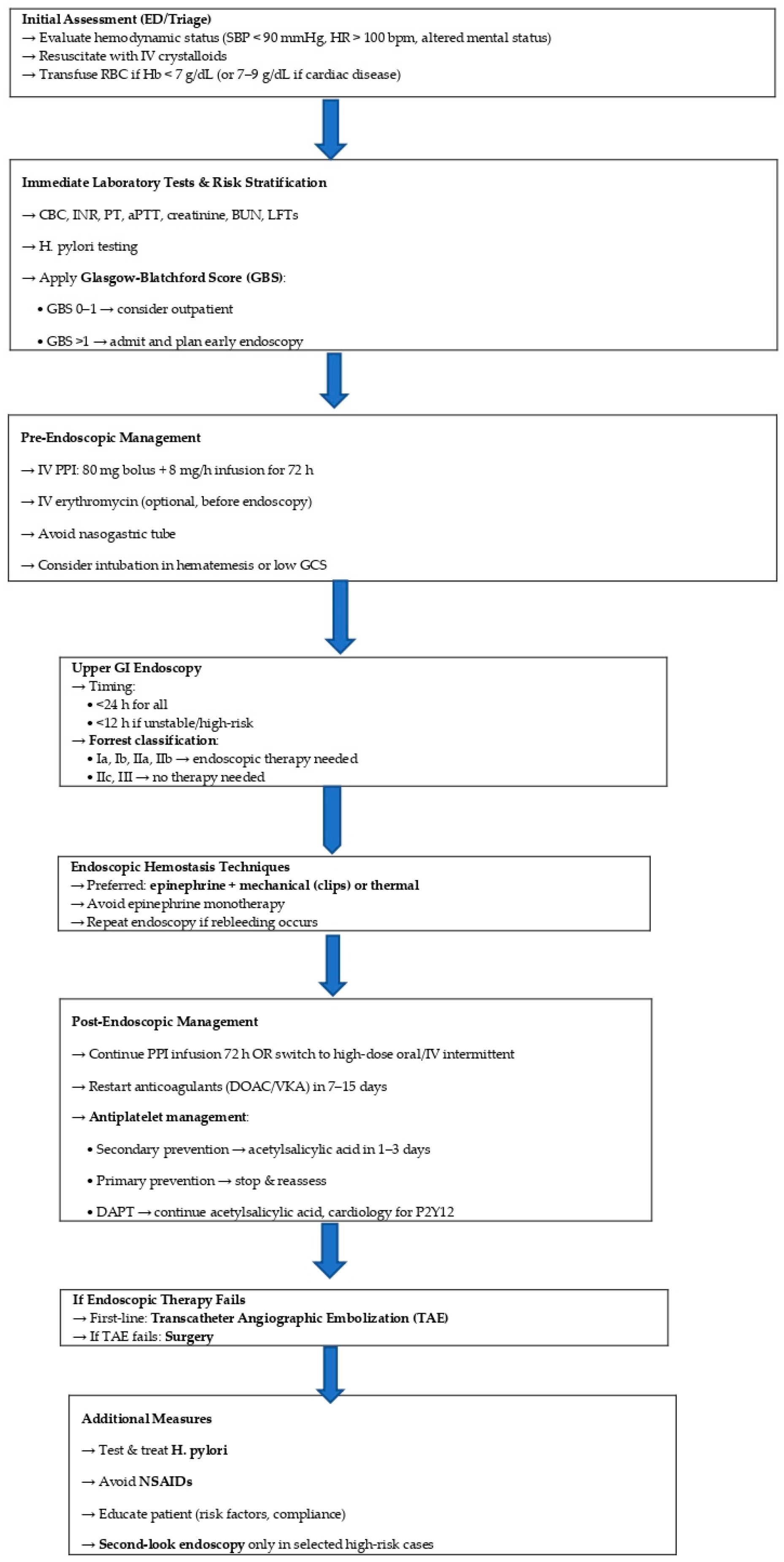
| Variable | Odds Ratio (OR) | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Age (years) | 1.04 | 1.01–1.07 | 0.011 |
| Hemoglobin (g/dL) | 0.82 | 0.69–0.97 | 0.017 |
| Alcohol use (Yes vs. No) | 2.15 | 1.18–3.91 | 0.013 |
| Anticoagulant use (Yes vs. No) | 2.45 | 1.21–4.98 | 0.012 |
| Surgical intervention (Yes) | 2.92 | 1.32–6.48 | 0.008 |
| Variable | Odds Ratio (OR) | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Age (years) | 1.01 | 0.97–1.05 | 0.510 |
| Hemoglobin (g/dL) | 0.75 | 0.61–0.93 | 0.008 |
| Rebleeding (Yes vs. No) | 3.90 | 2.00–7.60 | <0.001 |
| Ulcerative lesion (Yes/No) | 2.15 | 1.10–4.20 | 0.024 |
| Alcohol use (Yes vs. No) | 1.42 | 0.76–2.65 | 0.270 |
| Variable | Odds Ratio (OR) | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Age (years) | 1.04 | 1.01–1.07 | 0.011 |
| Hemoglobin (g/dL) | 0.82 | 0.69–0.97 | 0.017 |
| Alcohol use (Yes vs. No) | 2.15 | 1.18–3.91 | 0.013 |
| Anticoagulant use (Yes vs. No) | 2.45 | 1.21–4.98 | 0.012 |
| Surgical intervention (Yes) | 2.92 | 1.32–6.48 | 0.008 |
| Comparison | Test Type | Value | p-Value | Statistical Significance |
|---|---|---|---|---|
| Mean age: deceased vs. survivors | Student’s t-test | t = 3.51 | 0.0005 | significant |
| Mortality: alcohol users vs. non-users | Chi-square | χ2 = 2.70 | 0.1001 | not significant |
| Type of antisecretory therapy | Chi-square | χ2 = 19.36 | 0.0001 | significant |
| Type of endoscopic treatment | Chi-square | χ2 = 4.79 | 0.0911 | borderline |
| Surgery timing intervals | Chi-square | χ2 = 9.19 | 0.0565 | borderline |
| Types of surgical interventions | Chi-square | χ2 = 33.71 | <0.0001 | significant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbu, L.A.; Vasile, L.; Cercelaru, L.; Șurlin, V.; Mogoantă, S.-S.; Mogoș, G.F.R.; Țenea Cojan, T.S.; Mărgăritescu, N.-D.; Buliman, A. Non-Variceal Upper Gastrointestinal Bleeding: A Retrospective Cohort of 364 Cases, Historical Comparison, and Updated Management Algorithm. Life 2025, 15, 1320. https://doi.org/10.3390/life15081320
Barbu LA, Vasile L, Cercelaru L, Șurlin V, Mogoantă S-S, Mogoș GFR, Țenea Cojan TS, Mărgăritescu N-D, Buliman A. Non-Variceal Upper Gastrointestinal Bleeding: A Retrospective Cohort of 364 Cases, Historical Comparison, and Updated Management Algorithm. Life. 2025; 15(8):1320. https://doi.org/10.3390/life15081320
Chicago/Turabian StyleBarbu, Laurențiu Augustus, Liviu Vasile, Liliana Cercelaru, Valeriu Șurlin, Stelian-Stefaniță Mogoantă, Gabriel Florin Răzvan Mogoș, Tiberiu Stefăniță Țenea Cojan, Nicolae-Dragoș Mărgăritescu, and Anca Buliman. 2025. "Non-Variceal Upper Gastrointestinal Bleeding: A Retrospective Cohort of 364 Cases, Historical Comparison, and Updated Management Algorithm" Life 15, no. 8: 1320. https://doi.org/10.3390/life15081320
APA StyleBarbu, L. A., Vasile, L., Cercelaru, L., Șurlin, V., Mogoantă, S.-S., Mogoș, G. F. R., Țenea Cojan, T. S., Mărgăritescu, N.-D., & Buliman, A. (2025). Non-Variceal Upper Gastrointestinal Bleeding: A Retrospective Cohort of 364 Cases, Historical Comparison, and Updated Management Algorithm. Life, 15(8), 1320. https://doi.org/10.3390/life15081320







