Comparison of the Effects of Cold-Water Immersion Applied Alone and Combined Therapy on the Recovery of Muscle Fatigue After Exercise: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Quality Assessment
2.5. Sensitivity Analysis
2.6. Statistical Analysis
3. Results
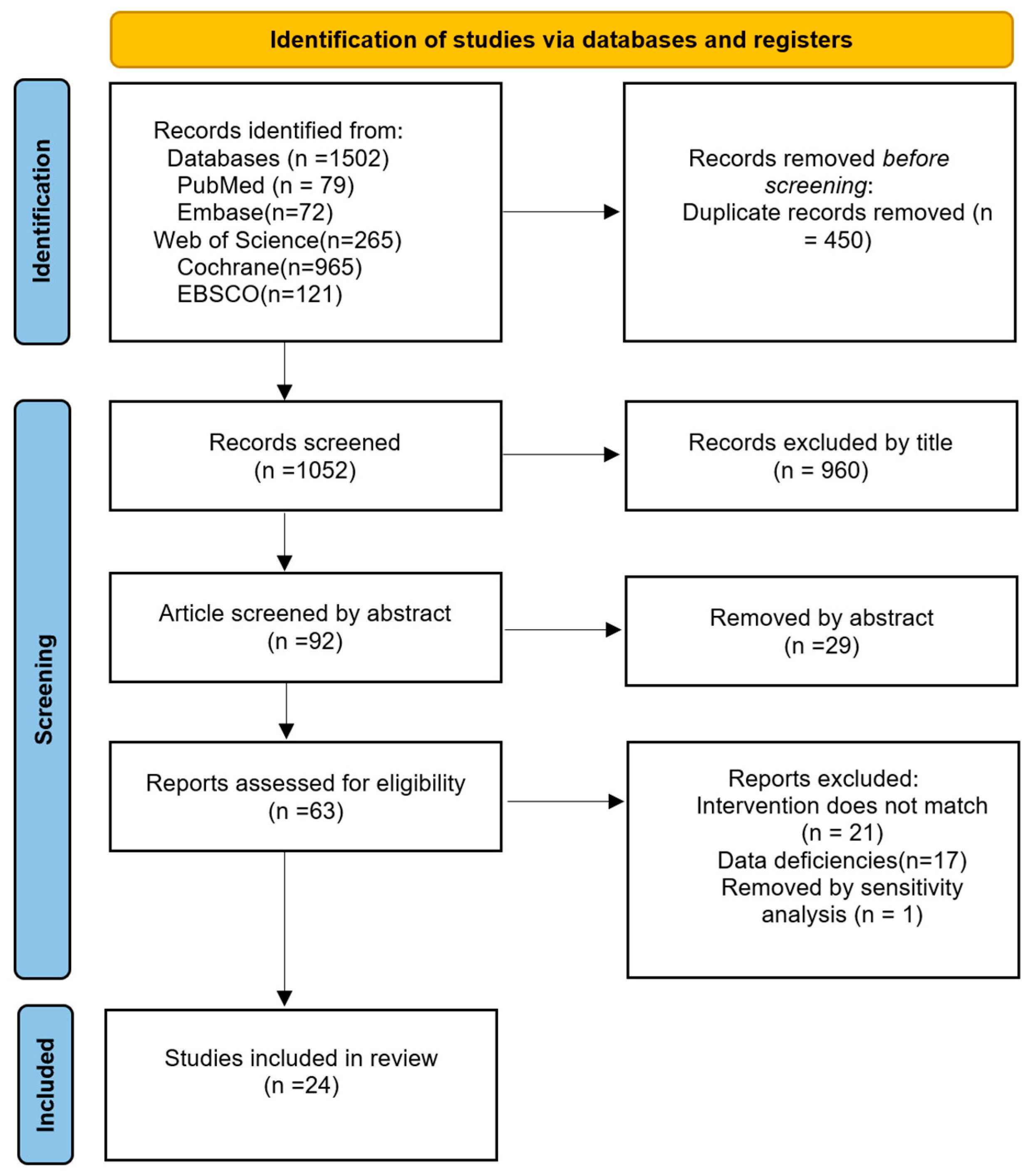
3.1. Included Studies
3.2. Description of the Included Trials
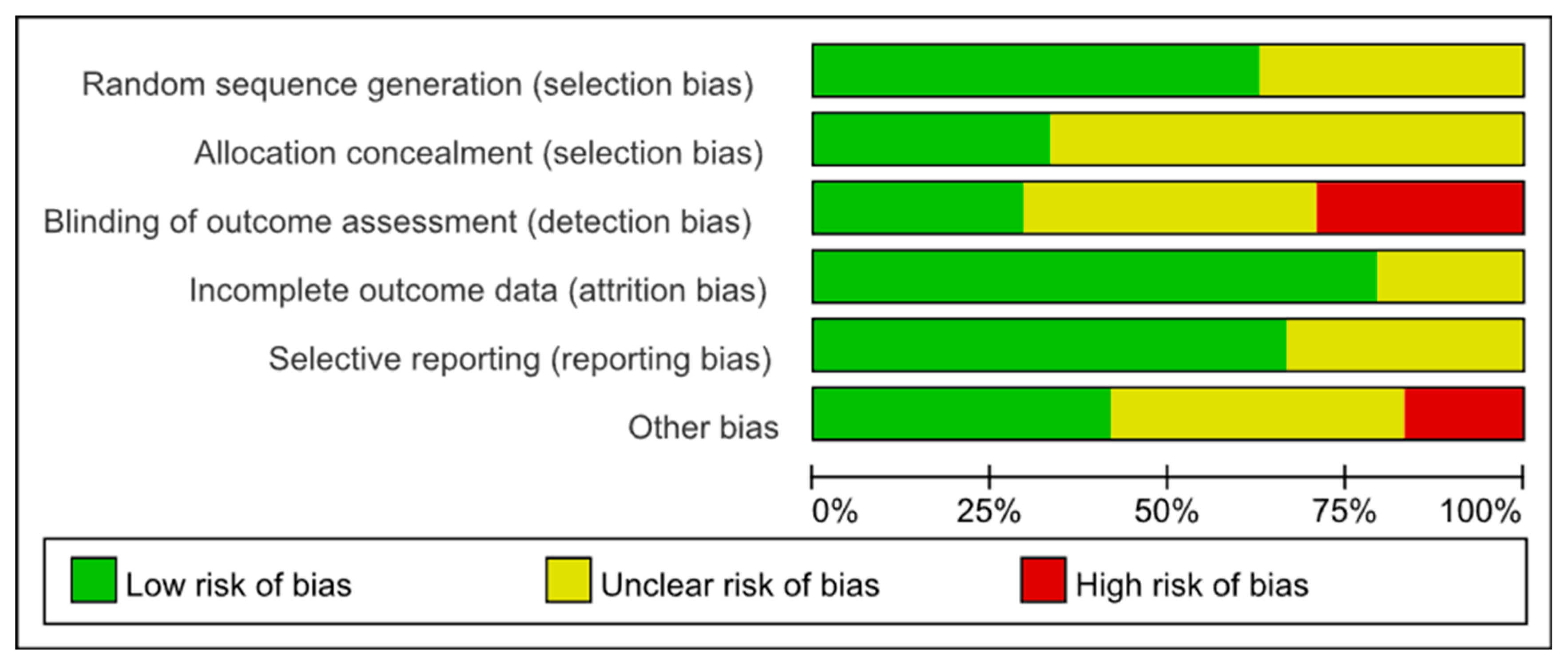
3.3. Risk of Bias Assessment
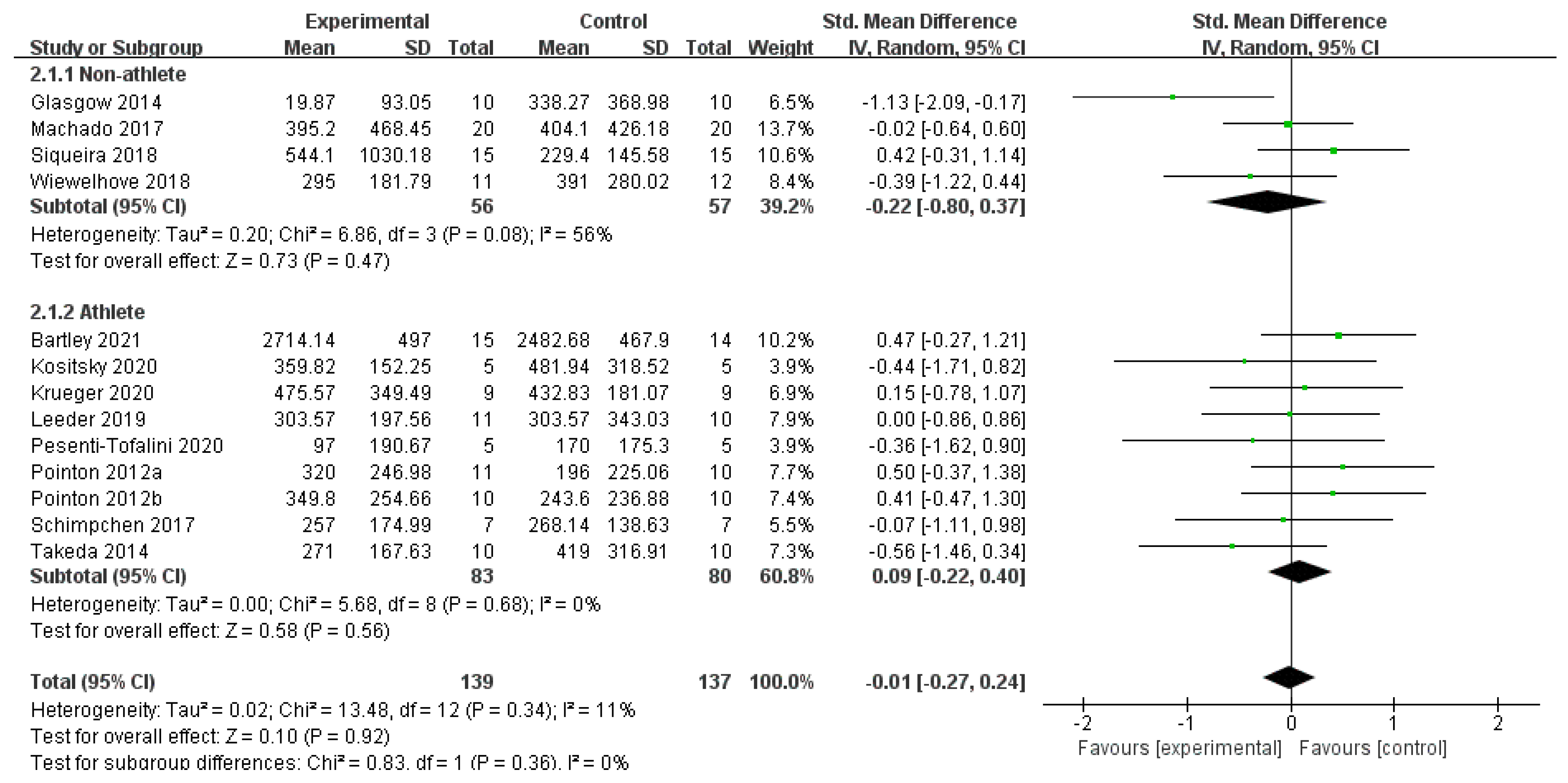
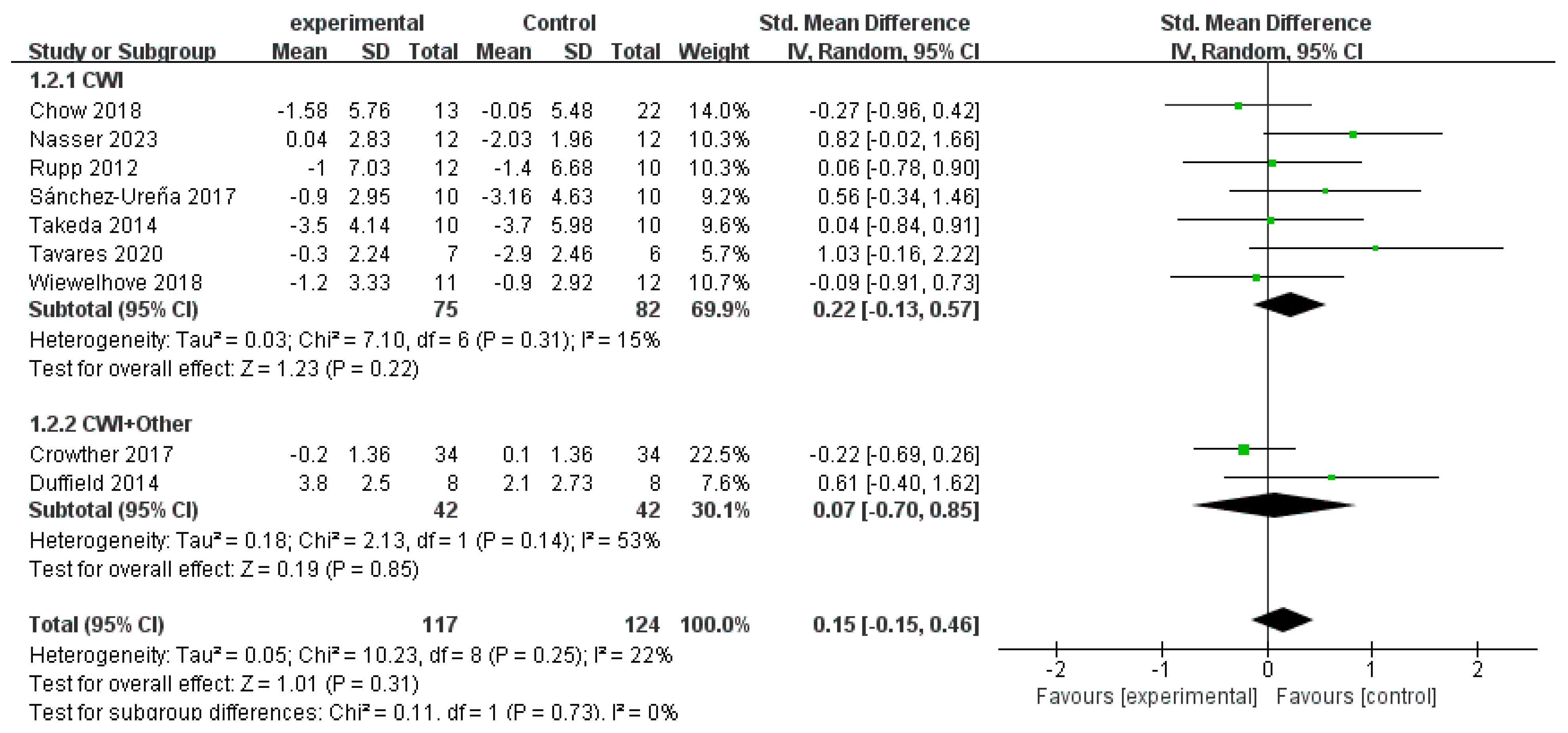
3.4. Synthesis of the Results
3.4.1. CK
3.4.2. CMJ
3.4.3. DOMS
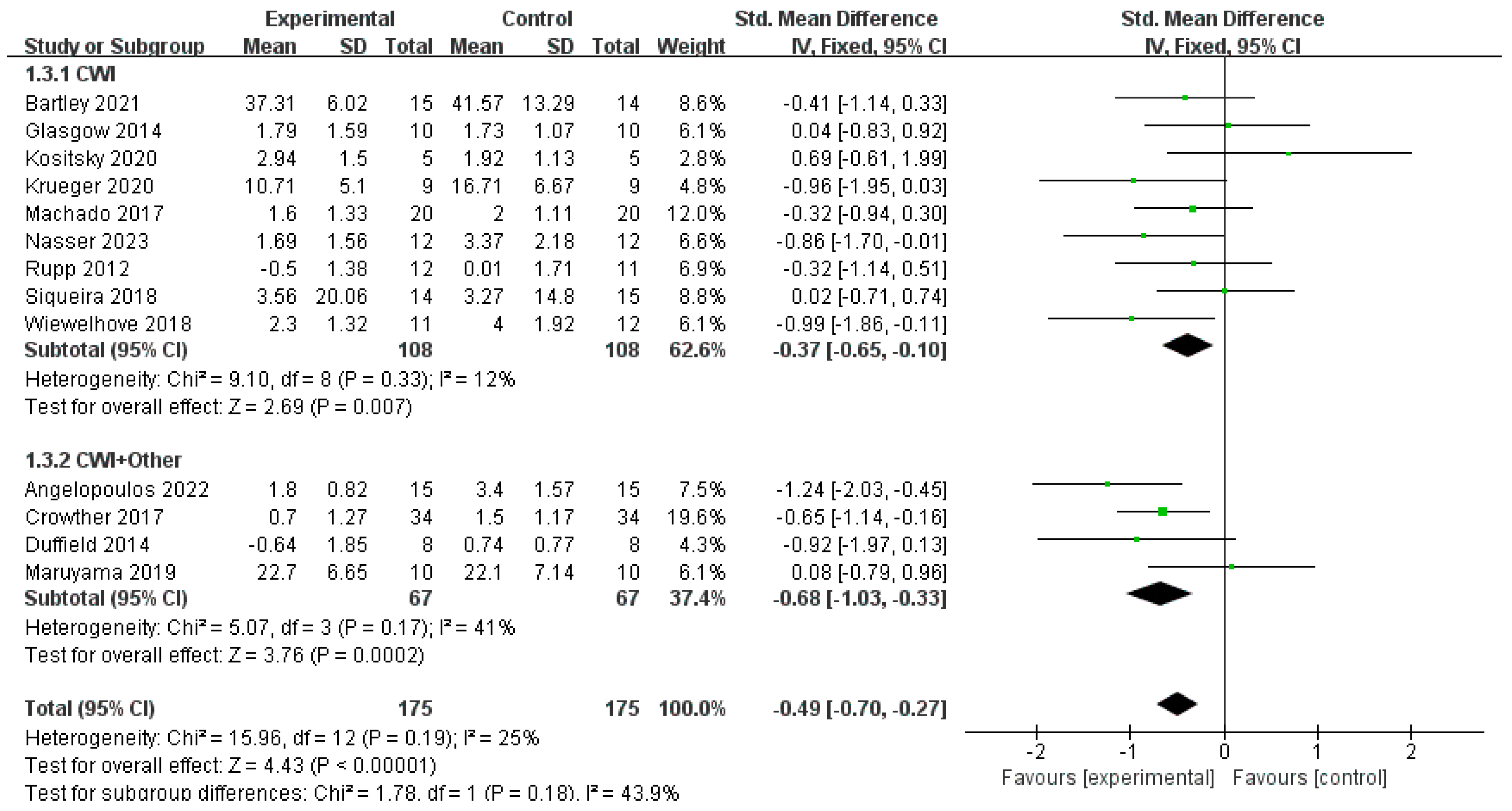
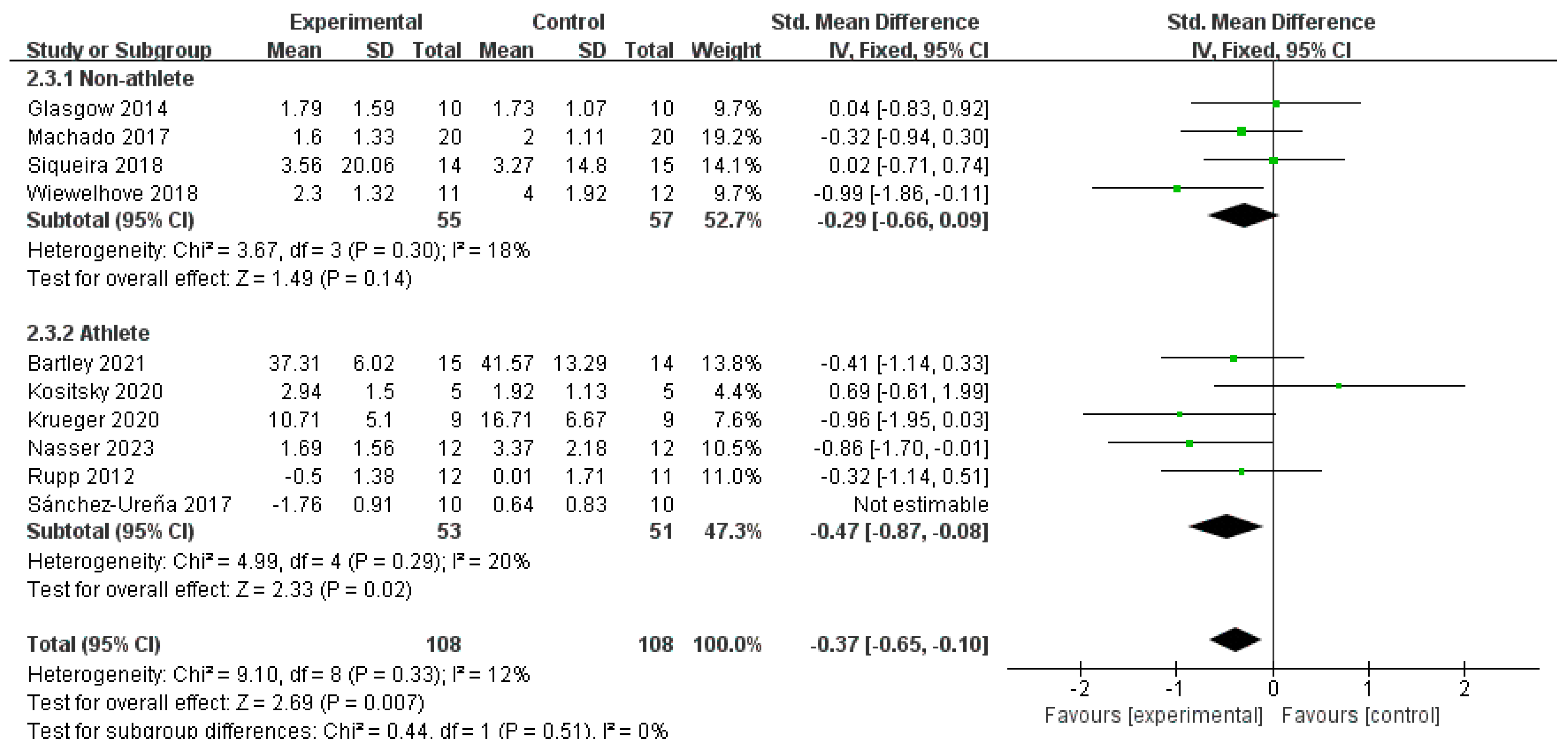
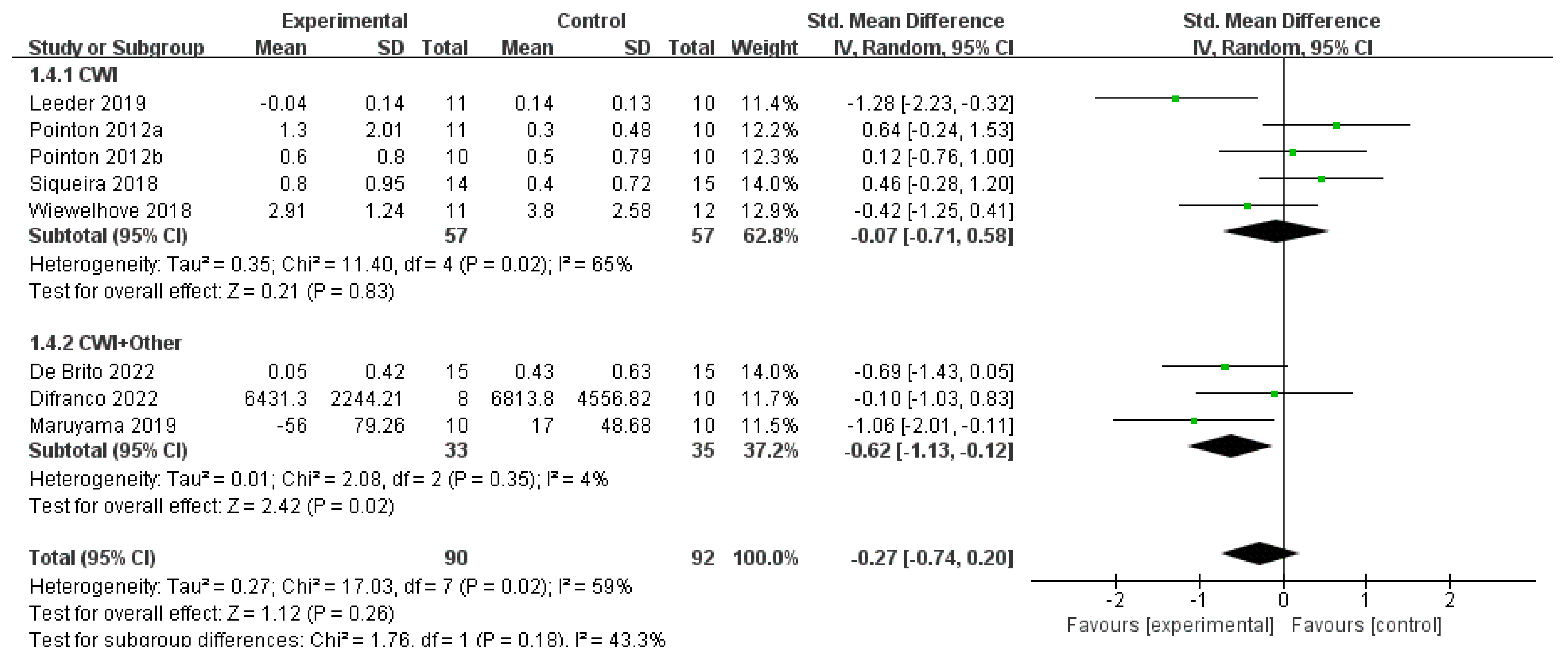
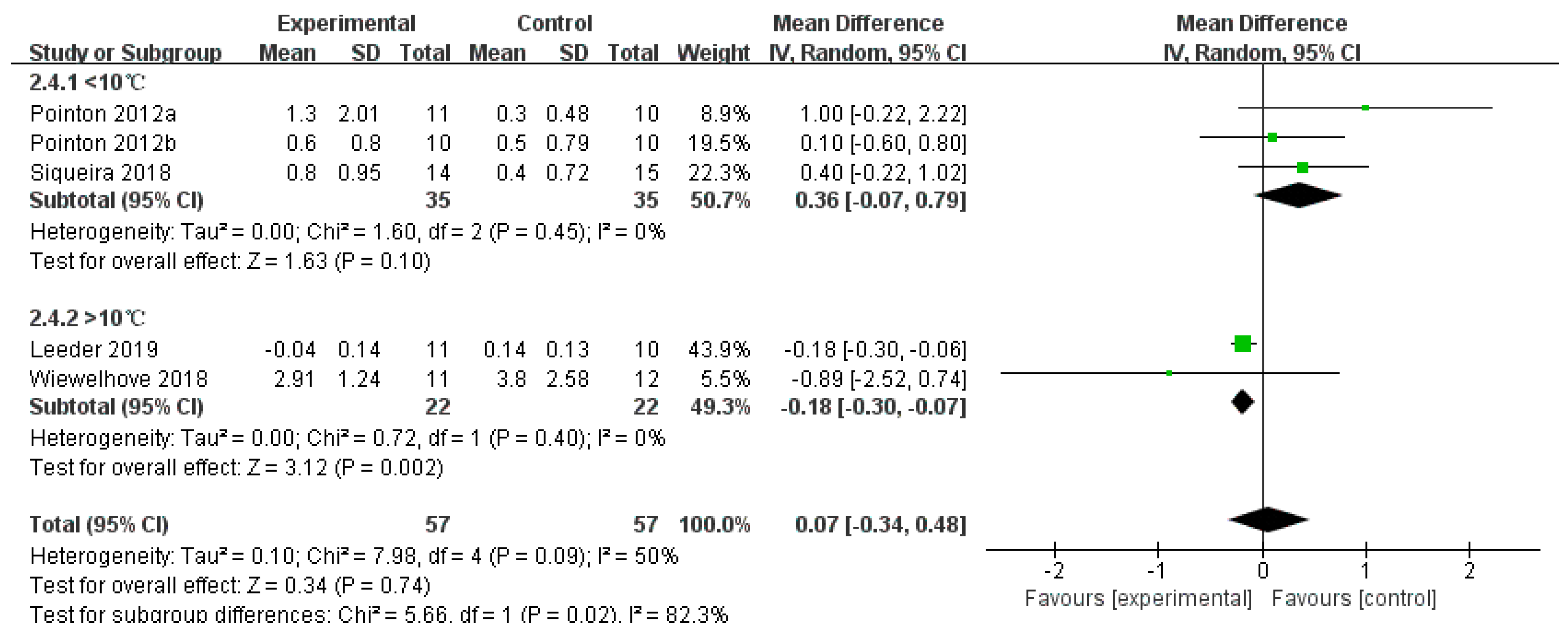
3.4.4. CRP
4. Discussion
4.1. Summary of Evidence
4.2. Comparison with Previous Studies
4.3. Mechanism Discussion
4.3.1. Core Mechanisms of CWI
4.3.2. Synergistic Mechanisms of CWI + Other
- (1)
- Pressure-Assisted Effects of Compression Garments: Wearing compression garments following exercise can modulate osmotic pressure through external pressure gradients, thereby reducing swelling and hematoma formation [49]. When combined with the vasoconstrictive effect of CWI, this may further suppress inflammatory exudation. In this study, CWI + Other in the athlete subgroup achieved an SMD of −1.13 (p = 0.0005), notably larger than that of CWI (SMD = −0.47), suggesting that compression intervention may exert an additive inhibitory effect on acute inflammatory responses following high-intensity exercise.
- (2)
- Antioxidant Synergy of Vitamins C and E: Vitamin C, a water-soluble antioxidant, scavenges cytoplasmic free radicals, while vitamin E, a lipid-soluble antioxidant, protects cell membranes from lipid peroxidation damage [25,50]. A combined supplementation can enhance antioxidant capacity through a regeneration cycle, thereby inhibiting NF-κB-mediated inflammatory responses [51]. In this study, the CRP-lowering effect of CWI + Other (SMD = −0.62) was consistent with that of CWI at water temperatures >10 °C (MD = −0.18), indicating that antioxidant supplementation may compensate for the incomplete suppression of inflammatory pathways by cold exposure alone, particularly in regulating biochemical markers less sensitive to temperature.
- (3)
- Massage-Induced Local Blood Flow Enhancement: Massage promotes local circulation through mechanical pressure, alleviating vasoconstriction-induced ischemia caused by CWI, enhancing the delivery of oxygen and nutrients and simultaneously relaxing tight muscle fibers and fascia, thereby improving muscle extensibility [38]. This explains the larger observed effect of the combined intervention in relieving DOMS by achieving more effective pain relief and functional restoration through a multi-pathway synergy of “cold-induced analgesia, compression-mediated anti-edema and massage-facilitated circulation recovery”.
4.3.3. Temperature-Dependent Mechanism of Inflammation Regulation
4.3.4. Potential Reasons for Non-Significant Effects on Certain Indicators
4.4. Sources of Heterogeneity
- (1)
- Sample Size Variability: The studies exhibited a wide range of sample sizes, with experimental group sizes varying from 5 to 34 participants. To formally test whether this variability was a source of heterogeneity, we conducted meta-regression analyses. These analyses showed no significant association between sample size and the treatment effect for either the DOMS outcome (p = 0.887) or the CRP outcome (p = 0.645).
- (2)
- Diverse Population Characteristics: The included studies comprised diverse populations, including both athletes and non-athletes, across all articles, with a broad age range (14–46 years old). To test the influence of age, a meta-regression was performed for the DOMS outcome, which found no significant association with the treatment effect (p = 0.526). A similar analysis for the CRP outcome was not feasible due to an insufficient number of studies reporting these data, preventing a conclusive determination of its influence on CRP heterogeneity.
- (3)
- Exercise Protocols: The studies encompassed a variety of exercise types, including triathlon, basketball, high-intensity interval training (HIIT), tennis and soccer. These exercises differed in intensity and duration, leading to varying levels of exercise-induced fatigue. Such differences in exercise protocols can significantly impact the efficacy of CWI and CWI + Other, thereby contributing to the observed heterogeneity in recovery effects.
4.5. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gandevia, S.C. Spinal and Supraspinal Factors in Human Muscle Fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [CrossRef]
- Li, N.; Zhou, R.; Krishna, B.; Pradhan, A.; Lee, H.; He, J.; Jiang, N. Non-Invasive Techniques for Muscle Fatigue Monitoring: A Comprehensive Survey. ACM Comput. Surv. 2024, 56, 1–40. [Google Scholar] [CrossRef]
- Brancaccio, P.; Maffulli, N.; Limongelli, F.M. Creatine Kinase Monitoring in Sport Medicine. Br. Med. Bull. 2007, 81–82, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Chen, P.; Zhang, W.; Luo, B.; Du, G.; Liao, T.; Zheng, C. A Evidence-Based Approach to Selecting Post-Exercise Cryostimulation Techniques for Improving Exercise Performance and Fatigue Recovery: A Systematic Review and Meta-Analysis. Heliyon 2024, 10, e32196. [Google Scholar] [CrossRef] [PubMed]
- Gathercole, R.J.; Stellingwerff, T.; Sporer, B.C. Effect of Acute Fatigue and Training Adaptation on Countermovement Jump Performance in Elite Snowboard Cross Athletes. J. Strength Cond. Res. 2015, 29, 37–46. [Google Scholar] [CrossRef]
- Lyu, M.; Chen, Z.; Tang, R.; Ding, L.; Deng, S.; Adams, R.; Han, J.; Li, Y. Lateral Shuffle-Induced Fatigue Effects on Ankle Proprioception and Countermovement Jump Performance. J. Sports Sci. Med. 2024, 21, 418–424. [Google Scholar] [CrossRef]
- Dakić, M.; Ilić, V.; Toskić, L.; Duric, S.; Šimenko, J.; Marković, M.; Dopsaj, M.; Cuk, I. Acute Effects of Short-Term Massage Procedures on Neuromechanical Contractile Properties of Rectus Femoris Muscle. Medicina 2024, 60, 125. [Google Scholar] [CrossRef]
- Chwała, W.; Pogwizd, P.; Rydzik, Ł.; Ambroży, T. Effect of Vibration Massage and Passive Rest on Recovery of Muscle Strength after Short-Term Exercise. Int. J. Environ. Res. Public Health 2021, 18, 11680. [Google Scholar] [CrossRef]
- Veqar, Z. Vibration Therapy in Management of Delayed Onset Muscle Soreness (DOMS). J. Clin. Diagn. Res. 2014, 8, LE01. [Google Scholar] [CrossRef]
- Carroll, T.J.; Taylor, J.L.; Gandevia, S.C. Recovery of Central and Peripheral Neuromuscular Fatigue after Exercise. J. Appl. Physiol. 2017, 122, 1068–1076. [Google Scholar] [CrossRef]
- Caballero-García, A.; Córdova-Martínez, A. Muscle Recovery and Nutrition. Nutrients 2022, 14, 2416. [Google Scholar] [CrossRef]
- Costello, J.T.; Algar, L.A.; Donnelly, A.E. Effects of Whole-body Cryotherapy (−110 °C) on Proprioception and Indices of Muscle Damage. Scand. J. Med. Sci. Sports 2012, 22, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Allan, R.; Malone, J.; Alexander, J.; Vorajee, S.; Ihsan, M.; Gregson, W.; Kwiecien, S.; Mawhinney, C. Cold for Centuries: A Brief History of Cryotherapies to Improve Health, Injury and Post-Exercise Recovery. Eur. J. Appl. Physiol. 2022, 122, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Bieuzen, F.; Bleakley, C.M.; Costello, J.T. Contrast Water Therapy and Exercise Induced Muscle Damage: A Systematic Review and Meta-Analysis. PLoS ONE 2013, 8, e62356. [Google Scholar] [CrossRef]
- Yoshida, R.; Nakamura, M.; Ikegami, R. The Effect of Single Bout Treatment of Heat or Cold Intervention on Delayed Onset Muscle Soreness Induced by Eccentric Contraction. Health Care 2022, 10, 2556. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Kabachkova, A.V.; Jiao, L.; Zhao, H.; Kapilevich, L.V. Effects of Cold Water Immersion after Exercise on Fatigue Recovery and Exercise Performance—Meta Analysis. Front. Physiol. 2023, 14, 1006512. [Google Scholar] [CrossRef]
- Moore, E.; Fuller, J.T.; Buckley, J.D.; Saunders, S.; Halson, S.L.; Broatch, J.R.; Bellenger, C.R. Impact of Cold-Water Immersion Compared with Passive Recovery Following a Single Bout of Strenuous Exercise on Athletic Performance in Physically Active Participants: A Systematic Review with Meta-Analysis and Meta-Regression. Sports Med. 2022, 52, 1667–1688. [Google Scholar] [CrossRef]
- Phillips, K.C.; Verbrigghe, D.; Gabe, A.; Jauquet, B.; Eischer, C.; Yoon, T. The Influence of Thermal Alterations on Prefrontal Cortex Activation and Neuromuscular Function during a Fatiguing Task. Int. J. Environ. Res. Public Health 2020, 17, 7194. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Sánchez-Ureña, B.; Martínez-Guardado, I.; Crespo, C.; Timón, R.; Calleja-González, J.; Ibañez, S.J.; Olcina, G. The Use of Continuous vs. Intermittent Cold Water Immersion as a Recovery Method in Basketball Players after Training: A Randomized Controlled Trial. Phys. Sportsmed. 2017, 45, 134–139. [Google Scholar] [CrossRef]
- McKinney, J.; Velghe, J.; Fee, J.; Isserow, S.; Drezner, J.A. Defining Athletes and Exercisers. Am. J. Cardiol. 2019, 123, 532–535. [Google Scholar] [CrossRef]
- Bartley, J.M.; Stearns, R.L.; Muñoz, C.X.; Nolan, J.K.; Radom-Aizik, S.; Maresh, C.M.; Casa, D.J.; Zaldivar, F.P.; Haddad, F.; Ganio, M.; et al. Effects of Cold Water Immersion on Circulating Inflammatory Markers at the Kona Ironman World Championship. Appl. Physiol. Nutr. Metab. 2021, 46, 719–726. [Google Scholar] [CrossRef]
- Chow, G.C.C.; Chung, J.W.Y.; Fong, S.S.M. Differential Effects of Post-Exercise Ice Water Immersion and Room Temperature Water Immersion on Muscular Performance, Vertical Jump, and Agility in Amateur Rugby Players: A Randomized Controlled Trial. Sci. Sports 2018, 33, e271–e279. [Google Scholar] [CrossRef]
- Duffield, R.; Murphy, A.; Kellett, A.; Reid, M. Recovery From Repeated On-Court Tennis Sessions: Combining Cold-Water Immersion, Compression, and Sleep Interventions. Int. J. Sports Physiol. Perform. 2014, 9, 273–282. [Google Scholar] [CrossRef] [PubMed]
- De Brito, E.; Teixeira, A.D.O.; Righi, N.C.; Paulitcth, F.D.S.; Da Silva, A.M.V.; Signori, L.U. Vitamins C and E Associated With Cryotherapy in the Recovery of the Inflammatory Response After Resistance Exercise: A Randomized Clinical Trial. J. Strength Cond. Res. 2022, 36, 135–141. [Google Scholar] [CrossRef]
- Pesenti-Tofalini, F.B.; Spartalis, E.R.; Okino, A.M.; Venturini, D.; Frisseli, A.; Macedo, C.D.S.G. Effect of Immersion in Cold Water on Creatine Kinase and Myoglobin Levels in Soccer Players. J. Health Sci. 2020, 22, 151–155. [Google Scholar] [CrossRef]
- Crowther, F.; Sealey, R.; Crowe, M.; Edwards, A.; Halson, S. Influence of Recovery Strategies upon Performance and Percep tions Following Fatiguing Exercise: A Randomized Controlled Trial. BMC Sports Sci. Med. Rehabil. 2017, 9, 25. [Google Scholar] [CrossRef]
- Tavares, F.; Simões, M.; Matos, B.; Smith, T.B.; Driller, M. The Acute and Longer-Term Effects of Cold Water Immersion in Highly-Trained Volleyball Athletes During an Intense Training Block. Front. Sports Act. Living 2020, 2, 568420. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, P.D.; Ferris, R.; Bleakley, C.M. Cold Water Immersion in the Management of Delayed-Onset Muscle Soreness: Is Dose Important? A Randomised Controlled Trial. Phys. Ther. Sport 2014, 15, 228–233. [Google Scholar] [CrossRef]
- Difranco, I.; Cockburn, E.; Dimitriou, L.; Paice, K.; Sinclair, S.; Faki, T.; Hills, F.A.; Gondek, M.B.; Wood, A.; Wilson, L.J. A Combination of Cherry Juice and Cold Water Immersion Does Not Enhance Marathon Recovery Compared to Either Treatment in Isolation: A Randomized Placebo-Controlled Trial. Front. Sports Act. Living 2022, 4, 957950. [Google Scholar] [CrossRef] [PubMed]
- Rupp, K.A.; Selkow, N.M.; Parente, W.R.; Ingersoll, C.D.; Weltman, A.L.; Saliba, S.A. The Effect of Cold Water Immersion on 48-Hour Performance Testing in Collegiate Soccer Players. J. Strength Cond. Res. 2012, 26, 2043–2050. [Google Scholar] [CrossRef]
- Kositsky, A.; Avela, J. The Effects of Cold Water Immersion on the Recovery of Drop Jump Performance and Mechanics: A Pilot Study in Under-20 Soccer Players. Front. Sports Act. Living 2020, 2, 17. [Google Scholar] [CrossRef]
- Krueger, M.; Costello, J.T.; Stenzel, M.; Mester, J.; Wahl, P. The Physiological Effects of Daily Cold-Water Immersion on 5-Day Tournament Performance in International Standard Youth Field-Hockey Players. Eur. J. Appl. Physiol. 2020, 120, 295–305. [Google Scholar] [CrossRef]
- Leeder, J.D.C.; Godfrey, M.; Gibbon, D.; Gaze, D.; Davison, G.W.; Van Someren, K.A.; Howatson, G. Cold Water Immersion Improves Recovery of Sprint Speed Following a Simulated Tournament. Eur. J. Sport Sci. 2019, 19, 1166–1174. [Google Scholar] [CrossRef]
- Machado, A.F.; Almeida, A.C.; Micheletti, J.K.; Vanderlei, F.M.; Tribst, M.F.; Netto Junior, J.; Pastre, C.M. Dosages of Cold-water Immersion Post Exercise on Functional and Clinical Responses: A Randomized Controlled Trial. Scand. J. Med. Sci. Sports 2017, 27, 1356–1363. [Google Scholar] [CrossRef]
- Takeda, M.; Sato, T.; Hasegawa, T.; Shintaku, H.; Kato, H.; Radak, Z. The Effects of Cold Water Immersion after Rugby Training on Muscle Power and Biochemical Markers. J. Sports Sci. Med. 2014, 13, 616–623. [Google Scholar]
- Nasser, N.; Zorgati, H.; Chtourou, H.; Guimard, A. Cold Water Immersion after a Soccer Match: Does the Placebo Effect Occur? Front. Physiol. 2023, 14, 1062398. [Google Scholar] [CrossRef]
- Angelopoulos, P.; Diakoronas, A.; Panagiotopoulos, D.; Tsekoura, M.; Xaplanteri, P.; Koumoundourou, D.; Saki, F.; Billis, E.; Tsepis, E.; Fousekis, K. Cold-Water Immersion and Sports Massage Can Improve Pain Sensation but Not Functionality in Athletes with Delayed Onset Muscle Soreness. Healthcare 2022, 10, 2449. [Google Scholar] [CrossRef]
- Pointon, M.; Duffield, R. Cold Water Immersion Recovery after Simulated Collision Sport Exercise. Med. Sci. Sports Exerc. 2012, 44, 206–216. [Google Scholar] [CrossRef]
- Pointon, M.; Duffield, R.; Cannon, J.; Marino, F.E. Cold Water Immersion Recovery Following Intermittent-Sprint Exercise in the Heat. Eur. J. Appl. Physiol. 2012, 112, 2483–2494. [Google Scholar] [CrossRef]
- Schimpchen, J.; Wagner, M.; Ferrauti, A.; Kellmann, M.; Pfeiffer, M.; Meyer, T. Can Cold Water Immersion Enhance Recovery in Elite Olympic Weightlifters? An Individualized Perspective. J. Strength Cond. Res. 2017, 31, 1569–1576. [Google Scholar] [CrossRef]
- Siqueira, A.F.; Vieira, A.; Bottaro, M.; Ferreira-Júnior, J.B.; Nóbrega, O.D.T.; De Souza, V.C.; Marqueti, R.D.C.; Babault, N.; Durigan, J.L.Q. Multiple Cold-Water Immersions Attenuate Muscle Damage but Not Alter Systemic Inflammation and Muscle Function Recovery: A Parallel Randomized Controlled Trial. Sci. Rep. 2018, 8, 10961. [Google Scholar] [CrossRef]
- Maruyama, T.; Mizuno, S.; Goto, K. Effects of Cold Water Immersion and Compression Garment Use after Eccentric Exercise on Recovery. J. Exerc. Nutr. Biochem. 2019, 23, 48–54. [Google Scholar] [CrossRef]
- Wiewelhove, T.; Schneider, C.; Döweling, A.; Hanakam, F.; Rasche, C.; Meyer, T.; Kellmann, M.; Pfeiffer, M.; Ferrauti, A. Effects of Different Recovery Strategies Following a Half-Marathon on Fatigue Markers in Recreational Runners. PLoS ONE 2018, 13, e0207313. [Google Scholar] [CrossRef]
- Machado, A.F.; Ferreira, P.H.; Micheletti, J.K.; De Almeida, A.C.; Lemes, Í.R.; Vanderlei, F.M.; Netto Junior, J.; Pastre, C.M. Can Water Temperature and Immersion Time Influence the Effect of Cold Water Immersion on Muscle Soreness? A Systematic Review and Meta-Analysis. Sports Med. 2016, 46, 503–514. [Google Scholar] [CrossRef]
- Wilson, L.J.; Cockburn, E.; Paice, K.; Sinclair, S.; Faki, T.; Hills, F.A.; Gondek, M.B.; Wood, A.; Dimitriou, L. Recovery Following a Marathon: A Comparison of Cold Water Immersion, Whole Body Cryotherapy and a Placebo Control. Eur. J. Appl. Physiol. 2018, 118, 153–163. [Google Scholar] [CrossRef]
- Madrid, R.; Donovan-Rodríguez, T.; Meseguer, V.; Acosta, M.C.; Belmonte, C.; Viana, F. Contribution of TRPM8 Channels to Cold Transduction in Primary Sensory Neurons and Peripheral Nerve Terminals. J. Neurosci. 2006, 26, 12512–12525. [Google Scholar] [CrossRef]
- Chung, Y.H.; Oh, K.W.; Kim, S.T.; Park, E.S.; Je, H.D.; Yoon, H.-J.; Sohn, U.D.; Jeong, J.H.; La, H.-O. Hypothermia Inhibits Endothelium-Independent Vascular Contractility via Rho-Kinase Inhibition. Biomol. Ther. 2018, 26, 139–145. [Google Scholar] [CrossRef]
- Hill, J.; Howatson, G.; Van Someren, K.; Leeder, J.; Pedlar, C. Compression Garments and Recovery from Exercise-Induced Muscle Damage: A Meta-Analysis. Br. J. Sports Med. 2014, 48, 1340–1346. [Google Scholar] [CrossRef]
- Wang, X. Vitamin E and Its Function in Membranes. Prog. Lipid Res. 1999, 38, 309–336. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]



| Intervention | Mean Age, Years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study, Year | Study Type | Number of Cases (E/C) | Sex (M/F) | Profession | Exercise Protocol | Outcome Measures | Time of Measure | E | C | E | C |
| Bartley, 2021 [22] | RCT | 15/14 | 22/7 | Athletes | Ironman game | CK DOMS | Baseline 24 h 48 h | CWI 10 °C 12 min | PAS | 42.5 ± 2.3 | 46.0 ± 3.2 |
| Sánchez-Ureña, 2017 [20] | Randomized, crossover | 10/10 | 10/0 | Athletes | Basketball technical and tactical skills training | CMJ DOMS | Baseline 24 h 48 h | CWI 12 °C 12 min | PAS | 14 ± 0.4 | 14 ± 0.4 |
| Chow, 2018 [23] | RCT | 13/22 | 13/22 | Non-athletes | HIIT | CMJ | PRE POST | IWI 5 °C 1 min | PAS | 22.9 ± 2.4 | 20.7 ± 2.4 |
| Duffield, 2014 [24] | Randomized, crossover | 8/8 | 8/0 | Athletes | Tennis training | CMJ DOMS | PRE POST | CWI 11 °C 15 min + compression garments | PAS | 20.9 ± 3.6 | 20.9 ± 3.6 |
| De Brito, 2022 [25] | Randomized, crossover | 14/14 | / | Non-athletes | 10 RM | CK CRP | Baseline 2 h | CWI 15 °C 10 min + vitamin C/E | PAS | 26.2 ± 5 | 26.2 ± 5 |
| Pesenti-Tofalini, 2020 [26] | RCT | 5/5 | 10/0 | Athletes | Soccer match | CK | Baseline POST 24 h 48 h | CWI 10 °C 10 min | PAS | 17.6 ± 0.54 | 16.8 ± 0.83 |
| Crowther, 2017 [27] | RCT | 34/34 | 34/0 | Non-athletes | Simulated team game | CMJ DOMS | Baseline POST 24 h 48 h | CWI 15 °C 14 min + low-intensity cyclic leg movement | PAS | 27 ± 6 | 27 ± 6 |
| Tavares, 2020 [28] | RCT | 7/6 | / | Athletes | Regular volleyball training and resistance training | CMJ | Baseline POST training camp | CWI | PAS | 19.2 ± 0.8 | 19.0 ± 1.3 |
| Glasgow, 2014 [29] | RCT | 10/10 | / | Non-athletes | Three sets of eccentric hamstring contractions to fatigue | CK DOMS | Baseline 24 h 48 h | CWI 10 °C 10 min | PAS | / | / |
| Difranco, 2022 [30] | RCT | 8/10 | 18/0 | Athletes | A competitive trail marathon run | CK CRP | Baseline 24 h 48 h | CWI 8 °C 10 min + cherry juice | Placebo + PAS | 42.7 ± 4.7 | 40.6 ± 7.2 |
| Rupp, 2012 [31] | RCT | 12/10 | 13/9 | Athletes | YO-YO test | CMJ DOMS | Baseline 24 h 48 h | CWI 12 °C 15 min | PAS | 19.8 ± 1.1 | 19.8 ± 1.1 |
| Kositsky, 2020 [32] | RCT | 5/5 | 10/0 | Athletes | Drop jumps | CK DOMS | Baseline 24 h 48 h | CWI 10 °C 20 min | PAS | 19.4 ± 0.9 | 18.4 ± 0.5 |
| Krueger, 2020 [33] | RCT | 9/9 | 18/0 | Athletes | Hockey game | CK DOMS | Baseline 24 h 48 h | CWI 6.4 ± 0.8 °C 5 min | PAS | 16.6 ± 0.6 | 16.6 ± 0.6 |
| Leeder, 2019 [34] | RCT | 11/10 | 21/0 | Athletes | Loughborough Intermittent Shuttle Test | CK CRP | PRE POST | CWI 14 °C 14 min | PAS | 20 ± 2 | 19 ± 1 |
| Machado, 2017 [35] | RCT | 20/20 | 40/0 | Non-athletes | Eccentric exercise | CK DOMS | Baseline 24 h 48 h | CWI 9 °C 15 min | PAS | 21.2 ± 2.0 | 20.4 ± 1.8 |
| Takeda, 2014 [36] | Randomized, crossover | 10/10 | 20/0 | Athletes | Rugby simulation training | CK CMJ | Baseline 24 h | CWI 15 °C 10 min | PAS | / | / |
| Nasser, 2023 [37] | Randomized, crossover | 12/12 | 24/0 | Athletes | Loughborough Intermittent Shuttle Test | CMJ DOMS | Baseline 24 h 48 h | CWI 11 °C 15 min | PAS | / | / |
| Angelopoulos, 2022 [38] | RCT | 15/15 | 30/0 | Athletes | Plyometric fatigue protocol | CK DOMS | Baseline POST 24 h 48 h | 20 min massage + CWI 10 °C 10 min | PAS | / | / |
| Pointon, 2012a [39] | RCT | 10/10 | 20/0 | Athletes | Loughborough Test (HIIT) | CK CRP | Baseline POST 2 h 24 h | CWI 9 °C 9 min + 1 min rest × 2 | PAS | / | / |
| Pointon, 2012b [40] | Randomized, crossover | 10/10 | 10/0 | Athletes | Intermittent-sprint exercise | CK CPR | Baseline POST 2 h 24 h | CWI 9 °C 9 min + 1 min rest × 2 | PAS | / | / |
| Schimpchen, 2017 [41] | Randomized, crossover | 7/7 | 7/0 | Athletes | Weightlifting training | CK | Baseline 24 h | CWI 12–15 °C 10 min | PAS | / | / |
| Siqueira, 2018 [42] | RCT | 14/15 | 29/0 | Non-athletes | Drop jumps | CK DOMS CRP | Baseline 24 h 48 h | CWI 10 °C 20 min | PAS | 20.5 ± 1.4 | 19.9 ± 1.4 |
| Maruyama, 2019 [43] | Randomized, crossover | 10/10 | 10/0 | Non-athletes | Eccentric exercise | CK DOMS CRP | Baseline 3 h 24 h | CWI 15 °C 15 min + lower-body compression garment | PAS | / | / |
| Wiewelhove, 2018 [44] | RCT | 11/12 | 23/0 | Non-athletes | Half-marathon race | CK CMJ DOMS CRP | Baseline POST 24 h | CWI 15 °C 15 min | PAS | 31.5 ± 10.2 | 31.3 ± 12.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Guo, C.; Luo, L.; Chen, X.; Zhang, K.; Liang, D.; Zhang, D. Comparison of the Effects of Cold-Water Immersion Applied Alone and Combined Therapy on the Recovery of Muscle Fatigue After Exercise: A Systematic Review and Meta-Analysis. Life 2025, 15, 1205. https://doi.org/10.3390/life15081205
Ma J, Guo C, Luo L, Chen X, Zhang K, Liang D, Zhang D. Comparison of the Effects of Cold-Water Immersion Applied Alone and Combined Therapy on the Recovery of Muscle Fatigue After Exercise: A Systematic Review and Meta-Analysis. Life. 2025; 15(8):1205. https://doi.org/10.3390/life15081205
Chicago/Turabian StyleMa, Junjie, Changfei Guo, Long Luo, Xiaoke Chen, Keying Zhang, Dongxue Liang, and Dong Zhang. 2025. "Comparison of the Effects of Cold-Water Immersion Applied Alone and Combined Therapy on the Recovery of Muscle Fatigue After Exercise: A Systematic Review and Meta-Analysis" Life 15, no. 8: 1205. https://doi.org/10.3390/life15081205
APA StyleMa, J., Guo, C., Luo, L., Chen, X., Zhang, K., Liang, D., & Zhang, D. (2025). Comparison of the Effects of Cold-Water Immersion Applied Alone and Combined Therapy on the Recovery of Muscle Fatigue After Exercise: A Systematic Review and Meta-Analysis. Life, 15(8), 1205. https://doi.org/10.3390/life15081205







