Enhancing Cardiovascular Autonomic Regulation in Parkinson’s Disease Through Non-Invasive Interventions
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Search Terms
2.3. Inclusion and Exclusion Criteria
- Original, peer-reviewed human studies published between January 2014 and December 2024 to capture the most recent evidence in emerging neuromodulation protocols.
- PD patients undergoing non-invasive interventions targeting cardiovascular autonomic function.
- Quantitative assessment of BRS or related cardiovascular outcomes (e.g., heart rate variability (HRV), blood pressure responses).
- Presence of an active treatment arm (single-arm or controlled design).
- Animal or in vitro studies.
- Reviews, meta-analyses or conference abstracts without full quantitative data.
- Studies evaluating exclusively invasive or pharmacological therapies.
- Interventions lacking a defined therapeutic component (e.g., observational, or purely diagnostic studies).
- Investigations including mixed patient cohorts with comorbidities other than PD, without separate PD-specific analysis.
- Participant populations outside adult age ranges (i.e., <18 years).
2.4. Screening and Selection
- Title and Abstract Screening: Two reviewers independently assessed titles and abstracts to exclude clearly irrelevant studies (e.g., those not involving PD, non-invasive interventions, or cardiovascular outcomes). Discrepancies were resolved by discussion.
- Full-Text Review: Full texts of potentially eligible articles were obtained and evaluated against the predefined inclusion and exclusion criteria (Section 2.3). Each study was confirmed to involve adult PD patients, non-invasive therapeutic interventions, and quantitative measures of BRS or related cardiovascular outcomes.
2.5. Risk of Bias Assessment
- Selection Bias: Assessed random sequence generation and allocation concealment methods.
- Performance and Detection Bias: Evaluated blinding of participants, personnel, and outcome assessors.
- Attrition Bias: Reviewed completeness of outcome data and the handling of missing data, as reported by studies (e.g., use of intention-to-treat analyses or specific imputation methods).
- Reporting Bias: Checked for selective outcome reporting by comparing published methods with reported results.
2.6. Data Extraction and Synthesis
- Participant Characteristics: Mean age, gender distribution, sample size, disease duration, and Hoehn and Yahr classification.
- Intervention Details: Type of non-invasive modality, study design (e.g., randomized controlled trial, single arm), intervention frequency and duration, stimulation or exercise parameters, and comparator conditions.
- Outcome Measures: Primary metrics of BRS (e.g., sequence method, transfer function analysis), secondary cardiovascular autonomic indices (e.g., HRV, low-frequency/high-frequency (LF/HF) ratio), and any reported adverse events.
3. Results
3.1. Articles Retrieved
3.2. Participants Characteristics
3.3. Rehabilitation Characteristics
- Effective Stimulation (ES): A randomized clinical trial (RCT) delivered four 6 s sessions of ES (total 2 min) in a single visit [16].
- Resistance Training (RT): In a 12-week RCT, participants completed 24 sessions (twice weekly) of progressive RT [21].
- Automated Mechanical Somatosensory Stimulation (AMSS): An interventional cohort study administered AMSS over 12 days, with two sessions per week (five total sessions) [17].
- Partial Weight-Supported Treadmill Training (PWSTT): A 4-week RCT comprised 16 sessions (four times weekly, 30 min each) [20].
- Dry Immersion (DI): A 4-week controlled clinical trial involved seven 45 min DI sessions (twice weekly) [19].
- Whole-Body Cryostimulation (WBC): A one-week pilot study delivered ten 2 min WBC sessions (twice daily) [18].
- Head-Up Tilt Sleeping (HUTS): A case report described daily HUTS without a specified session duration [24].
- Rigorous study designs: Most interventions (RT, PWSTT, DI) employed randomized clinical trials to strengthen evidence quality.
- Extended training periods: Interventions such as RT and RMT spanned 12 weeks, underscoring the necessity of sustained training for meaningful autonomic adaptation.
- Protocol diversity: Session lengths varied from seconds (ES, WBC) to weeks (RMT), complicating direct efficacy comparisons but illustrating the breadth of non-invasive approaches.
- Analogous modalities: ES and AMSS shared similar mechanistic goals of somatosensory activation, while PWSTT and RT both leveraged load-bearing exercise to induce cardiovascular adaptations.
3.4. Study Designs and Protocols
3.5. Rehabilitation Effects
3.6. Statistical Analysis
4. Discussion
4.1. Limitations
- ES: Focused on stimulation site specificity without systematically varying stimulus intensity, limiting insight into dose–response relationships.
- RT: Heterogeneity in concurrent medication regimens and absence of long-term follow-up assessments constrain interpretation of sustained autonomic effects.
- WBC: Small cohort size and lack of a control group reduce statistical power and external validity; age-related variability was not explored.
- PWSTT: Participants were not selected based on OH or impaired BRS, limiting applicability to the target dysautonomia population.
- DI: Recruitment challenges and multimorbidity in older subjects precluded an age-matched control group; the individual contributions of immersion versus thermoneutral temperature (32 °C) were not disaggregated.
- RMT: Absence of randomized, blinded, sham-controlled designs and control-group adherence issues in follow-up studies introduce potential bias; baseline differences in age, disease severity, and duration may confound outcomes.
- AMSS: No control or sham-stimulation arm was included, leaving placebo effects unaddressed.
- HUTS: Case-report design and lack of standardized tilt protocols limit generalizability and dose–response characterization.
4.2. Future Directions
- Standardization and Personalization
- Develop consensus protocols with clearly defined intervention parameters (e.g., stimulation sites, frequencies, durations).
- Incorporate individualized titration of stimulation intensity or exercise load based on patient-specific physiological markers (e.g., baseline BRS, hemodynamic responses).
- Intensive, Multimodal Rehabilitation
- Design studies that combine complementary interventions (e.g., ES + RT or RMT + HUTS) to target multiple facets of autonomic dysfunction simultaneously.
- Evaluate “dose–response” effects by comparing standard versus high-intensity regimens to identify optimal training volumes for maximal autonomic adaptation.
- Mechanistic and Neuromodulatory Investigations
- Pair clinical trials with mechanistic studies (e.g., neuroimaging, autonomic reflex testing) to uncover neural circuits and molecular pathways responsible for observed benefits.
- Explore biomarkers (e.g., neurotrophic factors, inflammatory mediators) to monitor treatment response and guide protocol refinement.
- Long-Term Efficacy and Disease-Modifying Potential
- Conduct extended follow-up assessments (≥12 months) to determine the durability of autonomic improvements and their impact on clinical outcomes such as fall rates and disease progression.
- Investigate whether sustained autonomic rehabilitation can modify the trajectory of PD-related cardiovascular decline or delay the onset of disabling symptoms.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PD | Parkinson’s Disease |
| OH | Orthostatic Hypotension |
| SBP | Systolic Blood Pressure |
| DBP | Diastolic Blood Pressure |
| BRS | Baroreflex Sensitivity |
| UPDRS | Unified Parkinson Disease Rating Scale |
| ES | Effective Stimulation |
| RT | progressive Resistant Training |
| WBC | Whole Body Cryostimulation |
| PWSTT | Partial Weight Supported Treadmill Training |
| DI | Dry Immersion |
| RMT | Respiratory Motor Training |
| HUTS | Head Up Tilt Sleeping |
| AMSS | Automated Mechanical Somatosensory Stimulation |
| RCT | Randomized Clinical Trial |
| ECG | Electrocardiograph |
| SAP | Systolic Arterial Pressure |
| HRV | Heart Rate Variability |
| HR | Heart Rate |
| LF | Low Frequency |
| HF | High Frequency |
References
- De Pablo-Fernandez, E.; Tur, C.; Revesz, T.; Lees, A.J.; Holton, J.L.; Warner, T.T. Association of Autonomic Dysfunction with Disease Progression and Survival in Parkinson Disease. JAMA Neurol. 2017, 74, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Szili-Török, T.; Kálmán, J.; Paprika, D.; Dibó, G.; Rózsa, Z.; Rudas, L. Depressed baroreflex sensitivity in patients with Alzheimer’s and Parkinson’s disease. Neurobiol. Aging 2001, 22, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Linden, D.; Diehl, R.; Berlit, P. Sympathetic cardiovascular dysfunction in long-standing idiopathic Parkinson’s disease. Clin. Auton. Res. 1997, 7, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Sabino-Carvalho, J.L.; Cartafina, R.A.; Guimarães, G.M.; Brandão, P.R.P.; Lang, J.A.; Vianna, L.C. Baroreflex function in Parkinson’s disease: Insights from the modified-Oxford technique. J. Neurophysiol. 2020, 124, 1144–1151. [Google Scholar] [CrossRef]
- Sabino-Carvalho, J.L.; Falquetto, B.; Takakura, A.C.; Vianna, L.C. Baroreflex dysfunction in Parkinson’s disease: Integration of central and peripheral mechanisms. J. Neurophysiol. 2021, 125, 1425–1439. [Google Scholar] [CrossRef]
- Kaufmann, H.; Goldstein, D.S. Autonomic dysfunction in Parkinson disease. Handb. Clin. Neurol. 2013, 117, 259–278. [Google Scholar] [CrossRef]
- Velseboer, D.C.; de Haan, R.J.; Wieling, W.; Goldstein, D.S.; de Bie, R.M. Prevalence of orthostatic hypotension in Parkinson’s disease: A systematic review and meta-analysis. Park. Relat. Disord. 2011, 17, 724–729. [Google Scholar] [CrossRef]
- Chen, Z.; Li, G.; Liu, J. Autonomic dysfunction in Parkinson’s disease: Implications for pathophysiology, diagnosis, and treatment. Neurobiol. Dis. 2020, 134, 104700. [Google Scholar] [CrossRef]
- Freeman, R.; Wieling, W.; Axelrod, F.B.; Benditt, D.G.; Benarroch, E.; Biaggioni, I.; Cheshire, W.P.; Chelimsky, T.; Cortelli, P.; Gibbons, C.H.; et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin. Auton. Res. 2011, 21, 69–72. [Google Scholar] [CrossRef]
- Freeman, R.; Abuzinadah, A.R.; Gibbons, C.; Jones, P.; Miglis, M.G.; Sinn, D.I. Orthostatic Hypotension: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 1294–1309. [Google Scholar] [CrossRef]
- Freeman, R. Neurogenic orthostatic hypotension. N. Engl. J. Med. 2008, 358, 615–624. [Google Scholar] [CrossRef]
- Masaki, K.H.; Schatz, I.J.; Burchfiel, C.M.; Sharp, D.S.; Chiu, D.; Foley, D.; Curb, J.D. Orthostatic hypotension predicts mortality in elderly men: The Honolulu Heart Program. Circulation 1998, 98, 2290–2295. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ferro, A.; Benito-León, J.; Gómez-Esteban, J.C. The management of orthostatic hypotension in Parkinson’s disease. Front. Neurol. 2013, 4, 00064. [Google Scholar] [CrossRef] [PubMed]
- Cani, I.; Giannini, G.; Guaraldi, P.; Barletta, G.; Sambati, L.; Baldelli, L.; Cortelli, P.; Calandra-Buonaura, G. Exploring Cardiovascular Autonomic Function before and after Chronic Deep Brain Stimulation in Parkinson’s Disease. Mov. Disord. Clin. Pract. 2024, 11, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.; Sayad, R.; Kedwany, A.M.; Sayed, H.H.; Caprara, A.L.F.; Rissardo, J.P. Cardiovascular dysautonomia and cognitive impairment in Parkinson’s disease (Review). Med. Int. 2024, 4, 70. [Google Scholar] [CrossRef]
- Barbic, F.; Galli, M.; Dalla Vecchia, L.; Canesi, M.; Cimolin, V.; Porta, A.; Bari, V.; Cerri, G.; Dipaola, F.; Bassani, T.; et al. Effects of mechanical stimulation of the feet on gait and cardiovascular autonomic control in Parkinson’s disease. J. Appl. Physiol. 2014, 116, 495–503. [Google Scholar] [CrossRef]
- Zamunér, A.R.; Shiffer, D.; Barbic, F.; Minonzio, M.; Andrade, C.P.; Corato, M.; Lalli, S.; Dipaola, F.; Cairo, B.; Albanese, A.; et al. Mechanical somatosensory stimulation decreases blood pressure in patients with Parkinson’s disease. J. Hypertens. 2019, 37, 1714–1721. [Google Scholar] [CrossRef]
- Piterà, P.; Cremascoli, R.; Bianchi, L.; Borghesi, F.; Verme, F.; Cattaldo, S.; Prina, E.; Mai, S.; Cipresso, P.; Galli, F.; et al. Autonomic Modulation in Parkinson’s Disease Using Whole-Body Cryostimulation: A Pilot Study. Biomedicines 2024, 12, 2467. [Google Scholar] [CrossRef]
- Gerasimova-Meigal, L.; Meigal, A.; Sireneva, N.; Saenko, I. Autonomic Function in Parkinson’s Disease Subjects Across Repeated Short-Term Dry Immersion: Evidence From Linear and Non-linear HRV Parameters. Front. Physiol. 2021, 12, 712365. [Google Scholar] [CrossRef]
- Ganesan, M.; Pal, P.K.; Gupta, A.; Sathyaprabha, T.N. Treadmill gait training improves baroreflex sensitivity in Parkinson’s disease. Clin. Auton. Res. 2014, 24, 111–118. [Google Scholar] [CrossRef]
- Kanegusuku, H.; Silva-Batista, C.; Peçanha, T.; Nieuwboer, A.; Silva, N.D., Jr.; Costa, L.A.; de Mello, M.T.; Piemonte, M.E.; Ugrinowitsch, C.; Forjaz, C.L. Effects of Progressive Resistance Training on Cardiovascular Autonomic Regulation in Patients With Parkinson Disease: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2017, 98, 2134–2141. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Lai, Y.-R.; Wu, F.-A.; Kuo, N.-Y.; Tsai, Y.-C.; Cheng, B.-C.; Tsai, N.-W.; Lu, C.-H. Simultaneously Improved Pulmonary and Cardiovascular Autonomic Function and Short-Term Functional Outcomes in Patients with Parkinson’s Disease after Respiratory Muscle Training. J. Clin. Med. 2020, 9, 316. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Lai, Y.-R.; Wu, F.-A.; Kuo, N.-Y.; Cheng, B.-C.; Tsai, N.-W.; Kung, C.-T.; Chiang, Y.-F.; Lu, C.-H. Detraining effect on pulmonary and cardiovascular autonomic function and functional outcomes in patients with Parkinson’s disease after respiratory muscle training: An 18-month follow-up study. Front. Neurol. 2021, 12, 735847. [Google Scholar] [CrossRef] [PubMed]
- Van der Stam, A.H.; Shmuely, S.; de Vries, N.M.; Thijs, R.D.; van Kesteren-Biegstraaten, M.; Bloem, B.R. Head-Up Tilt Sleeping to Treat Orthostatic Intolerance in a Patient with Advanced Parkinson’s Disease: A Case Report. Case Rep. Neurol. 2024, 16, 256–261. [Google Scholar] [CrossRef]
- Meigal, A.Y.; Tretyakova, O.G.; Gerasimova-Meigal, L.I.; Saenko, I.V. Vertical Spatial Orientation in Patients with Parkinsonism under the State of Single “Dry” Immersion and a Course of Immersions. Hum. Physiol. 2021, 47, 183–192. [Google Scholar] [CrossRef]
- Bernardi, L.; Bissa, M.; DeBarbieri, G.; Bharadwaj, A.; Nicotra, A. Arterial baroreflex modulation influences postural sway. Clin. Auton. Res. 2011, 21, 151–160. [Google Scholar] [CrossRef]
- Rau, H.; Brody, S.; Brunia, C.H.M.; Damen, E.; Elbert, T. Activation of carotid baroreceptors inhibits spinal reflexes in man. Electroencephalogr. Clin. Neurophysiol./Evoked Potentials Sect. 1993, 89, 328–334. [Google Scholar] [CrossRef][Green Version]
- Louis, J.; Theurot, D.; Filliard, J.R.; Volondat, M.; Dugué, B.; Dupuy, O. The use of whole-body cryotherapy: Time- and dose-response investigation on circulating blood catecholamines and heart rate variability. Eur. J. Appl. Physiol. 2020, 120, 1733–1743. [Google Scholar] [CrossRef]
- Leppäluoto, J.; Westerlund, T.; Huttunen, P.; Oksa, J.; Smolander, J.; Dugué, B.; Mikkelsson, M. Effects of long-term whole-body cold exposures on plasma concentrations of ACTH, beta-endorphin, cortisol, catecholamines and cytokines in healthy females. Scand. J. Clin. Lab. Investig. 2008, 68, 145–153. [Google Scholar] [CrossRef]
- Gerasimova-Meigal, L.I.; Sireneva, N.V.; Meigal, A.Y. Estimation of the Effect of the Course of Short-Term Sessions of Dry Immersion on Autonomic Regulation in Patients with Parkinsonism. Hum. Physiol. 2021, 47, 404–409. [Google Scholar] [CrossRef]
- Rocha, R.S.B.; De Oliveira Rocha, L.S.; Pena, E.S.M.; Caldas, L.C.P.; Moreno, M.A. Analysis of autonomic modulation of heart rate in patients with Parkinson’s disease and elderly individuals submitted to game therapy training. Geriatr. Gerontol. Int. 2018, 18, 20–25. [Google Scholar] [CrossRef]
- Ganesan, M.; Sathyaprabha, T.N.; Pal, P.K.; Gupta, A. Partial Body Weight-Supported Treadmill Training in Patients With Parkinson Disease: Impact on Gait and Clinical Manifestation. Arch. Phys. Med. Rehabil. 2015, 96, 1557–1565. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Chan, J.; Wu, Y.P.; Bernard, J.R.; Liao, Y.H. Effect of expiratory muscle strength training intervention on the maximum expiratory pressure and quality of life of patients with Parkinson disease. NeuroRehabilitation 2017, 41, 219–226. [Google Scholar] [CrossRef]
- Pitts, T.; Bolser, D.; Rosenbek, J.; Troche, M.; Okun, M.S.; Sapienza, C. Impact of expiratory muscle strength training on voluntary cough and swallow function in Parkinson disease. Chest 2009, 135, 1301–1308. [Google Scholar] [CrossRef]
- Troche, M.S.; Okun, M.S.; Rosenbek, J.C.; Musson, N.; Fernandez, H.H.; Rodriguez, R.; Romrell, J.; Pitts, T.; Wheeler-Hegland, K.M.; Sapienza, C.M. Aspiration and swallowing in Parkinson disease and rehabilitation with EMST: A randomized trial. Neurology 2010, 75, 1912–1919. [Google Scholar] [CrossRef]
- Reyes, A.; Castillo, A.; Castillo, J.; Cornejo, I. The effects of respiratory muscle training on peak cough flow in patients with Parkinson’s disease: A randomized controlled study. Clin. Rehabil. 2018, 32, 1317–1327. [Google Scholar] [CrossRef]
- Reyes, A.; Castillo, A.; Castillo, J.; Cornejo, I.; Cruickshank, T. The Effects of Respiratory Muscle Training on Phonatory Measures in Individuals with Parkinson’s Disease. J. Voice 2020, 34, 894–902. [Google Scholar] [CrossRef]
- Saleem, A.F.; Sapienza, C.M.; Okun, M.S. Respiratory muscle strength training: Treatment and response duration in a patient with early idiopathic Parkinson’s disease. NeuroRehabilitation 2005, 20, 323–333. [Google Scholar] [CrossRef]
- Rodrigues, G.D.; Dal Lago, P.; da Silva Soares, P.P. Time-dependent effects of inspiratory muscle training and detraining on cardiac autonomic control in older women. Exp. Gerontol. 2021, 150, 111357. [Google Scholar] [CrossRef]
- Omboni, S.; Smit, A.A.; van Lieshout, J.J.; Settels, J.J.; Langewouters, G.J.; Wieling, W. Mechanisms underlying the impairment in orthostatic tolerance after nocturnal recumbency in patients with autonomic failure. Clin. Sci. 2001, 101, 609–618. [Google Scholar] [CrossRef]
- Van Lieshout, J.J.; ten Harkel, A.D.; Wieling, W. Fludrocortisone and sleeping in the head-up position limit the postural decrease in cardiac output in autonomic failure. Clin. Auton. Res. 2000, 10, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Boscan, P.; Pickering, A.E.; Paton, J.F. The nucleus of the solitary tract: An integrating station for nociceptive and cardiorespiratory afferents. Exp. Physiol. 2002, 87, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Toney, G.M.; Mifflin, S.W. Sensory modalities conveyed in the hindlimb somatic afferent input to nucleus tractus solitarius. J. Appl. Physiol. 2000, 88, 2062–2073. [Google Scholar] [CrossRef] [PubMed]
- Werner, W.G.; DiFrancisco-Donoghue, J.; Lamberg, E.M. Cardiovascular response to treadmill testing in Parkinson disease. J. Neurol. Phys. Ther. 2006, 30, 68–73. [Google Scholar] [CrossRef]
- Sugawara, J.; Tomoto, T. Effects of short-term warm water immersion on cardiac baroreflex sensitivity in healthy men. J. Physiol. Sci. 2020, 70, 34. [Google Scholar] [CrossRef]
- Yu, L.; De Mazancourt, M.; Hess, A.; Ashadi, F.R.; Klein, I.; Mal, H.; Courbage, M.; Mangin, L. Functional connectivity and information flow of the respiratory neural network in chronic obstructive pulmonary disease. Hum. Brain Mapp. 2016, 37, 2736–2754. [Google Scholar] [CrossRef]
- Joosten, S.A.; O’Driscoll, D.M.; Berger, P.J.; Hamilton, G.S. Supine position related obstructive sleep apnea in adults: Pathogenesis and treatment. Sleep Med. Rev. 2014, 18, 7–17. [Google Scholar] [CrossRef]
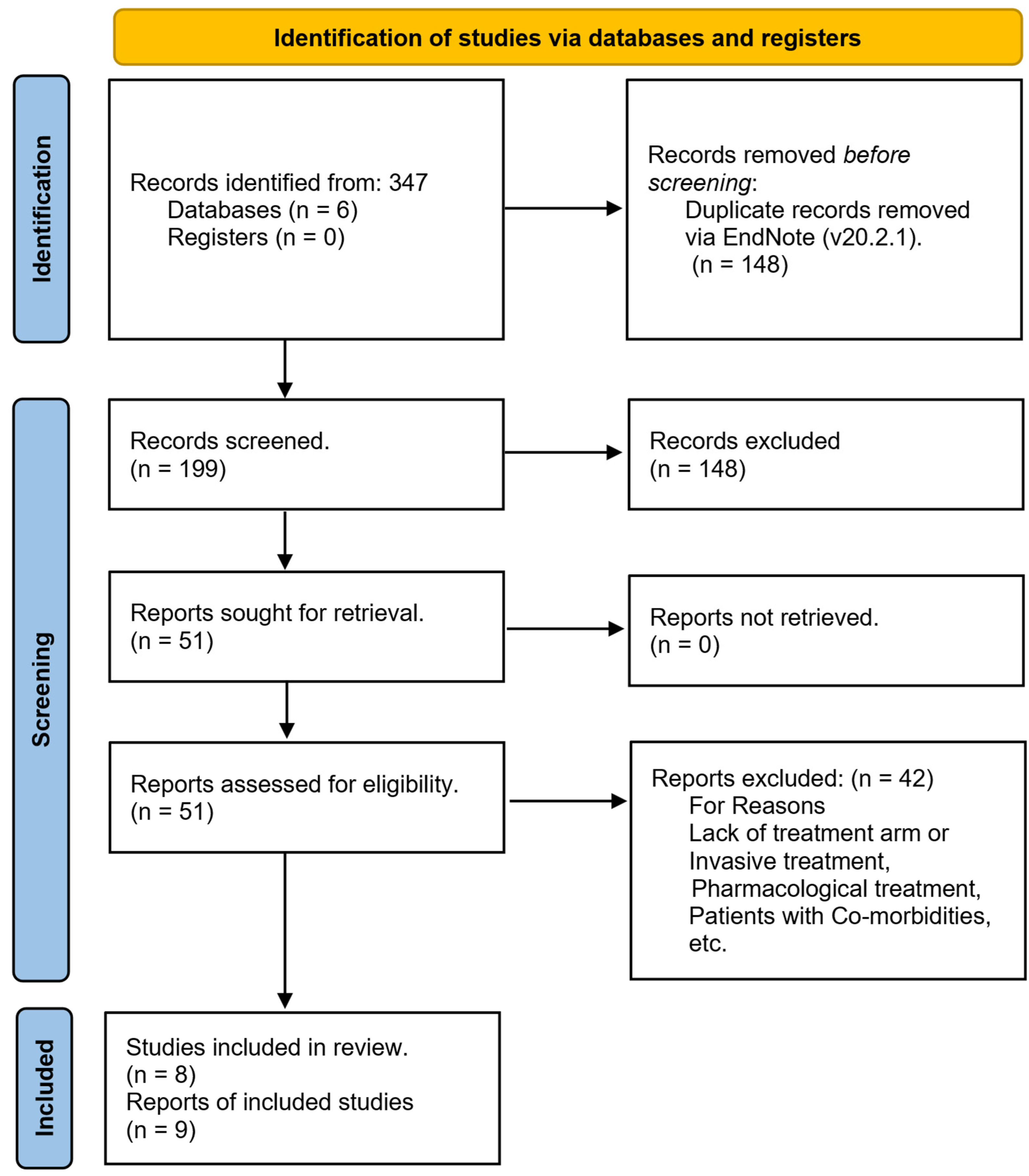

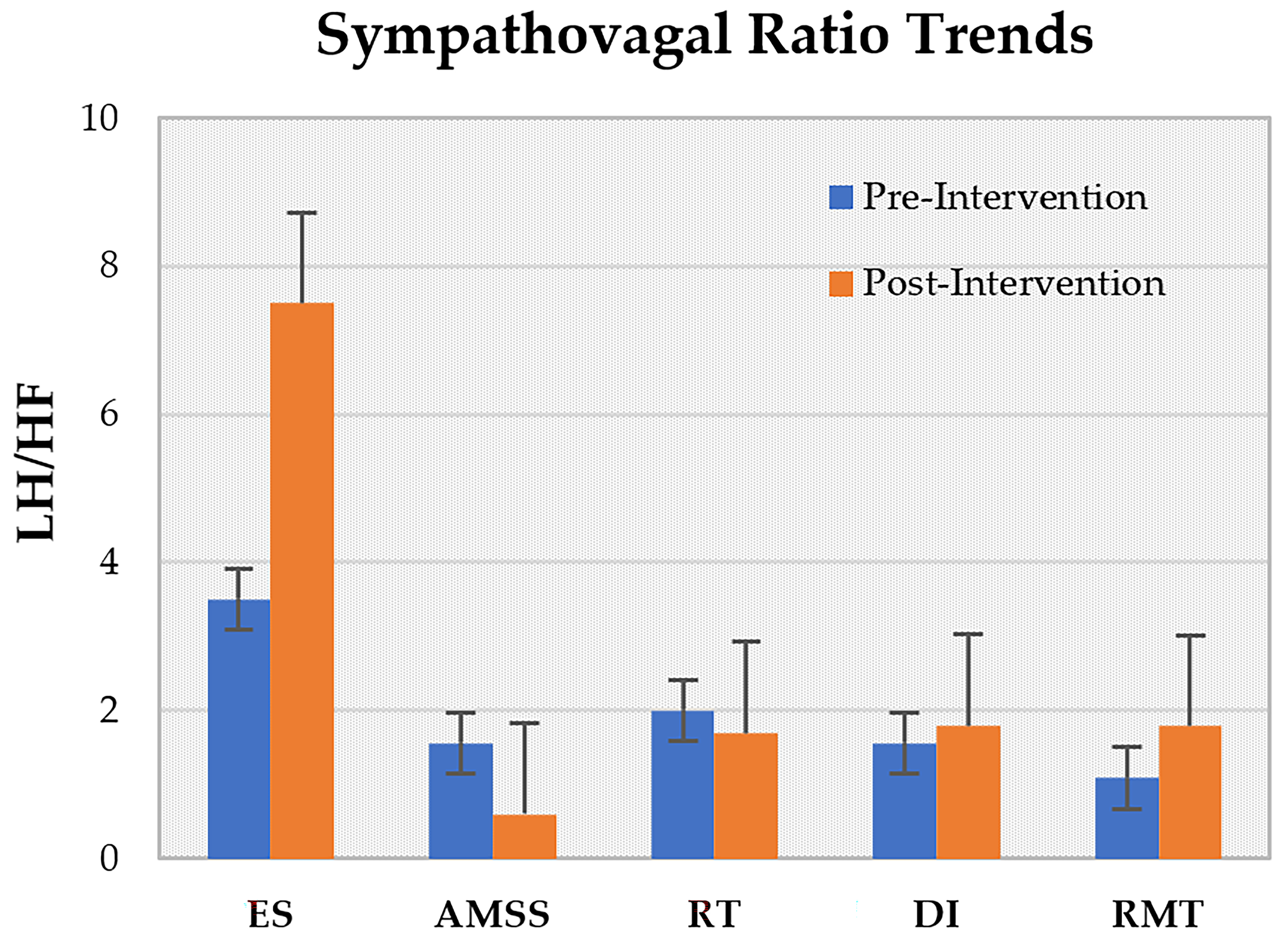
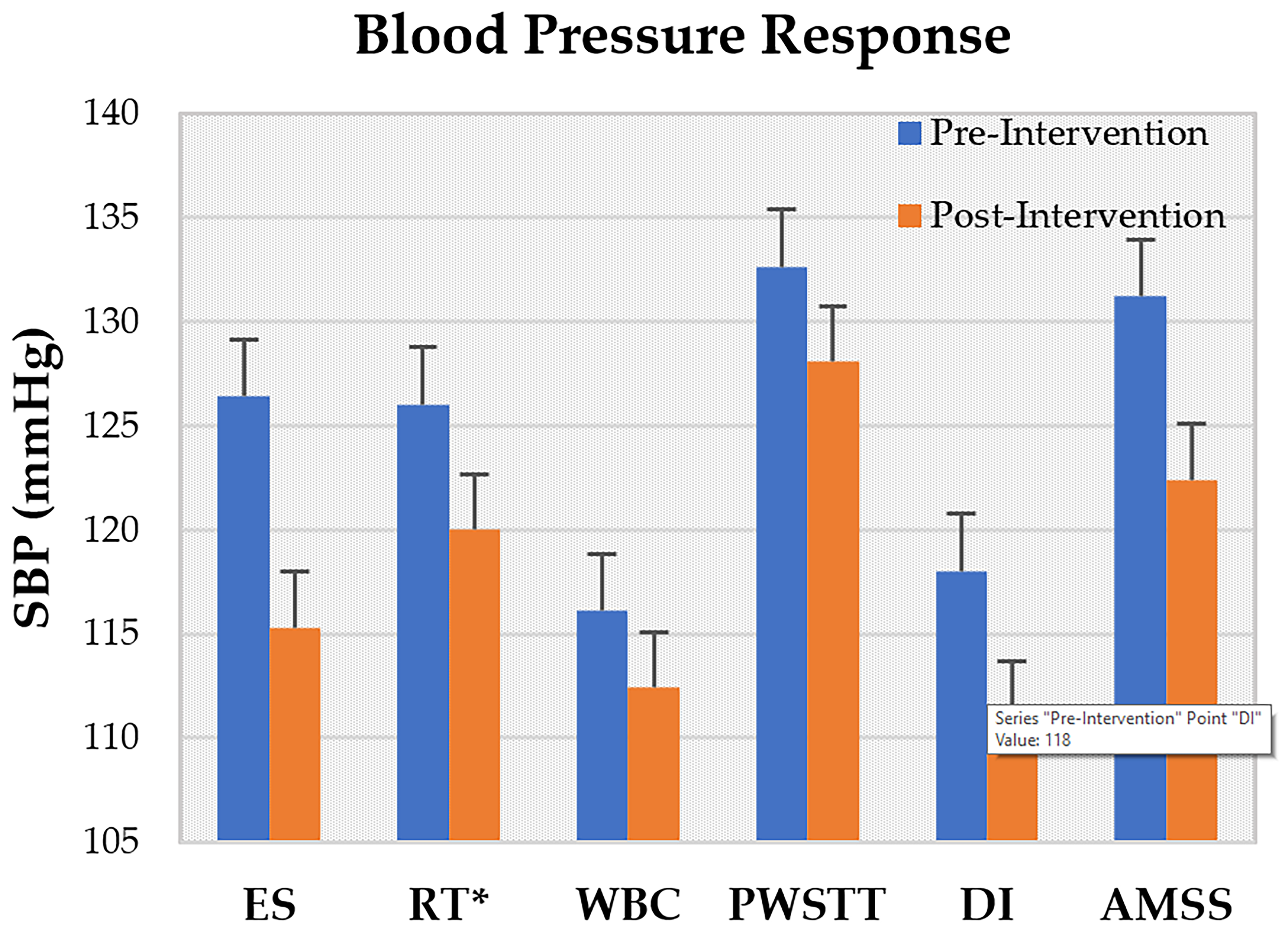
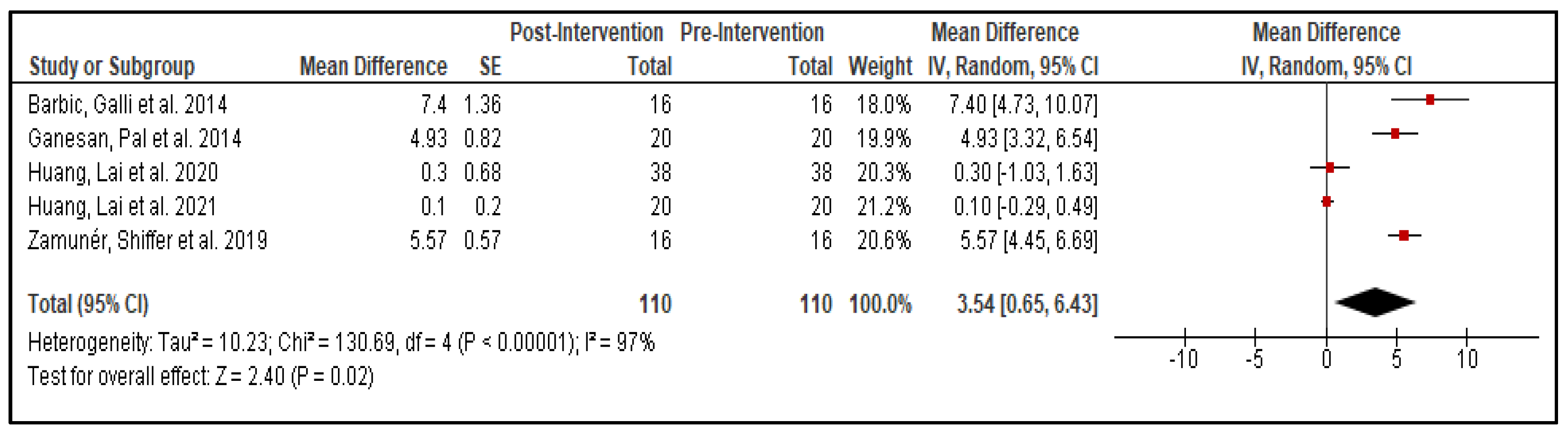
| Author, Year | No. of Participants | Drop Out | Gender (M/F) | Mean Age (Year) | Disease Duration (Year) | BMI (Kg/m2) | Hoehn and Yahr Scale |
|---|---|---|---|---|---|---|---|
| 1. Barbic, Galli et al., 2014 [16] | 16 | NA | 8/8 | 66 ± 3 | 13 ± 1 | 23 ± 1 | 2–3 |
| 2. Kanegusuku, Silva-Batista et al., 2017 [21] | 30 | 3 | 22/5 | 65 ± 8 | 8.7 ± 4.7 | 25.6 ± 3.6 | 2–3 |
| 3. Piterà, Cremascoli et al., 2024 [18] | 17 | 4 | 6/7 | 64.5 ± 9 | 5.4 ± 7.3 | 26.18 ± 3.91 | 1–3 |
| 4. Ganesan, Pal et al., 2014 [20] | 60 | NA | 46/14 | 58.15 ± 8.7 | 5.3 ± 3.4 | 23.57 ± 3.92 | 1–3 |
| 5. Gerasimova-Meigal, Meigal et al., 2021 [19] | 20 | NA | 13/7 | 61 ± 6 | 4.5 ± 1.1 | 27.42 ± 3.7 | 1–3 |
| 6. Huang, Lai et al., 2020 [22] | 75 | NA | 29/33 | 64.1 ± 9.9 | 5.4 ± 4.4 | 24.2 ± 4.35 | 2–3 |
| 7. Huang, Lai et al., 2021 [23] | 75 | 23 | 23/29 | 64.9 ± 9.85 | 5.25 ± | NA | 2–3 |
| 8. Zamunér, Shiffer et al., 2019 [17] | 23 | 7 | 6/10 | 66.2 ± 9.4 | 7 ± 3.5 | 24.2 ± 2.8 | 2–4 |
| 9. van der Stam, Shmuely et al., 2024 [24] | 1 | NA | M | 69.00 | 10 | NA | 2 |
| Author, Year | Study Design | Intervention | Length of Delivery | No. and Frequency of Sessions | Each Session Time | Quality Rating * |
|---|---|---|---|---|---|---|
| 1. Barbic, Galli et al., 2014 [16] | Randomized Clinical Trial | ES | 2 min | 4; 4 | 6 s | Moderate |
| 2. Kanegusuku, Silva-Batista et al., 2017 [21] | Randomized Clinical trial | RT | 12 weeks | 24; 2 days/week | NA | High |
| 3. Piterà, Cremascoli et al., 2024 [18] | Pilot Study | WBC | 1 week | 10; 2/day | 2 min | Moderate |
| 4. Ganesan, Pal et al., 2014 [20] | Randomized Clinical Trial | PWSTT | 4 weeks | 16; 4 days/week | 30 min | High |
| 5. Gerasimova-Meigal, Meigal et al., 2021 [19] | Controlled Clinical Trial | DI | 4 weeks | 7; 2/week | 45 min | Moderate |
| 6. Huang, Lai et al., 2020 [22] | Prospective Case–Control study | RMT | 12 weeks | 120; 2/day | 30 min | Moderate |
| 7. Huang, Lai et al., 2021 [23] | Prospective Case–Control study | RMT | 12 weeks | 120; 2/day | 30 min | Moderate |
| 8. Zamunér, Shiffer et al., 2019 [17] | Interventional Model | AMSS | 12 days | 5; 2/week | NA | Moderate |
| 9. van der Stam, Shmuely et al., 2024 [24] | Case Report | HUTS | ∞ | ∞; 1/day | NA | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suthar, A.; Zemmar, A.; Krassioukov, A.; Ovechkin, A. Enhancing Cardiovascular Autonomic Regulation in Parkinson’s Disease Through Non-Invasive Interventions. Life 2025, 15, 1244. https://doi.org/10.3390/life15081244
Suthar A, Zemmar A, Krassioukov A, Ovechkin A. Enhancing Cardiovascular Autonomic Regulation in Parkinson’s Disease Through Non-Invasive Interventions. Life. 2025; 15(8):1244. https://doi.org/10.3390/life15081244
Chicago/Turabian StyleSuthar, Aastha, Ajmal Zemmar, Andrei Krassioukov, and Alexander Ovechkin. 2025. "Enhancing Cardiovascular Autonomic Regulation in Parkinson’s Disease Through Non-Invasive Interventions" Life 15, no. 8: 1244. https://doi.org/10.3390/life15081244
APA StyleSuthar, A., Zemmar, A., Krassioukov, A., & Ovechkin, A. (2025). Enhancing Cardiovascular Autonomic Regulation in Parkinson’s Disease Through Non-Invasive Interventions. Life, 15(8), 1244. https://doi.org/10.3390/life15081244








