Dual-Task Gait Analysis: Combined Cognitive–Motor Demands Most Severely Impact Walking Patterns and Joint Kinematics
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Protocol
2.3. Statistical Analysis
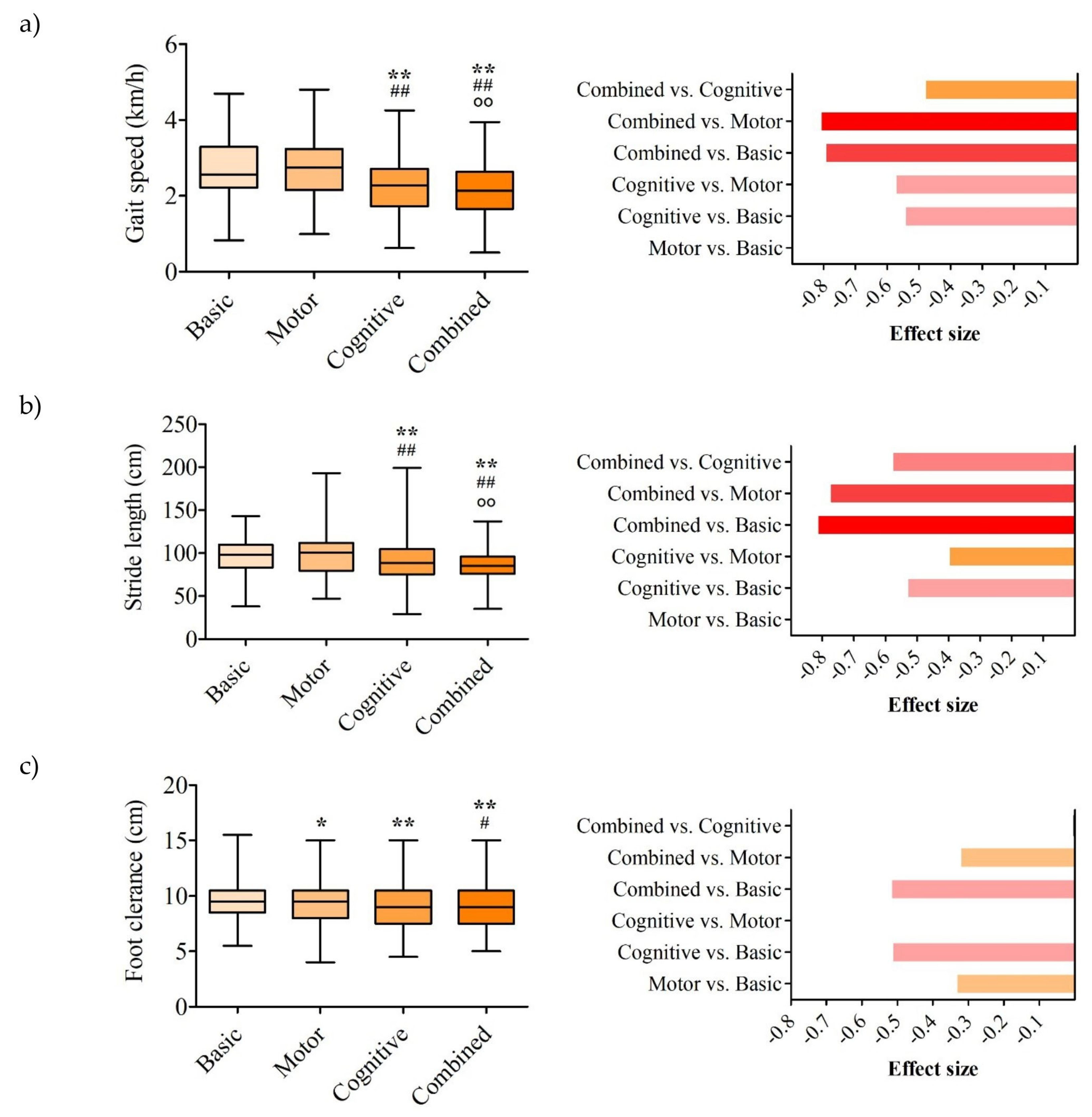
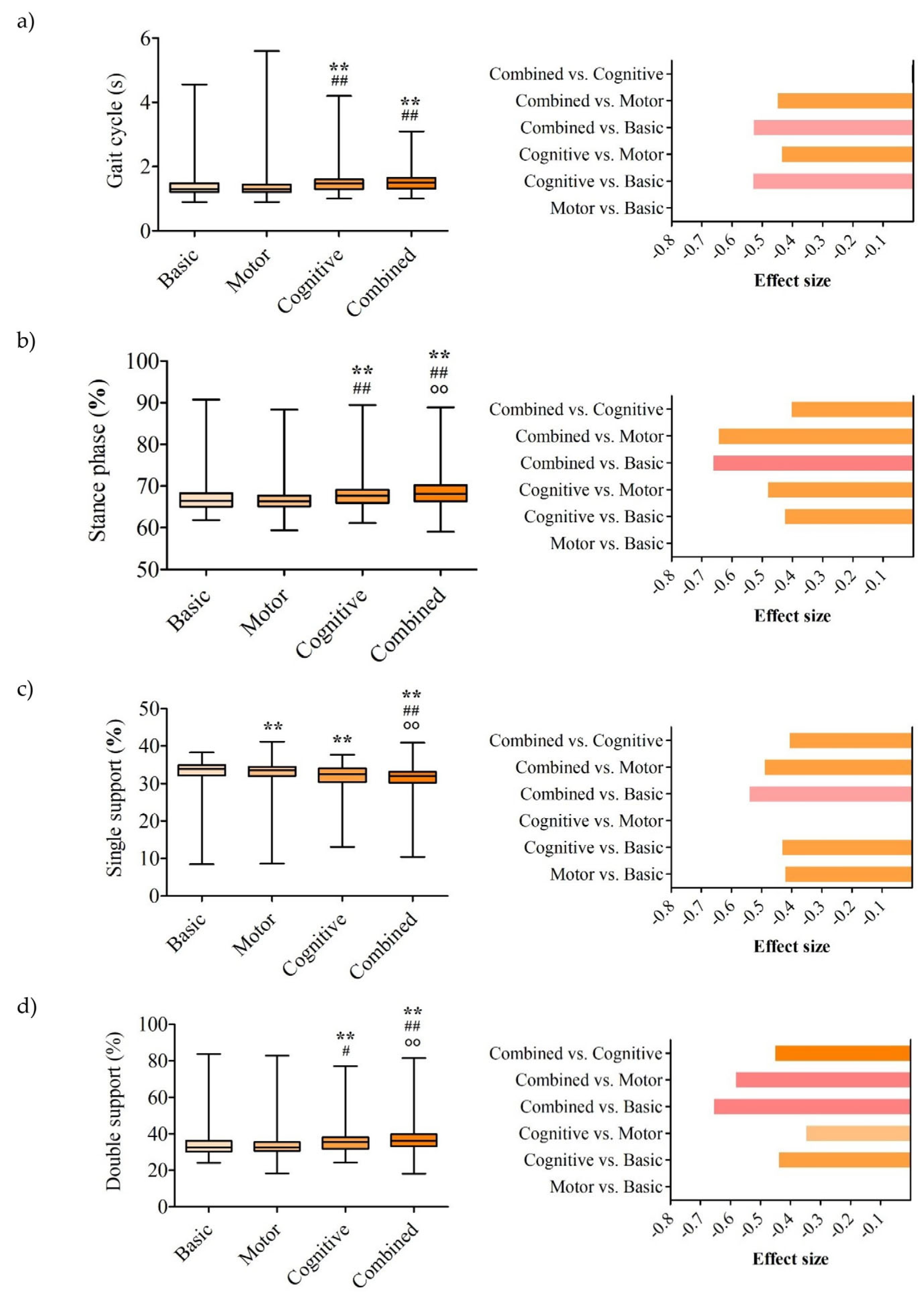
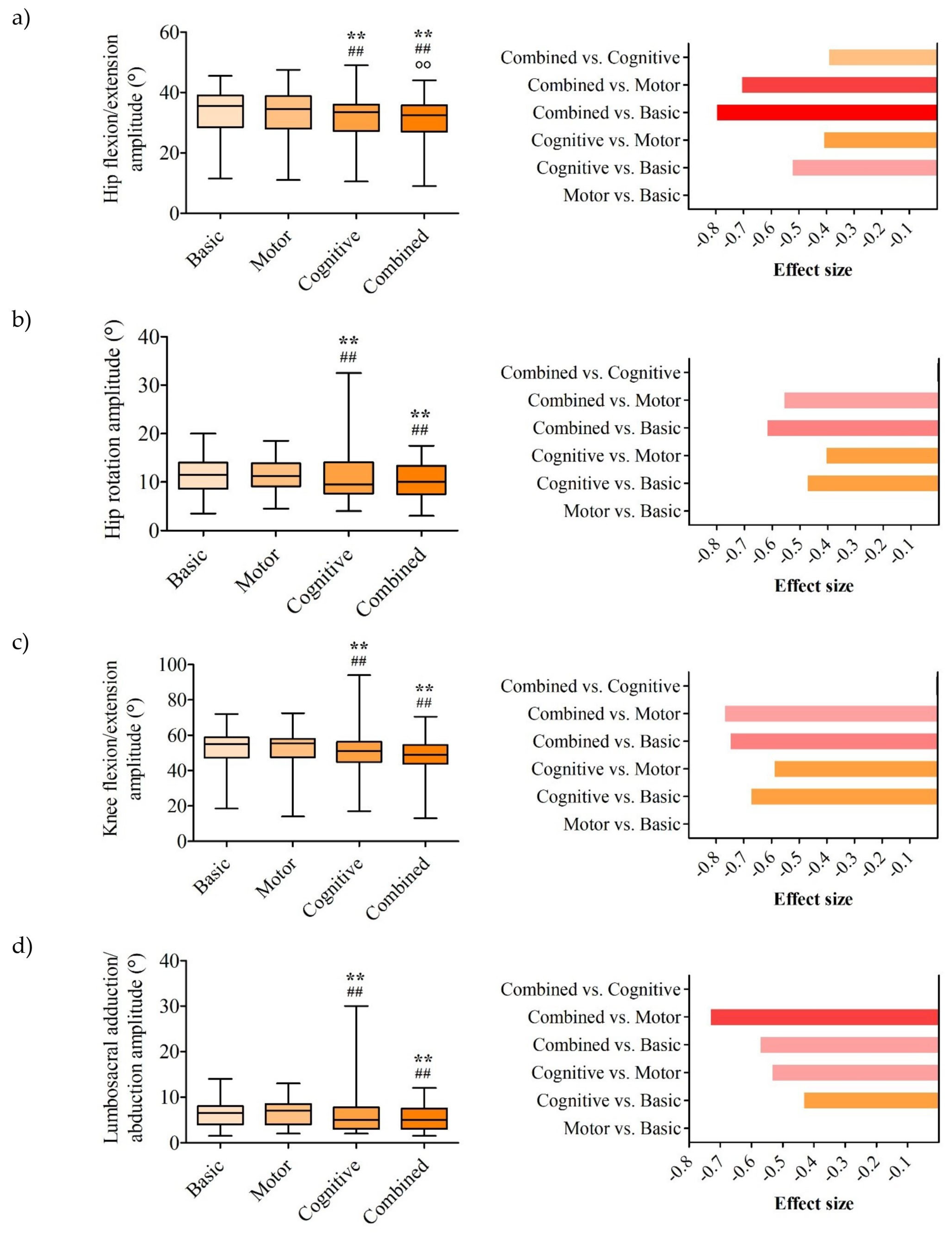
3. Results
3.1. Spatiotemporal Parameters
3.2. Kinematic Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SD | Standard Deviation |
| ROM | Range of Motion |
| CoM | Center of Mass |
| IMU | Inertial Measurement Unit |
References
- Pirker, W.; Katzenschlager, R. Gait disorders in adults and the elderly: A clinical guide. Wien. Klin. Wochenschr. 2017, 129, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Bortone, I.; Griseta, C.; Battista, P.; Castellana, F.; Lampignano, L.; Zupo, R.; Sborgia, G.; Lozupone, M.; Moretti, B.; Giannelli, G.; et al. Physical and cognitive profiles in motoric cognitive risk syndrome in an older population from southern Italy. Eur. J. Neurol. 2021, 28, 2565–2573. [Google Scholar] [CrossRef] [PubMed]
- Whittle, M. Gait Analysis, 5th ed.; Churchill Livingstone: London, UK, 2012. [Google Scholar]
- Callisaya, M.L.; Blizzard, L.; Schmidt, M.D.; McGinley, J.L.; Srikanth, V.K. Ageing and gait variability—A population-based study of older people. Age Ageing 2010, 39, 191–197. [Google Scholar] [CrossRef]
- Fritz, S.; Lusardi, M. White paper: “Walking speed: The sixth vital sign”. J. Ger. Phys. Ther. 2009, 32, 46–49. [Google Scholar] [CrossRef]
- Ble, A.; Volpato, S.; Zuliani, G.; Guralnik, J.M.; Bandinelli, S.; Lauretani, F.; Bartali, B.; Maraldi, C.; Fellin, R.; Ferrucci, L. Executive function correlates with walking speed in older persons: The InCHIANTI study. J. Am. Geriatr. Soc. 2005, 53, 410–415. [Google Scholar] [CrossRef]
- Martin, L.K.; Blizzard, L.; Wood, G.A.; Srikanth, V.; Thomson, R.; Sanders, M.L.; Callisaya, L.M. Cognitive Function, Gait, and Gait Variability in Older People: A Population-Based Study. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Ghai, S.; Ghai, I.; Effenberg, A.O. Effects of Dual Tasks and Dual-Task Training on Postural Stability: A Systematic Review and Meta-Analysis. Clin. Interv. Aging 2017, 12, 557–577. [Google Scholar] [CrossRef]
- Bridenbaugh, S.A.; Kressig, R.W. Motor cognitive dual tasking: Early detection of gait impairment, fall risk and cognitive decline. Z. Gerontol. Geriatr. 2015, 48, 15–21. [Google Scholar] [CrossRef]
- Yogev-Seligmann, G.; Hausdorff, J.M.; Giladi, N. The role of executive function and attention in gait. Mov. Disord. 2008, 23, 329–342. [Google Scholar] [CrossRef]
- Priest, A.W.; Salamon, K.B.; Hollman, J.H. Age-related differences in dual task walking: A cross sectional study. J. Neuroeng. Rehabil. 2008, 14, 5–29. [Google Scholar] [CrossRef]
- Hofheinz, M.; Schusterschitz, C. Dual task interference in estimating the risk of falls and measuring change: A comparative, psychometric study of four measurements. Clin. Rehab. 2010, 24, 831–842. [Google Scholar] [CrossRef]
- Martínez-Ramírez, A.; Martinikorena, I.; Lecumberri, P.; Gómez, M.; Millor, N.; Casas-Herrero, A.; Zambom-Ferraresi, F.; Izquierdo, M. Dual task gait performance in frail individuals with and without mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 2016, 42, 7–16. [Google Scholar] [CrossRef]
- Hollman, J.H.; Kovash, M.F.; Kubik, J.J.; Linbo, A.R. Age-related differences in spatiotemporal markers of gait stability during dual task walking. Gait Posture 2007, 26, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, B.W.; Brooks, D.M. Comparative Analysis of Optoelectronic Accuracy in the Laboratory Setting Versus Clinical Operative Environment: A Systematic Review. Glob. Spine J. 2022, 12 (Suppl. S2), 59S–74S. [Google Scholar] [CrossRef]
- Scataglini, S.; Abts, E.; Van Bocxlaer, C.; Van den Bussche, M.; Meletani, S.; Truijen, S. Accuracy, Validity, and Reliability of Markerless Camera-Based 3D Motion Capture Systems versus Marker-Based 3D Motion Capture Systems in Gait Analysis: A Systematic Review and Meta-Analysis. Sensors 2024, 24, 3686. [Google Scholar] [CrossRef]
- Camomilla, V.; Cappozzo, A.; Vannozzi, G. Handbook of Human Motion; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Ribeiro, N.F.; Santos, C.P. Inertial Measurement Units: A Brief State of the Art on Gait Analysis. In Proceedings of the 2017 IEEE 5th Portuguese Meeting on Bioengineering (ENBENG), Coimbra, Portugal, 16–18 February 2017; IEEE: Lisbon, Portugal, 2017; pp. 1–4. [Google Scholar]
- Skvortsov, D.; Kaurkin, S.; Akhpashev, A.; Altukhova, A.; Troitskiy, A.; Zagorodniy, N. Gait Analysis and Knee Kinematics in Patients with Anterior Cruciate Ligament Rupture: Before and After Reconstruction. Appl. Sci. 2020, 10, 3378. [Google Scholar] [CrossRef]
- de Gomes, G.; Teixeira-Salmela, L.F.; de Freitas, F.A.S.; Fonseca, M.L.M.; de Pinheiro, B.; de Morais, V.A.C.; Caramelli, P. Gait Performance of the Elderly Under Dual-Task Conditions: Review of Instruments Employed and Kinematic Parameters. Rev. Bras. Geriatr. Gerontol. 2016, 19, 165–182. [Google Scholar] [CrossRef][Green Version]
- Radovanović, S.; Milićev, M.; Perić, S.; Basta, I.; Kostić, V.; Stević, Z. Gait in amiotrophic lateral sclerosis: Is gait pattern differently affected in spinal and bulbar onset of the disease during dual task walking? Amyotroph. Lateral Scler. Front. Degener. 2014, 15, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Beauchet, O.; Dubost, V.; Aminian, K.; Gonthier, R.; Kressig, R.W. Dual-task-related gait changes in the elderly: Does the type of cognitive task matter? J. Mot. Behav. 2005, 37, 259–264. [Google Scholar]
- Yogev-Seligmann, G.; Giladi, N.; Brozgol, M.; Hausdorff, J.M. A training program to improve gait while dual tasking in patients with Parkinson’s disease: A pilot study. Arch. Phys. Med. Rehabil. 2012, 93, 176–181. [Google Scholar] [CrossRef]
- Asai, T.; Doi, T.; Hirata, S.; Ando, H. Dual tasking affects lateral trunk control in healthy younger and older adults. Gait Posture 2013, 38, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Schulz, B.W. A New Measure of Trip Risk Integrating Minimum Foot Clearance and Dynamic Stability across the Swing Phase of Gait. J. Biomech. 2017, 55, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, L.V.; Hassan, S.A.; Kasawara, K.T.; Reid, W.D. The effect of mental tracking task on spatiotemporal gait parameters in healthy younger and middle- and older aged participants during dual tasking. Exp. Brain Res. 2019, 237, 3123–3132. [Google Scholar] [CrossRef] [PubMed]
- Fallahtafti, F.; Boron, J.B.; Venema, D.M.; Kim, H.J.; Yentes, J.M. Task specificity impacts dual-task interference in older adults. Aging Clin. Exp. Res. 2021, 33, 581–587. [Google Scholar] [CrossRef]
- Springer, S.; Giladi, N.; Peretz, C.; Yogev-Seligmann, G.; Simon, E.S.; Hausdorff, J.M. Dual-tasking effects on gait variability: The role of aging, falls, and executive function. Mov. Disord. 2006, 21, 950–957. [Google Scholar] [CrossRef]
- Hausdorff, J.M.; Schweiger, A.; Herman, T.; Yogev-Seligmann, G.; Giladi, N. Dual-task decrements in gait: Contributing factors among healthy older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 1335–1343. [Google Scholar] [CrossRef]
- Tiedemann, A.; Sherrington, C.; Lord, S.R. Physiological and psychological predictors of walking speed in older community-dwelling people. Gerontology 2005, 51, 390–395. [Google Scholar] [CrossRef]
- Smith, E.; Cusack, T.; Cunningham, C.; Blake, C. The Influence of a Cognitive Dual Task on the Gait Parameters of Healthy Older Adults: A Systematic Review and Meta-Analysis. J. Aging Phys. Act. 2017, 25, 671–686. [Google Scholar] [CrossRef]
- Smith, B.A.; Kubo, M.; Ulrich, B.D. Gait parameter adjustments for walking on a treadmill at preferred, slower, and faster speeds in older adults with Down syndrome. Curr. Gerontol. Geriatr. Res. 2012, 2012, 782671. [Google Scholar] [CrossRef]
- Lempke, L.B.; Oh, J.; Johnson, R.S.; Schmidt, J.D.; Lynall, R.C. Single- Versus Dual-Task Functional Movement Paradigms: A Biomechanical Analysis. J. Sport Rehabil. 2021, 30, 774–785. [Google Scholar] [CrossRef]
- Pinto, C.; Salazar, A.P.; Hennig, E.M.; Kerr, G.; Pagnussat, A.S. Dual-Task Walking Reduces Lower Limb Range of Motion in Individuals with Parkinson’s Disease and Freezing of Gait: But Does It Happen during What Events through the Gait Cycle? PLoS ONE 2020, 15, e0243133. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.; Gonçalves, R.; Santos, J. Age-Related Deficits of Dual-Task Walking: A Review. J. Geriatr. Phys. Ther. 2011, 34, 63–73. [Google Scholar]
- Van Iersel, M.B.; Rispens, P.; Munneke, M.; Bloem, B.R.; Esselink, R.A.; Kessels, R.P. Executive Function and Dual-Task Performance in Older Adults: A Systematic Review. Int. J. Geriatr. Psychiatry 2007, 22, 723–732. [Google Scholar]

| Male (n = 11) | Female (n = 30) | Total (N = 41) | |
|---|---|---|---|
| Age (years) | 68.7 | 71.9 | 71.1 |
| Height (m) | 1.83 | 1.62 | 1.67 |
| Weight (kg) | 81.5 | 71.5 | 73.9 |
| BMI (kg/m2) | 24.4 | 27.5 | 26.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nedović, N.; Mutavdžin Krneta, S.; Jovanović, S.; Vujičić, D.; Kozinc, Ž.; Skvortsov, D. Dual-Task Gait Analysis: Combined Cognitive–Motor Demands Most Severely Impact Walking Patterns and Joint Kinematics. Life 2025, 15, 1009. https://doi.org/10.3390/life15071009
Nedović N, Mutavdžin Krneta S, Jovanović S, Vujičić D, Kozinc Ž, Skvortsov D. Dual-Task Gait Analysis: Combined Cognitive–Motor Demands Most Severely Impact Walking Patterns and Joint Kinematics. Life. 2025; 15(7):1009. https://doi.org/10.3390/life15071009
Chicago/Turabian StyleNedović, Nenad, Slavica Mutavdžin Krneta, Stevan Jovanović, Danilo Vujičić, Žiga Kozinc, and Dmitry Skvortsov. 2025. "Dual-Task Gait Analysis: Combined Cognitive–Motor Demands Most Severely Impact Walking Patterns and Joint Kinematics" Life 15, no. 7: 1009. https://doi.org/10.3390/life15071009
APA StyleNedović, N., Mutavdžin Krneta, S., Jovanović, S., Vujičić, D., Kozinc, Ž., & Skvortsov, D. (2025). Dual-Task Gait Analysis: Combined Cognitive–Motor Demands Most Severely Impact Walking Patterns and Joint Kinematics. Life, 15(7), 1009. https://doi.org/10.3390/life15071009







