Research Progress on the Mechanism of Action of Food-Derived ACE-Inhibitory Peptides
Abstract
1. Introduction
2. Materials and Methods
3. Angiotensin-Converting Enzyme (ACE) and Its Inhibitory Mechanisms

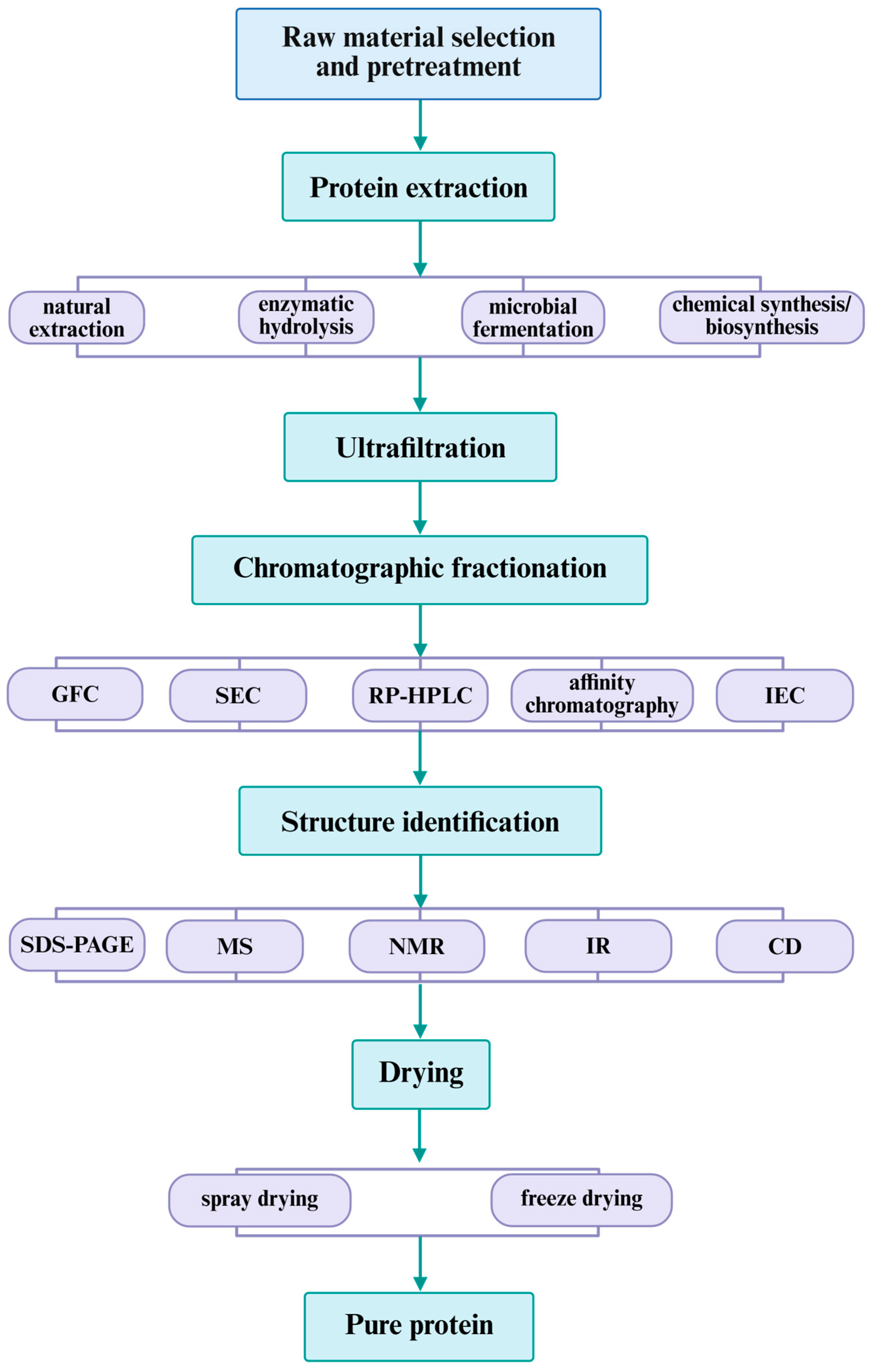
4. Production of Food-Derived ACE-Inhibitory Peptides
4.1. Enzymatic Hydrolysis for Obtaining ACE-Inhibitory Peptides from Various Foods
4.2. Fermentation Process
5. Isolation, Purification, and Sequencing of Peptides
6. Structure–Activity Relationship
7. Gastrointestinal Digestive Stability and Bioavailability of ACE-Inhibitory Peptides
7.1. In Vitro Studies
7.2. In Vivo Studies
8. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Report on Hypertension: The Race Against a Silent Killer; World Health Organization: Geneva, Switzerland, 2023; ISBN 978-92-4-008106-2.
- Giles, T.D.; Berk, B.C.; Black, H.R.; Cohn, J.N.; Kostis, J.B.; Izzo, J.L., Jr.; Weber, M.A. Expanding the Definition and Classification of Hypertension. J. Clin. Hypertens. 2005, 7, 505–512. [Google Scholar] [CrossRef]
- Leoncini, G.; Viazzi, F.; De Cosmo, S.; Russo, G.; Fioretto, P.; Pontremoli, R. Blood pressure reduction and RAAS inhibition in diabetic kidney disease: Therapeutic potentials and limitations. J. Nephrol. 2020, 33, 949–963. [Google Scholar] [CrossRef]
- Daskaya-Dikmen, C.; Yucetepe, A.; Karbancioglu-Guler, F.; Daskaya, H.; Ozcelik, B. Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from Plants. Nutrients 2017, 9, 316. [Google Scholar] [CrossRef]
- Jang, J.-H.; Jeong, S.-C.; Kim, J.-H.; Lee, Y.-H.; Ju, Y.-C.; Lee, J.-S. Characterisation of a new antihypertensive angiotensin I-converting enzyme inhibitory peptide from Pleurotus cornucopiae. Food Chem. 2011, 127, 412–418. [Google Scholar] [CrossRef]
- Li, Z.; He, Y.; He, H.; Zhou, W.; Li, M.; Lu, A.; Che, T.; Shen, S. Purification identification and function analysis of ACE inhibitory peptide from Ulva prolifera protein. Food Chem. 2023, 401, 134127. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Khan, H.; Haque, S.; Ahmad, S.; Srivastava, N.; Khan, A. Angiotensin-Converting Enzyme and Hypertension: A Systemic Analysis of Various ACE Inhibitors, Their Side Effects, and Bioactive Peptides as a Putative Therapy for Hypertension. J. Renin-Angiotensin-Aldosterone Syst. 2023, 2023, 7890188. [Google Scholar] [CrossRef]
- Flack, J.M.; Novikov, S.V.; Ferrario, C.M. Benefits of adherence to anti-hypertensive drug therapy. Eur. Heart J. 1996, 17, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Cheung, I.W.Y.; Nakayama, S.; Hsu, M.N.K.; Samaranayaka, A.G.P.; Li-Chan, E.C.Y. Angiotensin-I Converting Enzyme Inhibitory Activity of Hydrolysates from Oat (Avena sativa) Proteins by In Silico and In Vitro Analyses. J. Agric. Food Chem. 2009, 57, 9234–9242. [Google Scholar] [CrossRef]
- Hu, Y.-D.; Xi, Q.-H.; Kong, J.; Zhao, Y.-Q.; Chi, C.-F.; Wang, B. Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from the Collagens of Monkfish (Lophius litulon) Swim Bladders: Isolation, Characterization, Molecular Docking Analysis and Activity Evaluation. Mar. Drugs 2023, 21, 516. [Google Scholar] [CrossRef]
- Ghassem, M.; Arihara, K.; Babji, A.S.; Said, M.; Ibrahim, S. Purification and identification of ACE inhibitory peptides from Haruan (Channa striatus) myofibrillar protein hydrolysate using HPLC–ESI-TOF MS/MS. Food Chem. 2011, 129, 1770–1777. [Google Scholar] [CrossRef]
- Ye, S.; Chen, Q.; Li, D.; Zhou, H.; Chen, Y.; Meng, C.; Hong, J. Isolation and identification of novel angiotensin I-converting enzyme (ACE) inhibitory peptides from Pony Seed and evaluation of the inhibitory mechanisms. J. Funct. Foods 2022, 95, 105151. [Google Scholar] [CrossRef]
- Li, Z.; He, H.; Liu, J.; Gu, H.; Fu, C.; Zeb, A.; Che, T.; Shen, S. Preparation and Vasodilation Mechanism of Angiotensin-I-Converting Enzyme Inhibitory Peptide from Ulva prolifera Protein. Mar. Drugs 2024, 22, 398. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Zhang, J.; Sun, B.; Zhang, M.; Zhang, Y.; Li, K.; Zhang, Y. ACE inhibitory activity and gastrointestinal digestion stability of umami peptides IIVFGRQLL from yeast extract. LWT 2024, 203, 116308. [Google Scholar] [CrossRef]
- Farzaneh, S.; Entezari, A.A.; Kiana, B.; Kimia, H.; Zeinab, A.-T.; Ahmad, A.; Saberi, M.R.; Jamshidkhan, C. Characterizing the Binding of Angiotensin Converting Enzyme I Inhibitory Peptide to Human Hemoglobin: Influence of Electromagnetic Fields. Protein Pept. Lett. 2020, 27, 1007–1021. [Google Scholar] [CrossRef]
- Premkumar, J.; Malini, M.; Joshy, A. A critical review on food protein-derived antihypertensive peptides. Drug Invent. Today 2019, 12, 474. [Google Scholar]
- Abachi, S.; Bazinet, L.; Beaulieu, L. Antihypertensive and Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from Fish as Potential Cardioprotective Compounds. Mar. Drugs 2019, 17, 613. [Google Scholar] [CrossRef]
- Devecioglu, D.; Sarıakcalı, B.; Orhan, B.; Daskaya-Dikmen, C.; Ozcelik, B.; Karbancioglu-Guler, F. Releasing angiotensin I-converting enzyme inhibitory (ACE-I) peptides by Yamadazyma spp. in non-fat milk. Int. J. Food Sci. Technol. 2024, 59, 5598–5605. [Google Scholar] [CrossRef]
- Pearman, N.A.; Morris, G.A.; Smith, A.M. Angiotensin-Converting Enzyme (ACE)-Inhibitor Activity of Novel Peptides Derived from Porcine Liver and Placenta. Molecules 2025, 30, 754. [Google Scholar] [CrossRef]
- Chevillard, C.; Jouquey, S.; Bree, F.; Mathieu, M.-N.; Stepniewski, J.P.; Tillement, J.P.; Hamon, G.; Corvol, P. Compared Properties of Trandolapril, Enalapril, and Their Diacid Metabolites. J. Cardiovasc. Pharmacol. 1994, 23, S11. [Google Scholar] [CrossRef]
- Cao, X.; Zhou, H.; Xie, J.; Zhang, Z.; Guo, S.; Luo, J.; Chen, Q.; Meng, C.; Zhang, F.; Hong, J. A novel angiotensin I-converting enzyme (ACE) inhibitory peptide from Wuyi rock tea residue: Preparation, identification, and its potential molecular mechanism. LWT 2025, 216, 117353. [Google Scholar] [CrossRef]
- Dong, J.; Wang, S.; Yin, X.; Fang, M.; Gong, Z.; Wu, Y. Angiotensin I converting enzyme (ACE) inhibitory activity and antihypertensive effects of rice peptides. Food Sci. Hum. Wellness 2022, 11, 1539–1543. [Google Scholar] [CrossRef]
- Zhang, Y.; Cong, J.; Bao, G.; Gu, S.; Wang, X. In vitro gastrointestinal digestion study and identification of novel angiotensin i-converting enzyme inhibitory peptide from broccoli (Brassica oleracea). LWT 2022, 164, 113613. [Google Scholar] [CrossRef]
- Wang, R.; Yun, J.; Wu, S.; Bi, Y.; Zhao, F. Optimisation and Characterisation of Novel Angiotensin-Converting Enzyme Inhibitory Peptides Prepared by Double Enzymatic Hydrolysis from Agaricus bisporus Scraps. Foods 2022, 11, 394. [Google Scholar] [CrossRef] [PubMed]
- Paisansak, S.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Reamtong, O.; Choowongkomon, K.; Karnchanatat, A. Angiotensin-I converting enzyme inhibitory peptide derived from the shiitake mushroom (Lentinula edodes). J. Food Sci. Technol. 2021, 58, 85–97. [Google Scholar] [CrossRef]
- Pekkoh, J.; Kamngoen, A.; Wichaphian, A.; Zin, M.T.; Chaipoot, S.; Yakul, K.; Pathom-aree, W.; Maneechote, W.; Cheirsilp, B.; Khoo, K.S.; et al. Production of ACE inhibitory peptides via ultrasonic-assisted enzymatic hydrolysis of microalgal Chlorella protein: Process improvement, fractionation, identification, and in silico structure-activity relationship. Future Foods 2025, 11, 100548. [Google Scholar] [CrossRef]
- Song, X.; Liu, R.; Mu, Y.; Wang, S.; Su, G. Enhancement of angiotensin converting enzyme (ACE) inhibitory activity of walnut peptides: Insights into the effect of ultrasound on protein structure and peptide function. Food Chem. Adv. 2025, 6, 100931. [Google Scholar] [CrossRef]
- López-Huertas, E.; Rubí-Villegas, J.; Sánchez-Moreno, L.; Nieto, R. Olive Pomace Extract Contains Low Molecular Weight Peptides and Possesses ACE Inhibitory Activity. Int. J. Mol. Sci. 2024, 25, 3962. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, Y.; Zheng, H.; Xiang, X.; Xu, L. A novel angiotensin-I-converting enzyme inhibitory peptide from oyster: Simulated gastro-intestinal digestion, molecular docking, inhibition kinetics and antihypertensive effects in rats. Front. Nutr. 2022, 9, 981163. [Google Scholar] [CrossRef]
- Zhu, W.-Y.; Wang, Y.-M.; Ge, M.-X.; Wu, H.-W.; Zheng, S.-L.; Zheng, H.-Y.; Wang, B. Production, identification, in silico analysis, and cytoprotection on H2O2-induced HUVECs of novel angiotensin-I-converting enzyme inhibitory peptides from Skipjack tuna roes. Front. Nutr. 2023, 10, 1197382. [Google Scholar] [CrossRef]
- Cao, J.; Xiang, B.; Dou, B.; Hu, J.; Zhang, L.; Kang, X.; Lyu, M.; Wang, S. Novel Angiotensin-Converting Enzyme-Inhibitory Peptides Obtained from Trichiurus lepturus: Preparation, Identification and Potential Antihypertensive Mechanism. Biomolecules 2024, 14, 581. [Google Scholar] [CrossRef]
- Han, R.; Tian, J.; Han, Y.; Wang, G.; Zhou, G.; Dai, C.; Wang, C. Crucian Carp-Derived ACE-Inhibitory Peptides with In Vivo Antihypertensive Activity: Insights into Bioactivity, Mechanism, and Safety. Molecules 2025, 30, 2812. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Huang, H.; Shao, Y.; Hao, S.; Li, L.; Wei, Y.; Chen, S.; Zhao, Y. Angiotensin-I-converting enzyme inhibitory peptides from eel (Anguilla japonica) bone collagen: Preparation, identification, molecular docking, and protective function on HUVECs. Front. Nutr. 2024, 11, 1462656. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Zhao, Q.; Wang, D.; Fan, Y.; Shi, Y.; Huang, A. Novel ACE inhibitory, antioxidant and α-glucosidase inhibitory peptides identified from fermented rubing cheese through peptidomic and molecular docking. LWT 2022, 159, 113196. [Google Scholar] [CrossRef]
- Qiu, J.; Xu, M.; Ren, R.; Zhao, Y.; Liu, L.; Li, X.; Zhu, X.; Ji, H.; Geng, Y.; Huang, X.; et al. Identification, inhibition modes, and molecular docking of ACE inhibitory peptides derived from Cheddar cheese. LWT 2024, 203, 116326. [Google Scholar] [CrossRef]
- Murray, B.; FitzGerald, R. Angiotensin Converting Enzyme Inhibitory Peptides Derived from Food Proteins: Biochemistry, Bioactivity and Production. Curr. Pharm. Des. 2007, 13, 773–791. [Google Scholar] [CrossRef]
- Deddish, P.A.; Wang, J.; Michel, B.; Morris, P.W.; Davidson, N.O.; Skidgel, R.A.; Erdös, E.G. Naturally occurring active N-domain of human angiotensin I-converting enzyme. Proc. Natl. Acad. Sci. USA 1994, 91, 7807–7811. [Google Scholar] [CrossRef] [PubMed]
- Ondetti, M.A.; Rubin, B.; Cushman, D.W. Design of Specific Inhibitors of Angiotensin-Converting Enzyme: New Class of Orally Active Antihypertensive Agents. Science 1977, 196, 441–444. [Google Scholar] [CrossRef]
- Dević Pavlić, S.; Ristić, S.; Flego, V.; Kapović, M.; Radojčić Badovinac, A. Angiotensin-converting enzyme insertion/deletion gene polymorphism in lung cancer patients. Genet. Test. Mol. Biomark. 2012, 16, 722–725. [Google Scholar] [CrossRef]
- Hanafi, M.A.; Hashim, S.N.; Yea, C.S.; Ebrahimpour, A.; Zarei, M.; Muhammad, K.; Abdul-Hamid, A.; Saari, N. High angiotensin-I converting enzyme (ACE) inhibitory activity of Alcalase-digested green soybean (Glycine max) hydrolysates. Food Res. Int. 2018, 106, 589–597. [Google Scholar] [CrossRef]
- Kayashima, Y.; Smithies, O.; Kakoki, M. The Kallikrein-Kinin System and Oxidative Stress. Curr. Opin. Nephrol. Hypertens. 2012, 21, 92–96. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, H. Function and structure of bradykinin receptor 2 for drug discovery. Pharmacol. Sin. 2023, 44, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Kahn, R.; Hellmark, T.; Leeb-Lundberg, L.M.F.; Akbari, N.; Todiras, M.; Olofsson, T.; Wieslander, J.; Christensson, A.; Westman, K.; Bader, M.; et al. Neutrophil-derived proteinase 3 induces kallikrein-independent release of a novel vasoactive kinin. J. Immunol. 2009, 182, 7906–7915. [Google Scholar] [CrossRef]
- Ekdahl, K.N.; Teramura, Y.; Hamad, O.A.; Asif, S.; Duehrkop, C.; Fromell, K.; Gustafson, E.; Hong, J.; Kozarcanin, H.; Magnusson, P.U.; et al. Dangerous liaisons: Complement, coagulation, and kallikrein/kinin cross-talk act as a linchpin in the events leading to thromboinflammation. Immunol. Rev. 2016, 274, 245–269. [Google Scholar] [CrossRef] [PubMed]
- Michel, T.; Vanhoutte, P.M. Cellular signaling and NO production. Pflugers. Arch. 2010, 459, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Madeddu, P.; Emanueli, C.; El-Dahr, S. Mechanisms of Disease: The tissue kallikrein–kinin system in hypertension and vascular remodeling. Nat. Clin. Pract. Nephrol. 2007, 3, 208–221. [Google Scholar] [CrossRef]
- McGiff, J.C.; Quilley, J. Prostaglandins, Kinins and the Regulation of Blood Pressure. Clin. Exp. Hypertens. 2009, 2, 729–740. [Google Scholar] [CrossRef]
- Moreau, M.E.; Garbacki, N.; Molinaro, G.; Brown, N.J.; Marceau, F.; Adam, A. The Kallikrein-Kinin System: Current and Future Pharmacological Targets. J. Pharmacol. Sci. 2005, 99, 6–38. [Google Scholar] [CrossRef]
- Hecker, M.; Pörsti, I.; Bara, A.T.; Busse, R. Potentiation by ACE inhibitors of the dilator response to bradykinin in the coronary microcirculation: Interaction at the receptor level. Br. J. Pharmacol. 1994, 111, 238–244. [Google Scholar] [CrossRef]
- Carretero, O.A. Novel mechanism of action of ACE and its inhibitors. Am. J. Physiol. -Heart Circ. Physiol. 2005, 289, H1796–H1797. [Google Scholar] [CrossRef]
- Olalere, O.A.; Yap, P.-G.; Gan, C.-Y. Comprehensive review on some food-derived bioactive peptides with anti-hypertension therapeutic potential for angiotensin-converting enzyme (ACE) inhibition. J. Proteins Proteom. 2023, 14, 129–161. [Google Scholar] [CrossRef]
- Jo, D.-M.; Khan, F.; Park, S.-K.; Ko, S.-C.; Kim, K.W.; Yang, D.; Kim, J.-Y.; Oh, G.-W.; Choi, G.; Lee, D.-S.; et al. From Sea to Lab: Angiotensin I-Converting Enzyme Inhibition by Marine Peptides—Mechanisms and Applications. Mar. Drugs 2024, 22, 449. [Google Scholar] [CrossRef]
- Aluko, R.E. Antihypertensive Peptides from Food Proteins. Annu. Rev. Food Sci. Technol. 2015, 6, 235–262. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, C.; Sun-Waterhouse, D.; Zhao, T.; Waterhouse, G.I.N.; Zhao, M.; Su, G. Identification of post-digestion angiotensin-I converting enzyme (ACE) inhibitory peptides from soybean protein Isolate: Their production conditions and in silico molecular docking with ACE. Food Chem. 2021, 345, 128855. [Google Scholar] [CrossRef]
- Alhenc-Gelas, F.; Bouby, N.; Girolami, J.-P. Kallikrein/K1, Kinins, and ACE/Kininase II in Homeostasis and in Disease Insight from Human and Experimental Genetic Studies, Therapeutic Implication. Front. Med. 2019, 6, 136. [Google Scholar] [CrossRef]
- Kleekayai, T.; Harnedy, P.A.; O’Keeffe, M.B.; Poyarkov, A.A.; CunhaNeves, A.; Suntornsuk, W.; FitzGerald, R.J. Extraction of antioxidant and ACE inhibitory peptides from Thai traditional fermented shrimp pastes. Food Chem. 2015, 176, 441–447. [Google Scholar] [CrossRef]
- Gobbetti, M.; Ferranti, P.; Smacchi, E.; Goffredi, F.; Addeo, F. Production of angiotensin-I-converting-enzyme-inhibitory peptides in fermented milks started by Lactobacillus delbrueckii subsp. bulgaricus SS1 and Lactococcus lactis subsp. cremoris FT4. Appl. Environ. Microbiol. 2000, 66, 3898–3904. [Google Scholar] [CrossRef]
- Ma, M.; Feng, Y.; Miao, Y.; Shen, Q.; Tang, S.; Dong, J.; Zhang, J.Z.H.; Zhang, L. Revealing the Sequence Characteristics and Molecular Mechanisms of ACE Inhibitory Peptides by Comprehensive Characterization of 160,000 Tetrapeptides. Foods 2023, 12, 1573. [Google Scholar] [CrossRef] [PubMed]
- Anekthanakul, K.; Senachak, J.; Hongsthong, A.; Charoonratana, T.; Ruengjitchatchawalya, M. Natural ACE inhibitory peptides discovery from Spirulina (Arthrospira platensis) strain C1. Peptides 2019, 118, 170107. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; She, Z.; Feng, Y.; Zhang, J.; Han, R.; Qi, Y.; Sun, L.; Sun, H. Optimization of Extraction Process and Activity of Angiotensin-Converting Enzyme (ACE) Inhibitory Peptide from Walnut Meal. Foods 2024, 13, 1067. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Hur, S.J. Antihypertensive peptides from animal products, marine organisms, and plants. Food Chem. 2017, 228, 506–517. [Google Scholar] [CrossRef]
- Vásquez, P.; Zapata, J.E.; Chamorro, V.C.; García Fillería, S.F.; Tironi, V.A. Antioxidant and angiotensin I-converting enzyme (ACE) inhibitory peptides of rainbow trout (Oncorhynchus mykiss) viscera hydrolysates subjected to simulated gastrointestinal digestion and intestinal absorption. LWT 2022, 154, 112834. [Google Scholar] [CrossRef]
- Goyal, N.; Hajare, S.N.; Gautam, S. Release of an encrypted, highly potent ACE-inhibitory peptide by enzymatic hydrolysis of moth bean (Vigna aconitifolia) protein. Front. Nutr. 2023, 10, 1167259. [Google Scholar] [CrossRef]
- Bao, C.; Chen, H.; Chen, L.; Cao, J.; Meng, J. Comparison of ACE inhibitory activity in skimmed goat and cow milk hydrolyzed by alcalase, flavourzyme, neutral protease and proteinase K. Acta. Univ. Cibiniensis Ser. E Food Technol. 2016, 20, 77–84. [Google Scholar] [CrossRef]
- Lu, X.; Sun, Q.; Zhang, L.; Wang, R.; Gao, J.; Jia, C.; Huang, J. Dual-enzyme hydrolysis for preparation of ACE-inhibitory peptides from sesame seed protein: Optimization, separation, and identification. J. Food Biochem. 2021, 45, e13638. [Google Scholar] [CrossRef]
- Kumar, V.; Shakila, R.J.; Muzaddadi, A.U.; Jeyasekaran, G.; Sukumar, D.; Padmavathy, P.; Kumar, Y. Optimization of Enzymatic Extraction of ACE Inhibitory Peptide from Rohu (Labeo rohita) Fish Waste using RSM. Indian J. Anim. Res. 2022, 56, 673–679. [Google Scholar] [CrossRef]
- Zheng, S.-L.; Luo, Q.-B.; Suo, S.-K.; Zhao, Y.-Q.; Chi, C.-F.; Wang, B. Preparation, Identification, Molecular Docking Study and Protective Function on HUVECs of Novel ACE Inhibitory Peptides from Protein Hydrolysate of Skipjack Tuna Muscle. Mar. Drugs 2022, 20, 176. [Google Scholar] [CrossRef]
- Heydarian, A.; Falah, F.; Yazdi, F.T.; Mortazavi, S.A. Optimization of dairy sludge fermentation culture medium to produce extracts containing bioactive peptides using co-culture of Limosilactobacillus fermentum and Saccharomyces cerevisiae. J. Funct. Foods 2024, 112, 105982. [Google Scholar] [CrossRef]
- Cruz-Casas, D.E.; Aguilar, C.N.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R.; Chávez-González, M.L.; Flores-Gallegos, A.C. Enzymatic hydrolysis and microbial fermentation: The most favorable biotechnological methods for the release of bioactive peptides. Food Chem. Mol. Sci. 2021, 3, 100047. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.; García-Vaquero, M. Bioactive Compounds from Fermented Food Products. Nov. Food Ferment. Technol. 2016, 14, 293–310. [Google Scholar]
- Canon, F.; Nidelet, T.; Guédon, E.; Thierry, A.; Gagnaire, V. Understanding the Mechanisms of Positive Microbial Interactions That Benefit Lactic Acid Bacteria Co-cultures. Front. Microbiol. 2020, 11, 2088. [Google Scholar] [CrossRef]
- Xu, Y.; Che, J.; Wang, Y.; Xiao, B.; Wei, L.; Rong, L.; Li, R. ACE inhibitory peptides and flavor compounds from Se-enriched Bacillus natto fermented chickpea. LWT 2025, 215, 117190. [Google Scholar] [CrossRef]
- Puspitojati, E.; Indrati, R.; Cahyanto, M.N.; Marsono, Y. Effect of fermentation time on the molecular weight distribution of ACE inhibitory peptide from jack bean tempe. IOP Conf. Ser. Earth Environ. Sci. 2023, 1177, 012026. [Google Scholar] [CrossRef]
- Loghman, S.; Moayedi, A.; Mahmoudi, M.; Khomeiri, M.; Gómez-Mascaraque, L.G.; Garavand, F. Single and Co-Cultures of Proteolytic Lactic Acid Bacteria in the Manufacture of Fermented Milk with High ACE Inhibitory and Antioxidant Activities. Fermentation 2022, 8, 448. [Google Scholar] [CrossRef]
- Priyanto, A.D.; Putranto, A.W.; Hsu, J.L. The potential of various seeds as angiotensin-I converting enzyme inhibitory peptides derived from protein hydrolysate: A short review. IOP Conf. Ser. Earth Environ. Sci. 2021, 733, 012130. [Google Scholar] [CrossRef]
- Chen, H.-J.; Dai, F.-J.; Chen, C.-Y.; Fan, S.-L.; Zheng, J.-H.; Chau, C.-F.; Lin, Y.-S.; Chen, C.-S. Effects of molecular weight fraction on antioxidation capacity of rice protein hydrolysates. Sci. Rep. 2023, 13, 3464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, F.; Zhang, T.; Li, C.-Y.; Zhu, L.; Yan, S. Isolation, identification, and molecular docking analysis of novel ACE inhibitory peptides from Spirulina platensis. Eur. Food Res. Technol. 2022, 248, 1107–1115. [Google Scholar] [CrossRef]
- Sitanggang, A.B.; Putri, J.E.; Palupi, N.S.; Hatzakis, E.; Syamsir, E.; Budijanto, S. Enzymatic Preparation of Bioactive Peptides Exhibiting ACE Inhibitory Activity from Soybean and Velvet Bean: A Systematic Review. Molecules 2021, 26, 3822. [Google Scholar] [CrossRef]
- Nasir, S.N.A.M.; Sarbon, N.M. Angiotensin converting enzyme (ACE), antioxidant activity and functional properties of shortfin scad (Decapterus macrosoma) muscle protein hydrolysate at different molecular weight variations. Biocatal. Agric. Biotechnol. 2019, 20, 101254. [Google Scholar] [CrossRef]
- Dong, Y.; Yan, W.; Zhang, Y.-Q. Effects of Spray Drying and Freeze Drying on Physicochemical Properties, Antioxidant and ACE Inhibitory Activities of Bighead Carp (Aristichthys nobilis) Skin Hydrolysates. Foods 2022, 11, 2083. [Google Scholar] [CrossRef]
- Gan, J.Y.; Chang, L.S.; Mat Nasir, N.A.; Babji, A.S.; Lim, S.J. Evaluation of physicochemical properties, amino acid profile and bioactivities of edible Bird’s nest hydrolysate as affected by drying methods. LWT 2020, 131, 109777. [Google Scholar] [CrossRef]
- Hu, X.; Dai, Z.; Jin, R. Purification and Identification of a Novel Angiotensin Converting Enzyme Inhibitory Peptide from the Enzymatic Hydrolysate of Lepidotrigla microptera. Foods 2022, 11, 1889. [Google Scholar] [CrossRef]
- Wei, G.; Wang, T.; Li, Y.; He, R.; Huang, A.; Wang, X. Identification, structural characterization, and molecular dynamic simulation of ACE inhibitory peptides in whey hydrolysates from Chinese Rushan cheese by-product. Food Chem. X 2024, 21, 101211. [Google Scholar] [CrossRef]
- Auwal, S.M.; Zainal Abidin, N.; Zarei, M.; Tan, C.P.; Saari, N. Identification, structure-activity relationship and in silico molecular docking analyses of five novel angiotensin I-converting enzyme (ACE)-inhibitory peptides from stone fish (Actinopyga lecanora) hydrolysates. PLoS ONE 2019, 14, e0197644. [Google Scholar] [CrossRef] [PubMed]
- Antony, P.; Baby, B.; Rahma, A.; Samad, S.A.; Dhaheri, Y.A.; Vijayan, R. Molecular insights into the inhibition of angiotensin-converting enzyme 1 by hemopressin peptides. Sci. Rep. 2024, 14, 28726. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Liao, W.; Wu, J. Molecular interactions, bioavailability, and cellular mechanisms of angiotensin-converting enzyme inhibitory peptides. J. Food Biochem. 2019, 43, e12572. [Google Scholar] [CrossRef]
- Aluko, R.E. Structure and function of plant protein-derived antihypertensive peptides. Curr. Opin. Food Sci. 2015, 4, 44–50. [Google Scholar] [CrossRef]
- Li, J.; Huo, X.; Zheng, Y.; Guo, Y.; Feng, C. ACE-Inhibitory Peptides Identified from Quinoa Bran Glutelin-2 Hydrolysates: In Silico Screening and Characterization, Inhibition Mechanisms of ACE, Coordination with Zinc Ions, and Stability. Plant Foods Hum. Nutr. 2023, 78, 419–425. [Google Scholar] [CrossRef]
- Abdelhedi, O.; Nasri, M. Basic and recent advances in marine antihypertensive peptides: Production, structure-activity relationship and bioavailability. Trends Food Sci. Technol. 2019, 88, 543–557. [Google Scholar] [CrossRef]
- Zhang, J.; Toldrá, F.; Zhang, W.; Yin, Y.; Zhu, Z. Study on the effects and mechanisms of ultrasound on the peptide profile and taste of unsmoked bacon using peptidomics and bioinformatics. Food Chem. 2023, 414, 135764. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, C. Structure and Function of Angiotensin Converting Enzyme and Its Inhibitors. Chin. J. Biotechnol. 2008, 24, 171–176. [Google Scholar] [CrossRef]
- Song, C.; Qiao, B.; Zhang, Q.; Wang, C.; Fu, Y.; Zhu, B. Study on the domain selective inhibition of angiotensin-converting enzyme (ACE) by food-derived tyrosine-containing dipeptides. J. Food Biochem. 2021, 45, e13779. [Google Scholar] [CrossRef]
- Alves-Lopes, R.; Montezano, A.C.; Neves, K.B.; Harvey, A.; Rios, F.J.; Skiba, D.S.; Arendse, L.B.; Guzik, T.J.; Graham, D.; Poglitsch, M.; et al. Selective Inhibition of the C-Domain of ACE (Angiotensin-Converting Enzyme) Combined with Inhibition of NEP (Neprilysin): A Potential New Therapy for Hypertension. Hypertension 2021, 78, 604–616. [Google Scholar] [CrossRef]
- Byun, H.-G.; Kim, S.-K. Structure and Activity of Angiotensin I Converting Enzyme Inhibitory Peptides Derived from Alaskan Pollack Skin. BMB Rep. 2002, 35, 239–243. [Google Scholar] [CrossRef]
- Iwaniak, A.; Minkiewicz, P.; Darewicz, M. Food-Originating ACE Inhibitors, Including Antihypertensive Peptides, as Preventive Food Components in Blood Pressure Reduction. Compr. Rev. Food Sci. Food Saf. 2014, 13, 114–134. [Google Scholar] [CrossRef]
- Wang, R.; Lu, X.; Sun, Q.; Gao, J.; Ma, L.; Huang, J. Novel ACE Inhibitory Peptides Derived from Simulated Gastrointestinal Digestion in Vitro of Sesame (Sesamum indicum L.) Protein and Molecular Docking Study. Int. J. Mol. Sci. 2020, 21, 1059. [Google Scholar] [CrossRef]
- Kumar, V.; Shakila, R.J.; Muzaddadi, A.U.; Jeyasekaran, G.; Sukumar, D.; Padmavathy, P. In Vitro Stability of ACE-Inhibitory Peptides of Rohu Fish Waste to Heat, pH and Gastrointestinal Enzymes. Turk. J. Fish. Aquat. Sci. 2023, 24, TRJFAS23788. [Google Scholar] [CrossRef]
- Giromini, C.; Cheli, F.; Rebucci, R.; Baldi, A. Invited review: Dairy proteins and bioactive peptides: Modeling digestion and the intestinal barrier. J. Dairy Sci. 2019, 102, 929–942. [Google Scholar] [CrossRef]
- Peslerbes, M.; Fellenberg, A.; Jardin, J.; Deglaire, A.; Ibáñez, R.A. Manufacture of Whey Protein Hydrolysates Using Plant Enzymes: Effect of Processing Conditions and Simulated Gastrointestinal Digestion on Angiotensin-I-Converting Enzyme (ACE) Inhibitory Activity. Foods 2022, 11, 2429. [Google Scholar] [CrossRef]
- Cunningham, D.F.; O’Connor, B. Proline specific peptidases. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1997, 1343, 160–186. [Google Scholar] [CrossRef]
- Vermeirssen, V.; Camp, J.V.; Verstraete, W. Bioavailability of angiotensin I converting enzyme inhibitory peptides. Br. J. Nutr. 2004, 92, 357–366. [Google Scholar] [CrossRef]
- Ohsawa, K.; Satsu, H.; Ohki, K.; Enjoh, M.; Takano, T.; Shimizu, M. Producibility and Digestibility of Antihypertensive β-Casein Tripeptides, Val-Pro-Pro and Ile-Pro-Pro, in the Gastrointestinal Tract: Analyses Using an in Vitro Model of Mammalian Gastrointestinal Digestion. J. Agric. Food Chem. 2008, 56, 854–858. [Google Scholar] [CrossRef]
- Parada, J.; Aguilera, J.M. Food Microstructure Affects the Bioavailability of Several Nutrients. J. Food Sci. 2007, 72, R21–R32. [Google Scholar] [CrossRef]
- Li, R.; Zhuang, Y.; Lin, L.; Li, L.; Fan, X.; Sun, L. In vitro simulated gastrointestinal digestion stability and in vivo antihypertensive effect of the peptide KYPHVF and its network pharmacology. J. Funct. Foods 2023, 107, 105672. [Google Scholar] [CrossRef]
- Contreras, M.D.M.; Sanchez, D.; Sevilla, M.Á.; Recio, I.; Amigo, L. Resistance of casein-derived bioactive peptides to simulated gastrointestinal digestion. Int. Dairy J. 2013, 32, 71–78. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Y.; Tian, T.; Xu, Y. Antihypertensive and antioxidant effects of food-derived bioactive peptides in spontaneously hypertensive rats. Food Sci. Nutr. 2024, 12, 8200–8210. [Google Scholar] [CrossRef]
- Mirzapour-Kouhdasht, A.; Garcia-Vaquero, M. Cardioprotective Peptides from Milk Processing and Dairy Products: From Bioactivity to Final Products including Commercialization and Legislation. Foods 2022, 11, 1270. [Google Scholar] [CrossRef]
- Walquist, M.J.; Eilertsen, K.-E.; Elvevoll, E.O.; Jensen, I.-J. Marine-Derived Peptides with Anti-Hypertensive Properties: Prospects for Pharmaceuticals, Supplements, and Functional Food. Mar. Drugs 2024, 22, 140. [Google Scholar] [CrossRef]

| Synthetic Drug | IC50 | Reference |
|---|---|---|
| Captopril | 1.3 × 10−6 mg/mL | [18] |
| Captopril | 5 × 10−4 μM | [19] |
| Trandolapril (aortic ACE) | 2.5 × 10−3 μM | [20] |
| Trandolapril (renal ACE) | 0.015 μM | [20] |
| Enalapril (aortic ACE) | 0.24 μM | [20] |
| Enalapril (renal ACE) | 0.034 μM | [20] |
| Substrate | Production, Fractionation, Purification | Condition and Resin/Material | IC50 | Sequencing and Molecular Mass Determination | Reference | |
|---|---|---|---|---|---|---|
| Plant Sources | Peony seed | 1—Enzymatic hydrolysis 2—Gel filtration chromatography (GFC) and reversed-phase high-performance liquid chromatography (RP-HPLC) | 1—Neutral protease 2—Sephadex G-25 column, C18 column, and analytic RP-HPLC column | HWS: 1.38 μM LAGGF: 2.65 μM VLSGF: 0.536 μM LAGYV: 2.80 μM | UPLC-QTOF-MS/MS | [12] |
| Ulva prolifera | 1—Enzymatic hydrolysis 2—GFC 3—UF | 1—Neutral protease (DH: 33.59%) 2—Sephadex-G100 fltration column (4000–15,000 Da) | KAF: 0.63 μM | High-performance liquid chromatography–quadrupole time-of-flight mass spectrometry (HPLC-Q-TOF-MS) | [13] | |
| Wuyi rock tea residue | 1—Alkali solubilization 2—Enzymatic hydrolysis 3—Column chromatography and RP-HPLC | 1—0.24 mol/L NaOH (1:34 ratio) 2—Neutral protease 3—Sephadex G-15 column and Phenomenex Gemini C18 column | FPFPRPP: 0.276 μM PPPRGP: 0.801 μM PFPRPPH: 0.369 μM LGHPW: 1.50 μM LKFPDF: 0.517 μML | Ultra-performance liquid chromatography–quadrupole time-of-flight mass spectrometry (UPLC-QTOF-MS/MS) | [21] | |
| Rice | 1—Alkali solubilization 2—Enzymatic hydrolysis 3—RP-HPLC | 1—0.1% mol/L NaOH (1:6 ratio) 2—Alcalase and trypsin 3—Cat Ex resin column | - | Multi-angle laser light scattering combined with gel permeation chromatography (MALLS/GPC) | [22] | |
| Broccoli (brassica oleracea) | 1—Water extraction 2—Enzymatic hydrolysis 3—Ethanol extraction 4—GFC, semi-preparative RP-HPLC, and RP-HPLC | 1—Hot water (80 °C) 2—Pepsin 3—10% (v/v) ethanol 4—Sephadex G-15 column, YMC-Pack ODS-AQ column, and C18 column | IAYKPAG: 2.1 μM MRWRD: 0.6 μM MRW: 0.38 μM LRIVA: 4.2 μM | UPLC-QTOF-MS/MS | [23] | |
| Agaricus bisporus scraps | 1—Enzymatic hydrolysis 2—Macroporous resin | 1—Alcalase and compound protease 2—DA201-C, XAD1600, XAD7HP, and AB-8 | 1.50 μM | LC-MS/MS | [24] | |
| Shiitake mushroom (Lentinula edodes) | 1—Enzymatic hydrolysis 2—RP-HPLC | 1—Alcalase (DH: 28.88%) 2—Luna C18 column | 37.14 μM | LC-Q-TOF–MS/MS | [25] | |
| Microalgal Chlorella | 1—Enzymatic hydrolysis 2—UF | 1—Alcalase 2—3.0 kDa cutoff | 2.47 μM–110.2 μM | Q-TOF-LC-MS/MS | [26] | |
| Walnut | 1—Enzymatic hydrolysis 2—HPLC | 1—Alcalase (0.5%, w/w) and papain (0.5%, w/w) 2—X-Peonyx®C18 analytical column | LPVGP: 9.05 µM FPLQPHQP: 5.03 µM | LC-MS/MS | [27] | |
| Olive pomace | 1—Water extraction 2—HPLC 3—GFC | - | 2.64–4.59 μM | PAGE, MS | [28] | |
| Animal Source | Monkfish (Lophius litulon) swim bladders | 1—Enzymatic hydrolysis 2—Column chromatography and RP-HPLC | 1—Alcalase and neutral protease 2—Sephadex G-25 column | SEGPK: 1.07 μM FDGPY: 1.37 μM SPGPW: 1.16 μM | SDS-PAGE, ESI-Q-TOF-MS | [10] |
| Oyster (Crassostrea gigas) | 1—Enzymatic hydrolysis 2—HPLC and RP-HPLC | 1—Ex vivo digestion 2—ZORBAX Eclipse SB-C18 colum | 4287 μM | ESI-Q-TOF-MS | [29] | |
| Yamadazyma spp. in non-fat milk | 1—Fermentation 2—HPLC | 1—Three yeast strains (BO10, B514-1, and BO13-2) separately and their double and triple combinations 2—C18 column | BO10 and BO13-2: 0.92 mg/Ml BO10 and B514-1: 2.10 mg/mL B514-1 and BO13-2: 2.46 mg/mL | - | [18] | |
| Skipjack tuna (Katsuwonus pelamis) roe | 1—Enzymatic hydrolysis 2—UF 3—Column chromatography and RP-HPLC | 1—2% (w/w) flavourzyme 2—1.0, 3.5, and 5.0 kDa cutoffs 3—DEAE-52 cellulose column, Sephadex G-25 column, and Zorbax 300SB-C18 column | WGESF: 1.37 μM IKSW: 1.35 μM YSHM: 0.805 μM WSPGF: 1.01 μM | Protein sequencer, electrospray ionization–quadrupole time-of-flight mass spectrometry (ESI-Q-TOF-MS) | [30] | |
| Trichiurus lepturus | 1—Enzymatic hydrolysis 2—UF 3—GFC | 1—Alkaline protease 2—3.0 and 10.0 kDa cutoffs 3—Sephadex G-25 column | FAGDDAPRR: 262.98 μM QGPIGPR: 81.09 μM GPTGPAGP: 168.11 μM | LC-MS/MS | [31] | |
| Crucian carp | 1—Enzymatic hydrolysis 2—RP-HPLC | 1—Pepsin (4%, w/w) and trypsin(4%, w/w) 2—Sephadex G-25 column | GA-Hyp-GAR: 4.00 μM | UHPLC-LTQ-Orbitrap | [32] | |
| Porcine liver and placenta | 1—Enzymatic hydrolysis 2—RP-HPLC | 1—Cysteine protease papain 2—Ascentis Express Peptide ES-C18 | FWG: 470 μM MFLG: 70 μM SDPPLVFVG: 1160 μM FFNDA: 830 μM | HPLC MS/MS | [19] | |
| Eel (Anguilla japonica) bone collagen | 1—Enzymatic hydrolysis 2—UF | 1—Alcalase, trypsin, protamex, papain, and pepsin 2—1.0 and 3.0 kDa cutoffs | 535.84 μM–3663.82 μM | Nano-HPLC-MS/MS | [33] | |
| Fermented rubing cheese | 1—Aqueous extraction 2—UF | 1—Hot water (40 °C) 2—10 kDa cutoff | VAPFPE: 493 μM EKVNELSKD: 98 μM LHLPLPLLQ: 480 μM LQDKIHP: 396 μM | LC-MS/MS | [34] | |
| Rushan cheese whey | 1—Enzymatic hydrolysis 2—UF 3—RP-HPLC | 1—Rennet enzyme 2—3.0 and 10.0 kDa cutoffs 3—Thermo Hypersil Gold HPLC | FFVAPFPEVFGK: 52.00 μM VRYL: 24.10 μM YLGY: 41.86 μM TTMP: 51.00 μM RYL: 106.64 μM VYPFPGPIPN: 325.00 μM | MS | [35] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Du, W.; Huang, H.; Wan, L.; Shang, C.; Mao, X.; Kong, X. Research Progress on the Mechanism of Action of Food-Derived ACE-Inhibitory Peptides. Life 2025, 15, 1219. https://doi.org/10.3390/life15081219
Li T, Du W, Huang H, Wan L, Shang C, Mao X, Kong X. Research Progress on the Mechanism of Action of Food-Derived ACE-Inhibitory Peptides. Life. 2025; 15(8):1219. https://doi.org/10.3390/life15081219
Chicago/Turabian StyleLi, Ting, Wanjia Du, Huiyan Huang, Luzhang Wan, Chenglong Shang, Xue Mao, and Xianghui Kong. 2025. "Research Progress on the Mechanism of Action of Food-Derived ACE-Inhibitory Peptides" Life 15, no. 8: 1219. https://doi.org/10.3390/life15081219
APA StyleLi, T., Du, W., Huang, H., Wan, L., Shang, C., Mao, X., & Kong, X. (2025). Research Progress on the Mechanism of Action of Food-Derived ACE-Inhibitory Peptides. Life, 15(8), 1219. https://doi.org/10.3390/life15081219





