Phlogacanthus pulcherrimus Leaf Extract as a Functional Feed Additive: Influences on Growth Indices, Bacterial Challenge Survival, and Expression of Immune-, Growth-, and Antioxidant-Related Genes in Labeo chrysophekadion (Bleeker, 1849)
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations and Animal Use Authorization
2.2. Plant Materials and Chemicals
2.3. Preparation of P. pulcherrimus Extract
2.4. Phytochemical Screening of PPE
2.5. Determination of Total Phenolic Content in PPE
2.6. Determination of Total Flavonoid Content in PPE
2.7. Determination of Antioxidant Capacity of PPE
2.8. Experimental Fish and Acclimatization Conditions
2.9. Preparation of a PPE-Supplemented Diet
2.10. Experimental Design
2.11. Determination of Growth Performance
ADG = [{final weight (g) − initial weight (g)}/experimental days]
SGR = [{ln final weight (g) − ln initial weight (g)}/experimental days] × 100
FCR = total feed fed (g) / weight gain (g)
PER = WG (g)/crude protein fed (g)
SR = [number of survival fish/initial number of fish] × 100
2.12. Pathogenic Challenge Test
2.13. Gene Expressions
2.13.1. Primers
2.13.2. RT-qPCR Analysis
2.14. Statistical Analysis
3. Results
3.1. Phytochemical Profiles of PPE
3.2. Total Phenolic Content, Total Flavonoid Content, and Antioxidant Capacity of PPE
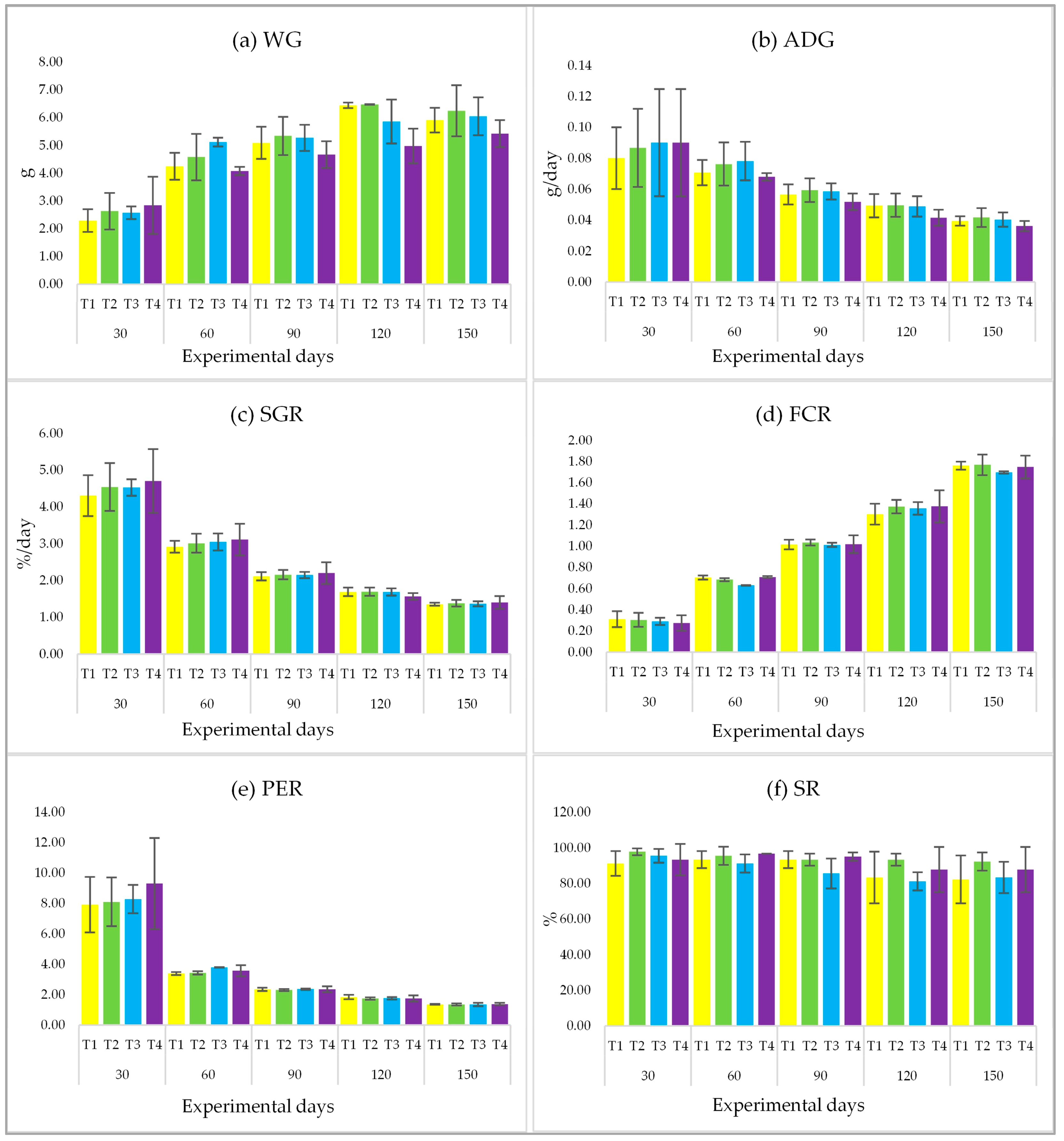
3.3. Growth Performance and Survival Rate
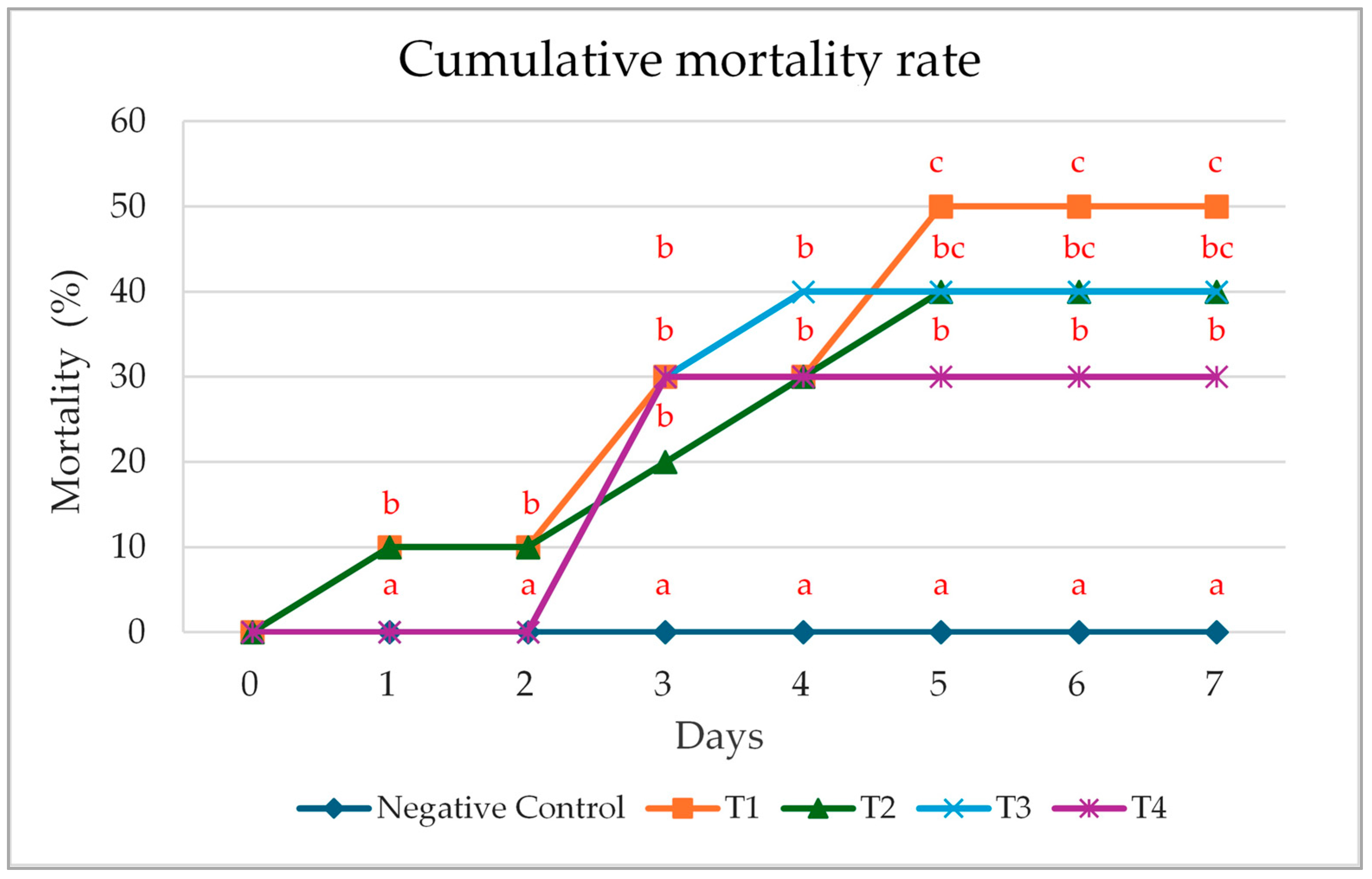
3.4. Cumulative Mortality Rate Following Pathogenic Exposure
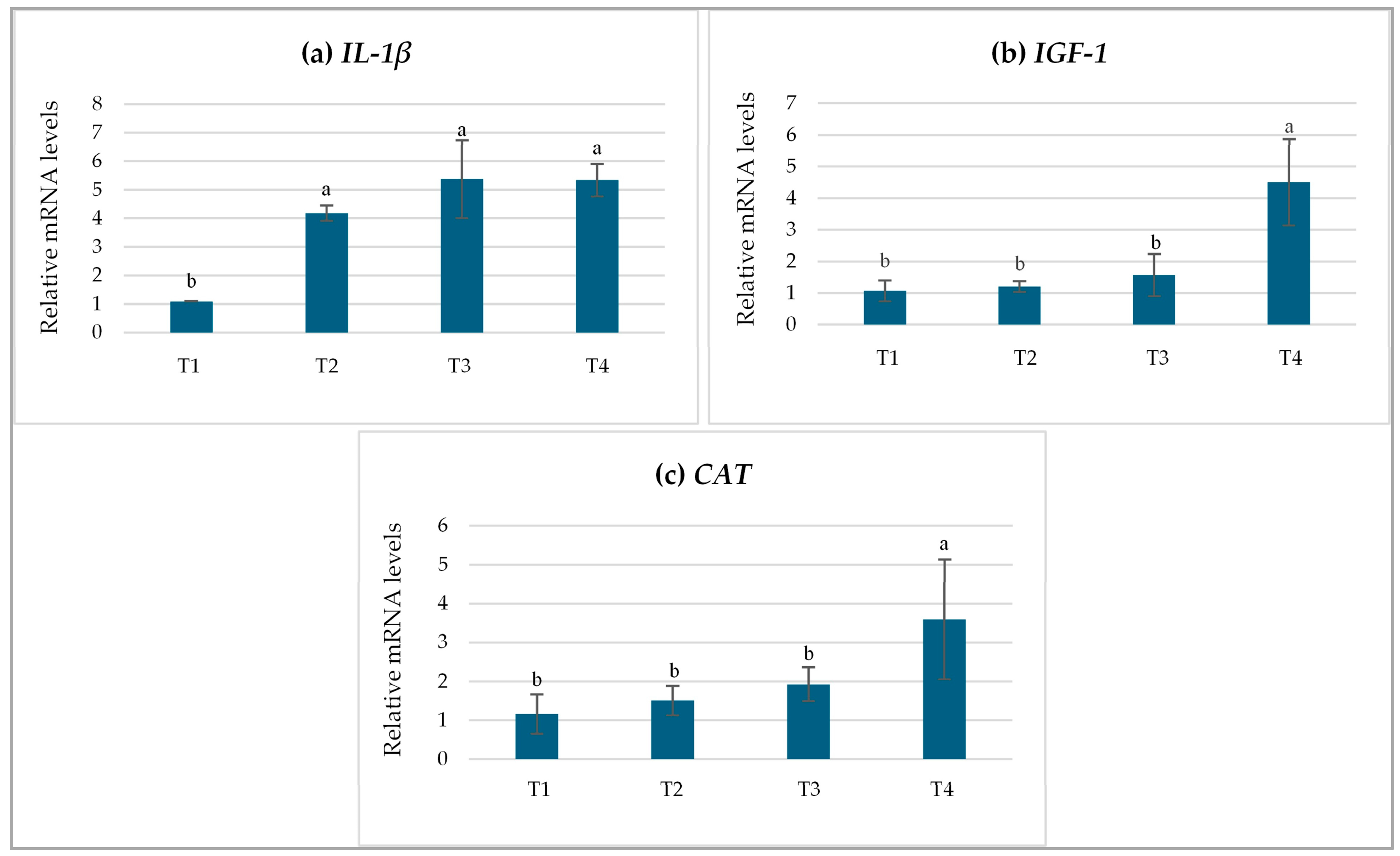
3.5. Relative Gene Expression Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PPE | Phlogacanthus pulcherrimus leaf ethanol extract |
| GAE | Gallic acid equivalent |
| QE | Quercetin equivalent |
| IC50 | Inhibition concentration 50% |
| IL-1β | Interleukin-1β |
| IGF-1 | Insulin-like growth factor 1 |
| CAT | Catalase |
| CFU | Colony-forming unit |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| RT-qPCR | Reverse transcription-quantitative polymerase chain reaction |
| DO | Dissolved oxygen |
| WG | Weight gain |
| ADG | Average daily gain |
| SGR | Specific growth rate |
| FCR | Feed conversion rate |
| PER | Protein efficiency ratio |
| SR | Survival rate |
| LD50 | Lethal dose 50% |
| SD | Standard deviation |
| H2O2 | Hydrogen peroxide |
References
- Froese, R.; Pauly, D. Fish Base. Available online: https://www.fishbase.org.au/summary/SpeciesSummary.php?ID=12102&genusname=Labeo&speciesname=chrysophekadion&AT=Labeo+chrysophekadion&lang=English (accessed on 19 April 2025).
- Freshwater Aquaculture Research and Development Center, Phrae, Freshwater Fisheries Research and Development Division, Department of Fisheries, Ministry of Agriculture and Cooperatives, Thailand. Breeding and Nursery of Black Sharkminnow (Labeo chrysophekadion). Available online: https://www4.fisheries.go.th/local/pic_activities/202009151826351_pic.pdf (accessed on 10 July 2024). (In Thai)
- Duangjai, E. The effect of stocking density on the growth and survival of Labeo chrysophekadion fish in the recirculating system using micro bubbles water. J. Innov. Tech. Res. 2020, 3, 33–43. (In Thai) [Google Scholar]
- Aquarium Glaser. Ornamental Fish from All over the World to Any Place Around the World, Labeo chrysophekadion. Available online: https://www.aquariumglaser.de/en/08-carp-like-fishes-2-barbs-minnows-carps-goldfish-etc/labeo_chrysophekadion_en/ (accessed on 19 April 2025).
- Research and Development Group for Ornamental Aquaculture and Aquatic Plants, Aquaculture Research and Development Division, Department of Fisheries, Ministry of Agriculture and Cooperatives, Thailand. Native Ornamental Fish and Aquatic Plants in Thailand: Knowledge Management on Research for Commercial Production and Sustainable Resource Utilization. Available online: https://www4.fisheries.go.th/local/index.php/main/view_activities/1378/238420 (accessed on 13 July 2025). (In Thai).
- Herbal Database, Faculty of Pharmaceutical Sciences, Ubon Ratchathani University. “Dee Pla Kang”. Available online: https://phar.ubu.ac.th/herb-DetailPhargarden/142#:~:text=%E0%B8%8A%E0%B8%B7%E0%B9%88%E0%B8%AD%E0%B8%AA%E0%B8%A1%E0%B8%B8%E0%B8%99%E0%B9%84%E0%B8%9E%E0%B8%A3,.th/herb%2Dthaiherbarium/ (accessed on 10 April 2025). (In Thai).
- Panyadee, P.; Balslev, H.; Wangpakapattanawong, P.; Inta, A. Medicinal plants in homegardens of four ethnic groups in Thailand. J. Ethnopharmacol. 2019, 239, 111927. [Google Scholar] [CrossRef]
- Wongnaya, N.; Maneerat, T.; Phousamanee, S. Conservation and restoration of food and herb diversity of Karen community in Klonglan National Park, Kamphaeng Phet. Gold. Teak Humanit. Soc. Sci. J. 2020, 26, 56–71. [Google Scholar]
- Boontha, S.; Buranrat, B.; Temkhitthawon, P.; Pitaksuteepong, T. Anticancer activities of Phlogacanthus pulcherrimus T. Anderson leaves extract on MCF-7 breast cancer cells. Key Eng. Mater. 2021, 901, 16–21. [Google Scholar] [CrossRef]
- Kheawchaum, S.; Mahidol, C.; Thongnest, S.; Boonsombat, J.; Batsomboon, P.; Sitthimonchai, S.; Ruchirawat, S.; Prawat, H. Ent-abietane diterpenoid lactone glycosides and a phenolic glycoside from Phlogacanthus pulcherrimus T. Anderson with cytotoxic and cancer chemopreventive activities. Phytochemistry 2022, 201, 113261. [Google Scholar] [CrossRef]
- Athipornchai, A.; Homvisasevongsa, S.; Semsri, S. Discovery and Development of Thai Medicinal Plants with Potential Antidiabetic Activity; Research Report; Faculty of Science, Burapha University: Saen Suk, Thailand, 2019; Available online: https://buuir.buu.ac.th/xmlui/bitstream/handle/1234567890/3970/2564_111.pdf?sequence=1&isAllowed=y (accessed on 1 June 2025). (In Thai)
- Kaewsoongnern, T.; Nukulkit, C.; Chan-ae, P.; Pongnaratorn, P.; Pakdee, N.; Wechvitan, P.; Chaweerak, S.; Jitcharoentham, A.; Prapawinee Padannok, P.; Hongwilai, C. Inhibitory effect on alpha glucosidase of herbs and local vegetables in Sakon Nakhon province. J. Thai. Trad. Alt. Med. 2021, 7, 15–28. (In Thai) [Google Scholar]
- Noontum, P. Antioxidant and α- Glucosidase Inhibitory Activities of Phlogacanthus pulcherrimus (T. Anderson) Leaf Extract. Master’s Thesis, Mahasarakham University, Mahasarakham, Thailand, August 2019. [Google Scholar]
- Chaichana, N. Nutrition information, element and antioxidant activity of native plant in Chiang Rai, Thailand. SWU Sci. J. 2020, 36, 144–153. [Google Scholar]
- Hoseinifar, S.H.; Fazelan, Z.; El-Haroun, E.; Yousefi, M.; Yazici, M.; Van Doan, H.; Paolucci, M. The effects of grapevine (Vitis vinifera L.) leaf extract on growth performance, antioxidant status, and immunity of zebrafish (Danio rerio). Fishes 2023, 8, 326. [Google Scholar] [CrossRef]
- Yue, R.; Dong, W.; Feng, Z.; Jin, T.; Wang, W.; Chen, Y.; He, Y.; Lin, S. Effects of Three Tested Medicinal Plant Extracts on Growth, Immune Function and Microflora in Juvenile Largemouth Bass (Micropterus salmoides). Aquac. Rep. 2024, 36, 102075. [Google Scholar] [CrossRef]
- Esen, R.; Öz, M.; Dikel, S. Effects of Artichoke (Cynara scolymus) Leaf Extract on the Growth, Blood, and Biochemistry Parameters of Nile Tilapia (Oreochromis niloticus). Trop. Anim. Health Prod. 2025, 57, 284. [Google Scholar] [CrossRef]
- Sookying, S.; Srisuttha, P.; Rodprasert, V.; Chaodon, C.; Phinrub, W.; Sutthi, N.; Panase, P. Utilizing invasive Pterygoplichthys pardalis as a sustainable fish meal substitute and Euphorbia hirta extract supplement: Effects on growth performance, organosomatic indices, hematological profiles, and serum biochemistry in Chinese Bullfrogs (Hoplobatrachus chinensis). Life 2025, 15, 115. [Google Scholar] [CrossRef]
- Pant, D.R.; Pant, N.D.; Saru, D.B.; Yadav, U.N.; Khanal, D.P. Phytochemical screening and study of antioxidant, antimicrobial, antidiabetic, anti-inflammatory and analgesic activities of extracts from stem wood of Pterocarpus marsupium Roxburgh. J. Intercult. Ethnopharmacol. 2017, 6, 170–176. [Google Scholar] [CrossRef]
- Hartanti, D.; Cahyani, A.N. Plant cyanogenic glycosides: An overview. Farmasains J. Farm. Dan Ilmu Kesehat. 2020, 5, 1–6. [Google Scholar]
- Shaikh, J.R.; Patil, M.K. Qualitative Tests for Preliminary Phytochemical Screening: An Overview. Int. J. Chem. Stud. 2020, 8, 603–608. [Google Scholar] [CrossRef]
- Chandra, S.; Khan, S.; Avula, B.; Lata, H.; Yang, M.H.; Elsohly, M.A.; Khan, I.A. Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: A comparative study. Evid.-Based Complement. Altern. Med. 2014, 2014, 253875. [Google Scholar] [CrossRef]
- Bello, A.A.; Katta, A.; Obaydo, R.H.; Jazmati, A. Phytochemical analysis and antioxidant efficacy of Chrysojasminum fruticans (L.) Banfi in Syrian flora. Heliyon 2024, 10, e37322. [Google Scholar] [CrossRef]
- Sutthi, N.; Panase, A.; Chitmanat, C.; Sookying, S.; Ratworawong, K.; Panase, P. Effects of dietary leaf ethanolic extract of Apium graveolens L. on growth performance, serum biochemical indices, bacterial resistance and lysozyme activity in Labeo chrysophekadion (Bleeker, 1849). Aquac. Rep. 2020, 18, 100551. [Google Scholar] [CrossRef]
- Bagenal, T. Methods for the Assessment of Fish Production in Fresh Waters, 3rd ed.; Blackwell Scientific Publication: Oxford, UK, 1978; 365p. [Google Scholar]
- Sievers, F.; Wilm, A.; Dineen, D.G.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Kole, S.; Anand, D.; Sharma, R.; Tripathi, G.; Makesh, M.; Rajendran, K.V.; Kadam Bedekar, M. Tissue specific expression profile of some immune related genes in Labeo rohita to Edwardsiella tarda infection. Fish Shellfish Immunol. 2017, 66, 575–582. [Google Scholar] [CrossRef]
- Kumar, S.; Sahu, N.P.; Ranjan, A. Feeding de-oiled rice bran (DORB) to Rohu, Labeo rohita: Effect of varying dietary protein and lipid level on growth, body composition, and insulin like growth factor (IGF) expression. Aquaculture 2018, 492, 59–66. [Google Scholar] [CrossRef]
- Parida, S.; Sahoo, P.K. Antioxidant defence in Labeo rohita to biotic and abiotic stress: Insight from mRNA expression, molecular characterization and recombinant protein-based ELISA of catalase, glutathione peroxidase, CuZn superoxide dismutase, and glutathione s-transferase. Antioxidants 2024, 13, 18. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sauceda, A.E.Q.; Sáyago-Ayerdi, S.G.; Ayala-Zavala, J.F.; Wall-Medrano, A.; de la Rosa, L.A.; González-Aguilar, G.A.; Álvarez-Parrilla, E. Biological actions of phenolic compounds. In Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd ed.; Elhadi, M., Yahia, E.M., Eds.; John Wiley & Sons Ltd.: New Jersey, UK, 2018; Volume 1, pp. 125–138. [Google Scholar] [CrossRef]
- Zheng, K.; Liang, M.; Yao, H.; Wang, J.; Chang, Q. Effect of size-fractionated fish protein hydrolysate on growth and feed utilization of turbot (Scophthalmus maximus L.). Aquac. Res. 2012, 44, 895–902. [Google Scholar] [CrossRef]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Phenolic compounds: Structure, classification, and antioxidant power. In Bioactive Compounds; Campos, M.R.S., Ed.; Woodhead Publishing: Cambridge, UK, 2019; Volume 1, pp. 33–50. [Google Scholar] [CrossRef]
- Hassanpour, S.H.; Doroudi, A. Review of the antioxidant potential of flavonoids as a subgroup of polyphenols and partial substitute for synthetic antioxidants. Avicenna J. Phytomed. 2023, 13, 354–376. [Google Scholar]
- Chen, X.; Li, H.; Zhang, B.; Deng, Z. The synergistic and antagonistic antioxidant interactions of dietary phytochemical combinations. Crit. Rev. Food. Sci. Nutr. 2022, 62, 5658–5677. [Google Scholar] [CrossRef]
- Sonam, K.S.; Guleria, S. Synergistic antioxidant activity of natural products. Ann. Pharmacol. Pharm. 2017, 2, 1086. [Google Scholar]
- Sawicki, T.; Jabłońska, M.; Danielewicz, A.; Przybyłowicz, K.E. Phenolic compounds profile and antioxidant capacity of plant-based protein supplements. Molecules 2024, 29, 2101. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313. [Google Scholar] [CrossRef]
- Li, N.; Sun, C.; Zhou, B.; Xing, H.; Ma, D.; Chen, G.; Weng, D. Low concentration of quercetin antagonizes the cytotoxic effects of anti-neoplastic drugs in ovarian cancer. PLoS ONE 2014, 9, e100314. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Bioactivities of phenolics by focusing on suppression of chronic diseases: A review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef]
- Kiokias, S.; Oreopoulou, V. A Review of the health orotective effects of phenolic acids against a range of severe pathologic conditions (including Coronavirus-based infections). Molecules 2021, 26, 5405. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Sun, Y.Z.; Wang, A.; Zhou, Z. Probiotics as means of diseases control in aquaculture, a review of current knowledge and future perspectives. Front. Microbiol. 2018, 9, 2429. [Google Scholar] [CrossRef]
- Dawood, M.A.; Koshio, S.; Esteban, M.Á. Beneficial roles of feed additives as immunostimulants in aquaculture: A review. Rev. Aquac. 2017, 10, 950–974. [Google Scholar] [CrossRef]
- Chakraborty, S.B.; Hancz, C. Application of phytochemicals as immunostimulant, antipathogenic and antistress agents in finfish culture. Rev. Aquac. 2011, 3, 103–119. [Google Scholar] [CrossRef]
- Awad, E.; Awaad, A. Role of medicinal plants on growth performance and immune status in fish. Fish Shellfish Immunol. 2017, 67, 40–54. [Google Scholar] [CrossRef]
- Van Doan, H.; Hoseinifar, S.H.; Ringø, E.; Ángeles Esteban, M.; Dadar, M.; Dawood, M.A.; Faggio, C. Host-associated probiotics: A key factor in sustainable aquaculture. Rev. Fish. Sci. Aquac. 2020, 28, 16–42. [Google Scholar] [CrossRef]
- Nafiqoh, N.; Sukenda, S.; Zairin, M., Jr.; Alimuddin, A.; Lusiastuti, A.; Sarter, S.; Caruso, D.; Avarre, J.C. Antimicrobial properties against Aeromonas hydrophila and immunostimulant effect on Clarias gariepinus of Piper betle, Psidium guajava, and Tithonia diversifolia plants. Aquac. Intern. 2020, 28, 1–13. [Google Scholar] [CrossRef]
- Abarike, E.D.; Jian, J.; Tang, J.; Cai, J.; Sakyi, E.M.; Kuebutornye, F.K. A mixture of Chinese herbs and a commercial probiotic Bacillus species improves hemato-immunological, stress, and antioxidant parameters, and expression of HSP70 and HIF-1α mRNA to hypoxia, cold, and heat stress in Nile tilapia, Oreochromis niloticus. Aquac. Rep. 2020, 18, 100438. [Google Scholar] [CrossRef]
- Olusola, S.E.; Nwokike, C.C. Effects of dietary leaves extracts of bitter (Vernonia amygdalina) and pawpaw (Carica papaya) on the growth, feed conversion efficiency and disease resistance on juveniles Clarias gariepinus. Aquac. Res. 2018, 49, 1858–1865. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics 2012, 13, 134. [Google Scholar] [CrossRef]
- Secombes, C.J.; Wang, T.; Bird, S. The interleukins of fish. Dev. Comp. Immunol. 2011, 35, 1336–1345. [Google Scholar] [CrossRef]
- Secombes, C.J.; Wang, T.; Hong, S.; Peddie, S.; Crampe, M.; Laing, K.J.; Cunningham, C.; Zou, J. Cytokines and innate immunity of fish. Dev. Comp. Immunol. 2001, 25, 713–723. [Google Scholar] [CrossRef]
- Reinecke, M. Insulin-like growth factors and fish reproduction. Biol. Reprod. 2010, 82, 656–661. [Google Scholar] [CrossRef]
- Midhun, S.J.; Arun, D.; Edatt, L.; Sruthi, M.V.; Thushara, V.V.; Oommen, O.V.; Kumar, V.B.S.; Divya, L. Modulation of digestive enzymes, GH, IGF-1 and IGF-2 genes in the teleost, tilapia (Oreochromis mossambicus) by dietary curcumin. Aquac. Int. 2016, 24, 1277–1286. [Google Scholar] [CrossRef]
- Aanyu, M.; Betancor, M.B.; Monroig, Ó. The effects of combined phytogenics on growth and nutritional physiology of Nile tilapia Oreochromis niloticus. Aquaculture 2020, 519, 734867. [Google Scholar] [CrossRef]
- Shehata, A.I.; Taha, S.A.; Elmaghraby, A.M.; Elhetawy, A.I.G.; Srour, T.M.; El Basuini, M.F.; Shahin, S.A. Effects of dietary bay leaf (Laurus nobilis) aqueous extract on growth performance, feed utilization, antioxidant activity, immunity, and gene expression in Nile tilapia (Oreochromis niloticus). Aquaculture 2025, 599, 742155. [Google Scholar] [CrossRef]
- Beckman, B.R. Perspectives on concordant and discordant relations between insulin-like growth factor 1 (IGF1) and growth in fishes. Gen. Comp. Endocrinol. 2011, 170, 233–252. [Google Scholar] [CrossRef]
- Von Ossowski, I.; Hausner, G.; Loewen, P.C. Molecular evolutionary analysis based on the amino acid sequence of catalase. J. Mol. Evol. 1993, 37, 71–76. [Google Scholar] [CrossRef]
- Monteiro, C.P.; Matias, C.N.; Bicho, M.; Santa-Clara, H.; Laires, M.J. Coordination between antioxidant defences might be partially modulated by magnesium status. Magnes. Res. 2016, 29, 161–168. [Google Scholar] [CrossRef]
- Hansen, B.H.; Rømma, S.; Garmo, Ø.A.; Olsvik, P.A.; Andersen, R.A. Antioxidative stress proteins and their gene expression in brown trout (Salmo trutta) from three rivers with different heavy metal levels. Comp. Biochem. Physiol. C 2006, 143, 263–274. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, X.; Shu, L.; Chen, L.; Sun, L.; Qian, H.; Liu, W.; Fu, Z. Oxidative stress response and gene expression with atrazine exposure in adult female zebrafish (Danio rerio). Chemosphere 2010, 78, 846–852. [Google Scholar] [CrossRef]
- Vinagre, C.; Madeira, D.; Narciso, L.; Cabral, H.N.; Diniz, M. Effect of temperature on oxidative stress in fish: Lipid peroxidation and catalase activity in the muscle of juvenile seabass, Dicentrarchus labrax. Ecol. Indic. 2012, 23, 274–279. [Google Scholar] [CrossRef]
| Gene | Primer Name | Sequence | Annealing Temperature (°C) | Amplicon Size (bp) | References |
|---|---|---|---|---|---|
| Immune-related genes | |||||
| Interleukin-1β (IL-1β) | IL-1β-qF | TTGAAGGCCGTGACACTGACT | 60 | 114 | [28] |
| IL-1β-qR | GATTCCCAGGCACACAGGTT | ||||
| Growth-related genes | |||||
| Insulin-like growth factors 1 (IGF-1) | F | GCAAACCGACAGGCTATGGGC | 60 | 166 | [29] |
| R | GTGTCTGTGTGCCGTTCCGC | ||||
| Antioxidant enzyme-related genes | |||||
| Catalase (CAT) | F | ACCTCTACAACGCCATCT | 57 | 95 | [30] |
| R | ATTCCACTTCCAGTTCTCAG | ||||
| Housekeeping gene | |||||
| β-actin | F | CACTGCTGCTTCCTCCTCCTCC | 60 | 139 | [29] |
| R | GATACCGCAAGACTCCATACCCAAG | ||||
| Phytochemicals | Results | Phytochemicals | Results |
|---|---|---|---|
| Alkaloids | − | Phenolics | + |
| Anthocyanins | − | Flavonoids | − |
| Anthraquinones | + | Hydrolysable tannins | − |
| Steroids | − | Condensed tannins | − |
| Saponins | − | Carbohydrates | + |
| Triterpenoids | + | Cyanogenic glycosides | − |
| Volatile coumarins | − | Cardiac glycosides | − |
| Nonvolatile coumarins | − |
| Analysis | Total Phenolics (mg GAE/g Extract) | Total Flavonoids (mg QE/g Extract) | Antioxidant Capacity (IC50) (μg/mL) |
|---|---|---|---|
| P. pulcherimus extract | 96.00 ± 14.58 | 17.55 ± 3.18 | 1314.08 ± 3.60 |
| Ascorbic acid | - | - | 7.53 ± 3.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sookying, S.; Auputinan, P.; Panprommin, D.; Panase, P. Phlogacanthus pulcherrimus Leaf Extract as a Functional Feed Additive: Influences on Growth Indices, Bacterial Challenge Survival, and Expression of Immune-, Growth-, and Antioxidant-Related Genes in Labeo chrysophekadion (Bleeker, 1849). Life 2025, 15, 1220. https://doi.org/10.3390/life15081220
Sookying S, Auputinan P, Panprommin D, Panase P. Phlogacanthus pulcherrimus Leaf Extract as a Functional Feed Additive: Influences on Growth Indices, Bacterial Challenge Survival, and Expression of Immune-, Growth-, and Antioxidant-Related Genes in Labeo chrysophekadion (Bleeker, 1849). Life. 2025; 15(8):1220. https://doi.org/10.3390/life15081220
Chicago/Turabian StyleSookying, Sontaya, Panitnart Auputinan, Dutrudi Panprommin, and Paiboon Panase. 2025. "Phlogacanthus pulcherrimus Leaf Extract as a Functional Feed Additive: Influences on Growth Indices, Bacterial Challenge Survival, and Expression of Immune-, Growth-, and Antioxidant-Related Genes in Labeo chrysophekadion (Bleeker, 1849)" Life 15, no. 8: 1220. https://doi.org/10.3390/life15081220
APA StyleSookying, S., Auputinan, P., Panprommin, D., & Panase, P. (2025). Phlogacanthus pulcherrimus Leaf Extract as a Functional Feed Additive: Influences on Growth Indices, Bacterial Challenge Survival, and Expression of Immune-, Growth-, and Antioxidant-Related Genes in Labeo chrysophekadion (Bleeker, 1849). Life, 15(8), 1220. https://doi.org/10.3390/life15081220









