Outcomes of Pregnancies with Absent or Hypoplastic Fetal Nasal Bone: A Retrospective Analysis of Prenatal Findings and Perinatal Outcomes
Abstract
1. Introduction
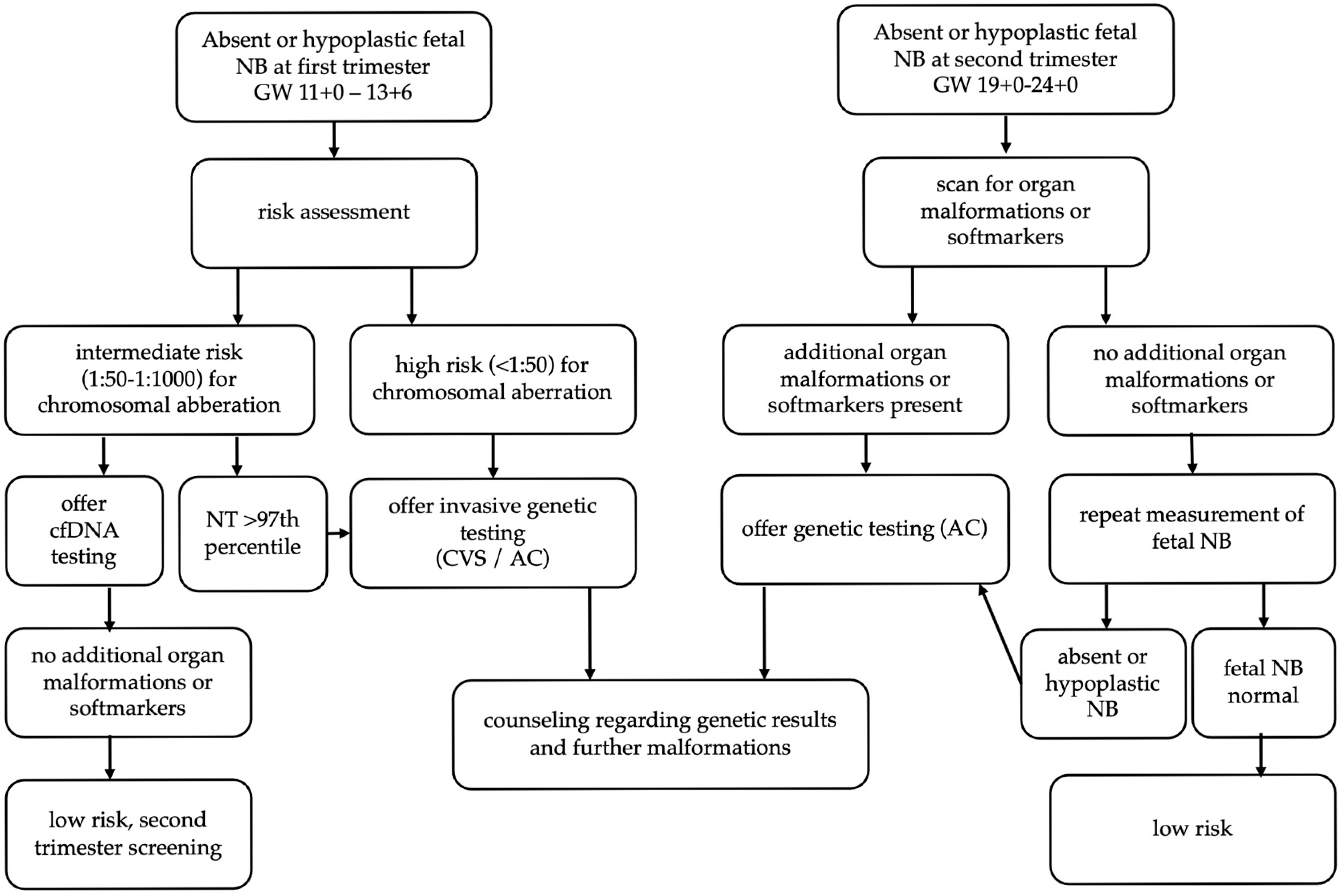
2. Material and Methods
Statistical Analysis
3. Results
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kagan, K.O.; Wright, D.; Spencer, K.; Molina, F.S.; Nicolaides, K.H. First-trimester screening for trisomy 21 by free beta-human chorionic gonadotropin and pregnancy-associated plasma protein-A: Impact of maternal and pregnancy characteristics. Ultrasound Obstet. Gynecol. 2008, 31, 493–502. [Google Scholar] [CrossRef]
- International Society of Ultrasound in Obstetrics and Gynecology; Bilardo, C.M.; Chaoui, R.; Hyett, J.A.; Kagan, K.O.; Karim, J.N.; Papageorghiou, A.T.; Poon, L.C.; Salomon, L.J.; Syngelaki, A.; et al. ISUOG Practice Guidelines (updated): Performance of 11–14-week ultrasound scan. Ultrasound Obstet. Gynecol. 2023, 61, 127–143. [Google Scholar]
- Cicero, S.; Curcio, P.; Papageorghiou, A.; Sonek, J.; Nicolaides, K. Absence of nasal bone in fetuses with trisomy 21 at 11–14 weeks of gestation: An observational study. Lancet 2001, 358, 1665–1667. [Google Scholar] [CrossRef]
- Cicero, S.; Rembouskos, G.; Vandecruys, H.; Hogg, M.; Nicolaides, K.H. Likelihood ratio for trisomy 21 in fetuses with absent nasal bone at the 11–14-week scan. Ultrasound Obstet. Gynecol. 2004, 23, 218–223. [Google Scholar] [CrossRef]
- Moreno-Cid, M.; Rubio-Lorente, A.; Rodríguez, M.J.; Bueno-Pacheco, G.; Tenías, J.M.; Román-Ortiz, C.; Arias, Á. Systematic review and meta-analysis of performance of second-trimester nasal bone assessment in detection of fetuses with Down syndrome. Ultrasound Obstet. Gynecol. 2014, 43, 247–253. [Google Scholar] [CrossRef]
- Society for Maternal-Fetal Medicine; Benacerraf, B.R.; Bromley, B.; Jelin, A.C. Absent nasal bone. Am. J. Obstet. Gynecol. 2019, 221, B6–B7. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Thakur, S.; Arora, N.; Khurana, D. Revisiting absent nasal bone in the second trimester. J. Clin. Ultrasound 2021, 49, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Liang, H.; Meng, Z.; Wang, J.; Zhang, R.; Wu, Y. Assessing the value of second-trimester nasal bone hypoplasia in predicting chromosomal abnormalities: A retrospective chromosomal microarray analysis of 351 fetuses. Arch. Gynecol. Obstet. 2022, 308, 1263–1270. [Google Scholar] [CrossRef]
- Moczulska, H.; Serafin, M.; Wojda, K.; Borowiec, M.; Sieroszewski, P. Fetal Nasal Bone Hypoplasia in the Second Trimester as a Marker of Multiple Genetic Syndromes. J. Clin. Med. 2022, 11, 1513. [Google Scholar] [CrossRef]
- Bromley, B.; Lieberman, E.; Shipp, T.D.; Benacerraf, B.R. Fetal Nose Bone Length: A Marker for Down Syndrome in the Second Trimester. J. Ultrasound Med. 2002, 21, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Gautier, M.; Gueneret, M.; Plavonil, C.; Jolivet, E.; Schaub, B. Normal Range of Fetal Nasal Bone Length during the Second Trimester in an Afro-Caribbean Population and Likelihood Ratio for Trisomy 21 of Absent or Hypoplastic Nasal Bone. Fetal Diagn. Ther. 2017, 42, 130–136. [Google Scholar] [CrossRef]
- Zelop, C.M.; Milewski, E.; Brault, K.; Benn, P.; Borgida, A.F.; Egan, J.F.X. Variation of Fetal Nasal Bone Length in Second-Trimester Fetuses According to Race and Ethnicity. J. Ultrasound Med. 2005, 24, 1487–1489. [Google Scholar] [CrossRef]
- The Fetal Medicine Foundation. Risk for Trisomies at 11–13 Weeks. (Risk Assessment). Available online: https://fetalmedicine.org/research/assess/trisomies (accessed on 8 July 2025).
- Santorum, M.; Wright, D.; Syngelaki, A.; Karagioti, N.; Nicolaides, K.H. Accuracy of first-trimester combined test in screening for trisomies 21, 18 and 13. Ultrasound Obstet. Gynecol. 2017, 49, 714–720. [Google Scholar] [CrossRef]
- Kagan, K.O.; Cicero, S.; Staboulidou, I.; Wright, D.; Nicolaides, K.H. Fetal nasal bone in screening for trisomies 21, 18 and 13 and Turner syndrome at 11–13 weeks of gestation. Ultrasound Obstet. Gynecol. 2009, 33, 259–264. [Google Scholar] [CrossRef]
- Groen, H.; Bouman, K.; Pierini, A.; Rankin, J.; Rissmann, A.; Haeusler, M.; Yevtushok, L.; Loane, M.; Erwich, J.J.H.M.; de Walle, H.E.K. Stillbirth and neonatal mortality in pregnancies complicated by major congenital anomalies: Findings from a large European cohort. Prenat. Diagn. 2017, 37, 1100–1111. [Google Scholar] [CrossRef]
- Hume, H.; Chasen, S.T. Trends in timing of prenatal diagnosis and abortion for fetal chromosomal abnormalities. Am. J. Obstet. Gynecol. 2015, 213, 545.e1–545.e4. [Google Scholar] [CrossRef] [PubMed]
- Salomon, L.J.; Alfirevic, Z.; Berghella, V.; Bilardo, C.M.; Chalouhi, G.E.; Da Silva Costa, F.; Hernandez-Andrade, E.; Malinger, G.; Munoz, H.; Paladini, D. ISUOG Practice Guidelines (updated): Performance of the routine mid-trimester fetal ultrasound scan. Ultrasound Obstet. Gynecol. 2022, 59, 840–856. [Google Scholar] [CrossRef] [PubMed]
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus definition of fetal growth restriction: A Delphi procedure: Consensus definition of FGR. Ultrasound Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef]
- Glenn, J.K.; Goldman, J. Task delegation to physician extenders—Some comparisons. Am. J. Public Health 1976, 66, 64–66. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Management of Stillbirth: Obstetric Care Consensus No, 10. Obstet. Gynecol. 2020, 135, e110–e1132. [Google Scholar] [CrossRef] [PubMed]
- Cicero, S.; Sonek, J.D.; McKenna, D.S.; Croom, C.S.; Johnson, L.; Nicolaides, K.H. Nasal bone hypoplasia in trisomy 21 at 15–22 weeks’ gestation. Ultrasound Obstet. Gynecol. 2003, 21, 15–18. [Google Scholar] [CrossRef]
- Dukhovny, S.; Wilkins-Haug, L.; Shipp, T.D.; Benson, C.B.; Kaimal, A.J.; Reiss, R. Absent Fetal Nasal Bone: What Does It Mean for the Euploid Fetus? J. Ultrasound Med. 2013, 32, 2131–2134. [Google Scholar] [CrossRef]
- Kagan, K.O.; Sonek, J.; Berg, X.; Berg, C.; Mallmann, M.; Abele, H.; Hoopmann, M.; Geipel, A. Facial markers in second- and third-trimester fetuses with trisomy 18 or 13, triploidy or Turner syndrome. Ultrasound Obstet. Gynecol. 2015, 46, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Bull, M.J. Down Syndrome. N. Engl. J. Med. 2020, 382, 2344–2352. [Google Scholar] [CrossRef]
- Hartman, R.J.; Rasmussen, S.A.; Botto, L.D.; Riehle-Colarusso, T.; Martin, C.L.; Cragan, J.D.; Shin, M.; Correa, A. The Contribution of Chromosomal Abnormalities to Congenital Heart Defects: A Population-Based Study. Pediatr. Cardiol. 2011, 32, 1147–1157. [Google Scholar] [CrossRef]
- Agathokleous, M.; Chaveeva, P.; Poon, L.C.Y.; Kosinski, P.; Nicolaides, K.H. Meta-analysis of second-trimester markers for trisomy 21. Ultrasound Obstet. Gynecol. 2013, 41, 247–261. [Google Scholar] [CrossRef]
- Fantasia, I.; Stampalija, T.; Sirchia, F.; Della Pietà, I.; Ottaviani Giammarco, C.; Guidolin, F.; Quadrifoglio, M.; Barresi, V.; Travan, L.; Faletra, F. First-trimester absent nasal bone: Is it a predictive factor for pathogenic CNV s in the low-risk population? Prenat. Diagn. 2020, 40, 1563–1568. [Google Scholar] [CrossRef] [PubMed]
- Lostchuck, E.; Hui, L. Should second-trimester hypoplastic nasal bone be sole indication for diagnostic testing with chromosomal microarray analysis? Ultrasound Obstet. Gynecol. 2019, 53, 848–850. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Lu, J.; Li, L.; Wei, R.; Wu, J. Prenatal chromosomal microarray analysis in foetuses with isolated absent or hypoplastic nasal bone. Ann. Med. 2022, 54, 1297–1302. [Google Scholar] [CrossRef]
- Zhang, F.; Long, W.; Zhou, Q.; Wang, J.; Shi, Y.; Liu, J.; Wang, Q. Is Prenatal Diagnosis Necessary for Fetal Isolated Nasal Bone Absence or Hypoplasia? Int. J. Gen. Med. 2021, 14, 4435–4441. [Google Scholar] [CrossRef]
- Prabhu, M.; Kuller, J.A.; Biggio, J.R. Society for Maternal-Fetal Medicine Consult Series #57: Evaluation and management of isolated soft ultrasound markers for aneuploidy in the second trimester. Am. J. Obstet. Gynecol. 2021, 225, B2–B15. [Google Scholar] [CrossRef]
- Prefumo, F.; Sairam, S.; Bhide, A.; Penna, L.; Hollis, B.; Thilaganathan, B. Maternal ethnic origin and fetal nasal bones at 11–14 weeks of gestation. BJOG 2004, 111, 109–112. [Google Scholar] [CrossRef]
- Papasozomenou, P.; Athanasiadis, A.P.; Zafrakas, M.; Panteris, E.; Loufopoulos, A.; Assimakopoulos, E.; Tarlatzis, B.C. Fetal nasal bone length in the second trimester: Comparison between population groups from different ethnic origins. J. Perinat. Med. 2016, 44, 229–235. [Google Scholar] [CrossRef]
- Franasiak, J.M.; Forman, E.J.; Hong, K.H.; Werner, M.D.; Upham, K.M.; Treff, N.R.; Scott, R.T., Jr. The nature of aneuploidy with increasing age of the female partner: A review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil. Steril. 2014, 101, 656–663.e1. [Google Scholar] [CrossRef]
- Najafi, K.; Gholami, S.; Moshtagh, A.; Bazrgar, M.; Sadatian, N.; Abbasi, G.; Rostami, P.; Khalili, S.; Babanejad, M.; Nourmohammadi, B.; et al. Chromosomal aberrations in pregnancy and fetal loss: Insight on the effect of consanguinity, review of 1625 cases. Molec. Gen. Gen. Med. 2019, 7, e820. [Google Scholar] [CrossRef] [PubMed]
- Staboulidou, I.; Wüstemann, M.; Vaske, B.; Scharf, A.; Hillemanns, P.; Schmidt, P. Interobserver Variability of the Measurement of Fetal Nasal Bone Length between 11 + 0 and 13 + 6 Gestation Weeks among Experienced and Inexperienced Sonographers. Ultraschall Med. 2008, 30, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Suwanrath, C.; Pruksanusak, N.; Kor-anantakul, O.; Suntharasaj, T.; Hanprasertpong, T.; Pranpanus, S. Reliability of fetal nasal bone length measurement at 11–14 weeks of gestation. BMC Pregnancy Childbirth 2013, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Yayla, M.; Nida Ergin, R.; Goynumer, G. Normative values of fetal nasal bone lengths of Turkish singleton pregnancies in the first trimester. J. Turk. Ger. Gynecol. Assoc. 2011, 12, 225–228. [Google Scholar] [CrossRef]
| Variable | N = 149 |
|---|---|
| Maternal age 1 | 34 (27–39) |
| Body mass index 1 | 23.5 (21–26) |
| Smoking 2 | 18 (12.1) |
| Body mass index > 24.9 kg/m2 | 49 (32.9) |
| Assisted reproductive techniques 2 | 7 (4.7) |
| Consanguinity | 5 (3.4) |
| Nulliparity 2 | 43 (28.9) |
| Gestational diabetes 2 | 13 (8.7) |
| Hypertensive pregnancy disorders 2 * | 8 (5.4) |
| Obstetric complications 2 ** | 13 (8.7) |
| Variable | All Cases N = 149 | No Chromosomal Aberrations N = 52 | Isolated Nasal Bone Anomaly N = 49 |
|---|---|---|---|
| Absent nasal bone | 104 (69.8) | 40 (76.9) | 38 (77.5) |
| Hypoplastic nasal bone | 38 (25.5) | 8 (15.4) | 11 (22.4) |
| Abnormal nasal bone | 7 (4.4) | 4 (7.7) | 0 (0) |
| Nuchal translucency > 3.5 mm | 41 (27.5) | 6 (11.5) | - |
| Tricuspid regurgitation | 9 (6) | 3 (5.8) | - |
| Abnormal ductus venosus | 28 (18.8) | 10 (19.2) | - |
| Malformations * | 82 (55) | 18 (34.6) | - |
| Heart defects | 52 (34.9) | 10 (19.2) | - |
| Facial dysmorphism | 43 (28.9) | 11 (21.2) | - |
| Brain malformations | 29 (19.5) | 5 (9.6) | - |
| Intestinal malformations | 26 (17.4) | 7 (13.5) | - |
| Urogenital malformations | 19 (12.8) | 5 (9.6) | - |
| Extremities anomalies | 15 (10.1) | 4 (7.7) | - |
| Isolated nasal bone anomaly | 49 (32.9) | 30 (57.7) | - |
| Soft marker | 56 (37.6) | 9 (17.3) | - |
| Fetal growth restriction | 13 (8.7) | 4 (7.7) | - |
| Fetal hydrops | 34 (22.8) | 2 (3.9) | - |
| Genetic testing conducted ** | 128 (85.9) | 52 (100) | 39 (79.6) |
| Invasive genetic testing conducted | 106 (71.6) | 35 (67.3) | 24 (48.98) |
| Amniocentesis | 35 (23.5) | 14 (26.9) | 8 (16.3) |
| Chorionic villous sampling | 65 (43.1) | 19 (36.5) | 18 (36.7) |
| Placental puncture | 10 (6.7) | 5 (9.6) | 0 (0) |
| Non-invasive genetic testing positive | 34 (22.8) | 18 (34.6) | 17 (34.7) |
| Chromosomal aberration | 76 (51) | - | 9 (18.4) |
| Trisomy 21 | 46 (30.9) | - | 6 (12.2) |
| Trisomy 18 | 7 (4.7) | - | 0 (0) |
| Trisomy 13 | 9 (6) | - | 0 (0) |
| Other chromosomal aberrations *** | 14 (9.4) | - | 3 (6.1) |
| Variable | All Cases N = 149 | No Chromosomal Aberrations N = 52 | Isolated Nasal Bone Anomaly N = 49 |
|---|---|---|---|
| TOP | 65 (43.6) | 6 (11.5) | 7 (14.3) |
| Abortion until 20 weeks GA | 9 (6) | 2 (3.8) | 1 (2) |
| Stillbirth | 3 (2) | 1 (1.92) | 1 (2) |
| Live births | 72 (48.3) | 43 (82.7) | 40 (81.6) |
| Neonatal death | 3(2) | 1 (1.92) | 0 (0) |
| Variable | N = 72 |
|---|---|
| GA at birth 1 | 38.57 (37.29–40.00) |
| Vaginal birth 2 | 34 (47.2) |
| Preterm birth 2 | 16 (22.2) |
| Male sex 2 | 29 (40.3) |
| Birth weight in grams 1 | 3000 (2670–3590) |
| Birth weight percentile 1 | 34 (9–70) |
| Birth length in centimeters | 50 (48.5–52.3) |
| APGAR 1 1 | 9 (8–9) |
| APGAR 5 1 | 10 (9–10) |
| APGAR 10 1 | 10 (9–10) |
| pH 1 | 7.27 (7.19–7.32) |
| Transfer to NICU 2 * | 20 (27.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karner, E.; Krepler, L.; Pateisky, P.; Grill, A.; Dremsek, P.; Yerlikaya-Schatten, G.; Springer, S. Outcomes of Pregnancies with Absent or Hypoplastic Fetal Nasal Bone: A Retrospective Analysis of Prenatal Findings and Perinatal Outcomes. Life 2025, 15, 1215. https://doi.org/10.3390/life15081215
Karner E, Krepler L, Pateisky P, Grill A, Dremsek P, Yerlikaya-Schatten G, Springer S. Outcomes of Pregnancies with Absent or Hypoplastic Fetal Nasal Bone: A Retrospective Analysis of Prenatal Findings and Perinatal Outcomes. Life. 2025; 15(8):1215. https://doi.org/10.3390/life15081215
Chicago/Turabian StyleKarner, Eva, Lara Krepler, Petra Pateisky, Agnes Grill, Paul Dremsek, Guelen Yerlikaya-Schatten, and Stephanie Springer. 2025. "Outcomes of Pregnancies with Absent or Hypoplastic Fetal Nasal Bone: A Retrospective Analysis of Prenatal Findings and Perinatal Outcomes" Life 15, no. 8: 1215. https://doi.org/10.3390/life15081215
APA StyleKarner, E., Krepler, L., Pateisky, P., Grill, A., Dremsek, P., Yerlikaya-Schatten, G., & Springer, S. (2025). Outcomes of Pregnancies with Absent or Hypoplastic Fetal Nasal Bone: A Retrospective Analysis of Prenatal Findings and Perinatal Outcomes. Life, 15(8), 1215. https://doi.org/10.3390/life15081215






