1. Introduction
Spirulina, classified under the genera
Arthrospira or
Limnospira, is a significant microalga renowned for its abundant nutritional content and diverse bioactive compounds [
1]. This rich composition positions it as an important functional food, a valuable component in animal feed, and a promising resource in medicinal applications [
2]. The diverse applications and nutritional profile of
Limnospira platensis underscore its significance as a valuable resource.
Limnospira is a blue-green microalga with a high growth rate, recognized for its significant polysaccharide content, contributing to its numerous health benefits. High-molecular-weight polysaccharides have demonstrated immunomodulatory, anti-inflammatory, and anticancer effects, as well as anti-aging properties [
2,
3,
4].
Limnospira has a higher protein content than soybeans (55–70%) [
5] and provides essential amino acids for the human body [
6], with a carbohydrate content of 15–25% [
7] and lipid content of 6–9% featuring essential fatty acids [
6,
8].
Limnospira also contains various pigments, minerals, vitamins, and phenolic compounds [
6,
9], making it a promising subject for exploration in health and wellness contexts, and polysaccharides derived from carbohydrates with glucose as a key component [
10].
The polysaccharide composition of
Limnospira is complex, consisting of various monosaccharides including rhamnose, mannose, xylose, and galactose, as well as two less common sugars [
11]. These polysaccharides are critical for the biological functions of
Limnospira and are currently undergoing investigation for their potential applications in health and medicine [
12]. Polysaccharides from natural sources are increasingly recognized for their various beneficial biological activities for health applications. These macromolecules exhibit physiological effects such as antioxidant, anti-inflammatory, immunomodulatory, antitumor, antidiabetic, and prebiotic activities, making them promising candidates for functional foods and dietary supplements [
12]. Polysaccharides from
L. platensis (
Spirulina) enhance the immune function, modulate gut microbiota, and improve insulin sensitivity. They also stimulate beneficial gut bacteria, regulate inflammation, and improve lipid and glucose metabolism, supporting their use in prebiotic formulations [
10]. However, the link between the structural characteristics and functions of polysaccharides remains inadequately understood, with further research into their chemical properties and mechanisms required to facilitate integration into evidence-based therapeutic and nutritional applications. The polysaccharides in
Limnospira provide an essential nutritional source for beneficial gut bacteria such as
Lactobacilli and
Bifidobacteria [
10]. These microorganisms play a pivotal role in maintaining a healthy gut microbiome, thereby contributing to gastrointestinal health and well-being [
13]. The extraction of polysaccharides from microalgae is a fundamental procedure for their isolation and subsequent applications. Various methodologies are available for polysaccharide extraction including water extraction, alkali extraction [
14], enzyme extraction [
12], and ultrasound-assisted methods [
15]. Each of these techniques possesses distinct advantages and limitations, and the selection of a particular method can significantly influence the yield of the extracted polysaccharides. Careful selection of appropriate safety solvents is paramount during the extraction process, particularly when considering the bioactivity of the resultant polysaccharides [
16]. Therefore, it is imperative to evaluate the effectiveness and safety of each extraction technique in the context of the intended applications of the functional polysaccharides.
Polysaccharides extracted from
Limnospira have attracted significant interest due to their various biological properties and potential health benefits. The characterization of these polysaccharides is crucial for a comprehensive understanding of their structural attributes and functionalities [
17]. This study investigated the extraction methods to characterize the biological properties associated with polysaccharides derived from
Limnospira. Our findings underscore the importance of these compounds in wide ranging applications including food, pharmaceuticals, and nutraceuticals. In contemporary agricultural and multidisciplinary science, the analysis of trace elements in food and feed is increasingly conducted using synchrotron X-ray fluorescence (XRF). This technique has garnered significant interest by providing detailed elemental composition with high sensitivity and spatial resolution. Synchrotron XRF facilitates a deeper understanding of nutrient content and contamination levels in agricultural products, ultimately contributing to enhanced food safety and optimized agricultural practices. Research in this field continues to advance, and XRF technology presents promising opportunities for improving the quality and safety of food and feed [
18,
19,
20,
21,
22,
23].
This study investigated the efficacy of various extraction techniques on the yield and biochemical composition of polysaccharides extracted from Spirulina (Limnospira platensis). The antioxidant, α-glucosidase inhibition, and probiotic properties of polysaccharides were also evaluated to determine their potential as functional ingredients in health applications.
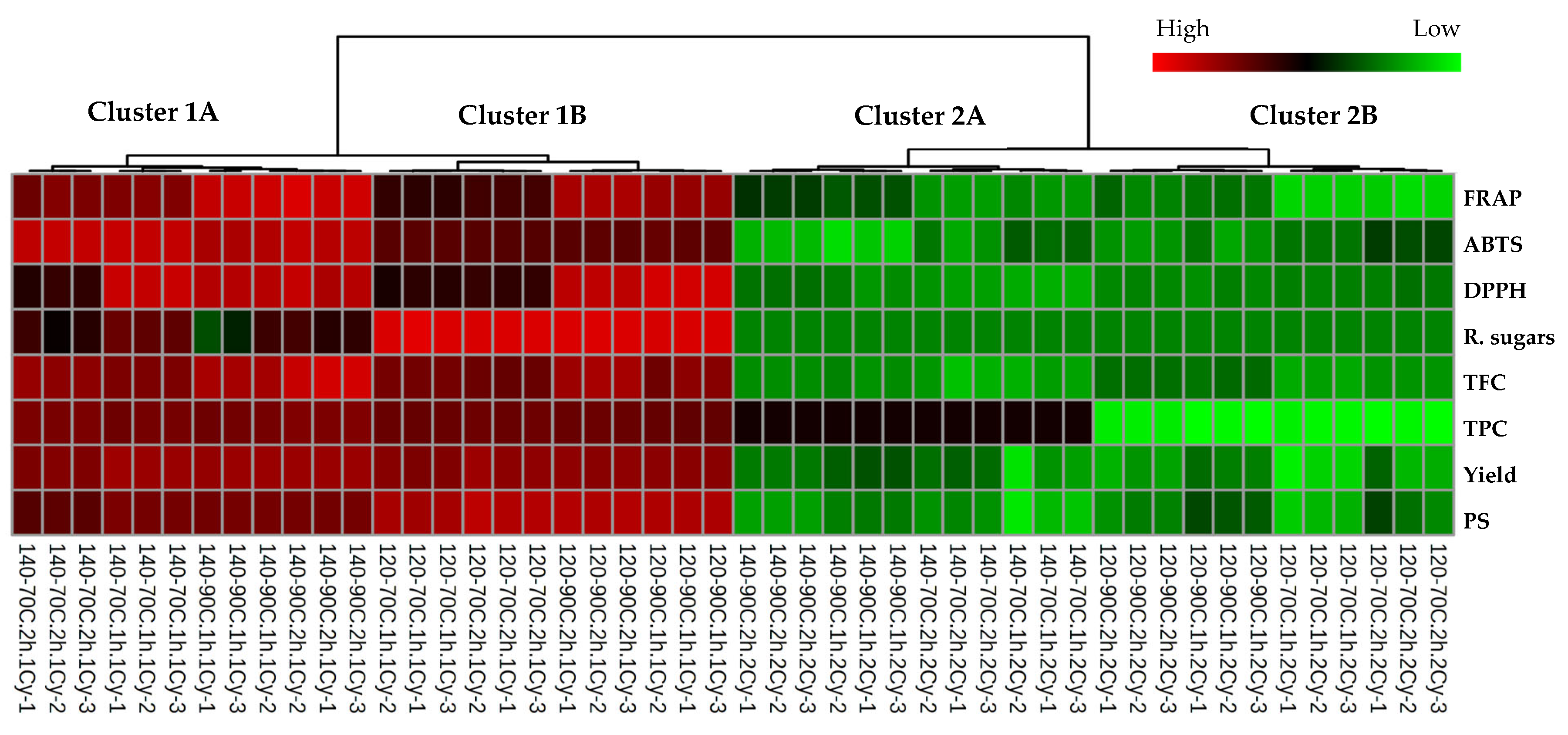
4. Discussion
Limnospira platensis, a blue-green alga, has a well-known nutritional composition and functional properties [
41]. A previous paper recorded the carbohydrate content in
L. platensis biomass to be 2.42 to 30.7% [
42]. Carbohydrate accumulation in
Limnospira platensis is influenced by multiple factors including the specific strain utilized, the cultivation methodology, the composition of the growth medium, and environmental variables such as light intensity, temperature, agitation rates, and carbon dioxide concentration [
43,
44,
45]. In this study, the carbohydrate content of
L. platensis was similar to previous reports at 10–14% [
46]. Polysaccharide extraction from
L. platensis was conducted utilizing water as a sustainable extraction method. The cell wall of
Limnospira sp. is predominantly composed of intricate heteropolysaccharides and glycoproteins, indicating that
Limnospira is void of cellulose, thereby rendering its cell wall more digestible and less rigid [
47,
48]. These polysaccharides are essential for maintaining cellular morphology, providing structural support and protecting the cell from environmental stressors [
49]. Our results indicated that water-soluble polysaccharides from
L. platensis were influenced by biomass concentration, extraction temperature and duration, and the number of extraction cycles. An increase in biomass concentration was associated with a higher level of polysaccharides. Enhancing the biomass/solvent ratio during polysaccharide extraction initially boosted the yield of polysaccharides [
50], while increasing the extraction temperature and duration significantly enhanced polysaccharide yield during the extraction process. Elevated temperatures within an appropriate range improved the solubility of polysaccharides and facilitated their release into the extraction solvent. However, excessively high temperatures or extended extraction times promoted the degradation of polysaccharides, adversely affecting their yield and structural integrity [
51]. Elevated temperatures enhanced the diffusion rates of polysaccharides, thereby facilitating their extraction from the biomass [
52]. Temperatures above a critical threshold of 180 °C led to the degradation of proteins and also the thermal degradation of polysaccharides [
53]. Extended extraction durations resulted in the breakdown of polysaccharides, ultimately decreasing the total yield [
54]. Results revealed an increase in polysaccharide concentration at a high biomass/water concentration of 1:20%
w/
v, particularly when the extraction temperature was set at 7 °C, producing polysaccharide concentration results similar to those observed at 90 °C. By contrast, the extraction process gave the highest output at a lower biomass concentration of 1:40%
w/
v, demonstrating comparable results across various extraction temperatures and time durations and showcasing the nuanced impact of these parameters on extraction efficiency. Our findings suggest that repetition of the extraction process during the second cycle is ineffective for polysaccharide extraction from
L. platensis, attributed to the absence of a cell wall, which only facilitates efficient extraction of polysaccharides in the initial cycle. Water extraction was selected as the primary method due to its alignment with green chemistry principles, cost-effectiveness, and safety for applications involving food, nutraceuticals, and probiotics. Unlike chemical (e.g., alkaline) or enzymatic methods, water extraction avoids the use of harsh reagents or costly enzymes, thereby preserving the native structure and functional integrity of bioactive compounds such as polysaccharides and pigments [
55,
56]. Moreover, water extraction minimizes residual contamination, making it particularly suitable for downstream biological or probiotic assays where purity and non-toxicity are critical [
57]. Ultrasound-assisted extraction (UAE), enzyme-assisted extraction (EAE), or alkali treatment enhance extraction efficiency and yield but may also lead to degradation or structural modification of sensitive compounds [
58,
59]. These methods often require the optimization of multiple parameters (e.g., enzyme type/concentration, pH, temperature), which increases processing complexity and cost. This study prioritized a clean-label and scalable approach, suitable for functional food applications.
A previous report detailed the extraction conditions for polysaccharides from
Limnospira using a range of methodologies. Each methodology has unique conditions that yield varying results. Conventional hot water extraction typically yields 8.35% polysaccharides from
L. platensis at 80 °C for 8 h [
60]. This extraction technique is notable for its efficacy in isolating valuable polysaccharide compounds, highlighting its relevance in the field of biopolymer research and applications. The controlled parameters of temperature and time play crucial roles in optimizing the yield and quality of the extracted polysaccharides, making this method popular within natural product chemistry [
61]. Alkali extraction significantly enhanced the yield of polysaccharides from
L. platensis, with 65% efficiency under optimal conditions. Key parameters for this process included biomass-to-solvent ratio 1:50 (%
w/
v), alkaline pH of 10.25, and extraction at a controlled temperature of 89.24 °C for 10 h. These conditions are crucial for maximizing polysaccharide extraction and demonstrate the effectiveness of alkali treatment in extracting valuable compounds from algal biomass [
14]. Ultrasonic-assisted extraction techniques have demonstrated significant efficacy in enhancing polysaccharide yields from various sources. A recent study involving
L. platensis reported a crude polysaccharide yield of 14.78% under optimized conditions utilizing a biomass-to-solvent ratio of 1:30%
w/
v at 81 °C for 30 min and an ultrasonic power setting of 92 W. These parameters indicate the potential of ultrasonic assistance in maximizing polysaccharide extraction efficiency from microalgal sources [
62]. The optimal conditions for polysaccharide extraction from microalgae are influenced by multiple factors and methods, which must be analyzed and considered for their implications in subsequent applications.
The extraction procedure employed for
L. platensis facilitated the isolation of polysaccharides and also various other bioactive compounds. Our investigation revealed that the water-soluble polysaccharides derived from
L. platensis contained significant levels of total phenolic and flavonoid contents, and a notable quantity of reducing sugars with antioxidant properties. These findings underscored the potential of
L. platensis as a source of bioactive substances with possible health benefits. A previous report indicated that increasing the extraction temperature led to higher phenolic content and enhanced antioxidant activity. Hot water extraction gave higher TPC, TFC, and antioxidant activity compared with extraction at room temperature [
63], supporting the effectiveness of our polysaccharide extraction method. Exploring the diverse chemical profiles of polysaccharides obtained from
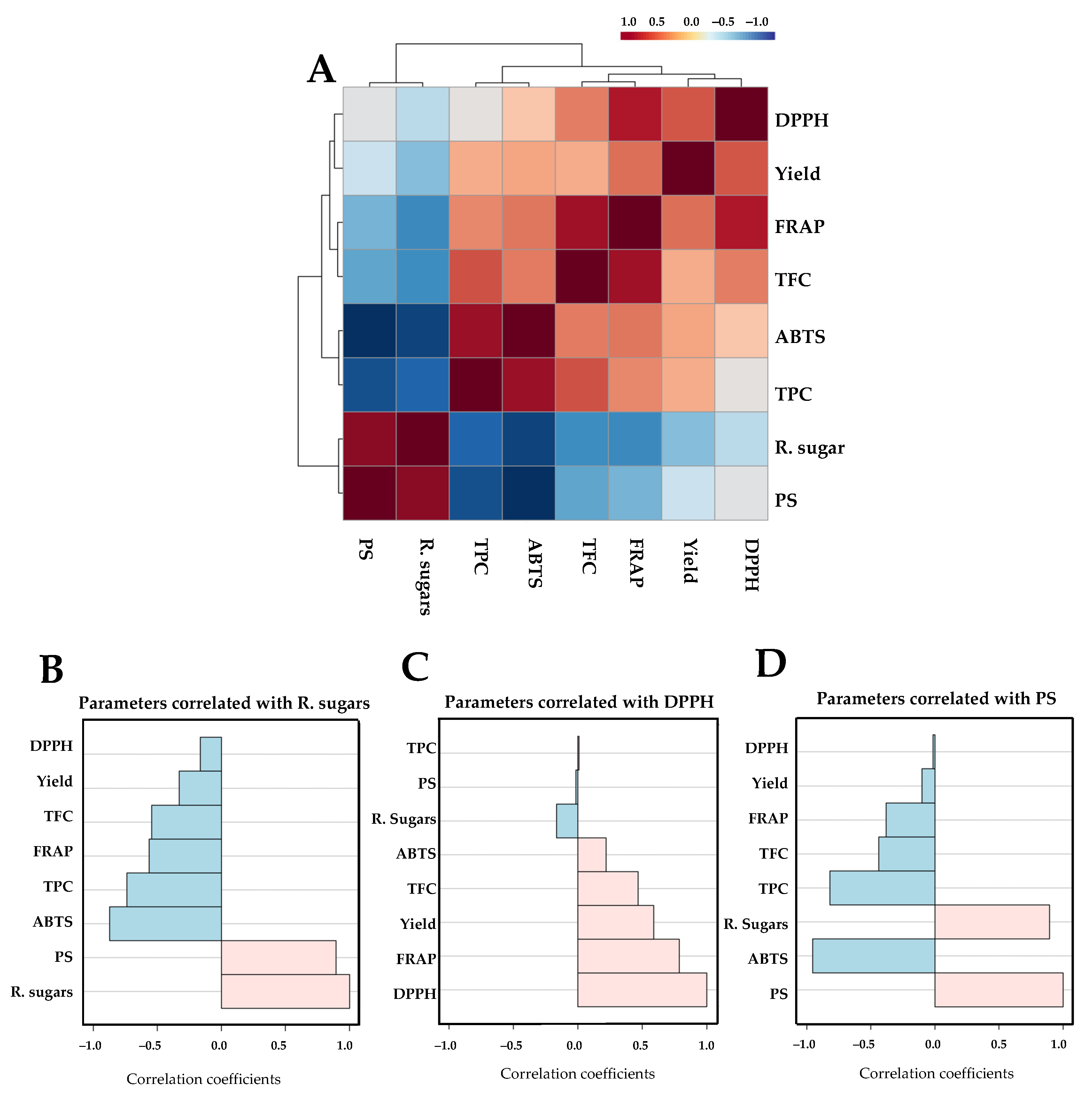
L. platensis unveiled fascinating insights. The levels of reducing sugars, DPPH, and polysaccharide content emerged as pivotal indicators. These parameters showed the potential to characterize the variations in extracted polysaccharides under different conditions throughout this study, highlighting the intricate relationship between extraction methods and the biochemical composition of these compounds.
The extraction yield of polysaccharides from
L. platensis obtained through hot water or mild method set up to 54.62 mg/g significantly exceeded other microalgae such as
Chlorella sp. (13–19%) [
64,
65], and was higher than the yields achieved through ultrasonication for
Caulerpa lentillifera (7.8%) [
66]. The bioactive compounds—TPC (3.66 mg GAE/g) and TFC (0.90 mg QE/g)—were similar to extracts from
Haematococcus pluvialis (around 4 mg GAE/g) and exceeded plant fiber extracts like inulin or pectin [
67]. Water-based extraction has moderate efficiency but is a low-cost, safe, and food-grade method that is competitive with more complex techniques such as microwave or pressure-assisted extraction. Specialized methods may enhance yield and selectivity, but our extraction approach provides eco-friendly, sustainable, and food-grade results with minimal processing.
The polysaccharides derived from
Limnospira were primarily characterized by their average molecular weight, the types of monosaccharides present, and the positioning of their glycosidic bonds. Results in
Table 8 show that the most prevalent monosaccharide in
Limnospira sp. from various locations was glucose, followed by rhamnose and galactose. The monosaccharide profile following acid hydrolysis of
L. platensis polysaccharide extracts aligned with findings from previous studies. The accumulation of glucose within the cell body is related to the photosynthetic activity of cyanobacteria, which convert carbon dioxide into sugars [
68]. By contrast, the production of other monosaccharides corresponds to the clumping behavior observed in cyanobacteria, particularly the significant amount of rhamnose [
69]. Variations in monosaccharide composition result in unique functional properties and food applications such as thickening agents, stabilizers, and flocculants [
68,
70,
71]. The presence of free monosaccharides in minimal amounts indicated that the sugar composition in spirulina is associated with polymer structures [
7]. A previous report identified a prebiotic oligosaccharide (SPO-1 fraction) featuring glucose molecules with an α-1,4-glycosidic linkage sourced from spirulina [
30]. To date, the biological activities attributed to
Limnospira include antioxidant, anticancer, anti-inflammatory, and antidiabetic properties as well as prebiotic potentials [
30,
72,
73].
The beneficial effects of oligosaccharides in
Limnospira, particularly in the context of probiotic activity, are well-documented in contemporary research. Short-chain oligosaccharides (with a degree of polymerization ranging from 3 to 10) play a crucial role in promoting the growth of health-beneficial bacteria through selective fermentation and the production of advantageous metabolites [
75,
76,
77]. Studies have indicated that polysaccharide extracts derived from spirulina encompass a diverse mixture of maltotriose, panose, and short-chain gluco-oligosaccharides (GlcOSs). Maltotriose, classified within the category of maltodextrins (MOSs), significantly enhances the viability of
Bifidobacterium breve, outperforming other oligosaccharides such as galacto-oligosaccharides (GOSs) and fructo-oligosaccharides (FOSs) after 24 h of fermentation [
78]. During the fermentation process, MOSs facilitate the production of short-chain fatty acids (SCFAs) in the intestine while simultaneously inhibiting pathogenic bacteria [
79,
80]. By contrast, panose, recognized as an isomalto-oligosaccharide, encourages the growth of various
Bifidobacterium species including
Bifidobacterium lactis. This oligosaccharide enhances the production of butyrate and acetate during colonic fermentation [
81,
82]. The structural complexity of GlcOSs—including parameters such as degree of polymerization, sugar composition, stereochemistry, and various glycosidic linkages—plays a pivotal role in determining their probiotic activity, influencing the non-digestibility and selective fermentability of these oligosaccharides [
77,
83,
84].
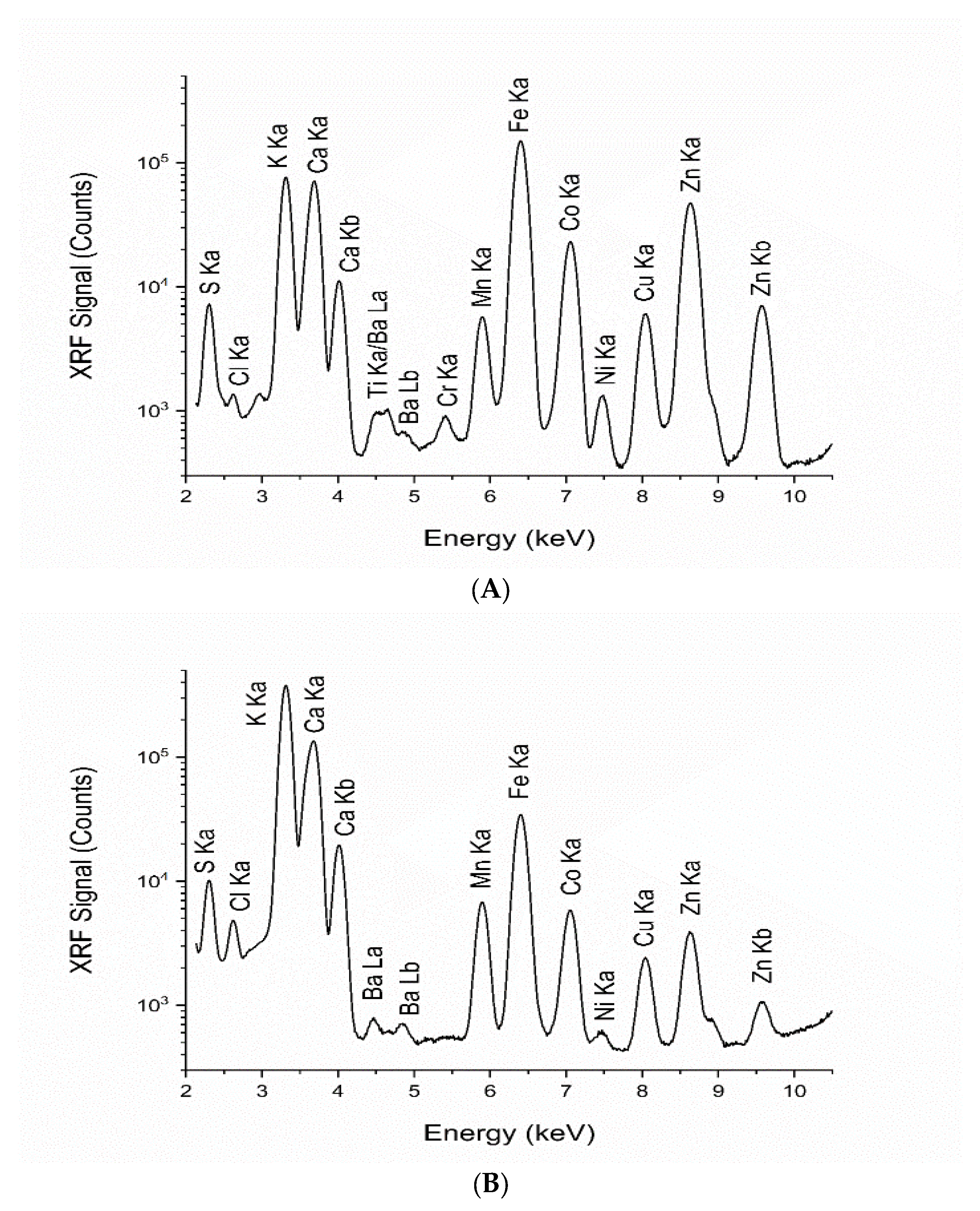
The elemental compositions of the microalgal biomass and polysaccharide extracts were analyzed using synchrotron X-ray fluorescence (XRF) at the Synchrotron Light Research Institute (SLRI). This advanced technique offers significant advantages in detecting inorganic mineral nutrients often present in trace amounts. Unlike traditional methods, synchrotron XRF can simultaneously identify a broad range of trace elements without the need for chemical pretreatment, providing a more accurate representation of their nutritional properties. The recent opening of Beamline 7.2 W has further expanded the potential for XRF experiments, making it possible to analyze a wide variety of elements in spirulina and its extracts. This comprehensive analysis underscores the importance of understanding the nutritional balance in this microalga, highlighting its potential as a valuable dietary component. The application of the XRF technique at BL7.2 in this study facilitated the simultaneous detection of a broad range of elements. Chemical analysis by synchrotron XRF yielded complementary information and served as a non-destructive method, enabling the examination of samples without the need for previous treatment. Our study identified elevated concentrations of sulfur, potassium, and calcium in
L. platensis and the polysaccharide powder derived from
L. platensis. The samples were dried, ground, pelletized, and subsequently analyzed using synchrotron XRF. The analysis also revealed essential inorganic mineral nutrients including manganese, iron, cobalt, nickel, copper, zinc, and barium in both samples, underscoring the significant advantage of the beamline in detecting inorganic mineral nutrients, which are found in considerable amounts. Elemental compositions in
Spirulina powder samples vary in different countries including China, Bulgaria, Belgium, and the USA [
85]. Elemental analysis of
Spirulina samples by synchrotron XRF differed depending on sample origin, cultivating protocol, processing method, and environmental conditions. This technique identifies a wide variety of minor and trace elements and also evaluates inorganic minerals and nutrients, thereby providing valuable insights for enhancing nutritional knowledge in practical food applications.
Lactobacillus rhamnosus and
Bifidobacterium longum were selected for this study due to their importance as key members of the human gut microbiota and their common use in prebiotic research [
86,
87]. These strains have been well-studied for their probiotic properties including improving gut barrier function, modulating immune responses, and producing beneficial metabolites such as short-chain fatty acids (SCFAs) [
88]. Both species effectively respond to non-digestible oligosaccharides and polysaccharides, making them ideal for evaluating the prebiotic potential of new compounds like
L. platensis-derived polysaccharides [
89].
L. rhamnosus has shown notable growth in response to microalgal polysaccharides [
90], while
B. longum serves as a common biomarker in functional food research due to its sensitivity to dietary fibers [
91]. Thus, their inclusion allowed for a targeted assessment of the extracted polysaccharides’ selective stimulation capacity. The evaluation of polysaccharide powder from
L. platensis to support the growth of
Lactobacillus rhamnosus ATCC 53103 and
Bifidobacterium longum TISTR 2195 was compared with glucose and inulin as controls.
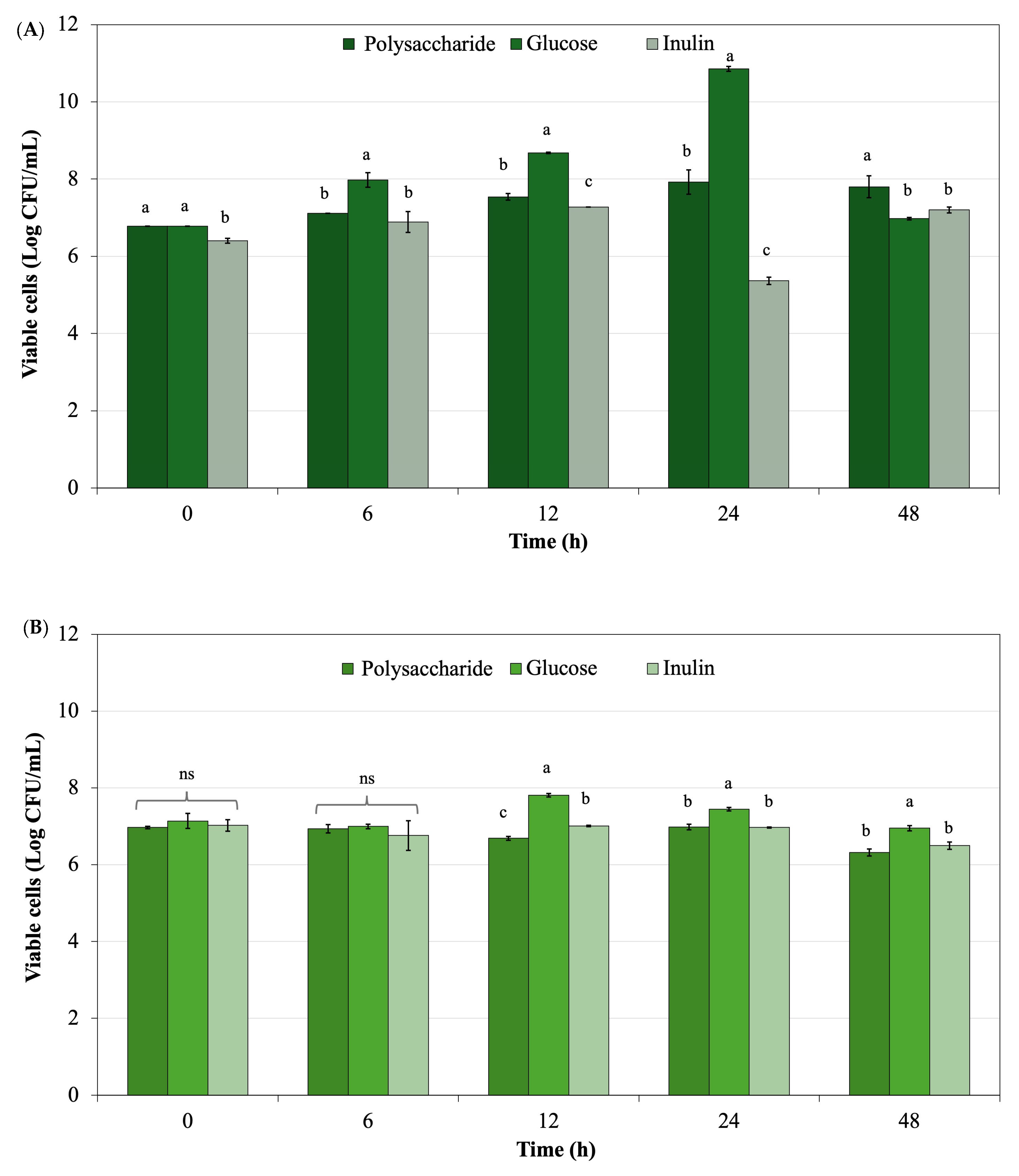
Figure 8 shows the probiotic growth with various supplemented ingredients including polysaccharide powder derived from
L. platensis, glucose, and inulin during 48 h of culture. Viable cells of
L. rhamnosus ATCC 53103, obtained from polysaccharide powder supplemented in the culture, were present in lower quantities than those derived from glucose, the standard medium. Viable cells from the polysaccharide powder were superior when compared with inulin, a recognized commercial prebiotic. For
B. longum TISTR 2195, the highest viable cell count was observed in glucose culture, while polysaccharide powder derived from
L. platensis exhibited a trend similar to inulin when supplemented in the culture.
The chemical analysis of polysaccharides derived from
L. platensis revealed functional groups that suggested a range of health benefits. The presence of hydroxyl, carboxyl, and glycosidic linkages indicated strong antioxidant potential, capable of scavenging free radicals and reducing oxidative stress linked to chronic diseases [
89]. These polysaccharides also have anti-inflammatory effects by influencing cytokine production and immune cell activation [
12,
92]. Their immunomodulatory properties, associated with molecular weight and structural complexity, enhance host defenses [
93]. The ability to inhibit α-glucosidase also points to potential antidiabetic effects, relevant for glycemic control [
94]. Overall, these bioactivities position
L. platensis polysaccharides as promising candidates for functional foods and therapeutic agents, warranting further structural elucidation and in vivo studies to fully explore their potential.
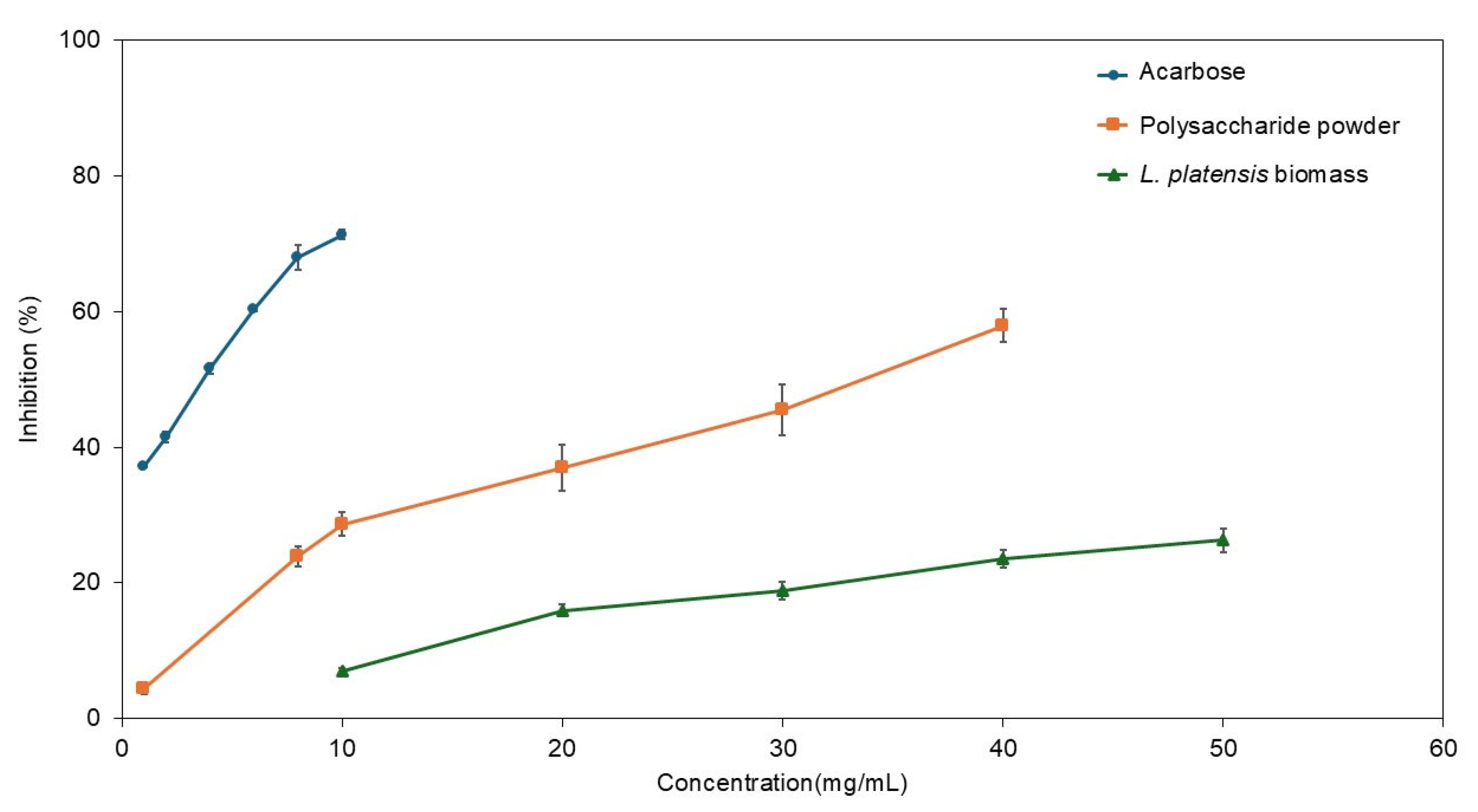
The inhibitory effects of
Limnospira on α-glucosidase are noteworthy. Carbohydrate metabolism relies heavily on the activity of α-amylase and α-glucosidase. Disruptions in the release and absorption of monosaccharides into the bloodstream contribute to type 2 diabetes (T2DM), which significantly affects lifestyles and has a rising mortality rate worldwide [
95,
96]. Targeting the inhibition of α-glucosidase is a crucial step in screening for new sources of antidiabetic compounds. Microalgae and their bioactive constituents have demonstrated considerable antidiabetic potential [
97,
98]. Various extracts from spirulina have been shown to suppress α-glucosidase such as water-extracted peptides (IC
50 at 134.2 µg/mL), 80% methanol extract (IC
50 at 9.56 mg/mL), butanol fraction (IC
50 at 23.0 µg/mL), ethyl acetate fraction (IC
50 at 35.7 µg/mL), and
p-coumaric acid fraction (IC
50 at 3.28 mg/mL) [
95,
99,
100]. Our results indicated that polysaccharides derived from
L. platensis can inhibit α-glucosidase activity utilizing safety and simplicity extraction technology. Future research should focus on the purification and fractionation of polysaccharides to enhance the α-glucosidase suppression effects of
Limnospira microalgae.
In this study, Polysaccharides extracted from
L. platensis exhibited promising bioactive properties and high yields—potentially exceeding those of other microalgae and plant sources—with significant practical applications. Their moderate α-glucosidase inhibitory activity suggests that they could serve as natural agents for managing postprandial blood glucose levels, making them valuable in diabetic supplement development [
67,
101]. Furthermore, their elevated phenolic and flavonoid content, along with prebiotic effects on beneficial gut bacteria like
Lactobacillus rhamnosus and
Bifidobacterium longum, highlights their potential in promoting gut health and metabolic balance [
89,
102]. Therefore, these polysaccharides demonstrate considerable promise for nutritional and therapeutic applications, with ongoing research likely to expand their scope of use.