Beneficial Effects of Water-Based Exercise Alone and in Combination with Cognitive Training on Cardiovascular Fitness and Arterial Stiffness in Older Adults with Mild Cognitive Impairment: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
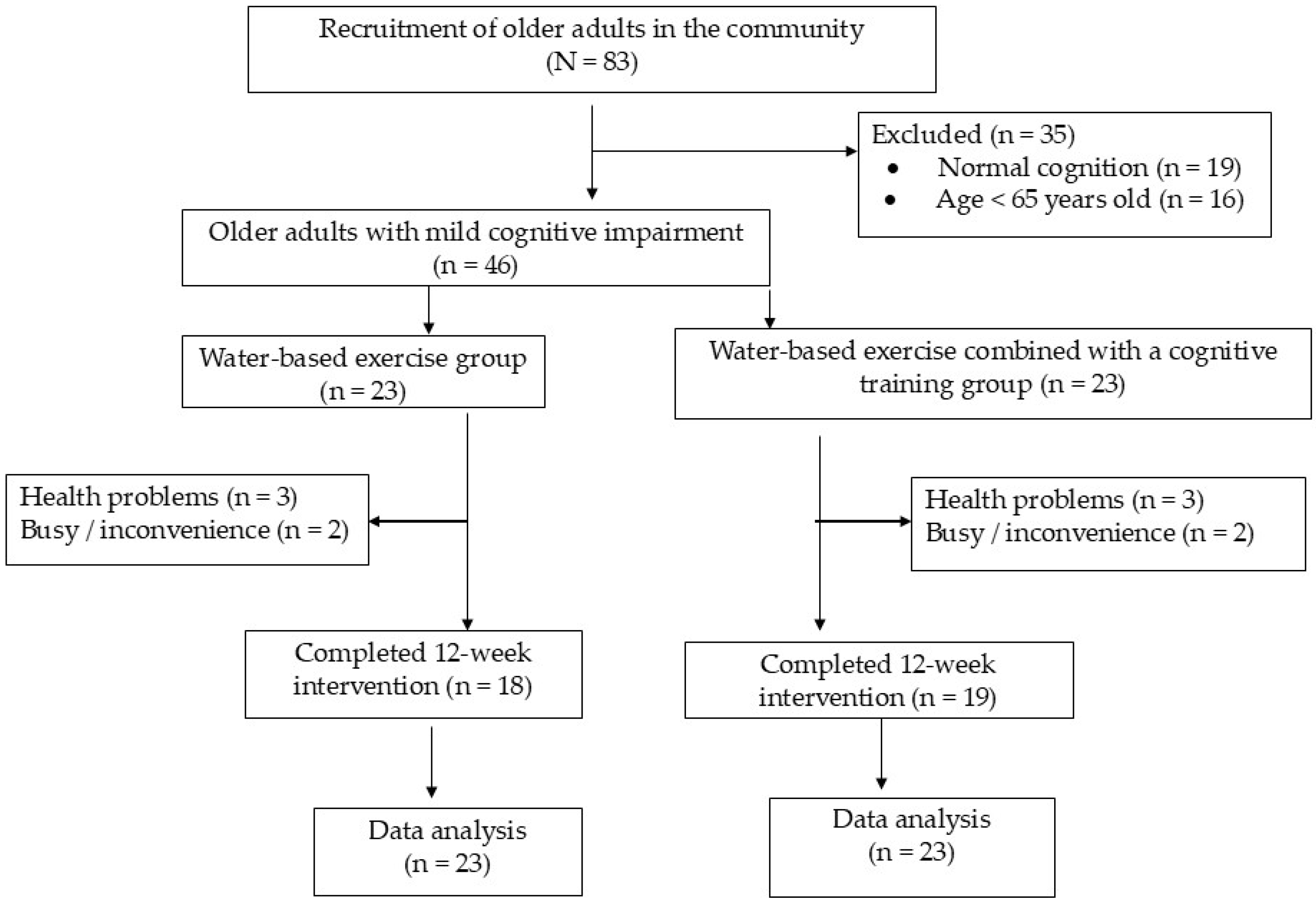
2.1. Participants and Design
2.2. Procedures
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.; Kim, H.J. Normal Aging Induces Changes in the Brain and Neurodegeneration Progress: Review of the Structural, Biochemical, Metabolic, Cellular, and Molecular Changes. Front. Aging Neurosci. 2022, 14, 931536. [Google Scholar] [CrossRef]
- Blum, L.; Rosenbaum, D.; Röben, B.; Dehnen, K.; Maetzler, W.; Suenkel, U.; Fallgatter, A.J.; Ehlis, A.-C.; Metzger, F.G. Age-related deterioration of performance and increase of cortex activity comparing time- versus item-controlled fNIRS measurement. Sci. Rep. 2021, 11, 6766. [Google Scholar] [CrossRef]
- Langa, K.M.; Levine, D.A. The diagnosis and management of mild cognitive impairment: A clinical review. JAMA 2014, 312, 2551–2561. [Google Scholar] [CrossRef]
- Farias, S.T.; Mungas, D.; Reed, B.R.; Harvey, D.; DeCarli, C. Progression of mild cognitive impairment to dementia in clinic- vs. community-based cohorts. Arch. Neurol. 2009, 66, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Bonarjee, V.V.S. Arterial Stiffness: A Prognostic Marker in Coronary Heart Disease. Available Methods and Clinical Application. Front. Cardiovasc. Med. 2018, 5, 64. [Google Scholar] [CrossRef]
- Triantafyllias, K.; Thiele, L.E.; Cavagna, L.; Baraliakos, X.; Bertsias, G.; Schwarting, A. Arterial Stiffness as a Surrogate Marker of Cardiovascular Disease and Atherosclerosis in Patients with Arthritides and Connective Tissue Diseases: A Literature Review. Diagnostics 2023, 13, 1870. [Google Scholar] [CrossRef]
- Boutouyrie, P.; Chowienczyk, P.; Humphrey, J.D.; Mitchell, G.F. Arterial stiffness and cardiovascular risk in hypertension. Circ. Res. 2021, 128, 864–886. [Google Scholar] [CrossRef]
- Zhong, W.; Cruickshanks, K.J.; Schubert, C.R.; Carlsson, C.M.; Chappell, R.J.; Klein, B.E.; Klein, R.; Acher, C.W. Pulse wave velocity and cognitive function in older adults. Alzheimer Dis. Assoc. Disord. 2014, 28, 44–49. [Google Scholar] [CrossRef]
- Bareiro, F.A.Q.; Carnicero, J.A.; Acha, A.A.; Artalejo, C.R.; Jimenez, M.C.G.; Mañas, L.R.; García García, F.J. Carotid-femoral pulse wave velocity score, an estimator of cognitive performance in the elderly: Results from the Toledo Study for Healthy Aging. Geroscience 2024, 46, 5711–5723. [Google Scholar] [CrossRef]
- Hirasawa, A.; Nagai, K.; Miyazawa, T.; Koshiba, H.; Tamada, M.; Shibata, S.; Kozaki, K. Relationship between arterial stiffness and cognitive function in outpatients with dementia and mild cognitive impairment compared with community residents without dementia. J. Geriatr. Cardiol. 2022, 19, 594–602. [Google Scholar] [CrossRef]
- Li, X.; Lyu, P.; Ren, Y.; An, J.; Dong, Y. Arterial stiffness and cognitive impairment. J. Neurol. Sci. 2017, 380, 1–10. [Google Scholar] [CrossRef]
- Araghi, M.; Shipley, M.J.; Wilkinson, I.B.; McEniery, C.M.; Valencia-Hernández, C.A.; Kivimaki, M.; Sabia, S.; Singh-Manoux, A.; Brunner, E.J. Association of aortic stiffness with cognitive decline: Whitehall II longitudinal cohort study. Eur. J. Epidemiol. 2020, 35, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Bueno, C.; Cunha, P.G.; Martinez-Vizcaino, V.; Pozuelo-Carrascosa, D.P.; Visier-Alfonso, M.E.; Jimenez-Lopez, E.; Cavero-Redondo, I. Arterial Stiffness and Cognition Among Adults: A Systematic review and meta-analysis of observational and longitudinal Studies. J. Am. Heart Assoc. 2020, 9, e014621. [Google Scholar] [CrossRef]
- Triantafyllidi, H.; Arvaniti, C.; Lekakis, J.; Ikonomidis, I.; Siafakas, N.; Tzortzis, S.; Trivilou, P.; Zerva, L.; Stamboulis, E.; Kremastinos, D.T. Cognitive impairment is related to increased arterial stiffness and microvascular damage in patients with never-treated essential hypertension. Am. J. Hypertens. 2009, 22, 525–530. [Google Scholar] [CrossRef]
- Yamamoto, N.; Yamanaka, G.; Ishikawa, M.; Takasugi, E.; Murakami, S.; Yamanaka, T.; Ishine, M.; Matsubayashi, K.; Hanafusa, T.; Otsuka, K. Cardio-ankle vascular index as a predictor of cognitive impairment in community-dwelling elderly people: Four-year follow-up. Dement. Geriatr. Cogn. Disord. 2009, 28, 153–158. [Google Scholar] [CrossRef]
- Yukutake, T.; Yamada, M.; Fukutani, N.; Nishiguchi, S.; Kayama, H.; Tanigawa, T.; Adachi, D.; Hotta, T.; Morino, S.; Tashiro, Y.; et al. Arterial stiffness determined according to the cardio-ankle vascular index (CAVI) is associated with mild cognitive decline in community-dwelling elderly subjects. J. Atheroscler. Thromb. 2014, 21, 49–55. [Google Scholar] [CrossRef]
- Amjad, I.; Toor, H.; Niazi, I.K.; Afzal, H.; Jochumsen, M.; Shafique, M.; Allen, K.; Haavik, H.; Ahmed, T. Therapeutic effects of aerobic exercise on EEG parameters and higher cognitive functions in mild cognitive impairment patients. Int. J. Neurosci. 2019, 129, 551–562. [Google Scholar] [CrossRef]
- Mandolesi, L.; Polverino, A.; Montuori, S.; Foti, F.; Ferraioli, G.; Sorrentino, P.; Sorrentino, G. Effects of physical exercise on cognitive functioning and wellbeing: Biological and psychological benefits. Front. Psychol. 2018, 9, 509. [Google Scholar] [CrossRef]
- Law, C.K.; Lam, F.M.; Chung, R.C.; Pang, M.Y. Physical exercise attenuates cognitive decline and reduces behavioural problems in people with mild cognitive impairment and dementia: A systematic review. J. Physiother. 2020, 66, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Bherer, L.; Erickson, K.I.; Liu-Ambrose, T. A Review of the effects of physical activity and exercise on cognitive and brain functions in older adults. J. Aging Res. 2013, 2013, 657508. [Google Scholar] [CrossRef] [PubMed]
- Blondell, S.J.; Hammersley-Mather, R.; Veerman, J.L. Does physical activity prevent cognitive decline and dementia?: A systematic review and meta-analysis of longitudinal studies. BMC Public Health 2014, 14, 510. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef]
- Okamoto, T.; Hashimoto, Y. Decreases in Arterial stiffness and wave reflection after isometric handgrip training are associated with improvements in cognitive function in older adults. Int. J. Environ. Res. Public Health 2022, 19, 9585. [Google Scholar] [CrossRef]
- Tomoto, T.; Liu, J.; Tseng, B.Y.; Pasha, E.P.; Cardim, D.; Tarumi, T.; Hynan, L.S.; Munro, C.C.; Zhang, R. One-year aerobic exercise reduced carotid arterial stiffness and increased cerebral blood flow in amnestic mild cognitive impairment. J. Alzheimers Dis. 2021, 80, 841–853. [Google Scholar] [CrossRef]
- Shimada, H.; Makizako, H.; Doi, T.; Park, H.; Tsutsumimoto, K.; Verghese, J.; Suzuki, T. Effects of combined physical and cognitive exercises on Cognition and mobility in patients with mild cognitive impairment: A randomized clinical trial. J. Am. Med. Dir. Assoc. 2018, 19, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Delbroek, T.; Vermeylen, W.; Spildooren, J. The effect of cognitive-motor dual task training with the biorescue force platform on cognition, balance and dual task performance in institutionalized older adults: A randomized controlled trial. J. Phys. Ther. Sci. 2017, 29, 1137–1143. [Google Scholar] [CrossRef]
- Yang, C.; Moore, A.; Mpofu, E.; Dorstyn, D.; Li, Q.; Yin, C. Effectiveness of combined cognitive and physical interventions to enhance functioning in older adults with mild cognitive impairment: A systematic review of randomized controlled trials. Gerontologist 2020, 60, 633–642. [Google Scholar] [CrossRef]
- Torres-Ronda, L.; Del Alcázar, X.S. The properties of water and their applications for training. J. Hum. Kinet. 2014, 44, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Carayannopoulos, A.G.; Han, A.; Burdenko, I.N. The benefits of combining water and land-based therapy. J. Exerc. Rehabil. 2020, 16, 20–26. [Google Scholar] [CrossRef]
- Moreira, N.B.; da Silva, L.P.; Rodacki, A.L.F. Aquatic exercise improves functional capacity, perceptual aspects, and quality of life in older adults with musculoskeletal disorders and risk of falling: A randomized controlled trial. Exp. Gerontol. 2020, 142, 111135. [Google Scholar] [CrossRef]
- Ayán, C.; Carvalho, P.; Varela, S.; Cancela, J.M. Effects of Water-Based Exercise Training on the Cognitive Function and Quality of Life of Healthy Adult Women. J. Phys. Act. Health 2017, 14, 899–904. [Google Scholar] [CrossRef]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild cognitive impairment: Clinical characterization and outcome. Arch. Neurol. 1999, 56, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Rikli, R.E.; Jones, C.J. Development and validation of a functional fitness test for community-residing older adults. J. Aging Phys. 1999, 7, 129–161. [Google Scholar] [CrossRef]
- Munakata, M.; Ito, N.; Nunokawa, T.; Yoshinaga, K. Utility of automated brachial ankle pulse wave velocity measurements in hypertensive patients. Am. J. Hypertens. 2003, 16, 653–657. [Google Scholar] [CrossRef][Green Version]
- Donnezan, L.C.; Perrot, A.; Belleville, S.; Bloch, F.; Kemoun, G. Effects of simultaneous aerobic and cognitive training on executive functions, cardiovascular fitness and functional abilities in older adults with mild cognitive impairment. Ment. Health Phys. Act. 2018, 15, 78–87. [Google Scholar] [CrossRef]
- Fedor, A.; Garcia, S.; Gunstad, J. The effects of a brief, water-based exercise intervention on cognitive function in older adults. Arch. Clin. Neuropsychol. 2015, 30, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Nishiguchi, S.; Yamada, M.; Tanigawa, T.; Sekiyama, K.; Kawagoe, T.; Suzuki, M.; Yoshikawa, S.; Abe, N.; Otsuka, Y.; Nakai, R.; et al. A 12-week physical and cognitive exercise program can improve cognitive function and neural efficiency in community-dwelling older adults: A randomized controlled trial. J. Am. Geriatr. Soc. 2015, 63, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Farinha, C.; Teixeira, A.M.; Serrano, J.; Santos, H.; Campos, M.J.; Oliveiros, B.; Silva, F.M.; Cascante-Rusenhack, M.; Luís, P.; Ferreira, J.P. Impact of different aquatic exercise programs on body composition, functional fitness and cognitive function of non-institutionalized elderly adults: A randomized controlled trial. Int. J. Environ. Res. Public Health 2021, 18, 8963. [Google Scholar] [CrossRef]
- Mulser, L.; Moreau, D. Effect of acute cardiovascular exercise on cerebral blood flow: A systematic review. Brain Res. 2023, 1809, 148355. [Google Scholar] [CrossRef]
- Vigorito, C.; Giallauria, F. Effects of exercise on cardiovascular performance in the elderly. Front. Physiol. 2014, 5, 51. [Google Scholar] [CrossRef]
- Xu, L.; Gu, H.; Cai, X.; Zhang, Y.; Hou, X.; Yu, J.; Sun, T. The effects of exercise for cognitive function in older adults: A systematic review and meta-analysis of randomized controlled trials. Int. J. Environ. Res. Public Health 2023, 20, 1088. [Google Scholar] [CrossRef]
- Kang, D.W.; Bressel, E.; Kim, D.Y. Effects of aquatic exercise on insulin-like growth factor-1, brain-derived neurotrophic factor, vascular endothelial growth factor, and cognitive function in elderly women. Exp. Gerontol. 2020, 132, 110842. [Google Scholar] [CrossRef]
- Gómez-Pinilla, F.; Ying, Z.; Roy, R.R.; Molteni, R.; Edgerton, V.R. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J. Neurophysiol. 2002, 88, 2187–2195. [Google Scholar] [CrossRef]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. Elife 2016, 5, e15092. [Google Scholar] [CrossRef] [PubMed]
- Tomoto, T.; Verma, A.; Kostroske, K.; Tarumi, T.; Patel, N.R.; Pasha, E.P.; Riley, J.; Tinajero, C.D.; Hynan, L.S.; Rodrigue, K.M.; et al. One-year aerobic exercise increases cerebral blood flow in cognitively normal older adults. J. Cereb. Blood Flow Metab. 2023, 43, 404–418. [Google Scholar] [CrossRef]
- Sherlock, L.; Fournier, S.; DeVallance, E.; Lee, K.; Carte, S. Effects of shallow water aerobic exercise training on arterial stiffness and pulse wave analysis in older individuals. Int. J. Aquatic. Res. Educ. 2014, 8, 310–320. [Google Scholar] [CrossRef]
- Ho, L.Y.W.; Kwan, R.Y.C.; Yuen, K.M.; Leung, W.C.; Tam, P.N.; Tsim, N.M.; Ng, S.S.M. The Effect of Aerobic Exercises on Arterial Stiffness in Older People: A Systematic Review and Meta-Analysis. Gerontologist 2024, 64, gnad123. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.S.; Kim, J.H.; Kim, Y.S.; Kim, D.Y. Effects of aquarobic exercise and burdock intake on serum blood lipids and vascular elasticity in Korean elderly women. Exp. Gerontol. 2018, 101, 63–68. [Google Scholar] [CrossRef]
- Li, G.; Lv, Y.; Su, Q.; You, Q.; Yu, L. The effect of aerobic exercise on pulse wave velocity in middle-aged and elderly people: A systematic review and meta-analysis of randomized controlled trials. Front. Cardiovasc. Med. 2022, 9, 960096. [Google Scholar] [CrossRef] [PubMed]
- Yiming, G.; Zhou, X.; Lv, W.; Peng, Y.; Zhang, W.; Cheng, X.; Li, Y.; Xing, Q.; Zhang, J.; Zhou, Q.; et al. Reference values of brachial-ankle pulse wave velocity according to age and blood pressure in a central Asia population. PLoS ONE 2017, 12, e0171737. [Google Scholar] [CrossRef]
- Sang, Y.; Wu, X.; Miao, J.; Cao, M.; Ruan, L.; Zhang, C. Determinants of Brachial-Ankle Pulse Wave Velocity and Vascular Aging in Healthy Older Subjects. Med. Sci. Monit. 2020, 26, e923112. [Google Scholar] [CrossRef] [PubMed]
- DuPont, J.J.; Kenney, R.M.; Patel, A.R.; Jaffe, I.Z. Sex differences in mechanisms of arterial stiffness. Br. J. Pharmacol. 2019, 176, 4208–4225. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Kiechl, S.J.; Wang, J.; Xu, Q.; Kiechl, S.; Pechlaner, R. Global Pulse Wave Velocity Study Group. Global distributions of age- and sex-related arterial stiffness: Systematic review and meta-analysis of 167 studies with 509,743 participants. EBioMedicine 2023, 92, 104619. [Google Scholar] [CrossRef] [PubMed]
- Manly, J.J.; Jones, R.N.; Langa, K.M.; Ryan, L.H.; Levine, D.A.; McCammon, R.; Heeringa, S.G.; Weir, D. Estimating the prevalence of dementia and mild cognitive impairment in the US: The 2016 Health and Retirement Study Harmonized Cognitive Assessment Protocol Project. JAMA Neurol. 2022, 79, 1242–1249. [Google Scholar] [CrossRef]
- Kehmeier, M.N.; Walker, A.E. Sex Differences in Large Artery Stiffness: Implications for Cerebrovascular Dysfunction and Alzheimer’s Disease. Front. Aging. 2021, 2, 791208. [Google Scholar] [CrossRef]
- Dao, E.; Barha, C.K.; Santos, M.; Welch, M.; Liu-Ambrose, T. Sex Differences in the Relationship Between Arterial Stiffness and Cognitive Function in Older Adults. J. Stroke Cerebrovasc. Dis. 2022, 31, 106175. [Google Scholar] [CrossRef]
| Total (n = 46) | W Group (n = 23) | W-COG Group (n = 23) | X2 | p Value | |
|---|---|---|---|---|---|
| Sex | |||||
| Male (%) | 3 (6.52) | 2 (66.67) | 1 (33.33) | 0.357 | 1.000 |
| Female (%) | 43 (93.48) | 21 (43.84) | 22 (51.16) | ||
| Educational level | 5.248 | 0.155 | |||
| Primary (%) | 30 (65.22) | 16 (53.33) | 14 (46.67) | ||
| Secondary (%) | 6 (13.04) | 1 (16.67) | 5 (83.33) | ||
| High school (%) | 5 (10.87) | 4 (80.00) | 1 (20.00) | ||
| Bachelor’s degrees or over (%) | 6 (13.04) | 2 (33.33) | 4 (66.67) | ||
| Underlying disease (%) | 2.580 | 0.461 | |||
| 0 (%) | 10 (21.74) | 3 (30.00) | 7 (70.00) | ||
| 1 (%) | 18 (39.13) | 9 (50.00) | 9 (50.00) | ||
| 2 (%) | 10 (21.74) | 5 (50.00) | 5 (50.00) | ||
| ≥3 (%) | 9 (19.57) | 6 (66.67) | 3 (33.33) | ||
| Exercise | 0.521 | 0.471 | |||
| <3 days/week (%) | 22 (47.83) | 12 (54.55) | 10 (45.45) | ||
| ≥3 days/week (%) | 25 (54.35) | 11 (44.00) | 14 (56.00) | ||
| Mean ± SD | Mean ± SD | Mean ± SD | t(test) | p value | |
| Age (years) | 68.74 ± 3.52 | 68.26 ± 3.28 | 69.43 ± 3.65 | −1.147 | 0.258 |
| BMI (kg/m2) | 24.25 ± 3.30 | 24.50 ± 3.91 | 23.85 ± 2.61 | 0.661 | 0.512 |
| MoCA (scores) | 22.00 ± 2.19 | 21.70 ± 2.32 | 22.29 ± 2.05 | −0.933 | 0.356 |
| W Group (n = 23) | W-COG Group (n = 23) | Mean Difference ± SE W Group vs. W-COG Group | p Value Between Groups | |
|---|---|---|---|---|
| MoCA | ||||
| Before mean ± SD | 21.70 ± 2.32 | 22.48 ± 1.89 | −0.78 ± 0.62 | F (1,44) = 1.576, p = 0.216, np2 = 0.035 |
| After mean± SD | 25.48 ± 3.13 | 26.57 ± 2.95 | −1.09 ± 0.90 | F (1,44) = 1.467, p = 0.232, np2 = 0.032 |
| Mean difference (after-before) ± SE | 3.78 ± 0.56 | 4.09 ± 0.56 | ||
| p value within group | F (1,44) = 46.142, p < 0.001, np2 = 0.512 | F (1,44) = 53.868, p < 0.001, np2 = 0.512 | ||
| Two MST | ||||
| Before mean ± SD | 131.87 ± 39.15 | 135.91 ± 27.43 | −4.04 ± 9.97 | F (1,44) = 0.167, p = 0.687, np2 = 0.004 |
| After mean ± SD | 154.74 ± 41.10 | 154.30 ± 28.64 | 0.44 ± 10.45 | F (1,44) = 0.002, p = 0.967, np2 = 0.000 |
| Mean difference (after-before) ± SE | 22.87 ± 5.34 | 18.39 ± 5.34 | ||
| p-value within group | F (1,44) = 18.374, p < 0.001, np2 = 0.295 | F (1,44) = 11.884, p = 0.001, np2 = 0.213 | ||
| BaPWV (cm/s) | ||||
| Before mean ± SD | 1523.91 ± 212.26 | 1653.00 ± 295.44 | −129.09 ± 75.86 | F (1,44) = 2.896, p = 0.096, np 2 = 0.062 |
| After mean ± SD | 1531.09 ± 258.19 | 1695.61 ± 349.23 | −164.52 ± 90.56 | F (1,44) = 3.300, p = 0.076, np2 = 0.070 |
| Mean difference (after-before) ± SE | 7.17 ± 28.98 | 42.61 ± 28.98 | ||
| p value within group | F (1,44) = 0.061, p = 0.806, np2 = 0.001 | F (1,44) = 2.163, p = 0.149, np2 = 0.047 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kooncumchoo, P.; Meekum, S.; Harnmanop, S.; Luangpon, N.; Yuenyongchaiwat, K. Beneficial Effects of Water-Based Exercise Alone and in Combination with Cognitive Training on Cardiovascular Fitness and Arterial Stiffness in Older Adults with Mild Cognitive Impairment: A Randomized Controlled Trial. Life 2025, 15, 1195. https://doi.org/10.3390/life15081195
Kooncumchoo P, Meekum S, Harnmanop S, Luangpon N, Yuenyongchaiwat K. Beneficial Effects of Water-Based Exercise Alone and in Combination with Cognitive Training on Cardiovascular Fitness and Arterial Stiffness in Older Adults with Mild Cognitive Impairment: A Randomized Controlled Trial. Life. 2025; 15(8):1195. https://doi.org/10.3390/life15081195
Chicago/Turabian StyleKooncumchoo, Patcharee, Sutaya Meekum, Somrudee Harnmanop, Nongnuch Luangpon, and Kornanong Yuenyongchaiwat. 2025. "Beneficial Effects of Water-Based Exercise Alone and in Combination with Cognitive Training on Cardiovascular Fitness and Arterial Stiffness in Older Adults with Mild Cognitive Impairment: A Randomized Controlled Trial" Life 15, no. 8: 1195. https://doi.org/10.3390/life15081195
APA StyleKooncumchoo, P., Meekum, S., Harnmanop, S., Luangpon, N., & Yuenyongchaiwat, K. (2025). Beneficial Effects of Water-Based Exercise Alone and in Combination with Cognitive Training on Cardiovascular Fitness and Arterial Stiffness in Older Adults with Mild Cognitive Impairment: A Randomized Controlled Trial. Life, 15(8), 1195. https://doi.org/10.3390/life15081195






