Efficacy and Safety of 5-Aminolevulinic Acid Hydrochloride Combined with Sodium Ferrous Citrate in Pediatric Patients with Leigh Syndrome and Central Nervous System Disorders: An Initial Exploratory Trial with a Double-Blind Placebo-Controlled Period, Followed by an Open-Label Period and a Subsequent Long-Term Administration Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Drug and Placebo
2.2. Patients
2.3. LS Causative Gene Analysis
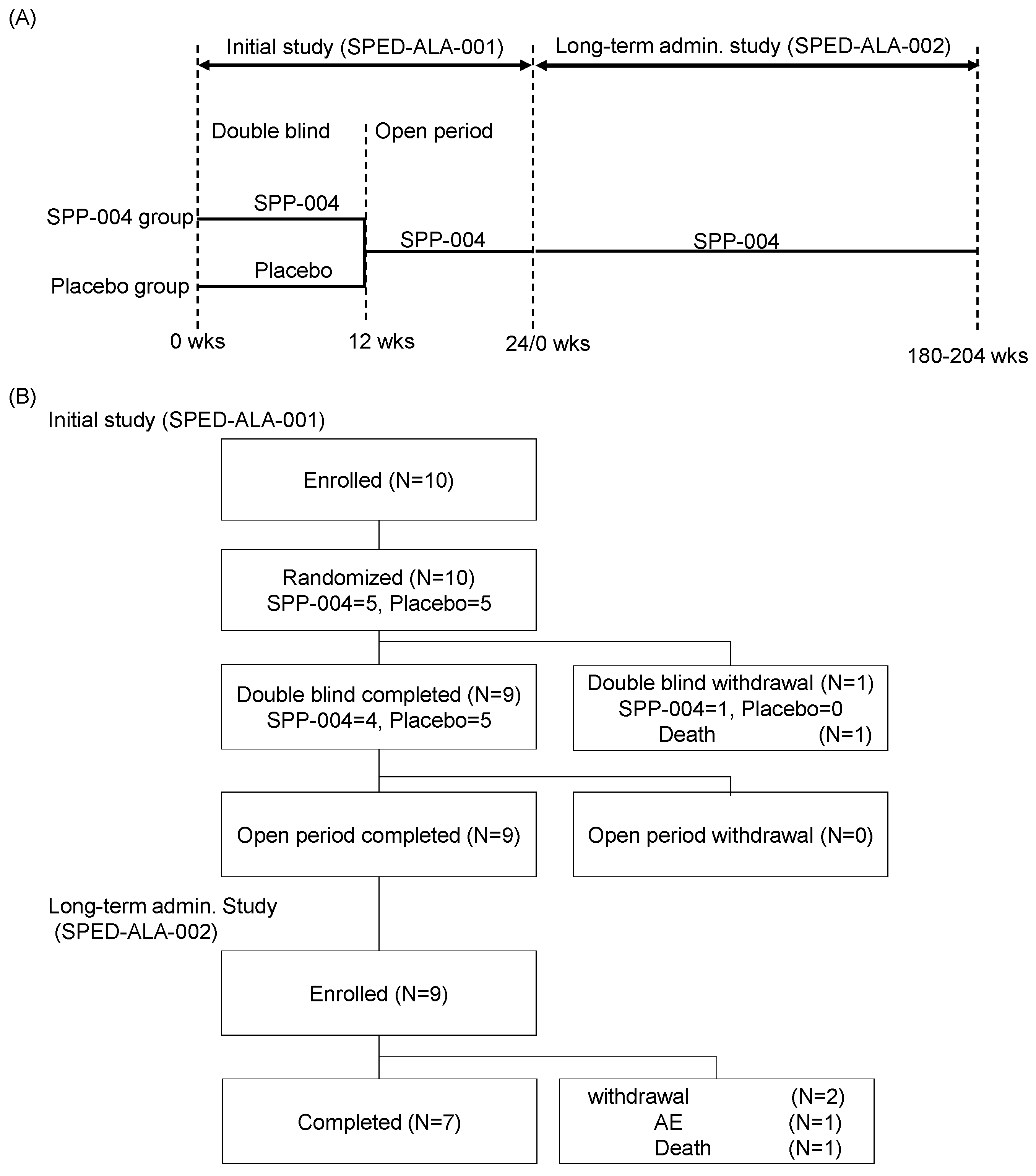
2.4. Study Design
2.5. Drug Administration Protocol
2.6. Dose Setting
2.7. Pharmacokinetic Analysis of the Significance of the Simultaneous Administration of 5-ALA and SFC
2.8. Pharmacokinetic Analysis
2.9. Efficacy and Safety
2.10. Statistical Analysis
2.11. Clinical Trial Institution
3. Results
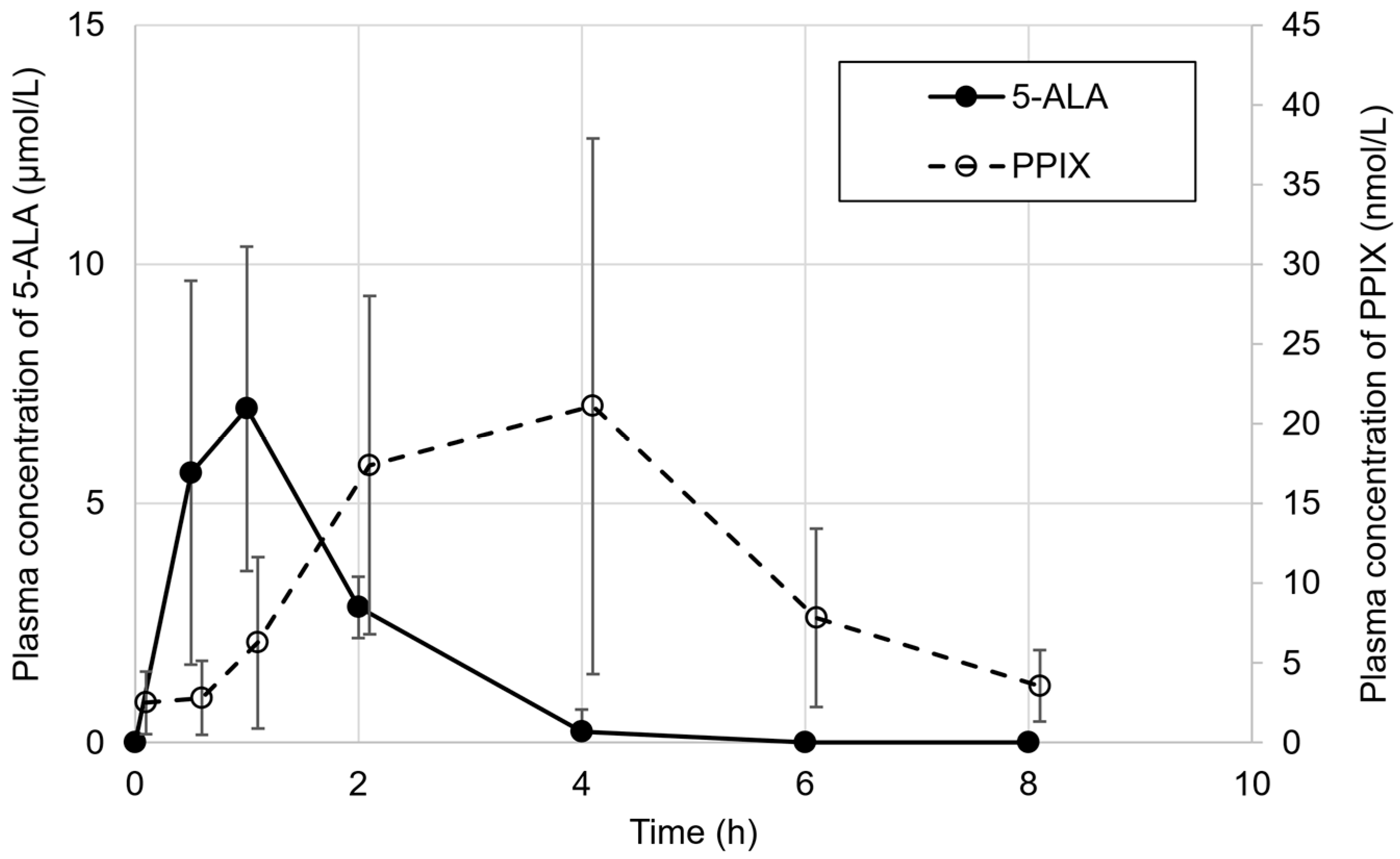
3.1. Patient Enrolment and Pharmacokinetics of 5-ALA
3.2. Safety Results
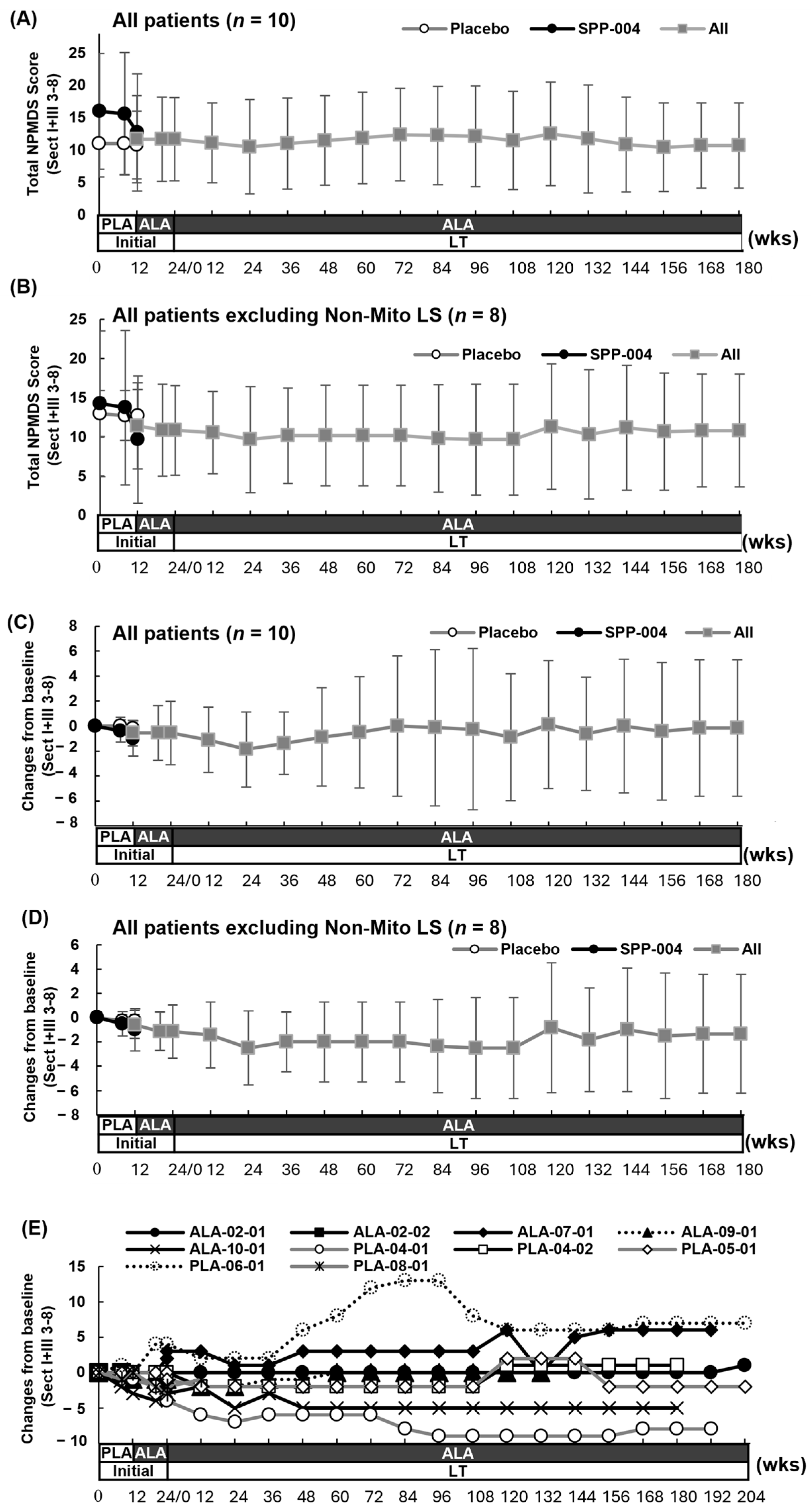
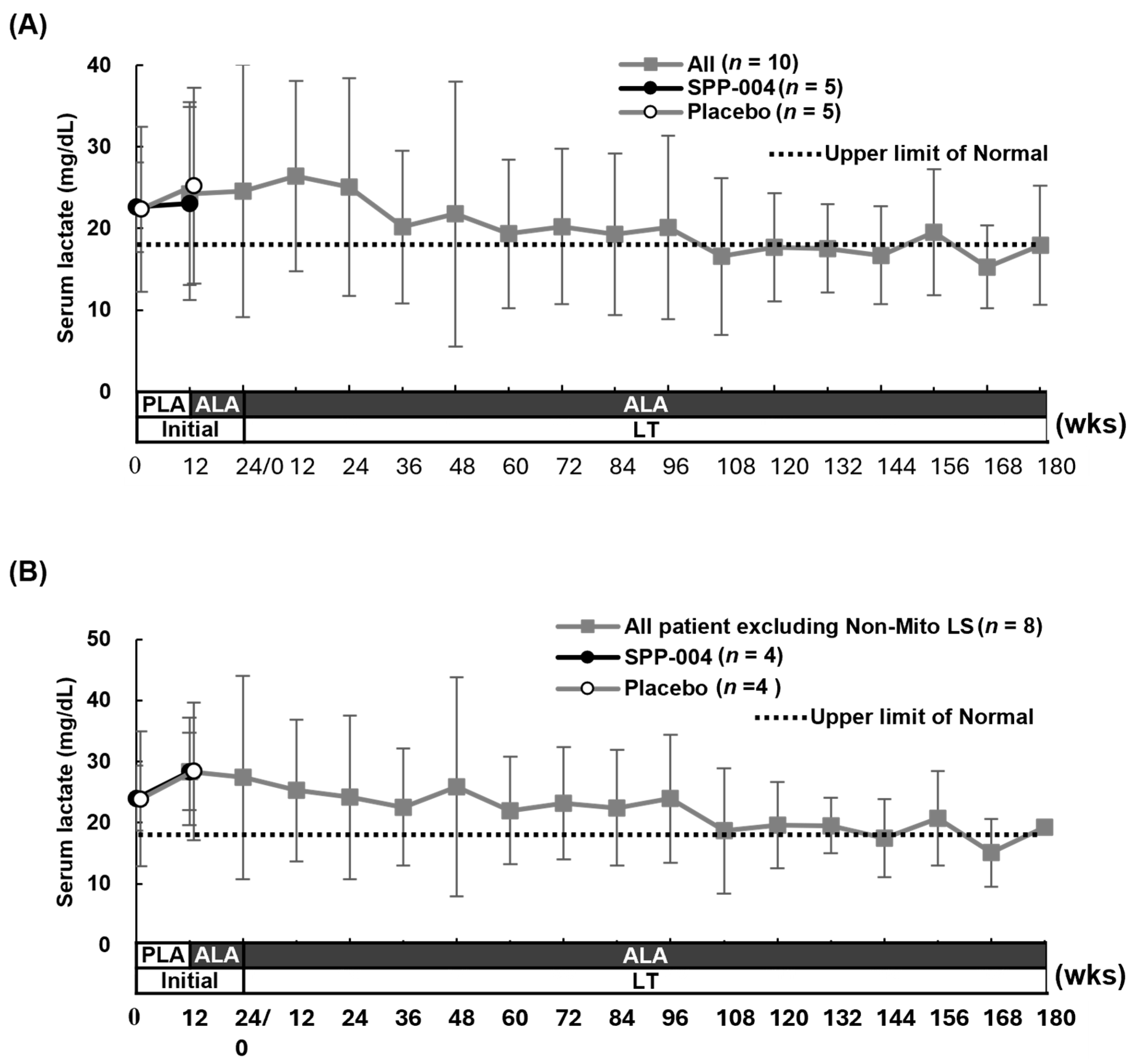
3.3. Efficacy Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADR | Adverse drug reaction |
| AE | Adverse event |
| 5-ALA | 5-Aminolevulinic acid |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| ATR-X | Alpha thalassemia/mental retardation syndrome X-linked |
| FAS | Full analysis set |
| FGF21 | Fibroblast growth factor 21 |
| FXTAS | Fragile X-associated tremor/ataxia syndrome |
| GGT | Gamma-glutamyl transferase |
| LS | Leigh syndrome |
| MD | Mitochondrial disease |
| MELAS | Mitochondrial myopathy encephalopathy, lactic acidosis, and stroke-like episodes |
| MRC | Mitochondrial respiratory chain |
| MTD | Maximally tolerated dose |
| Non-Mito LS | Leigh syndrome due to a non-mitochondrial gene variant |
| NPMDS | Newcastle Pediatric Mitochondrial Disease Scale |
| SAE | Serious adverse event |
| SD | Standard deviation |
| SFC | Sodium ferrous citrate |
| SOC | System organ class |
| PPIX | Protoporphyrin IX |
| PPS | Per protocol set |
| PT | Preferred term |
Appendix A
Appendix A.1
| Caucasian | Japanese | |||||||
|---|---|---|---|---|---|---|---|---|
| Dose of 5-ALA/SFC | AEs | SAEs | AEs | SAEs | ||||
| Cases | % (Cases) | Events | Cases | % (Cases) | Events | |||
| Placebo | (n = 6) | 50.0% (3/6) | 5 | 0 | (n = 4) | 50.0% (2/4) | 2 | 0 |
| 150 mg/235 mg | (n = 6) | 50.0% (3/6) | 4 | 0 | (n = 6) | 50.0% (3/6) | 3 | 0 |
| 300 mg/470 mg | (n = 6) | 66.7% (4/6) | 11 | 0 | (n = 6) | 0.0% (0/6) | 0 | 0 |
| 600 mg/940 mg | (n = 6) | 83.3% (5/6) | 19 | 0 | ||||
Appendix A.2
| Caucasian | Japanese | ||||||
|---|---|---|---|---|---|---|---|
| Placebo | 150 mg/235 mg | 300 mg /470 mg | 600 mg /940 mg | Placebo | 150 mg /235 mg | 300 mg /470 mg | |
| (n = 6) | (n = 6) | (n = 6) | (n = 6) | (n = 6) | (n = 6) | (n = 6) | |
| Abdominal distension | 2 | 2 | |||||
| Nausea | 1 | 2 | 1 | ||||
| Feces discolored | 2 | 1 | |||||
| Headache | 1 | 1 | 2 | ||||
| Dizziness | 1 | 1 | |||||
| Chest discomfort | 2 | ||||||
| Myalgia | 1 | 1 | |||||
Appendix A.3
| Body Weight | Daily Dose (5-ALA/SFC) | Number of Doses and Number of Capsules Taken for 5-ALA and SFC |
|---|---|---|
| >20 kg | 50 mg/78.44 mg | Twice a day, 1 capsule in the morning and 1 capsule in the evening |
| ≥20 kg and <30 kg | 75 mg/117.66 mg | Twice a day, 2 capsules in the morning and 1 capsule in the evening |
| ≥30 kg and <40 kg | 100 mg/156.88 mg | Twice a day, 2 capsules in the morning and 2 capsules in the evening |
| ≥40 kg and <50 kg | 125 mg/196.1 mg | Twice a day, 3 capsules in the morning and 2 capsules in the evening |
| ≥50 kg | 150 mg/235.32 mg | Twice a day, 3 capsules in the morning and 3 capsules in the evening |
References
- Russell, O.M.; Gorman, G.S.; Lightowlers, R.N.; Turnbull, D.M. Mitochondrial Diseases: Hope for the Future. Cell 2020, 181, 168–188. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.; Collier, J.J.; Glasgow, R.I.C.; Robertson, F.M.; Pyle, A.; Blakely, E.L.; Alston, C.L.; Oláhová, M.; McFarland, R.; Taylor, R.W. Recent advances in understanding the molecular genetic basis of mitochondrial disease. J. Inherit. Metab. Dis. 2020, 43, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Rodenburg, R.J. Biochemical diagnosis of mitochondrial disorders. J. Inherit. Metab. Dis. 2011, 34, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Saneto, R.P.; Friedman, S.D.; Shaw, D.W. Neuroimaging of mitochondrial disease. Mitochondrion 2008, 8, 396–413. [Google Scholar] [CrossRef] [PubMed]
- Schubert Baldo, M.; Vilarinho, L. Molecular basis of Leigh syndrome: A current look. Orphanet J. Rare Dis. 2020, 15, 31. [Google Scholar] [CrossRef] [PubMed]
- Diodato, D.; Schiff, M.; Cohen, B.H.; Bertini, E.; Rahman, S.; Workshop participants. 258th ENMC international workshop Leigh syndrome spectrum: Genetic causes, natural history and preparing for clinical trials 25–27 March 2022, Hoofddorp, Amsterdam, The Netherlands. Neuromuscul. Disord. 2023, 33, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, I.; Shadiack, E.; Ganetzky, R.D.; Falk, M.J. Mitochondrial medicine therapies: Rationale, evidence, and dosing guidelines. Curr. Opin. Pediatr. 2020, 32, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Almannai, M.; El-Hattab, A.W.; Ali, M.; Soler-Alfonso, C.; Scaglia, F. Clinical trials in mitochondrial disorders, an update. Mol. Genet. Metab. 2020, 131, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.; Goldstein, A.; Koenig, M.K.; Scaglia, F.; Enns, G.M.; Saneto, R.; Anselm, I.; Cohen, B.H.; Falk, M.J.; Greene, C.; et al. Diagnosis and management of mitochondrial disease: A consensus statement from the Mitochondrial Medicine Society. Genet. Med. 2015, 17, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Koga, Y.; Povalko, N.; Inoue, E.; Nakamura, H.; Ishii, A.; Suzuki, Y.; Yoneda, M.; Kanda, F.; Kubota, M.; Okada, H.; et al. Therapeutic regimen of L-arginine for MELAS: 9-year, prospective, multicenter, clinical research. J. Neurol. 2018, 265, 2861–2874. [Google Scholar] [CrossRef] [PubMed]
- Ikawa, M.; Povalko, N.; Koga, Y. Arginine therapy in mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Nishio, Y.; Fujino, M.; Zhao, M.; Ishii, T.; Ishizuka, M.; Ito, H.; Takahashi, K.; Abe, F.; Nakajima, M.; Tanaka, T.; et al. 5-Aminolevulinic acid combined with ferrous iron enhances the expression of heme oxygenase-1. Int. Immunopharmacol. 2014, 19, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Shimura, M.; Nozawa, N.; Ogawa-Tominaga, M.; Fushimi, T.; Tajika, M.; Ichimoto, K.; Matsunaga, A.; Tsuruoka, T.; Kishita, Y.; Ishii, T.; et al. Effects of 5-aminolevulinic acid and sodium ferrous citrate on fibroblasts from individuals with mitochondrial diseases. Sci. Rep. 2019, 9, 10549. [Google Scholar] [CrossRef] [PubMed]
- Ogura, S.-I.; Maruyama, K.; Hagiya, Y.; Sugiyama, Y.; Tsuchiya, K.; Takahashi, K.; Abe, F.; Tabata, K.; Okura, I.; Nakajima, M.; et al. The effect of 5-aminolevulinic acid on cytochrome c oxidase activity in mouse liver. BMC Res. Notes 2011, 4, 66. [Google Scholar] [CrossRef] [PubMed]
- Shioda, N.; Yabuki, Y.; Yamaguchi, K.; Onozato, M.; Li, Y.; Kurosawa, K.; Tanabe, H.; Okamoto, N.; Era, T.; Sugiyama, H.; et al. Targeting G-quadruplex DNA as cognitive function therapy for ATR-X syndrome. Nat. Med. 2018, 24, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Asamitsu, S.; Yabuki, Y.; Ikenoshita, S.; Kawakubo, K.; Kawasaki, M.; Usuki, S.; Nakayama, Y.; Adachi, K.; Kugoh, H.; Ishii, K.; et al. CGG repeat RNA G-quadruplexes interact with FMRpolyG to cause neuronal dysfunction in fragile X-related tremor/ataxia syndrome. Sci. Adv. 2021, 7, eabd9440. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Masuki, S.; Morikawa, A.; Ogawa, Y.; Kamijo, Y.I.; Takahashi, K.; Nakajima, M.; Nose, H. Effects of 5-aminolevulinic acid supplementation on home-based walking training achievement in middle-aged depressive women: Randomized, double-blind, crossover pilot study. Sci. Rep. 2018, 8, 7151. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.H.; Shintani, T.T.; Rodriguez, B.L.; Davis, J.; Harrigan, R.C. The Role of 5-aminolevulinic acid (5-ALA) and sleep. Int. J. Clin. Med. 2013, 4, 1–7. [Google Scholar] [CrossRef]
- Aquino, R.K.; Perez, M.; Sil, P.; Shintani, T.; Harrigan, R.; Rodriguez, B. The Relationship of 5-Aminolevulinic Acid on Mood and Coping Ability in Prediabetic Middle Aged and Older Adults. Geriatrics 2018, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Kohda, M.; Tokuzawa, Y.; Kishita, Y.; Nyuzuki, H.; Moriyama, Y.; Mizuno, Y.; Hirata, T.; Yatsuka, Y.; Yamashita-Sugahara, Y.; Nakachi, Y.; et al. A Comprehensive Genomic Analysis Reveals the Genetic Landscape of Mitochondrial Respiratory Chain Complex Deficiencies. PLoS Genet. 2016, 12, e1005679. [Google Scholar] [CrossRef] [PubMed]
- Mingone, C.J.; Gupte, S.A.; Chow, J.L.; Ahmad, M.; Abraham, N.G.; Wolin, M.S. Protoporphyrin IX generation from delta-aminolevulinic acid elicits pulmonary artery relaxation and soluble guanylate cyclase activation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L337–L344. [Google Scholar] [CrossRef] [PubMed]
- Juzenas, P.; Juzeniene, A. Reduction of cutaneous photosensitivity by application of ointment containing ferrous or cobaltous ions concomitant with the use of topical protoporphyrin IX precursors. Photodiagnosis Photodyn. Ther. 2010, 7, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Fukuhara, H.; Inoue, K.; Shuin, T.; Hagiya, Y.; Nakajima, M.; Tanaka, T.; Ogura, S.-I.; Missirlis, F. The effect of iron ion on the specificity of photodynamic therapy with 5-aminolevulinic acid. PLoS ONE 2015, 10, e0122351. [Google Scholar] [CrossRef] [PubMed]
- Ota, U.; Fukuhara, H.; Ishizuka, M.; Abe, F.; Kawada, C.; Tamura, K.; Tanaka, T.; Inoue, K.; Ogura, S.-I.; Shuin, T. Plasma protoporphyrin IX following administration of 5-aminolevulinic acid as a potential tumor marker. Mol. Clin. Oncol. 2015, 3, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Phoenix, C.; Schaefer, A.; Elson, J.; Morava, E.; Bugiani, M.; Uziel, G.; Smeitink, J.; Turnbull, D.; McFarland, R. A scale to monitor progression and treatment of mitochondrial disease in children. Neuromuscul. Disord. 2006, 16, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Baertling, F.; Rodenburg, R.J.; Schaper, J.; Smeitink, J.A.; Koopman, W.J.H.; Mayatepek, E.; Morava, E.; Distelmaier, F. A guide to diagnosis and treatment of Leigh syndrome. J. Neurol. Neurosurg. Psychiatry 2014, 85, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.Z.; Ng, Y.S.; Blain, A.; Jiminez-Moreno, C.; Alston, C.L.; Nesbitt, V.; Simmons, L.; Santra, S.; Wassmer, E.; Blakely, E.L.; et al. Natural History of Leigh Syndrome: A Study of Disease Burden and Progression. Ann. Neurol. 2022, 91, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, E.; Fushimi, T.; Ogawa-Tominaga, M.; Shimura, M.; Tajika, M.; Ichimoto, K.; Matsunaga, A.; Tsuruoka, T.; Ishige, M.; Fuchigami, T.; et al. Mortality of Japanese patients with Leigh syndrome: Effects of age at onset and genetic diagnosis. J. Inherit. Metab. Dis. 2020, 43, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Suomalainen, A.; Elo, J.M.; Pietiläinen, K.H.; Hakonen, A.H.; Sevastianova, K.; Korpela, M.; Isohanni, P.; Marjavaara, S.K.; Tyni, T.; Kiuru-Enari, S.; et al. FGF-21 as a biomarker for muscle-manifesting mitochondrial respiratory chain deficiencies: A diagnostic study. Lancet Neurol. 2011, 10, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Su, S.-L.; Wang, W.-F.; Wu, S.-L.; Wu, H.-M.; Chang, J.-C.; Huang, C.-S.; Cheng, W.-L.; Soong, B.-W.; Lee, Y.-C.; Li, J.-Y.; et al. FGF21 in ataxia patients with spinocerebellar atrophy and mitochondrial disease. Clin. Chim. Acta 2012, 414, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, J.M.; Forsström, S.; Bottani, E.; Viscomi, C.; Baris, O.R.; Isoniemi, H.; Höckerstedt, K.; Österlund, P.; Hurme, M.; Jylhävä, J.; et al. FGF21 is a biomarker for mitochondrial translation and mtDNA maintenance disorders. Neurology 2016, 87, 2290–2299. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Qiu, Y.; Zhou, M.; Chen, M.; Hu, Y.; Hong, S.; Jiang, L.; Guo, Y. Circulating FGF21 and GDF15 as Biomarkers for Screening, Diagnosis, and Severity Assessment of Primary Mitochondrial Disorders in Children. Front. Pediatr. 2022, 10, 851534. [Google Scholar] [CrossRef] [PubMed]
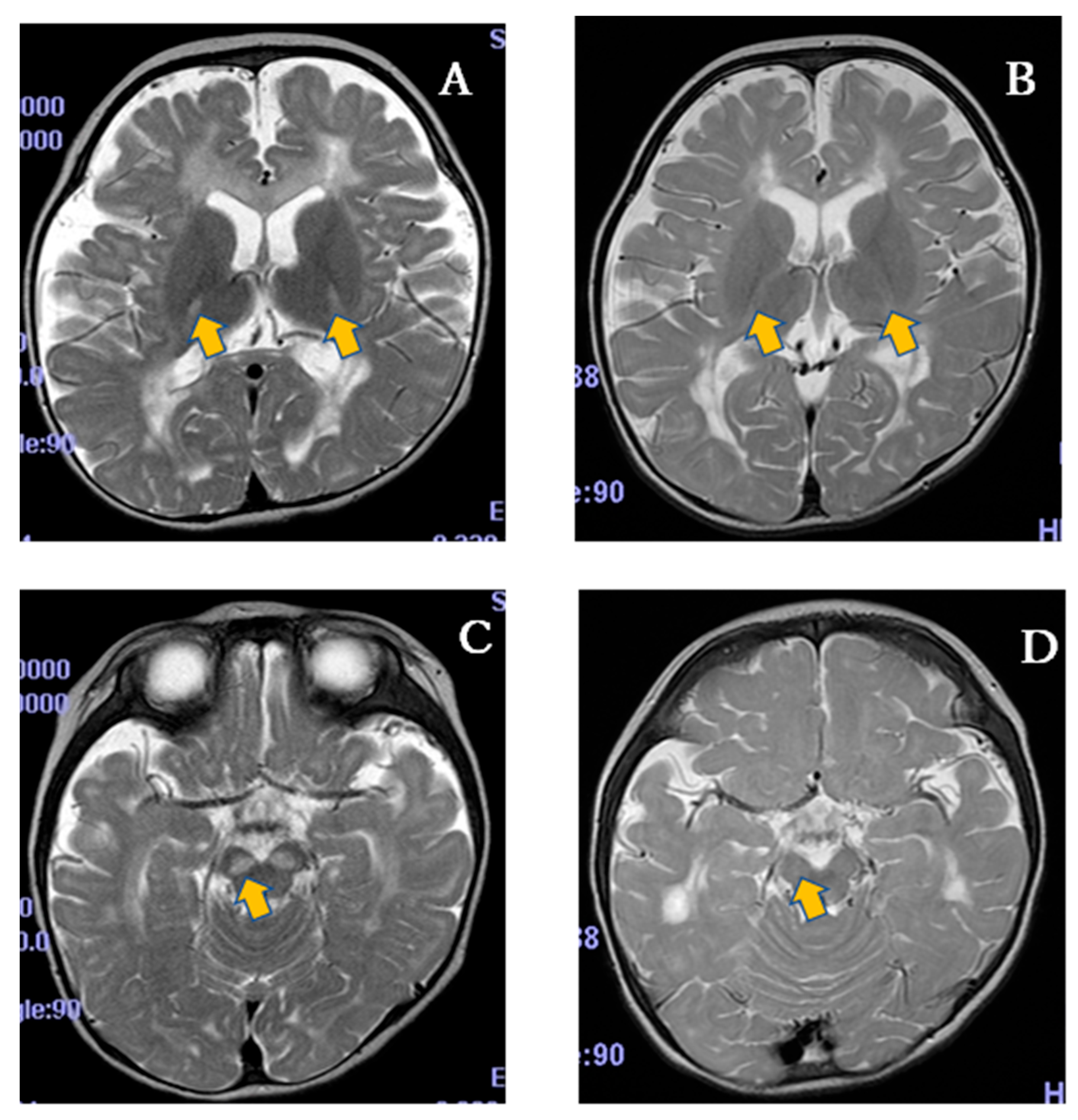
| SPP-004 (n = 5) | Placebo (n = 5) | |
|---|---|---|
| Sex—n (%) | ||
| Male | 3 (60.0) | 1 (20.0) |
| Female | 2 (40.0) | 4 (80.0) |
| Age at informed consent—months | ||
| n | 5 | 4 |
| Mean ± SD | 19.4 ± 2.88 | 19.5 ± 3.11 |
| Median [Min, Max] | 20.0 [15, 23] | 19.5 [16, 23] |
| Presence of preterm birth—n (%) | ||
| None | 5 (100.0) | 4 (80.0) |
| Yes | 0 (0.0) | 1 (20.0) |
| Adjusted age—months | ||
| n | 0 | 1 |
| Mean | - | 16 |
| Median [Min, Max] | - | 16.0 [16, 16] |
| Primary disease—n (%) | ||
| Chronic progressive external ophthalmoplegia | 0 (0.0) | 0 (0.0) |
| Myoclonus epilepsy syndrome | 0 (0.0) | 0 (0.0) |
| Leigh syndrome | 5 (100.0) | 5 (100.0) |
| Leber hereditary optic neuropathy | 0 (0.0) | 0 (0.0) |
| Pearson syndrome | 0 (0.0) | 0 (0.0) |
| Presence of consanguineous marriage—n (%) | ||
| None | 5 (100.0) | 5 (100.0) |
| Yes | 0 (0.0) | 0 (0.0) |
| Family history—n (%) | ||
| None | 2 (40.0) | 3 (60.0) |
| Yes | 3 (60.0) | 2 (40.0) |
| Medical history—n (%) | ||
| None | 5 (100.0) | 3 (60.0) |
| Yes | 0 (0.0) | 2 (40.0) |
| Presence of complications—n (%) | ||
| None | 0 (0.0) | 2 (40.0) |
| Yes | 5 (100.0) | 3 (60.0) |
| SPP-004 | Placebo | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pt No. | ALA-02-01 | ALA-02-02 * | ALA-07-01 | ALA-09-01 | ALA-10-01 | PLA-04-01 | PLA-04-02 | PLA-05-01 | PLA-06-01 | PLA-08-01 |
| Sex | M | F | F | M | M | F | M | F | F | F |
| Primary disease | LS | LS | LS | Non-Mito LS | LS | LS | LS | LS | Non-Mito LS | LS |
| Onset age | 0Y 2M | 0Y 1M | 0Y 11M | 0Y 2M | 1Y 1M | 0Y10M | 0Y 5M | 0Y 10M | 0Y 10M | 0Y 1M |
| Age at start of trial | 1Y 7M | 1Y 3M | 1Y 11M | 1Y 9M | 1Y 9M | 1Y 10M | 1Y 10M | 2Y 2M | 1Y 8M | 2Y 0M |
| MRC activity | n.d. | |||||||||
| Sample tissue | Fb | Fb | Fb | Mb | Mb | Fb | Mb | Mb | Fb | |
| Abnormality | - | I | I | IV | I | I | II | IV | IV | |
| Gene | MT-ND6 (T14487C) | MT-ND3 (T10158C) | ECHS1 | SCN8A | MT-ND5 (T13094C) | NDUFV2 | TRMT5 | SURF1 | n.d. | MT-ATP6 (T8993C) |
| MRI abnormality | Basal ganglia lesion MRS lactate peak | Basal ganglia lesion | Basal ganglia lesion | Basal ganglia lesion | Basal ganglia lesion | Basal ganglia lesion | Basal ganglia lesion | Basal ganglia lesion | Basal ganglia lesion | Basal ganglia lesion |
| NPMDS I+III3-8 | 19 | 25 | 6 | 23 | 7 | 10 | 15 | 16 | 3 | 11 |
| Lactate (mmol/L) | 2.1 + | 3.0 + | 2.1 + | 1.9 | 3.2 + | 4.1 + | 1.5 | 1.8 | 1.8 | 3.0 + |
| Pyruvate (mmol/L) | 0.02 | 0.09 | 0.13 | 0.07 | 0.15 | 0.21 | 0.06 | 0.09 | 0.08 | 0.13 |
| L/P ratio | 97.0 + | 35.5 + | 16.2 | 27.9 + | 21.0 + | 19.8 | 27.8 + | 20.3 + | 23.3 + | 22.7 + |
| FGF21 (pg/mL) | 891.640 | 10262.091 | ≤60 | 96.747 | ≤60 | 312.731 | 569.603 | 833.855 | ≤60 | ≤60 |
| n | Mean ± SD | Median [Min, Max] | |
|---|---|---|---|
| 5-ALA | |||
| Cmax (μmol/L) | 8 | 7.00 ± 3.42 | 6.92 [2.48, 12.3] |
| tmax (h) | 8 | 0.9 ± 0.2 | 1.0 [0.5, 1.0] |
| t1/2 (h) | - | n.c. | n.c. |
| AUC0-8 (h·μmol/L) | 8 | 12.8 ± 4.14 | 11.7 [8.13, 18.6] |
| AUC0-t (h·μmol/L) | 8 | 10.4 ± 3.58 | 10.3 [6.63, 15.7] |
| AUC0-∞ (h·μmol/L) | - | n.c. | n.c. |
| PPIX | |||
| Cmax (nmol/L) | 8 | 22.8 ± 16.3 | 17.0 [6.58, 49.2] |
| tmax (h) | 8 | 3.0 ± 1.1 | 3.0 [2.0, 4.0] |
| t1/2 (h) | 4 | 1.8 ± 0.3 | 1.9 [1.4, 2.2] |
| AUC0-8 (h·nmol/L) | 8 | 94.3 ± 64.5 | 68.3 [31.2, 197] |
| AUC0-t (h·nmol/L) | 8 | 94.3 ± 64.5 | 68.3 [31.2, 197] |
| AUC0-∞ (h·nmol/L) | 4 | 76.3 ± 35.7 | 68.1 [43.5, 125] |
| Initial study (SPED-ALA-001) | ||||||||||||
| SPP-004 | Placebo | |||||||||||
| Double-blind period (n= 5) | Open-label period (n = 4) | Total (n = 5) | Double-blind period (n = 5) | Open-label period (n = 5) | Total (n = 5) | |||||||
| Cases n (%) | Events n | Cases n (%) | Events n | Cases n (%) | Events n | Cases n (%) | Events n | Cases n (%) | Events n | Cases n (%) | Events n | |
| All AEs | 5 (100.0) | 17 | 4 (100.0) | 12 | 5 (100.0) | 29 | 5 (100.0) | 21 | 5 (100.0) | 24 | 5 (100.0) | 45 |
| Severity | ||||||||||||
| Mild | 2 (40.0) | 7 | 3 (75.0) | 9 | 2 (40.0) | 16 | 2 (40.0) | 18 | 2 (40.0) | 17 | 1 (20.0) | 35 |
| Moderate | 2 (40.0) | 6 | 1 (25.0) | 3 | 2 (40.0) | 9 | 3 (60.0) | 3 | 3 (60.0) | 7 | 4 (80.0) | 10 |
| Severe | 1 (20.0) | 4 | 0 (0.0) | 0 | 1 (20.0) | 4 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 |
| Relationship with investigational drug | ||||||||||||
| Related | 2 (40.0) | 4 | 2 (50.0) | 2 | 4 (80.0) | 6 | 1 (20.0) | 1 | 3 (60.0) | 3 | 3 (60.0) | 4 |
| Unrelated | 3 (60.0) | 13 | 2 (50.0) | 10 | 1 (20.0) | 23 | 4 (80.0) | 20 | 2 (40.0) | 21 | 2 (40.0) | 41 |
| Death | 1 (20.0) | - | 0 (0.0) | - | 1 (20.0) | - | 0 (0.0) | - | 0 (0.0) | - | 0 (0.0) | - |
| Serious AEs | 2 (40.0) | 7 | 2 (50.0) | 5 | 3 (60.0) | 12 | 3 (60.0) | 3 | 1 (20.0) | 5 | 3 (60.0) | 8 |
| AEs leading to discontinuation | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 |
| Long-term administration study (SPED-ALA-002) | ||||||||||||
| All (n = 9) | ||||||||||||
| Cases—n (%) | Events—n | |||||||||||
| All AEs | 9 (100.0) | 255 | ||||||||||
| Severity | ||||||||||||
| Mild | 1 (11.1) | 168 | ||||||||||
| Moderate | 3 (33.3) | 79 | ||||||||||
| Severe | 5 (55.6) | 8 | ||||||||||
| Relationship with investigational drugs | ||||||||||||
| Unelated | 7 (77.8) | 251 | ||||||||||
| Related | 2 (22.2) | 4 | ||||||||||
| Death | 1 (11.1) | - | ||||||||||
| Serious AEs | 7 (77.8) | 61 | ||||||||||
| AEs leading to discontinuation | 2 (22.2) | 2 | ||||||||||
| Initial Study (SPED-ALA-001) | ||||||||||||||
| SPP-004 | Placebo | |||||||||||||
| Double-blind period | Open-label period | Total | Double-blind period | Open-label period | Total | |||||||||
| n = 5 | n = 4 | n = 5 | n = 5 | n = 5 | n = 5 | |||||||||
| SOC | Cases | Events | Cases | events | Cases | events | Cases | events | Cases | events | Cases | events | ||
| PT (Med DRA/J Ver 19.0) | Severity | n (%) | n | n (%) | n | n (%) | n | n (%) | n | n (%) | n | n (%) | n | |
| All | 2 (40.0) | 4 | 2 (50.0) | 2 | 4 (80.0) | 6 | 1 (20.0) | 1 | 3 (60.0) | 3 | 3 (60.0) | 4 | ||
| Cardiac disorders | 1 (20.0) | 1 | 0 (0.0) | 0 | 1 (20.0) | 1 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | ||
| Left ventricular failure | Severe | 1 (20.0) | 1 | 0 (0.0) | 0 | 1 (20.0) | 1 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | |
| Gastrointestinal disorder | 1 (20.0) | 1 | 1 (25.0) | 1 | 2 (40.0) | 2 | 1 (20.0) | 1 | 1 (20.0) | 1 | 2 (40.0) | 2 | ||
| Diarrhea | Mild | 1 (20.0) | 1 | 0 (0.0) | 0 | 1 (20.0) | 1 | 1 (20.0) | 1 | 0 (0.0) | 0 | 1 (20.0) | 1 | |
| Hematochezia | Mild | 0 (0.0) | 0 | 1 (25.0) | 1 | 1 (20.0) | 1 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | |
| Tooth discoloration | Mild | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 1 (20.0) | 1 | 1 (20.0) | 1 | |
| Investigations | 0 (0.0) | 0 | 1 (25.0) | 1 | 1 (20.0) | 1 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | ||
| Electroencephalogram abnormal | Mild | 0 (0.0) | 0 | 1 (25.0) | 1 | 1 (20.0) | 1 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | |
| Respiratory, thoracic, and mediastinal disorders | 1 (20.0) | 2 | 0 (0.0) | 0 | 1 (20.0) | 2 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | ||
| Pulmonary alveolar hemorrhage | Severe | 1 (20.0) | 1 | 0 (0.0) | 0 | 1 (20.0) | 1 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | |
| Respiratory failure | Severe | 1 (20.0) | 1 | 0 (0.0) | 0 | 1 (20.0) | 1 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | |
| Skin and subcutaneous tissue disorders | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 2 (40.0) | 2 | 2 (40.0) | 2 | ||
| Urticarias | Moderate | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 2 (40.0) | 2 | 2 (40.0) | 2 | |
| Long-term administration study (SPED-ALA-002) | ||||||||||||||
| SOC | Cases | Events | ||||||||||||
| PT (MedDRA/J Ver. 22.1) | Severity | n (%) | n | |||||||||||
| All | 2 (22.2) | 4 | ||||||||||||
| Skin and subcutaneous tissue disorders | ||||||||||||||
| Urticaria | Moderate | 2 (22.2) | 2 | |||||||||||
| Mild | 2 (22.2) | 2 | ||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abe, Y.; Hamasaki, T.; Natsume, J.; Mogami, Y.; Murayama, K.; Shiraishi, H.; Abe, Y.; Kumada, S.; Tanaka, R.; Ihara, K.; et al. Efficacy and Safety of 5-Aminolevulinic Acid Hydrochloride Combined with Sodium Ferrous Citrate in Pediatric Patients with Leigh Syndrome and Central Nervous System Disorders: An Initial Exploratory Trial with a Double-Blind Placebo-Controlled Period, Followed by an Open-Label Period and a Subsequent Long-Term Administration Study. Life 2025, 15, 1168. https://doi.org/10.3390/life15081168
Abe Y, Hamasaki T, Natsume J, Mogami Y, Murayama K, Shiraishi H, Abe Y, Kumada S, Tanaka R, Ihara K, et al. Efficacy and Safety of 5-Aminolevulinic Acid Hydrochloride Combined with Sodium Ferrous Citrate in Pediatric Patients with Leigh Syndrome and Central Nervous System Disorders: An Initial Exploratory Trial with a Double-Blind Placebo-Controlled Period, Followed by an Open-Label Period and a Subsequent Long-Term Administration Study. Life. 2025; 15(8):1168. https://doi.org/10.3390/life15081168
Chicago/Turabian StyleAbe, Yuichi, Toshimitsu Hamasaki, Jun Natsume, Yukiko Mogami, Kei Murayama, Hideaki Shiraishi, Yuki Abe, Satoko Kumada, Ryuta Tanaka, Kenji Ihara, and et al. 2025. "Efficacy and Safety of 5-Aminolevulinic Acid Hydrochloride Combined with Sodium Ferrous Citrate in Pediatric Patients with Leigh Syndrome and Central Nervous System Disorders: An Initial Exploratory Trial with a Double-Blind Placebo-Controlled Period, Followed by an Open-Label Period and a Subsequent Long-Term Administration Study" Life 15, no. 8: 1168. https://doi.org/10.3390/life15081168
APA StyleAbe, Y., Hamasaki, T., Natsume, J., Mogami, Y., Murayama, K., Shiraishi, H., Abe, Y., Kumada, S., Tanaka, R., Ihara, K., Sakakibara, T., Okazaki, Y., Nakagawa, H., Takahashi, K., Yamauchi, M., Nakajima, M., & Ohtake, A. (2025). Efficacy and Safety of 5-Aminolevulinic Acid Hydrochloride Combined with Sodium Ferrous Citrate in Pediatric Patients with Leigh Syndrome and Central Nervous System Disorders: An Initial Exploratory Trial with a Double-Blind Placebo-Controlled Period, Followed by an Open-Label Period and a Subsequent Long-Term Administration Study. Life, 15(8), 1168. https://doi.org/10.3390/life15081168






