Comparison of LC-PUFAs Biosynthetic Characteristics in Male and Female Tilapia at Different Ontogenetic Stages
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets and Fish
2.2. Fish Husbandry, Weighing and Sampling
2.3. Analysis of Proximate and Fatty Acid Compositions
2.4. Quantitative Real-Time PCR (qRT-PCR)
2.5. Histological Observation
2.6. Statistical Analysis
3. Results
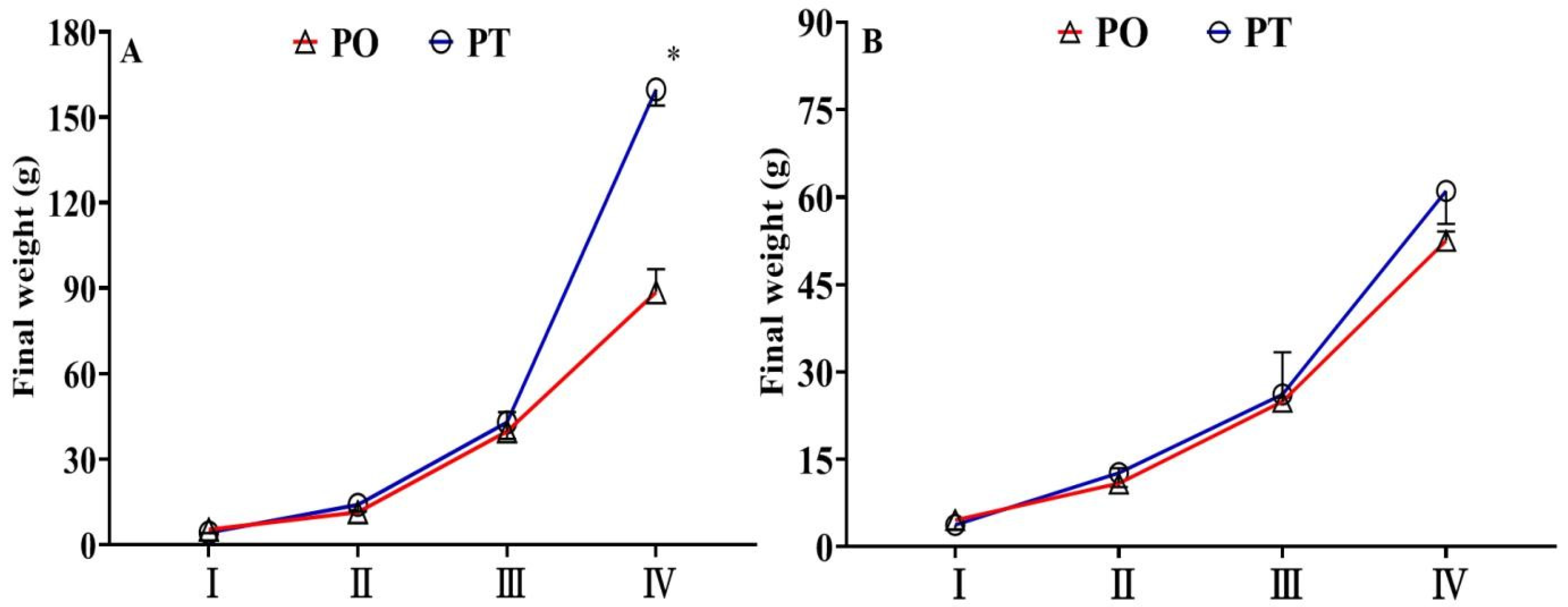
3.1. Final Weight of Fish Across Four Growth Stages
3.2. Initial Fatty Acid Compositions of Whole Fish
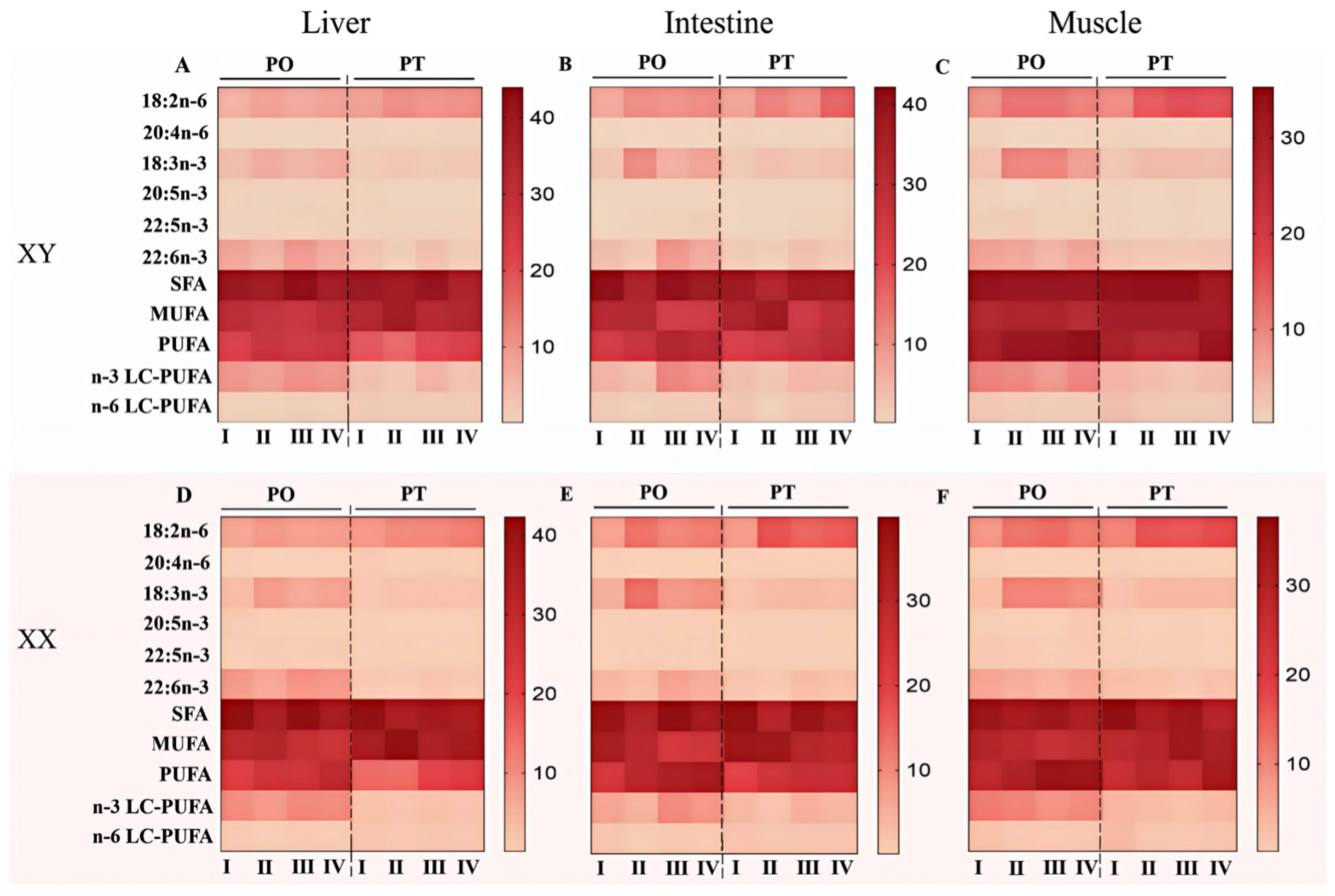
3.3. Fatty Acid Composition in the Liver, Intestine and Muscle of Tilapia Across Four Stages
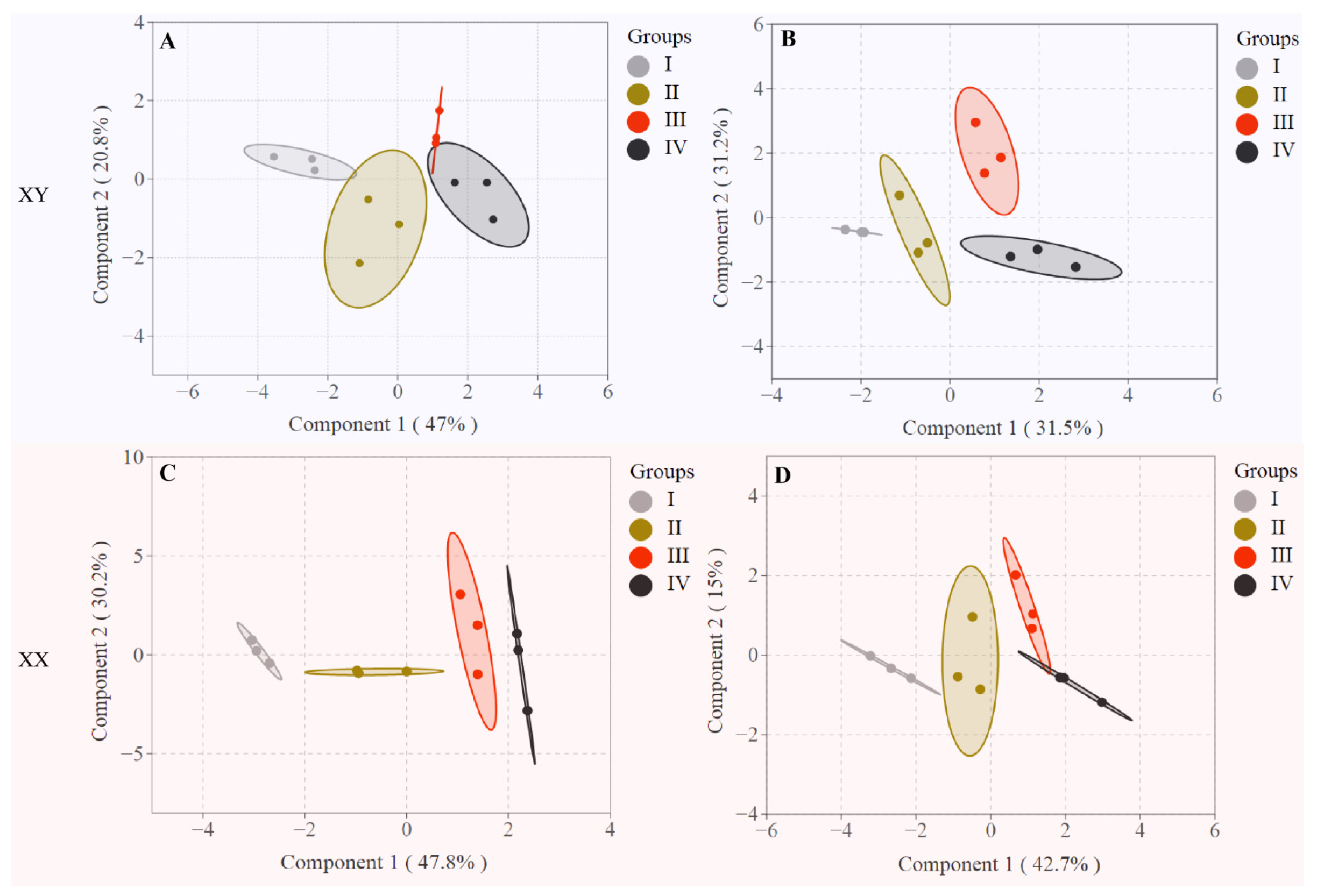
3.4. Differentiation of Fish Across Different Growth Stages Based on the LC-PUFA Contents
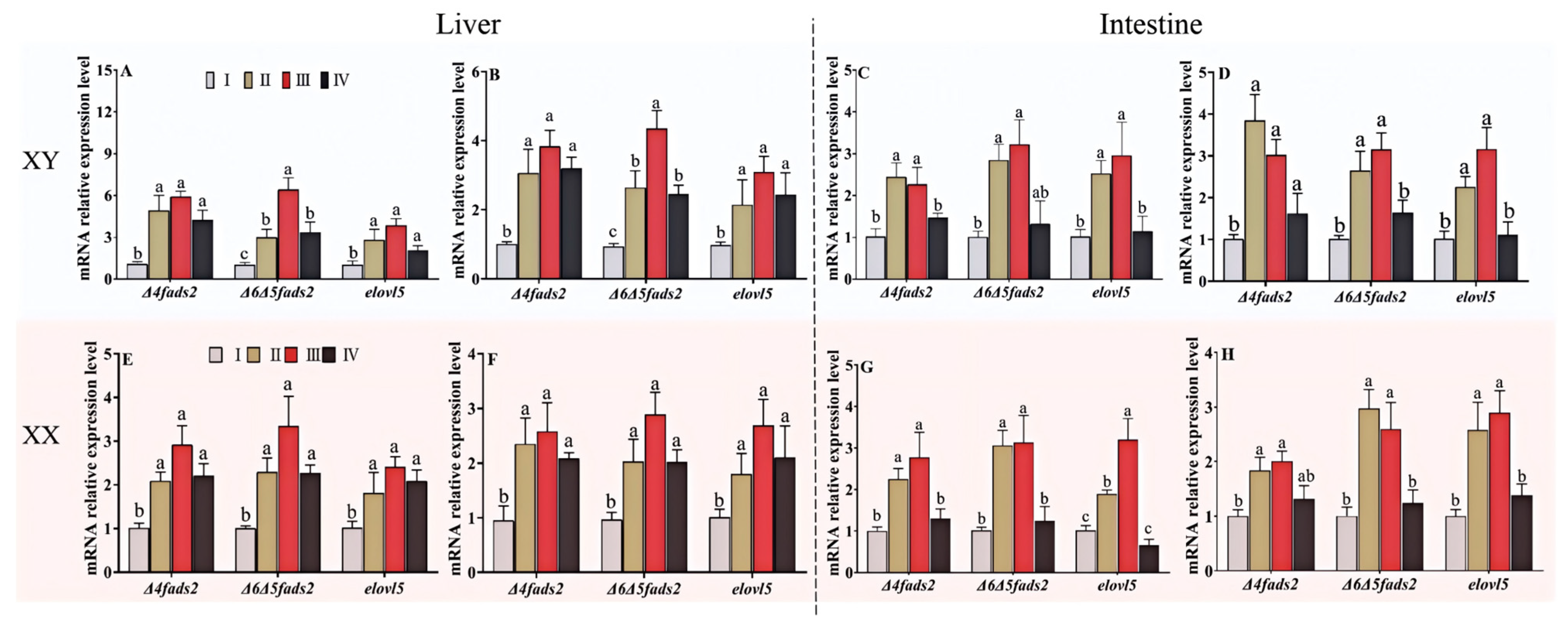
3.5. Expression of Genes Involved in LC-PUFA Biosynthesis in Fish at Four Different Growth Stages
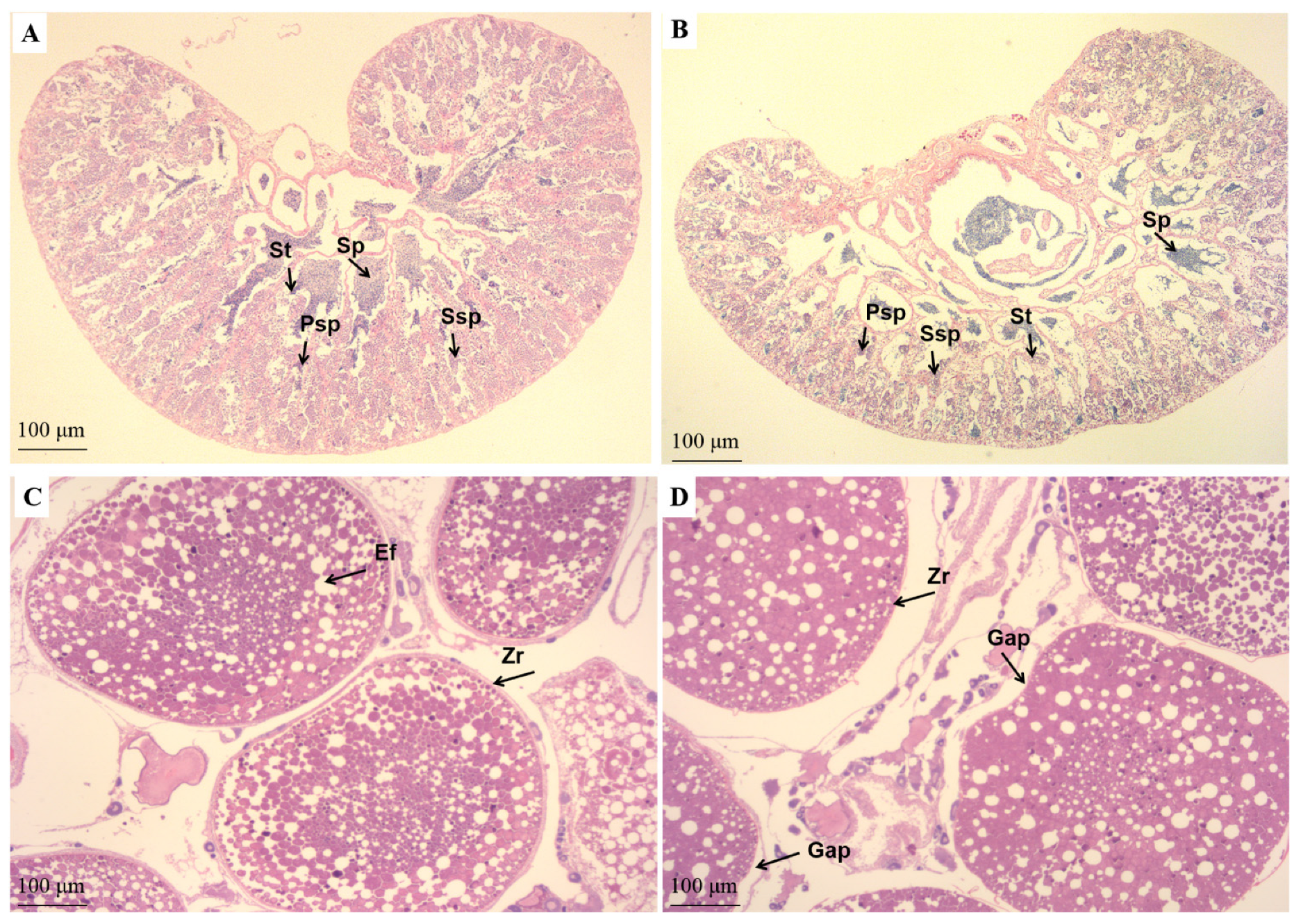
3.6. Gonad Fatty Acid Compositions and Morphology at Stage IV
4. Discussion
4.1. EFA Requirements of Tilapia Vary at Different Growth Stages
4.2. Variations in the Biosynthetic Capacity of LC-PUFA in Tilapia at Different Growth Stages
4.3. Comparison of the Biosynthetic Capacity of LC-PUFA in Male and Female Tilapia
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALA | α-Linolenic acid |
| ARA | Arachidonic acid |
| DHA | Docosahexaenoic acid |
| EFA | Essential fatty acids |
| Elovl | Elongation of very long-chain fatty acids |
| EPA | Eicosapentaenoic acid |
| Fads | Fatty acid desaturase |
| LA | Linoleic acid |
| LC-PUFA | Long-chain polyunsaturated fatty acid |
| VO | Vegetable oil |
References
- Ferosekhan, S.; Xu, H.L.; Turkmen, S.; Gómez, A.; Afonso, J.M.; Fontanillas, R.; Rosenlund, G.; Kaushik, S.; Izquierdo, M. Reproductive performance of gilthead seabream (Sparus aurata) broodstock showing different expression of fatty acyl desaturase 2 and fed two dietary fatty acid profiles. Sci. Rep. 2020, 10, 15547. [Google Scholar] [CrossRef]
- Tocher, D.R. Omega-3 long-chain polyunsaturated fatty acids and aquaculture in perspective. Aquaculture 2015, 449, 94–107. [Google Scholar] [CrossRef]
- Glencross, B.D.; Baily, J.; Berntssen, M.H.G.; Hardy, R.; Mackenzie, S.; Tocher, D.R. Risk assessment of the use of alternative animal and plant raw material resources in aquaculture feeds. Rev. Aquac. 2020, 12, 703–758. [Google Scholar] [CrossRef]
- Tocher, D.R.; Betancor, M.B.; Sprague, M.; Olsen, R.E. Omega-3 long-chain polyunsaturated fatty acids, EPA and DHA: Bridging the gap between supply and demand. Nutrients 2019, 11, 89. [Google Scholar] [CrossRef]
- An, W.; Xu, J.; Chen, F.; Ma, Y.; Su, Z.; Guang, J.; Su, N.; Zhang, G.; Xu, C.; Li, Y.; et al. Effects of dietary n-3 LC-PUFA levels on the growth, immunity, and lipid metabolism of freshwater carnivorous teleost largemouth bass (Micropterus salmoides) juveniles. Aquac. Rep. 2023, 32, 101704. [Google Scholar] [CrossRef]
- Qin, Y.; He, L.; Wang, Y.; Li, D.; Chen, W.; Ye, J. Growth performance, fatty acid composition, and lipid metabolism are altered in groupers (Epinephelus coioides) by dietary fish oil replacement with palm oil. Anim. Nutr. 2022, 8, 102–113. [Google Scholar] [CrossRef]
- Sánchez-Moya, A.; García-Meilán, I.; Riera-Heredia, N.; Vélez, E.J.; Lutfi, E.; Fontanillas, R.; Gutiérrez, J.; Capilla, E.; Navarro, I. Effects of different dietary vegetable oils on growth and intestinal performance, lipid metabolism and flesh quality in gilthead sea bream. Aquaculture 2020, 519, 734881. [Google Scholar] [CrossRef]
- Xie, D.; Chen, C.; Dong, Y.; You, C.; Wang, S.; Monroig, Ó.; Tocher, D.R.; Li, Y. Regulation of long-chain polyunsaturated fatty acid biosynthesis in teleost fish. Prog. Lipid Res. 2021, 82, 101095. [Google Scholar] [CrossRef]
- Zhang, Z.; Miar, Y.; Huyben, D.; Colombo, S.M. Omega-3 long-chain polyunsaturated fatty acids in Atlantic salmon: Functions, requirements, sources, de novo biosynthesis and selective breeding strategies. Rev. Aquac. 2024, 16, 1030–1041. [Google Scholar] [CrossRef]
- De la Cruz-Alvarado, F.J.; Álvarez-González, C.A.; Llera-Herrera, R.; Monroig, Ó.; Kabeya, N.; Rodríguez-Morales, S.; Concha-Frias, B.; Guerrero-Zárate, R.; Jiménez-Martínez, L.D.; Peña-Marín, E.S. Expression of long-chain polyunsaturated fatty acids biosynthesis genes during the early life-cycle stages of the tropical gar Atractosteus tropicus. Comp. Biochem. Phys. B 2021, 256, 110628. [Google Scholar] [CrossRef]
- Torres, M.; Navarro, J.C.; Varó, I.; Monroig, Ó.; Hontoria, F. Nutritional regulation of genes responsible for long-chain (C20-24) and very long-chain (>C24) polyunsaturated fatty acid biosynthesis in post-larvae of gilthead seabream (Sparus aurata) and Senegalese sole (Solea senegalensis). Aquaculture 2020, 525, 735314. [Google Scholar] [CrossRef]
- You, C.; Miao, S.; Lin, S.; Wang, S.; Waiho, K.; Li, Y. Expression of long-chain polyunsaturated fatty acids (LC-PUFA) biosynthesis genes and utilization of fatty acids during early development in rabbitfish Siganus canaliculatus. Aquaculture 2017, 479, 774–779. [Google Scholar] [CrossRef]
- Oboh, A.; Kabeya, N.; Carmona-Antonanzas, G.; Castro, L.F.C.; Dick, J.R.; Tocher, D.R.; Monroig, O. Two alternative pathways for docosahexaenoic acid (DHA, 22:6n-3) biosynthesis are widespread among teleost fish. Sci. Rep. 2017, 7, 3889. [Google Scholar] [CrossRef]
- Chen, C.; Guan, W.; Xie, Q.; Chen, G.; He, X.; Zhang, H.; Guo, W.; Chen, F.; Tan, Y.; Pan, Q. n-3 essential fatty acids in Nile tilapia, Oreochromis niloticus: Bioconverting LNA to DHA is relatively efficient and the LC-PUFA biosynthetic pathway is substrate limited in juvenile fish. Aquaculture 2018, 495, 513–522. [Google Scholar] [CrossRef]
- Nobrega, R.O.; Banze, J.F.; Batista, R.O.; Fracalossi, D.M. Improving winter production of Nile tilapia: What can be done? Aquac. Rep. 2020, 18, 100453. [Google Scholar] [CrossRef]
- Xie, D.; Guan, J.; Huang, X.; Xu, C.; Pan, Q.; Li, Y. Tilapia can be a beneficial n-3 LC-PUFA source due to its high biosynthetic capacity in the liver and intestine. J. Agric. Food Chem. 2022, 70, 2701–2711. [Google Scholar] [CrossRef]
- You, C.; Lu, F.; Wang, S.; Chen, C.; Li, Y. Comparison of the growth performance and long-chain PUFA biosynthetic ability of the genetically improved farmed tilapia (Oreochromis niloticus) reared in different salinities. Br. J. Nutr. 2019, 121, 374–383. [Google Scholar] [CrossRef]
- Yu, J.; Wen, X.; You, C.; Wang, S.; Chen, C.; Tocher, D.R.; Li, Y. Comparison of the growth performance and long-chain polyunsaturated fatty acids (LC-PUFA) biosynthetic ability of red tilapia (Oreochromis mossambicus♀× O. niloticus♂) fed fish oil or vegetable oil diet at different salinities. Aquaculture 2021, 542, 736899. [Google Scholar] [CrossRef]
- Hajizadeh, A.; Jauncey, K.; Rana, K. Effects of dietary lipid source on egg and larval quality of Nile tilapia, Oreochromis niloticus (L.). In Proceedings of the Eight International Symposium on Tilapia in Aquaculture, Cairo, Egypt, 12–14 October 2008; Volume 2, pp. 965–977. [Google Scholar]
- Ng, W.K.; Romano, N. A review of the nutrition and feeding management of farmed tilapia throughout the culture cycle. Rev. Aquac. 2013, 5, 220–254. [Google Scholar] [CrossRef]
- Işik, O.; Sarihan, E.; Kuşvuran, E.; Gül, Ö.; Erbatur, O. Comparison of the fatty acid composition of the freshwater fish larvae Tilapia zillii, the rotifer Brachionus calyciflorus, and the microalgae Scenedesmus abundans, Monoraphidium minitum and Chlorella vulgaris in the algae-rotifer-fish larvae food chains. Aquaculture 1999, 174, 299–311. [Google Scholar] [CrossRef]
- Lim, C.; Yildirim-Aksoy, M.; Klesius, P. Lipid and fatty acid requirements of tilapias. N. Am. J. Aquac. 2011, 73, 188–193. [Google Scholar] [CrossRef]
- Stickney, R.R.; Hardy, R.W. Lipid requirements of some warmwater species. Aquaculture 1989, 79, 145–156. [Google Scholar] [CrossRef]
- Moreno-Fernandez, J.; Ochoa, J.J.; Lopez-Frias, M.; Diaz-Castro, J. Impact of early nutrition, physical activity and sleep on the fetal programming of disease in the pregnancy: A narrative review. Nutrients 2020, 12, 3900. [Google Scholar] [CrossRef] [PubMed]
- Fei, S.; Liu, C.; Xia, Y.; Liu, H.; Han, D.; Ji, J.; Yang, Y.; Zhu, X.; Xie, S. The effects of dietary linolenic acid to linoleic acid ratio on growth performance, tissues fatty acid profile and sex steroid hormone synthesis of yellow catfish Pelteobagrus fulvidraco. Aquac. Rep. 2020, 17, 100361. [Google Scholar] [CrossRef]
- Kottmann, J.S.; Tomkiewicz, J.; Butts, I.A.; Lund, I.; Jacobsen, C.; Støttrup, J.G.; Holst, L. Effects of essential fatty acids and feeding regimes on egg and offspring quality of European eel: Comparing reproductive success of farm-raised and wild-caught broodstock. Aquaculture 2020, 529, 735581. [Google Scholar] [CrossRef]
- Santamaría-Miranda, A.; Dumas, S.; Pérez-Urbiola, J.C.; Peña, R.; Treviño, L.; Moguel-Hernández, I.; Arjona, O.; Palacios, E. Fatty acid composition and spawning quality in wild and captive broodstock of Pacific red snapper Lutjanus peru. Aquaculture 2021, 538, 736577. [Google Scholar] [CrossRef]
- Stuart, K.; Johnson, R.; Armbruster, L.; Drawbridge, M. Arachidonic acid in the diet of captive yellowtail and its effects on egg quality. N. Am. J. Aquac. 2018, 80, 97–106. [Google Scholar] [CrossRef]
- Tocher, D.R. Fatty acid requirements in ontogeny of marine and freshwater fish. Aquac. Res. 2008, 41, 717–732. [Google Scholar] [CrossRef]
- Lall, S.P.; Kaushik, S.J. Nutrition and metabolism of minerals in fish. Animals 2021, 11, 2711. [Google Scholar] [CrossRef]
- Xu, H.; Turchini, G.M.; Francis, D.S.; Liang, M.; Mock, T.S.; Rombenso, A.; Ai, Q. Are fish what they eat? A fatty acid’s perspective. Prog. Lipid Res. 2020, 80, 101064. [Google Scholar] [CrossRef]
- Monroig, Ó.; Tocher, D.R.; Castro, L.F.C. Polyunsaturated fatty acid biosynthesis and metabolism in fish. In Polyunsaturated Fatty Acid Metabolism; Academic Press and AOCS: London, UK, 2018; pp. 31–60. [Google Scholar]
- Steinberg, C.E.W. LC-PUFAs in Reproduction and Behavior—‘Good Cop–Bad Cop?’. In Aquatic Animal Nutrition: Organic Macro-and Micro-Nutrients; Springer: Berlin/Heidelberg, Germany, 2022; pp. 753–772. [Google Scholar]
- Monroig, Ó.; Webb, K.; Ibarra-Castro, L.; Holt, G.J.; Tocher, D.R. Biosynthesis of long-chain polyunsaturated fatty acids in marine fish: Characterization of an Elovl4- like elongase from cobia Rachycentron canadum and activation of the pathway during early life stages. Aquaculture 2011, 312, 145–153. [Google Scholar] [CrossRef]
- Morais, S.; Castanheira, F.; Martinez-Rubio, L.; Conceicao, L.E.; Tocher, D.R. Long chain polyunsaturated fatty acid synthesis in a marine vertebrate: Ontogenetic and nutritional regulation of a fatty acyl desaturase with Δ4 activity. BBA-Mol. Cell Biol. L. 2013, 1821, 660–671. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Miao, S.; Xie, Z.; Lin, S.; Wang, S.; Chen, C.; Lin, L.; Huang, Y.; Zhou, M.; Dong, Y.; et al. Cloning, tissue specificity and regulation of expression of genes of four key enzymes related to long-chain polyunsaturated fatty acids (LC-PUFA) biosynthesis by ambient salinity during embryogenesis in the marine teleost Siganus guttatus. Comp. Biochem. Phys. B 2024, 269, 110903. [Google Scholar] [CrossRef] [PubMed]
- Péron, M.; Gonzalvez, R.; Hue, S.; Soudant, P.; Le Grand, F.; Mazurais, D.; Vagner, M. Spatial and ontogenetic modulation of fatty acid composition in juvenile European sea bass (Dicentrarchus labrax) from two French estuaries. Mar. Environ. Res. 2024, 197, 106456. [Google Scholar] [CrossRef]
- Rigaud, C.; Kahilainen, K.K.; Calderini, M.L.; Pilecky, M.; Kainz, M.J.; Tiirola, M.; Taipale, S.J. Preparing for the future offspring: European perch (Perca fluviatilis) biosynthesis of physiologically required fatty acids for the gonads happens already in the autumn. Oecologia 2023, 203, 477–489. [Google Scholar] [CrossRef]
- Childs, C.E.; Hoile, S.P.; Burdge, G.C.; Calder, P.C. Changes in rat n-3 and n-6 fatty acid composition during pregnancy are associated with progesterone concentrations and hepatic FADS2 expression. Prostaglandins Leukot. Essent. Fat. Acids 2012, 86, 141–147. [Google Scholar] [CrossRef]
- Xu, H.; Ferosekhan, S.; Turkmen, S.; Afonso, J.M.; Zamorano, M.J.; Izquierdo, M. High broodstock fads2 expression combined with nutritional programing through broodstock diet improves the use of low fishmeal and low fish oil diets in gilthead seabream (Sparus aurata) progeny. Aquaculture 2021, 535, 736321. [Google Scholar] [CrossRef]
- Chaguaceda, F.; Eklöv, P.; Scharnweber, K. Regulation of fatty acid composition related to ontogenetic changes and niche differentiation of a common aquatic consumer. Oecologia 2020, 193, 325–336. [Google Scholar] [CrossRef]
- Jeong, B.; Jeong, W.; Moon, S.; Ohshima, T. Preferential accumulation of fatty acitureds in the testis and ovary of cultured and wild sweet smelt Plecoglossus altivelis. Comp. Biochem. Phys. B 2002, 131, 251–259. [Google Scholar] [CrossRef]
- Ma, X.; Wang, L.; Xie, D.; Tian, X.; Zhang, Y.; Wu, L.; Liu, H.; Wang, L.; Dong, C.; Li, X.; et al. Effect of dietary linolenic/linoleic acid ratios on growth performance, ovarian steroidogenesis, plasma sex steroid hormone, and tissue fatty acid accumulation in juvenile common carp, Cyprinus carpio. Aquac. Rep. 2020, 18, 100452. [Google Scholar] [CrossRef]
- Jiao, J.; Liu, Y.; Zhang, H.; Li, L.; Qiao, F.; Chen, L.; Zhang, M.; Du, Z. Metabolism of linoleic and linolenic acids in hepatocytes of two freshwater fish with different n-3 or n-6 fatty acid requirements. Aquaculture 2019, 515, 734595. [Google Scholar] [CrossRef]
- Decsi, T.; Kennedy, K. Sex-specific differences in essential fatty acid metabolism. Am. J. Clin. Nutr. 2011, 94, S1914–S1919. [Google Scholar] [CrossRef]
- Lin, Y.; Brown, J.A.; DiMartino, C.; Dahms, I.; Salem, N., Jr.; Hibbeln, J.R. Differences in long chain polyunsaturates composition and metabolism in male and female rats. Prostaglandins Leukot. Essent. Fat. Acids 2016, 113, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Pontifex, M.G.; Vauzour, D.; Muller, M. Sexual dimorphism in the context of nutrition and health. Proc. Nutr. Soc. 2024, 83, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Wassef, E.A.; Wahbi, O.M.; Shalaby, S.H. Effects of dietary vegetable oils on liver and gonad fatty acid metabolism and gonad maturation in gilthead seabream (Sparus aurata) males and females. Aquac. Int. 2012, 20, 255–281. [Google Scholar] [CrossRef]
- Nogueira, N.; Fernandes, I.; Fernandes, T.; Cordeiro, N. A comparative analysis of lipid content and fatty acid composition in muscle, liver and gonads of Seriola fasciata Bloch 1793 based on gender and maturation stage. J. Food Compos. Anal. 2017, 59, 68–73. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, F.; Ma, Q.; Wei, Y.; Liang, M.; Xu, H. Sex differences in fatty acid composition of Chinese tongue sole (Cynoglossus semilaevis) tissues. Fishes 2023, 8, 421. [Google Scholar] [CrossRef]
- Manor, M.L.; Cleveland, B.M.; Brett, K.P.; Yao, J.; Leeds, T. Differences in growth, fillet quality, and fatty acid metabolism-related gene expression between juvenile male and female rainbow trout. Fish. Physiol. Biochem. 2015, 41, 533–547. [Google Scholar] [CrossRef]
| Ingredient (%) | Dietary Groups | |
|---|---|---|
| PO | PT | |
| Fermented soybean meal | 20 | 20 |
| Cottonseed protein concentrate | 10 | 10 |
| Chicken powder | 10 | 10 |
| Meat and bone meal | 6 | 6 |
| Soybean protein concentrate | 6 | 6 |
| Spray-dried blood cells | 4 | 4 |
| Beer yeast meal | 3 | 3 |
| Pregelatinized starch | 15 | 15 |
| Wheat flour | 6.5 | 6.5 |
| Calcium dihydrogen phosphate | 3 | 3 |
| Choline chloride | 2 | 2 |
| Lecithin | 2 | 2 |
| Lysine | 2 | 2 |
| Taurine | 1 | 1 |
| Bacillus subtilis | 1 | 1 |
| Perilla oil 1 | 6 | - |
| Peanut oil 2 | - | 6 |
| Vitamin–mineral premix 3 | 2.5 | 2.5 |
| Proximate composition (%) | ||
| Dry matter | 89.32 | 90.15 |
| Ash | 11.24 | 10.78 |
| Crude protein | 36.72 | 36.66 |
| Crude lipid | 7.56 | 7.23 |
| Main fatty acids (% total fatty acids) | ||
| 16:0 | 16.78 | 13.33 |
| 18:0 | 5.48 | 4.66 |
| SFA | 19.12 | 22.91 |
| 16:1n-7 | 0.84 | 0.49 |
| 18:1n-9 | 24.05 | 38.12 |
| MUFA | 24.88 | 38.60 |
| 18:2n-6 (LA) | 24.96 | 34.91 |
| 18:3n-3 (LNA) | 20.32 | 2.21 |
| PUFA | 45.28 | 37.12 |
| Genes | Primers | Sequence 5′—3′ | Accession Number |
|---|---|---|---|
| Δ5Δ6 fads2 | Δ5Δ6 fads2-F | CTGGTCATCGATCGAAAGGT | NM_001279623.1 |
| Δ5Δ6 fads2-R | GCGGCTTCAGAAACTTATGC | ||
| Δ4 fads2 | Δ4 fads2-F | CTTACTGTGCTCGGTGATT | XM_003440472.4 |
| Δ4 fads2-R | GGTCCTTGCTGAAGATGTT | ||
| elovl5 | elovl5-F | GGCTTCCTCCTCCGTCTAAA | NM_001279460.1 |
| elovl5-R | GTGCAAAGGTTGGTGGGTAG | ||
| β-actin | β-actin-F | CAGGATGCAGAAGGAGATCACA | KJ126772.1 |
| β-actin-R | CGATCCAGACGGAGTATTTACG |
| Main Fatty Acid | Groups | |
|---|---|---|
| Male | Female | |
| 18:2n-6 | 7.27 | 8.93 |
| 20:3n-6 | 0.43 | 0.35 |
| 20:4n-6 | 2.56 | 2.14 |
| 18:3n-3 | 2.66 | 2.66 |
| 20:5n-3 | 2.40 | 2.62 |
| 22:5n-3 | 4.22 | 4.12 |
| 22:6n-3 | 16.01 | 14.84 |
| SFA | 33.31 | 32.40 |
| MUFA | 23.30 | 24.33 |
| PUFA | 35.04 | 35.54 |
| n-3 LC-PUFA | 23.07 | 22.00 |
| n-6 LC-PUFA | 3.69 | 3.62 |
| Dietary Groups | ||
|---|---|---|
| PO | PT | |
| XY | ||
| 18:2n-6 | 7.65 ± 0.31 b | 12.39 ± 1.26 a |
| 20:4n-6 | 2.24 ± 0.26 b | 4.38 ± 0.07 a |
| 18:3n-3 | 4.39 ± 0.16 a | 2.66 ± 0.34 b |
| 20:5n-3 | 1.49 ± 0.21 a | 0.56 ± 0.09 b |
| 22:5n-3 | 2.13 ± 0.32 a | 0.68 ± 0.11 b |
| 22:6n-3 | 7.53 ± 0.39 a | 3.45 ± 0.91 b |
| SFA | 39.49 ± 1.44 | 38.29 ± 2.11 |
| MUFA | 14.27 ± 1.85 b | 20.10 ± 2.83 a |
| PUFA | 30.15 ± 1.58 | 28.79 ± 2.20 |
| n-3 LC-PUFA | 12.42 ± 0.48 a | 4.66 ± 0.96 b |
| n-6 LC-PUFA | 3.74 ± 0.15 b | 6.17 ± 0.07 a |
| XX | ||
| 18:2n-6 | 10.56 ± 0.73 b | 16.17 ± 0.29 a |
| 20:4n-6 | 0.85 ± 0.04 b | 1.88 ± 0.03 a |
| 18:3n-3 | 12.04 ± 1.21 a | 2.02 ± 0.09 b |
| 20:5n-3 | 0.91 ± 0.08 a | 0.26 ± 0.04 b |
| 22:5n-3 | 1.44 ± 0.07 a | 0.48 ± 0.06 b |
| 22:6n-3 | 7.05 ± 0.12 a | 2.34 ± 0.04 b |
| SFA | 32.19 ± 0.90 | 30.35 ± 0.60 |
| MUFA | 24.89 ± 1.05 b | 32.95 ± 1.47 a |
| PUFA | 36.87 ± 2.21 a | 27.70 ± 0.53 b |
| n-3 LC-PUFA | 10.89 ± 1.56 a | 3.27 ± 0.49 b |
| n-6 LC-PUFA | 1.81 ± 0.32 b | 2.30 ± 0.18 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Gao, L.; Guan, J.; Xu, C.; Wang, D.; Li, Y.; Xie, D. Comparison of LC-PUFAs Biosynthetic Characteristics in Male and Female Tilapia at Different Ontogenetic Stages. Life 2025, 15, 1167. https://doi.org/10.3390/life15081167
Chen F, Gao L, Guan J, Xu C, Wang D, Li Y, Xie D. Comparison of LC-PUFAs Biosynthetic Characteristics in Male and Female Tilapia at Different Ontogenetic Stages. Life. 2025; 15(8):1167. https://doi.org/10.3390/life15081167
Chicago/Turabian StyleChen, Fang, Liuling Gao, Junfeng Guan, Chao Xu, Deshou Wang, Yuanyou Li, and Dizhi Xie. 2025. "Comparison of LC-PUFAs Biosynthetic Characteristics in Male and Female Tilapia at Different Ontogenetic Stages" Life 15, no. 8: 1167. https://doi.org/10.3390/life15081167
APA StyleChen, F., Gao, L., Guan, J., Xu, C., Wang, D., Li, Y., & Xie, D. (2025). Comparison of LC-PUFAs Biosynthetic Characteristics in Male and Female Tilapia at Different Ontogenetic Stages. Life, 15(8), 1167. https://doi.org/10.3390/life15081167








