Outcomes of Acute Kidney Injury in Melioidosis: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol Registration
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
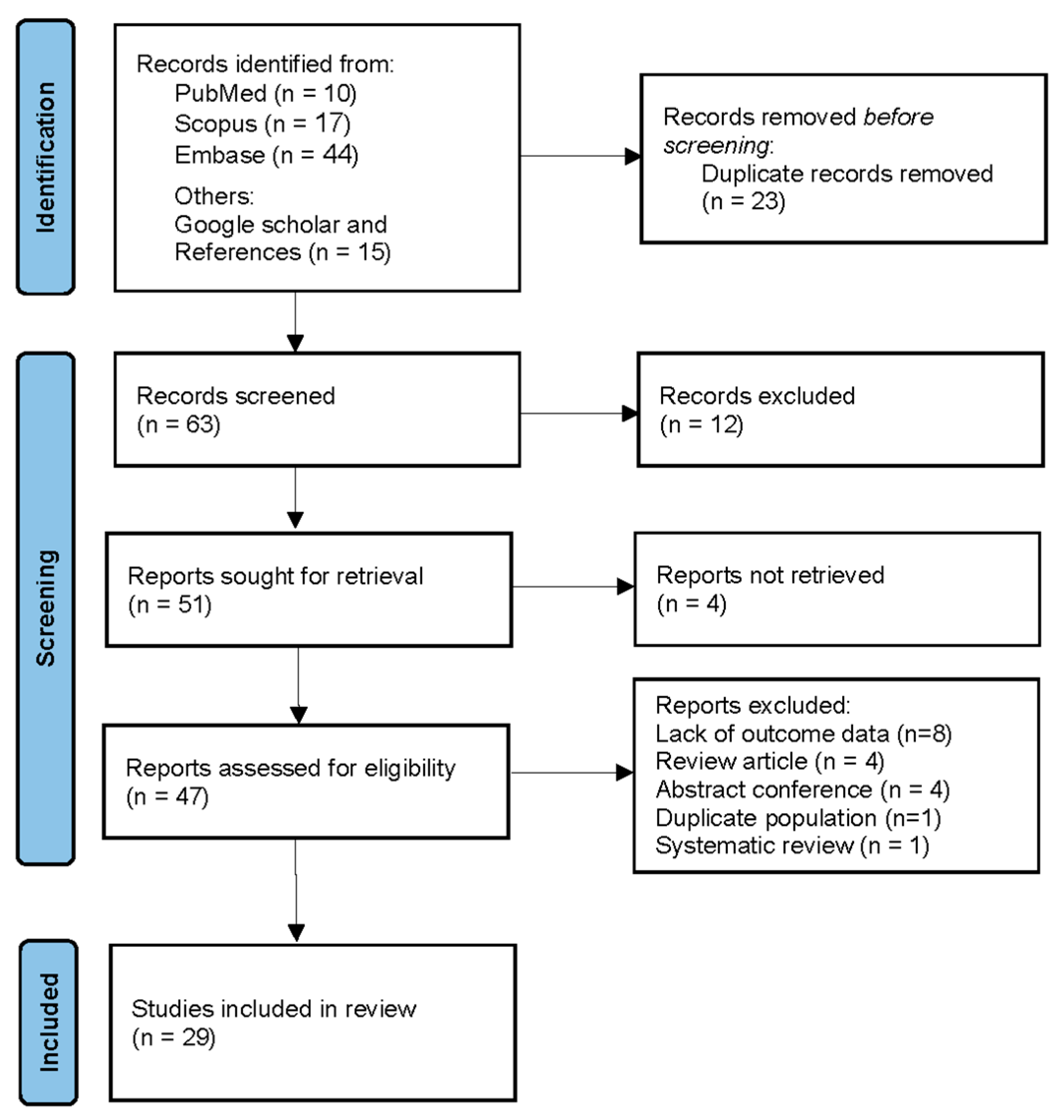
2.4. Study Selection
2.5. Data Extraction
2.6. Quality Assessment
2.7. Statistical Analysis
2.8. Bayesian Re-Analysis of Risk Difference (RD)
3. Results
3.1. Included Studies
3.2. Characteristics of Included Studies
| Author (Year) [Ref.] | Country | Sample Size (n = 380) | AKI Cases (n = 123) | AKI % | Mortality (AKI) | Mortality (no AKI) | ICU Admission | RRT Required | Organ Dysfunction | Risk Factor | AKI Definition | Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alhatmi (2020) [11] | Saudi Arabia | 2 | 1 | 50 | 100% (Case 1) | 0% (Case 2) | 1 | 1 | Multi-organ failure | Travel to Thailand (Case 1), India (Case 2) | Not explicitly defined, clinical evidence | Case 1: Fulminant sepsis, ECMO support; Case 2: Treated successfully with antibiotics. |
| Amali (2024) [12] | Singapore | 1 | 1 | 100 | 0% (Survived) | Not applicable | Yes (ECMO support) | Yes | Multi-organ dysfunction, including lungs, spleen, liver (hepatosplenic abscesses) | CASP4 mutation (R344W), no diabetes or other comorbidities | Acute presentation with renal dysfunction requiring ECMO support | Persistent B. pseudomallei infection, treated with recombinant IFN-γ leading to successful outcome and discharge. |
| Arya (2021) [13] | United States | 1 | 1 | 100 | 0% (Survived) | Not applicable | Yes | Not specified | Multiple organ involvement, including lungs, spleen, kidneys (severe sepsis) | Type-2 diabetes, hyperlipidemia, non-alcoholic fatty liver disease, prior SARS-CoV-2 infection, recent travel to Bangladesh | Clinical evidence of acute renal dysfunction | Complex case with delayed diagnosis, multiple organ abscesses, treated with meropenem and TMP-SMX, eventual recovery. |
| Boyle (2024) [14] | Australia | 8 | 2 | 25 | 0/2 (0%) | 0/5 (0%) | 6/8 (75%) | Not specified | Multiple organ systems, including aortic, renal, pulmonary, splenic involvement | Vascular disease, diabetes, chronic kidney disease, chronic lung disease, alcohol use | Not explicitly defined, clinical diagnosis of acute renal dysfunction | Complex cases of mycotic aneurysm due to Burkholderia pseudomallei, delayed diagnosis common, management includes surgical intervention and long-term antibiotics, high morbidity and mortality noted. |
| Chang (2020) [15] | Malaysia | 1 | 1 | 100 | 0% (Survived) | Not applicable | Not specified | No | Splenic abscess, renal dysfunction, sepsis | Pregnancy, no other comorbidities | Mild renal impairment with raised serum creatinine at 129 µmol/L | Case of a young pregnant woman with bacteraemic melioidosis and splenic abscesses, eventual spontaneous abortion. |
| Chanvitan (2019) [16] | Thailand | 27 | 1 | 3.7 | 100% (1/1) | 9/26 (34.6%) | Not specified | Not specified | Sepsis, pneumonia, soft tissue infection, splenic and hepatic abscesses | Diabetes, thalassemia, renal disease in a minority of cases | Increase in serum creatinine ≥ 0.3 mg/dL or 1.5-fold from baseline | Pediatric cohort, significant hepatic and splenic abscesses as diagnostic clues, AKI rare but fatal in one case. |
| Che Rahim (2019) [17] | Malaysia | 1 | 1 | 100 | 0% (Survived) | Not applicable | Yes | Yes (dialysis) | Septic shock, respiratory failure, hepatic dysfunction, renal dysfunction | Systemic lupus erythematosus, immunosuppression | Acute renal failure requiring dialysis | Young female patient with SLE, developed severe sepsis and multi-organ failure, successfully recovered with intensive treatment including immunosuppression and antibiotics. |
| Chou (2007) [4] | Taiwan | 30 | 19 | 63.3 | 9/19 (47.4%) | 0% (0/11) | 14/30 (46.7%) | Not specified | Sepsis, pneumonia, respiratory failure, renal failure | Diabetes mellitus, chronic renal disease, excessive alcohol consumption, malignancy, cardiovascular disease | Reduction in estimated creatinine clearance of 50% or need for RRT | Study of bacteremic melioidosis in Taiwan post-typhoon outbreak; high mortality in patients with AKI, often associated with pneumonia and septic shock. |
| Cossaboom (2020) [18] | United States | 1 | 1 | 100 | 0% (Survived) | Not applicable | Yes | Yes (CRRT) | Respiratory failure, sepsis | Type 2 diabetes, unilateral renal agenesis, rural water exposure | Clinical evidence with renal failure | Melioidosis acquired from environmental exposure in Texas; patient recovered with treatment. |
| Fairhead (2020) [19] | Australia | 1 | 1 | 100 | 0% (Survived) | Not applicable | Yes | Not specified | Pneumonia, discitis, osteomyelitis | Rheumatoid arthritis, chronic lung disease, immunosuppression with etanercept | Elevated creatinine on admission | Polymicrobial bacteremia (melioidosis and Acinetobacter), improved with antibiotics. |
| Ganesan (2019) [20] | India | 7 | 6 | 85.7 | 50% (3/6) | 0% (0/1) | Not specified | Not specified | Renal dysfunction, hepatic dysfunction, sepsis, metabolic derangements | Diabetes, alcoholism | Elevated renal parameters and clinical evidence | Case series of melioidosis with high mortality among AKI cases; slow microbiological clearance and persisting radiological abnormalities were noted. |
| Gouse (2017) [21] | India | 24 | 2 | 8.3 | 50% (1/2) | 0% (0/22) | Not specified | Not specified | Musculoskeletal involvement, septic arthritis, osteomyelitis, intramuscular abscesses | Diabetes, thalassemia, sickle cell anemia, chronic renal disease | Clinical evidence with acute renal failure | Largest musculoskeletal melioidosis series from India, surgical management resulted in good outcomes. |
| Gulati (2022) [22] | United States (Vietnam origin) | 1 | 1 | 100 | 100% (Died) | Not applicable | Yes | No (died before) | Sepsis, multi-organ dysfunction, ARDS | Diabetes, HIV infection, history of travel to Vietnam | Acute renal failure with sepsis | First reported case of latent melioidosis activation by COVID-19; rapid deterioration despite treatment. |
| Gunasena (2023) [23] | Sri Lanka | 1 | 1 | 100 | 0% (Survived) | Not applicable | Yes | Yes (intermittent hemodialysis) | Pulmonary hemorrhage, sepsis, jaundice | Farmer, environmental exposure | Acute renal dysfunction, oliguric AKI | Co-infection with leptospirosis and melioidosis, recovered with antibiotics, dialysis, and plasma exchange. |
| Gupta (2021) [24] | India | 11 | 3 | 27.3 | 33.3% (1/3) | 0% (0/8) | Not specified | Not specified | Osteoarticular melioidosis with systemic involvement | Diabetes, trauma, immunosuppression | Clinical evidence of acute renal dysfunction | Combination of osteomyelitis and arthritis, some patients had pulmonary involvement, treated with meropenem/ceftazidime and cotrimoxazole. |
| Hin (2012) [25] | Malaysia | 4 | 1 | 25 | 100% (1/1) | 66.7% (2/3) | Yes | Yes (hemodialysis) | Sepsis, multi-organ failure, pneumonia, hepatic dysfunction | Diabetes, leptospirosis co-infection, rescue operation exposure | Acute renal failure with sepsis | Cluster of cases among rescuers exposed to contaminated water, co-infection with leptospirosis confirmed by PCR. |
| Jagtap (2017) [26] | India | 9 | 1 | 11.1 | 100% (1/1) | 14.3% (1/7) | Not specified | Not specified | Liver abscess, splenic abscess, pancreatic abscess, empyema, SBP | Diabetes, alcoholism | Acute-on-chronic liver failure with renal dysfunction | GI manifestations of melioidosis, unusual presentation including pancreatitis and SBP, high mortality with severe liver disease. |
| Jang (2015) [27] | Korea | 1 | 1 | 100 | 0% (Survived) | Not applicable | Yes | Yes (due to worsening renal function) | Mycotic aneurysm with multi-organ embolism | Travel to Thailand, IgA nephropathy, gout, hypertension | Acute renal dysfunction during hospital course | Identified B. pseudomallei by 16S rRNA sequencing, surgical intervention with aortic repair, good recovery. |
| Lim (2022) [28] | Malaysia | 1 | 1 | 100 | 100% (Died) | Not applicable | Yes | Yes (hemodialysis) | Multi-organ failure, sepsis, ARDS, hepatic dysfunction | Uncontrolled diabetes, co-infection with leptospirosis, panhypopituitarism | Acute renal failure with sepsis and shock | Late diagnosis due to non-specific presentation, death from multi-organ failure despite aggressive treatment. |
| Liu (2014) [29] | Singapore | 74 | 9 | 12.2 | 15.9% (Approx.) | 3.3% (Approx.) | Not specified | Not specified | Sepsis, hypotension, respiratory distress, renal impairment | Type II diabetes, sulfonylurea treatment | Renal impairment with need for RRT | Sulfonylurea usage linked to severe septic complications and immune suppression. |
| Loh (2017) [30] | Australia | 1 | 1 | 100 | 0% (Survived) | Not applicable | Yes | Yes (dialysis) | Sepsis, respiratory failure, cerebral abscess, temporal lobe involvement | Pig hunting, exposure to soil and environmental pathogens | Acute renal failure with raised creatinine (241 µmol/L) | Complex case with delayed diagnosis, eventually recovered with prolonged antibiotics. |
| Meraj (2019) [31] | United States (Filipino origin) | 1 | 1 | 100 | 0% (Survived) | Not applicable | Yes | Yes (meropenem failure) | Persistent bacteremia sepsis | Travel to endemic areas, ceftazidime-resistant strain | Persistent bacteremia with renal involvement | Prolonged meropenem treatment required, eventual recovery. |
| Morelli (2015) [32] | Netherlands (Gambia travel) | 1 | 1 | 100 | 0% (Survived) | Not applicable | Yes | Yes (eventual hemodialysis) | Sepsis, prostatic abscess, ESRD | Travel to Gambia, environmental exposure | Acute renal failure progressing to ESRD | Prostatic abscess, recovery with ceftazidime, but renal failure persisted. |
| Prabhu (2021) [5] | India | 164 | 59 | 35.98 | 32.2% | 5.7% | 37.3% (AKI), 13.3% (non-AKI) | 8/59 (13.6%) | Sepsis, bacteremia, shock, multi-organ dysfunction | CKD, bacteremia, shock | AKIN criteria | AKI associated with higher mortality and ICU care; survivors showed kidney recovery. |
| Stewart (2021) [33] | United States (Arizona) | 1 | 1 | 100 | 0% (Survived) | Not applicable | Yes | Not specified | Sepsis, pneumonia, multiple abscesses | Unknown environmental exposure | Not directly defined, clinical evidence | First autochthonous case in the U.S., delayed diagnosis, recovery with antibiotics. |
| Tamtami (2017) [34] | Oman | 1 | 1 | 100 | 100% (Died) | Not applicable | Yes | Yes | Severe sepsis, ARDS, multi-organ failure | Occupational exposure in Laos/Cambodia, diabetes mellitus | Acute renal failure with multi-organ dysfunction | Imported case of melioidosis with B. pseudomallei isolated from blood; rapid deterioration despite intensive therapy. |
| Tran (2022) [35] | Vietnam | 3 | 2 | 66.7 | 100% (2/2) | 100% (1/1) | Yes (for all) | Not specified | Liver dysfunction, renal dysfunction, sepsis | Contaminated borehole water (B. pseudomallei ST541) | Elevated creatinine, clinical evidence | Cluster of 3 children from 1 family; B. pseudomallei traced to household borehole water; severe outcomes with liver and kidney involvement. |
| Wadwekar (2018) [36] | India | 1 | 1 | 100 | 0% (Survived) | Not applicable | Yes | Not specified | Sepsis, multiple abscesses, splenic rupture | Diabetes mellitus, soil exposure | Acute renal dysfunction with sepsis | Case report of melioidosis presenting with sepsis and splenic rupture, survived after aggressive treatment. |
| Warapitiya (2021) [37] | Sri Lanka | 1 | 1 | 100 | 100% (Died) | Not applicable | Yes | Yes (dialysis) | Severe sepsis; multi-organ failure, including renal and hepatic failure | Cut injury in paddy field, environmental exposure | Acute renal failure requiring dialysis | Severe sepsis with melioidosis, leading to multi-organ failure and death despite ICU support and broad-spectrum antibiotics. |
3.3. Pooled Odd and Heterogeneity
3.4. Sensitivity Analysis
3.5. Qualities of Included Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKI | acute kidney injury |
| AKIN | Acute Kidney Injury Network |
| ARDS | Acute Respiratory Distress Syndrome |
| CKD | chronic kidney disease |
| ECMO | Extracorporeal Membrane Oxygenation |
| ICU | intensive care unit |
| KDIGO | Kidney Disease: Improving Global Outcomes |
| MeSH | Medical Subject Heading |
| OR | odds ratio |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| SLE | Systemic Lupus Erythematosus |
References
- Wiersinga, W.J.; Currie, B.J.; Peacock, S.J. Melioidosis. N. Engl. J. Med. 2018, 378, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Limmathurotsakul, D.; Golding, N.; Dance, D.A.B.; Messina, J.P.; Pigott, D.M.; Moyes, C.L.; Rolim, D.B.; Bertherat, E.; Day, N.P.; Peacock, S.J.; et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat. Microbiol. 2016, 1, 15008. [Google Scholar] [CrossRef] [PubMed]
- Currie, B.J.; Ward, L.; Cheng, A.C. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl. Trop. Dis. 2010, 4, e900. [Google Scholar] [CrossRef] [PubMed]
- Chou, D.W.; Chung, K.M.; Chen, C.H.; Cheung, B.M.H. Bacteremic melioidosis in southern Taiwan: Clinical characteristics and outcome. J. Formos. Med. Assoc. 2007, 106, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, R.A.; Shaw, T.; Rao, I.R.; Kalwaje Eshwara, V.; Nagaraju, S.P.; Shenoy, S.V.; Mukhopadhyay, C. Acute kidney injury and its outcomes in melioidosis. J. Nephrol. 2021, 34, 1941–1948. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024, 105 (Suppl. S4), S117–S314. [Google Scholar] [CrossRef] [PubMed]
- Peerapornratana, S.; Priyanka, P.; Wang, S.; Smith, A.; Singbartl, K.; Palevsky, P.M.; Chawla, L.S.; Yealy, D.M.; Angus, D.C.; Kellum, J.A. ProCESS and ProGReSS-AKI investigators. Sepsis-associated acute kidney disease. Kidney Int. Rep. 2020, 5, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An up-dated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Goligher, E.C.; Heath, A.; Harhay, M.O. Bayesian statistics for clinical research. Lancet 2024, 404, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, F.G.; Casey, J.D.; Shankar-Hari, M.; Harrell, F.E., Jr.; Harhay, M.O. Using bayesian methods to augment the interpretation of critical care trials. An overview of theory and example reanalysis of the alveolar recruitment for acute respiratory distress syndrome trial. Am. J. Respir. Crit. Care Med. 2021, 203, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Alhatmi, H.; Alharbi, A.; Bosaeed, M.; Aldosary, O.; Aljohani, S.; Alalwan, B.; Alsaeedi, A.; Almahmoud, S.; Alothman, A. Melioidosis: Case reports of confirmed Burkholderia pseudomallei in Saudi Arabia. J. Infect. Public Health 2020, 13, 824–826. [Google Scholar] [CrossRef] [PubMed]
- Amali, A.A.; Ravikumar, S.; Chew, W.L.; Tan, Z.; Sam, Q.H.; Chen, K.W.; Boucher, D.; MacLaren, G.; Chai, L.Y.A. Extracorporeal membrane oxygenation-dependent fulminant melioidosis from caspase 4 mutation reversed by interferon gamma therapy. Clin. Infect. Dis. 2024, 78, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Arya, A.; Shaikh, H.; Weber, D.; Pettengill, M.; Moss, S. Fever in a returning traveler: A case and literature review of melioidosis. IDCases 2021, 26, e01340. [Google Scholar] [CrossRef] [PubMed]
- Boyle, R.; Withey, G.; Smith, S.; Hanson, J. Mycotic aneurysms due to Burkholderia pseudomallei in Far North Queensland, tropical Australia: A case series and review of the literature. Acta Trop. 2024, 260, 107480. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Lau, N.L.J.; Currie, B.J.; Podin, Y. Disseminated melioidosis in early pregnancy—An unproven cause of foetal loss. BMC Infect. Dis. 2020, 20, 201. [Google Scholar] [CrossRef] [PubMed]
- Chanvitan, S.; Geater, A.; Laoprasopwattana, K. Hepatic/splenic abscess and/or skin and soft tissue infection as predictors of melioidosis in children. J. Infect. Dev. Ctries. 2019, 13, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Che Rahim, M.J.; Mohammad, N.; Kamaruddin, M.I.; Wan Ghazali, W.S. Systemic lupus erythematosus and melioidosis. BMJ Case Rep. 2019, 12, e229974. [Google Scholar] [CrossRef] [PubMed]
- Cossaboom, C.M.; Marinova-Petkova, A.; Strysko, J.; Rodriguez, G.; Maness, T.; Ocampo, J.; Gee, J.E.; Elrod, M.G.; Gulvik, C.A.; Liu, L.; et al. Melioidosis in a resident of texas with no recent travel history, united states. Emerg. Infect. Dis. 2020, 26, 1295–1299. [Google Scholar] [CrossRef] [PubMed]
- Fairhead, L.; Vardanega, J.; Pandey, R.; Smith, S. Polymicrobial community-acquired Acinetobacter baumannii and Burkholderia pseudomallei bacteremia: Opportunistic infections with similar risk factors in northern Australia. IDCases 2020, 21, e00833. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, V.; Sundaramoorthy, R.; Subramanian, S. Melioidosis-Series of seven cases from Madurai, Tamil Nadu, India. Indian J. Crit. Care Med. 2019, 23, 149–151. [Google Scholar] [PubMed]
- Gouse, M.; Jayasankar, V.; Patole, S.; Veeraraghavan, B.; Nithyananth, M. Clinical outcomes in musculoskeletal involvement of Burkholderia pseudomallei infection. Clin. Orthop. Surg. 2017, 9, 386–391. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gulati, U.; Nanduri, A.C.; Juneja, P.; Kaufman, D.; Elrod, M.G.; Kolton, C.B.; Gee, J.E.; Garafalo, K.; Blaney, D.D. Case report: A fatal case of latent melioidosis activated by COVID-19. Am. J. Trop. Med. Hyg. 2022, 106, 1170–1172. [Google Scholar] [CrossRef] [PubMed]
- Gunasena, J.B.; De Silva, S.T. Double-trouble: A rare case of co-infection with melioidosis and leptospirosis from Sri Lanka. Trop. Doct. 2023, 53, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Bhat, S.N.; Reddysetti, S.; Kadavigere, R.; Godkhindi, V.M.; Mukhopadhyay, C.; Saravu, K. Osteoarticular melioidosis: A retrospective cohort study of a neglected disease. Infez. Med. 2021, 29, 574–582. [Google Scholar] [PubMed]
- Hin, H.S.; Ramalingam, R.; Chunn, K.Y.; Ahmad, N.; Ab Rahman, J.; Mohamed, M.S. Fatal co-infection--melioidosis and leptospirosis. Am. J. Trop. Med. Hyg. 2012, 87, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, N.; Shah, H.; Kancharla, A.; Tandan, M.; Pal, P.; Lakhtakia, S.; Ramchandani, M.; Reddy, D.N. Gastrointestinal manifestations of melioidosis: A single center experience. Indian J. Gastroenterol. 2017, 36, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.R.; Lee, C.W.; Ok, S.J.; Kim, M.J.; Bae, M.J.; Song, S.; Yi, J.; Kim, K.H. Melioidosis presenting as a mycotic aneurysm in a Korean patient, diagnosed by 16S rRNA sequencing and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Int. J. Infect. Dis. 2015, 38, 62–64. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lim, K.; Shukeri, W.; Mazlan, M.; Rafiqi, M.; Abidin, H. Fulminant septic shock from melioidosis and leptospirosis co-infections. Anaesth. Pain Intensive Care 2022, 26, 257–259. [Google Scholar] [CrossRef]
- Liu, X.; Foo, G.; Lim, W.P.; Ravikumar, S.; Sim, S.H.; Win, M.S.; Goh, J.G.; Lim, J.H.; Ng, Y.H.; Fisher, D.; et al. Sulphonylurea usage in melioidosis is associated with severe disease and suppressed immune response. PLoS Negl. Trop. Dis. 2014, 8, e2795. [Google Scholar] [CrossRef] [PubMed]
- Loh, T.L.; Latis, S.; Crossland, G.; Patel, H. Disseminated melioidosis in the head and neck. BMJ Case Rep. 2017, 2017, bcr2016218606. [Google Scholar] [CrossRef] [PubMed]
- Meraj, S.; Rodenberg, B.; Thannum, S.; Sheley, J.; Foreman, J. Persistent Burkholderia pseudomallei Bacteremia in A Filipino Immigrant to the United States: A Case Report. Trop. Med. Infect. Dis. 2019, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Morelli, F.; Smeets, L.; Hobijn, M.; Boom, H. Melioidosis and renal failure in a Dutch man after a trip to Gambia. Neth. J. Med. 2015, 73, 296–298. [Google Scholar] [PubMed]
- Stewart, T.; Engelthaler, D.M.; Blaney, D.D.; Tuanyok, A.; Wangsness, E.; Smith, T.L.; Pearson, T.; Komatsu, K.K.; Keim, P.; Currie, B.J.; et al. Epidemiology and investigation of melioidosis, Southern Arizona. Emerg. Infect. Dis. 2011, 17, 1286–1288. [Google Scholar] [CrossRef] [PubMed]
- Tamtami, N.A.; Khamis, F.; Al-Jardani, A. Imported Case of Melioidosis in Oman: Case Report. Oman. Med. J. 2017, 32, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.T.L.; Phan, P.H.; Bui, L.N.H.; Bui, H.T.V.; Hoang, N.T.B.; Tran, D.M.; Trinh, T.T. Child melioidosis deaths caused by Burkholderia pseudomallei-Contaminated borehole water, Vietnam, 2019. Emerg. Infect. Dis. 2022, 28, 1689–1693. [Google Scholar] [CrossRef] [PubMed]
- Wadwekar, B.; Suresh Ninan, R.; Bhat, S.; Sheela, D.; Ramaya, S.; Kanungo, R. Lid abscess: An unusual presentation of melioidosis. Australas. Med. J. 2018, 11, 322–325. [Google Scholar] [CrossRef]
- Warapitiya, D.S.; Subasinghe, S.; de Silva, R.F.; Piyarisi, D.L.; Jayatilleke, K. Severe sepsis with multiorgan failure due to melioidosis: A lesson to learn. Case Rep. Med. 2021, 2021, 5563214. [Google Scholar] [CrossRef] [PubMed]
- Daher, E.D.F.; de Abreu, K.L.; da Silva Junior, G.B. Leptospirosis-associated acute kidney injury. J. Bras. Nefro. 2010, 32, 400–407. [Google Scholar]
- Osorio-Rodríguez, E.; Rodelo-Barrios, D.; Rebolledo-Maldonado, C.; Polo-Barranco, A.; Patiño-Patiño, J.; Aldana-Roa, M.; Sánchez-Daza, V.; Sierra-Ordoñez, E.; Bettin-Martínez, A. Acute kidney injury associated with severe leptospirosis: Fatal re-emerging disease in Latin America. Kidney Dial. 2024, 4, 78–92. [Google Scholar] [CrossRef]
- Bignardi, P.R.; Pinto, G.R.; Boscarioli, M.L.N.; Lima, R.A.A.; Delfino, V.D.A. Acute kidney injury associated with dengue virus infection: A review. J. Bras. Nefro. 2022, 44, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.F.; Burdmann, E.A. Dengue-associated acute kidney injury. Clin. Kidney J. 2015, 8, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Levi, T.M.; de Souza, S.P.; de Magalhães, J.G.; de Carvalho, M.S.; Cunha, A.L.; Dantas, J.G.; Cruz, M.G.; Guimarães, Y.L.; Cruz, C.M. Comparison of the RIFLE, AKIN and KDIGO criteria to predict mortality in critically ill patients. Rev. Bras. Ter. Intensiv. 2013, 25, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Rao, I.R.; Shaw, T.; Prabhu, R.A.; Eshwara, V.K.; Nagaraju, S.P.; Rangaswamy, D.; Shenoy, S.V.; Bhojaraja, M.V.; Mukhopadhyay, C. Hyponatremia in Melioidosis: Analysis of 10-year Data from a Hospital-Based Registry. J. Glob. Infect. Dis. 2022, 14, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.W.J. The Great Mimicker or the Great Masquerader? Am. J. Med. 2022, 135, e27–e30. [Google Scholar] [CrossRef] [PubMed]
- Luvira, U.; Sukahatya, M.; Alano, F.A.; Danguilan, R.A.; Thang, N.T.; Lin, C.H.; Lee, G.; Morad, Z.; Thirakhupt, P. Clinical features of renal diseases in South-East Asia. Nephrology 1998, 4, S9–S11. [Google Scholar] [CrossRef]
- Mackintosh, D.; Mantha, M.; Oliver, K. Goodpasture disease as a consequence of melioidosis. Intern. Med. J. 2016, 46, 1446–1449. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.E.; Bramwell, J.; Gadil, E.; Woerle, C.; Ewin, T.; Davies, J.; Janson, S.; Currie, B.J. Adverse reactions to trimethoprim/sulfamethoxazole for melioidosis eradication therapy: An evaluation of frequency and risk factors. Int. J. Infect. Dis. 2025, 150, 107283. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Lee, K.H.; Tambyah, P.A. Bacteraemic melioidosis pneumonia: Impact on outcome, clinical and radiological features. J. Infect. 2004, 48, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Nandurkar, D.; Lau, K. Melioidosis as a cause of multifocal osteomyelitis. Clin. Nucl. Med. 2006, 31, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Laklaeng, S.N.; Songsri, J.; Wisessombat, S.; Mala, W.; Phothaworn, P.; Senghoi, W.; Nuinoon, M.; Tangphatsornruang, S.; Wongtawan, T.; Hayakijkosol, O.; et al. Multi-locus sequence typing and genetic diversity of antibiotic-resistant genes and virulence-associated genes in Burkholderia pseudomallei: Insights from whole genome sequencing of animal and environmental isolates in Thailand. Vet. Microbiol. 2024, 298, 110236. [Google Scholar] [CrossRef] [PubMed]
- Patamatamkul, S.; Klungboonkrong, V.; Praisarnti, P.; Jirakiat, K. A case-control study of community-acquired Acinetobacter baumannii pneumonia and melioidosis pneumonia in northeast Thailand: An emerging fatal disease with unique clinical features. Diagn. Microbiol. Infect. Dis. 2017, 87, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, Z.; Currie, B.J. Melioidosis and the kidney. Nephrology 2013, 18, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Meumann, E.M.; Currie, B.J. Approach to melioidosis. CMI Commun. 2024, 1, 100008. [Google Scholar] [CrossRef]
- Norman, F.F.; Blair, B.M.; Chamorro-Tojeiro, S.; González-Sanz, M.; Chen, L.H. The Evolving Global Epidemiology of Human Melioidosis: A Narrative Review. Pathogens 2024, 13, 926. [Google Scholar] [CrossRef] [PubMed]
- Raja, N.S. Melioidotic septic arthritis: A case report and literature review. J. Microbiol. Immunol. Infect. 2007, 40, 178–182. [Google Scholar] [PubMed]
- Keragala, K.A.R.K.; Gunathilaka, M.G.R.S.S.; Senevirathna, R.M.I.S.K.; Jayaweera, J.A.A.S. Efficacy and safety of co-trimoxazole in eradication phase of melioidosis; systematic review. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 74. [Google Scholar] [CrossRef] [PubMed]
- Aravan, L.; Mohamad, N.I. Pos-165 Acute kidney injury and its outcome among melioidosis patients in a tertiary hospital of a north-eastern state of Malaysia. Kidney Int. Rep. 2022, 7, S71–S72. [Google Scholar] [CrossRef]
- Grace, S.; Currie, B.; Kumar, S. Parathyroid Hormone Independent Hypercalcaemia Secondary to Granulomatous Inflammation: Could This Be Melioidosis? J. Endocr. Soc. 2022, 6, A172. [Google Scholar] [CrossRef]
- Janelle, P.; Maeve, O.; Robert, H.; Anoushka, K.; Hemant, K.; Rajalingam, S.; Sze, A.W. Atypical anti-gbm disease in association with systemic melioidosis. In Proceedings of the Australian and New Zealand Society of Nephrology (ANZSN) 2023, Christchurch, New Zealand, 2–6 September 2023. [Google Scholar]
- Tan, Y.L.; Ming, L.J.; Wei, K.W. Burkholderia pseudomallei (melioidosis) peritoneal dialysis peritonitis. In Proceedings of the World Congress of Nephrology WCN′22. WCN′22, Kuala Lumpur, Malaysia, 24–27 February 2022. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klangbud, W.K.; Chatatikun, M.; Laklaeng, S.-n.; Tangpong, J.; Wongyikul, P.; Phinyo, P.; Thanasai, J.; Khemla, S.; Chanthot, C.; Phongphithakchai, A. Outcomes of Acute Kidney Injury in Melioidosis: A Systematic Review and Meta-Analysis. Life 2025, 15, 1108. https://doi.org/10.3390/life15071108
Klangbud WK, Chatatikun M, Laklaeng S-n, Tangpong J, Wongyikul P, Phinyo P, Thanasai J, Khemla S, Chanthot C, Phongphithakchai A. Outcomes of Acute Kidney Injury in Melioidosis: A Systematic Review and Meta-Analysis. Life. 2025; 15(7):1108. https://doi.org/10.3390/life15071108
Chicago/Turabian StyleKlangbud, Wiyada Kwanhian, Moragot Chatatikun, Sa-ngob Laklaeng, Jitabanjong Tangpong, Pakpoom Wongyikul, Phichayut Phinyo, Jongkonnee Thanasai, Supphachoke Khemla, Chaimongkhon Chanthot, and Atthaphong Phongphithakchai. 2025. "Outcomes of Acute Kidney Injury in Melioidosis: A Systematic Review and Meta-Analysis" Life 15, no. 7: 1108. https://doi.org/10.3390/life15071108
APA StyleKlangbud, W. K., Chatatikun, M., Laklaeng, S.-n., Tangpong, J., Wongyikul, P., Phinyo, P., Thanasai, J., Khemla, S., Chanthot, C., & Phongphithakchai, A. (2025). Outcomes of Acute Kidney Injury in Melioidosis: A Systematic Review and Meta-Analysis. Life, 15(7), 1108. https://doi.org/10.3390/life15071108








