Congee Containing Carotenoids-Enriched Functional Ingredient from Tomato Improves Cognition, Serum α-Synuclein, Monoaminergic Function, and Gut-Brain Axis Functions in the Elderly Volunteers
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Carotenoid-Rich Functional Congee
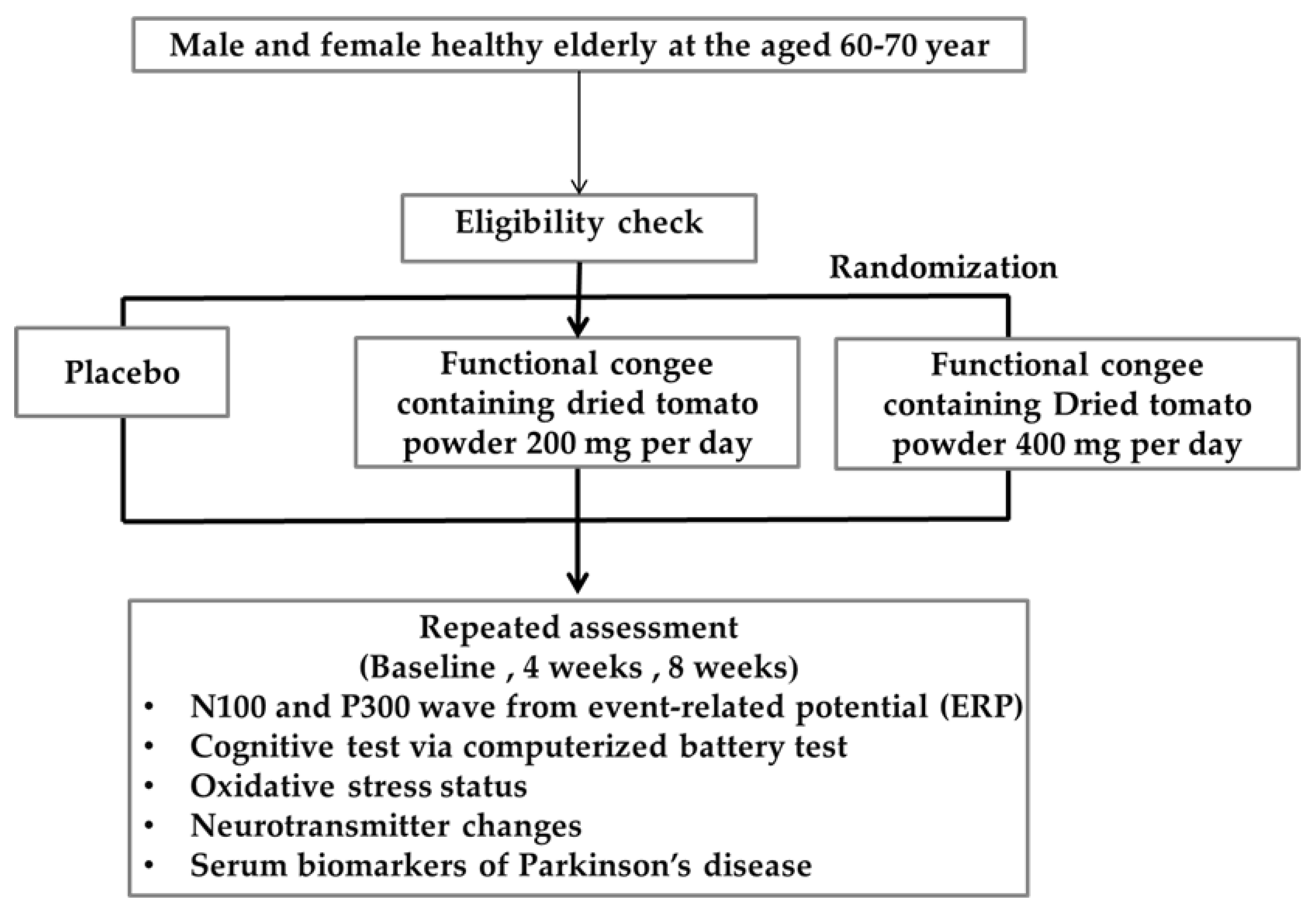
2.2. Study Design
- Sample size
- Inclusion and exclusion criteria
- Study protocol
2.3. Event-Related Potential
2.4. Working Memory Assessment
- Word Presentation
- Picture Presentation
- Simple Reaction Time
- Digit Vigilance Task
- Choice Reaction Time
- Spatial Working Memory
- Numeric Working Memory
2.5. Blood Collection and Preparation
2.6. Biochemical Assessments
2.6.1. Acetylcholinesterase (AChE) Activity Assessment
2.6.2. Monoamine Oxidase (MAO) Assessment
2.6.3. Assessments of Serum Biomarkers of Parkinson’s Disease
2.6.4. Determination of Oxidative Stress Markers
2.7. Assessment of Lactobacillus spp. and Bifidobacterium spp.
2.8. Statistical Analysis
3. Results
3.1. Demographic Data, Vital Signs, Body Weight, Height, and Body Mass Index (BMI)
3.2. Effect of Functional Congee Containing Dried Tomato Powder on Cognitive Function
3.3. Effect of Functional Congee Containing Dried Tomato Powder on Biochemical Changes
3.4. Changes in Lactobacillus and Bifidobacterium spp.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Population Reference Bureau. 2024 World Population Data Sheet. Available online: https://2024-wpds.prb.org/ (accessed on 3 December 2024).
- Wyss-Coray, T. Ageing, neurodegeneration and brain rejuvenation. Nature 2016, 539, 180–186. [Google Scholar] [CrossRef]
- Van Schependom, J.; D’haeseleer, M. Advances in Neurodegenerative Diseases. J. Clin. Med. 2023, 12, 1709. [Google Scholar] [CrossRef]
- Kiaei, M. New hopes and challenges for treatment of neurodegenerative disorders: Great opportunities for young neuroscientists. Basic Clin. Neurosci. 2013, 4, 3–4. [Google Scholar] [PubMed]
- Chou, S.C.; Aggarwal, A.; Dawson, V.L.; Dawson, T.M.; Kam, T.I. Recent advances in preventing neurodegenerative diseases. Fac. Rev. 2021, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, A.; Mercuri, N.B.; Venneri, A.; Faustini, G.; Longhena, F.; Pizzi, M.; Missale, C.; Spano, P. Review: Parkinson’s disease: From synaptic loss to connectome dysfunction. Neuropathol. Appl. Neurobiol. 2016, 42, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Stefanis, L. α-Synuclein in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009399. [Google Scholar] [CrossRef]
- Stewart, T.; Liu, C.; Ginghina, C.; Cain, K.C.; Auinger, P.; Cholerton, B.; Shi, M.; Zhang, J.; Parkinson Study Group DATATOP Investigators. Cerebrospinal fluid α-synuclein predicts cognitive decline in Parkinson disease progression in the DATATOP cohort. Am. J. Pathol. 2014, 184, 966–975. [Google Scholar] [CrossRef]
- Chang, C.W.; Yang, S.Y.; Yang, C.C.; Chang, C.W.; Wu, Y.R. Plasma and Serum Alpha-Synuclein as a Biomarker of Diagnosis in Patients With Parkinson’s Disease. Front. Neurol. 2020, 10, 1388. [Google Scholar] [CrossRef]
- Murakami, H.; Ono, K.; Shiraishi, T.; Umehara, T.; Omoto, S.; Iguchi, Y. Mini Review: Correlations of Cognitive Domains with Cerebrospinal Fluid α-Synuclein Levels in Patients with Parkinson’s Disease. Front. Aging Neurosci. 2021, 12, 616357. [Google Scholar] [CrossRef]
- Wang, H.; Atik, A.; Stewart, T.; Ginghina, C.; Aro, P.; Kerr, K.F.; Seibyl, J.; Jennings, D.; PARS Investigators; Jensen, P.H.; et al. Plasma α-synuclein and cognitive impairment in the Parkinson’s Associated Risk Syndrome: A pilot study. Neurobiol. Dis. 2018, 116, 53–59. [Google Scholar] [CrossRef]
- Teil, M.; Arotcarena, M.L.; Faggiani, E.; Laferriere, F.; Bezard, E.; Dehay, B. Targeting α-synuclein for PD Therapeutics: A Pursuit on All Fronts. Biomolecules 2020, 10, 391. [Google Scholar] [CrossRef] [PubMed]
- Fields, C.R.; Bengoa-Vergniory, N.; Wade-Martins, R. Targeting Alpha-Synuclein as a Therapy for Parkinson’s Disease. Front. Mol. Neurosci. 2019, 12, 299. [Google Scholar] [CrossRef]
- Pasquini, J.; Brooks, D.J.; Pavese, N. The Cholinergic Brain in Parkinson’s Disease. Mov. Disord. Clin. Pract. 2021, 8, 1012–1026. [Google Scholar] [CrossRef]
- Bohnen, N.I.; Yarnall, A.J.; Weil, R.S.; Moro, E.; Moehle, M.S.; Borghammer, P.; Bedard, M.A.; Albin, R.L. Cholinergic system changes in Parkinson’s disease: Emerging therapeutic approaches. Lancet Neurol. 2022, 21, 381–392. [Google Scholar] [CrossRef]
- Davinelli, S.; Ali, S.; Solfrizzi, V.; Scapagnini, G.; Corbi, G. Carotenoids and Cognitive Outcomes: A Meta-Analysis of Randomized Intervention Trials. Antioxidants 2021, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Tanprasertsuk, J.; Scott, T.M.; Barbey, A.K.; Barger, K.; Wang, X.D.; Johnson, M.A.; Poon, L.W.; Vishwanathan, R.; Matthan, N.R.; Lichtenstein, A.H.; et al. Carotenoid-Rich Brain Nutrient Pattern is Positively Correlated with Higher Cognition and Lower Depression in the Oldest Old with No Dementia. Front. Nutr. 2021, 8, 704691. [Google Scholar] [CrossRef]
- Wattanathorn, J.; Thukham-mee, W.; Muchimapura, S.; Tong-un, T.; Wannanon, P. Tomato, a potential yin food, protects against stroke. Chin. Med. 2012, 3, 144–150. [Google Scholar] [CrossRef]
- di Matteo, V.; Pierucci, M.; Di Giovanni, G.; Dragani, L.K.; Murzilli, S.; Poggi, A.; Esposito, E. Intake of tomato-enriched diet protects from 6-hydroxydopamine-induced degeneration of rat nigral dopaminergic neurons. J. Neural. Transm. Suppl. 2009, 73, 333–341. [Google Scholar][Green Version]
- Frosini, M.; Marcolongo, P.; Gamberucci, A.; Tamasi, G.; Pardini, A.; Giunti, R.; Fiorenzani, P.; Aloisi, A.M.; Rossi, C.; Pessina, F. Effects of Aqueous Extract of Lycopersicum esculentum L. var. “Camone” Tomato on Blood Pressure, Behavior and Brain Susceptibility to Oxidative Stress in Spontaneously Hypertensive Rats. Pathophysiology 2021, 28, 189–201. [Google Scholar] [CrossRef]
- Oboh, G.; Bakare, O.O.; Ademosun, A.O.; Akinyemi, A.J.; Olasehinde, T.A. Inhibition of Cholinesterases and Some Pro-Oxidant induced Oxidative Stress in Rats Brain by Two Tomato (Lycopersicon esculentum) Varieties. Int. J. Biomed. Sci. 2015, 11, 48–53. [Google Scholar] [CrossRef]
- Yoon, K.N.; Cui, Y.; Quan, Q.L.; Lee, D.H.; Oh, J.H.; Chung, J.H. Tomato and lemon extracts synergistically improve cognitive function by increasing brain-derived neurotrophic factor levels in aged mice. Br. J. Nutr. 2024, 131, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Muralidhara, G.K. Oral supplements of aqueous extract of tomato seeds alleviate motor abnormality, oxidative impairments and neurotoxicity induced by rotenone in mice: Relevance to Parkinson’s disease. Neurochem. Res. 2014, 39, 1382–1394. [Google Scholar]
- Chapman, N.H.; Fisk, I.; Craigon, J.; Towey, C.; Grant, I.; Brewer, J. Exploring the Effects of Tomato Extract Supplementation on Cognitive Function during Exercise and at Rest. J. Nutr. Health Sci. 2019, 6, 203. [Google Scholar]
- Sim, J.; Lewis, M. The size of a pilot study for a clinical trial should be calculated in relation to considerations of precision and efficiency. J. Clin. Epidemiol. 2012, 65, 301–308. [Google Scholar] [CrossRef]
- Browne, R.H. On the use of a pilot sample for sample size determination. Stat. Med. 1995, 14, 1933–1940. [Google Scholar] [CrossRef]
- In, J. Introduction of a pilot study. Korean J. Anesthesiol. 2017, 70, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Kieser, M.; Wassmer, G. On the use of the upper confidence limit for the variance from a pilot sample for sample size determination. Biom. J. 1996, 8, 941–949. [Google Scholar] [CrossRef]
- Wattanathorn, J.; Thukham-mee, W.; Tong-un, T.; Sangartit, W.; Somboonporn, W.; Paholpak, P. A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, 8-Week Pilot Study of Tuna-Byproduct-Derived Novel Supplements for Managing Cellular Senescence and Cognitive Decline in Perimenopausal and Postmenopausal Women. Antioxidants 2025, 14, 520. [Google Scholar] [CrossRef]
- Wattanathorn, J.; Somboonporn, W.; Thukham-Mee, W.; Sungkamnee, S. Memory-Enhancing Effect of 8-Week Consumption of the Quercetin-Enriched Culinary Herbs-Derived Functional Ingredients: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Foods 2022, 11, 2678. [Google Scholar] [CrossRef]
- Wattanathorn, J.; Tong-Un, T.; Thukham-Mee, W.; Paholpak, P.; Rangseekhajee, P. A Randomized, Double-Blind, Placebo-Controlled Study of an Anthocyanin-Rich Functional Ingredient on Cognitive Function and Eye Dryness in Late Adulthood Volunteers: Roles of Epigenetic and Gut Microbiome Modulations. Nutrients 2023, 15, 3499. [Google Scholar] [CrossRef]
- Elmann, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Holt, A.; Sharman, D.F.; Baker, G.B.; Palcic, M.M. A continuous spectrophotometric assay for monoamine oxidase and related enzymes in tissue homogenates. Anal. Biochem. 1997, 244, 384–392. [Google Scholar] [CrossRef]
- Stafford, G.I.; Pederson, P.D.; Jäger, A.K.; Van Staden, J. Monoamine oxidase inhibition by southern African traditional medicinal plants. S. Afr. J. Bot. 2007, 73, 384–390. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Sun, Y.; Oberley, L.W.; Li, Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef]
- Goldblith, S.A.; Proctor, B.E. Photometric determination of catalase activity. J. Biol. Chem. 1950, 187, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Ohnon, W.; Wattanathorn, J.; Thukham-Mee, W.; Muchimapura, S.; Wannanon, P.; Tong-Un, T. The combined extract of black sticky rice and dill improves poststroke cognitive impairment in metabolic syndrome condition. Oxid. Med. Cell. Longev. 2019, 2019, 9089035. [Google Scholar] [CrossRef]
- Wattanathorn, J.; Palachai, N.; Thukham-Mee, W.; Muchimapura, S. Memory-enhancing effect of a phytosome containing the combined extract of mulberry fruit and ginger in an animal model of ischemic stroke with metabolic syndrome. Oxid. Med. Cell. Longev. 2020, 2020, 3096826. [Google Scholar] [CrossRef] [PubMed]
- Thornton, A.R.; Harmer, M.; Lavoie, B.A. Selective attention increases the temporal precision of the auditory N100 event-related potential. Hear. Res. 2007, 230, 73–79. [Google Scholar] [CrossRef]
- Clark, C.R.; Geffen, G.M.; Geffen, L.B. Role of monoamine pathways in the control of attention: Effects of droperidol and methylphenidate in normal adult humans. Psychopharmacology 1986, 90, 28–34. [Google Scholar] [CrossRef]
- Lawson, D.C.; Turic, D.; Langley, K.; Pay, H.M.; Govan, C.F.; Norton, N.; Hamshere, M.L.; Owen, M.J.; O′Donovan, M.C.; Thapar, A. Association analysis of monoamine oxidase A and attention deficit hyperactivity disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2003, 116B, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.; Yamao, S.; Fukuda, H.; Mimori, Y.; Nakamura, S. The P300 event-related potentials in dementia of the Alzheimer type. Correlations between P300 and monoamine metabolites. Electroencephalogr. Clin. Neurophysiol. 1990, 77, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Holland, N.; Robbins, T.W.; Rowe, J.B. The role of noradrenaline in cognition and cognitive disorders. Brain 2021, 144, 2243–2256. [Google Scholar] [CrossRef]
- Ellis, K.A.; Nathan, P.J. The pharmacology of human working memory. Int. J. Neuropsychopharmacol. 2001, 4, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Yang, S.Y.; Horng, H.E.; Yang, C.C.; Chieh, J.J.; Chen, H.H.; Liu, B.H.; Chiu, M.J. Plasma α-synuclein predicts cognitive decline in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2017, 88, 818–824. [Google Scholar] [CrossRef]
- Liao, J.F.; Cheng, Y.F.; You, S.T.; Kuo, W.C.; Huang, C.W.; Chiou, J.J.; Hsu, C.C.; Hsieh-Li, H.M.; Wang, S.; Tsai, Y.C. Lactobacillus plantarum PS128 alleviates neurodegenerative progression in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse models of Parkinson’s disease. Brain Behav. Immun. 2020, 90, 26–46. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Z.; Zhao, L.; Zhao, Y.; Yang, G.; Wang, C.; Gao, L.; Niu, C.; Li, S. Lactobacillus plantarum DP189 Reduces α-SYN Aggravation in MPTP-Induced Parkinson’s Disease Mice via Regulating Oxidative Damage, Inflammation, and Gut Microbiota Disorder. J. Agric. Food Chem. 2022, 70, 1163–1173. [Google Scholar] [CrossRef]
- Park, S.J.; Jaiswal, V.; Lee, H.J. Dietary Intake of Flavonoids and Carotenoids Is Associated with Anti-Depressive Symptoms: Epidemiological Study and In Silico-Mechanism Analysis. Antioxidants 2021, 11, 53. [Google Scholar] [CrossRef]
- Gandla, K.; Babu, A.K.; Unnisa, A.; Sharma, I.; Singh, L.P.; Haque, M.A.; Dashputre, N.L.; Baig, S.; Siddiqui, F.A.; Khandaker, M.U.; et al. Carotenoids: Role in Neurodegenerative Diseases Remediation. Brain Sci. 2023, 13, 457. [Google Scholar] [CrossRef]
- Kim, J.H.; Hwang, J.; Shim, E.; Chung, E.J.; Jang, S.H.; Koh, S.B. Association of serum carotenoid, retinol, and tocopherol concentrations with the progression of Parkinson’s Disease. Nutr. Res. Pract. 2017, 11, 114–120. [Google Scholar] [CrossRef]
- Hu, P.; Bretsky, P.; Crimmins, E.M.; Guralnik, J.M.; Reuben, D.B.; Seeman, T.E. Association between serum beta-carotene levels and decline of cognitive function in high-functioning older persons with or without apolipoprotein E 4 alleles: MacArthur studies of successful aging. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 616–620. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, R.; Zhao, N.; Xu, J.; Liu, F.; Wei, X.; Fan, M. Rational design of lycopene emulsion-based nanofood for Lactobacillus plantarum to enhance the growth and flavor production. Food Hydrocoll. 2022, 127, 107518. [Google Scholar] [CrossRef]
- Wiese, M.; Bashmakov, Y.; Chalyk, N.; Nielsen, D.S.; Krych, Ł.; Kot, W.; Klochkov, V.; Pristensky, D.; Bandaletova, T.; Chernyshova, M.; et al. Prebiotic effect of lycopene and dark chocolate on gut microbiome with systemic changes in liver metabolism, skeletal muscles and skin in moderately obese persons. Biomed Res. Int. 2019, 2019, 4625279. [Google Scholar] [CrossRef] [PubMed]
- Dingeo, G.; Brito, A.; Samouda, H.; Iddir, M.; La Frano, M.R.; Bohn, T. Phytochemicals as modifiers of gut microbial communities. Food Funct. 2020, 11, 8444–8471. [Google Scholar] [CrossRef]
- Hansenne, M. Le potentiel évoqué cognitif P300 (I): Aspects théorique et psychobiologique [The p300 cognitive event-related potential. I. Theoretical and psychobiologic perspectives]. Neurophysiol. Clin. 2000, 30, 191–210. [Google Scholar] [CrossRef] [PubMed]
- Eyamu, J.; Kim, W.S.; Kim, K.; Lee, K.H.; Kim, J.U. Prefrontal event-related potential markers in association with mild cognitive impairment. Front. Aging Neurosci. 2023, 15, 1273008. [Google Scholar]
- Sokhadze, E.M.; Casanova, M.F.; Casanova, E.L.; Lamina, E.; Kelly, D.P.; Khachidze, I. Event-related Potentials (ERP) in Cognitive Neuroscience Research and Applications. NeuroRegulation 2017, 4, 14–27. [Google Scholar] [CrossRef]
- Wesnes, K.A.; Simpson, P.M.; White, L.A.; Pinker, S. The Cognitive Drug Research Computerized Assessment Systems for Elderly, AAMI & Demented Patients: Cognitive Drug Research, 13 The Grove, Reading RG1 4RB. J. Psychopharmacol. 1992, 6, 108. [Google Scholar]
- Wesnes, K.A.; Pincock, C.; Richardson, D.; Helm, G.; Hails, S. Breakfast reduces declines in attention and memory over the morning in schoolchildren. Appetite 2003, 41, 329–331. [Google Scholar] [CrossRef]
- Mardini, H.; Saxby, B.K.; Record, C.O. Computerized psychometric testing in minimal encephalopathy and modulation by nitrogen challenge and liver transplant. Gastroenterology 2008, 135, 1582–1590. [Google Scholar] [CrossRef]
- Gaweł, S.; Wardas, M.; Niedworok, E.; Wardas, P. Dialdehyd malonowy (MDA) jako wskaźnik procesów peroksydacji lipidów w organizmie [Malondialdehyde (MDA) as a lipid peroxidation marker]. Wiad Lek. 2004, 57, 453–455. [Google Scholar] [PubMed]
- Shichiri, M. The role of lipid peroxidation in neurological disorders. J. Clin. Biochem. Nutr. 2014, 54, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Yahia, S.; Tahari, Z.; Medjdoub, A.; Bessaih, N.; Messatfa, M.; Raiah, M.; Ouldcadi, H.; Saadi Ouslim, A.S.; Seddiki, S.; Sahraoui, T. Expression Analysis of Oxidative Stress Markers 8-hydroxydeoxyguanosine and Protein Carbonyl in Breast Cancer and Their associations with Certain Immunological and Tumor Markers. Asian Pac. J. Cancer Prev. 2025, 26, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, E.; Léjard, V.; Ezzine, C.; Govindin, P.; Morat, A.; Giat, M.; Lapaque, N.; Doré, J.; Blottière, H.M. An Insight into Functional Metagenomics: A High-Throughput Approach to Decipher Food-Microbiota-Host Interactions in the Human Gut. Int. J. Mol. Sci. 2023, 24, 17630. [Google Scholar] [CrossRef]
- Qi, J.; Liu, Y.; Wu, J.; Kawagishi, H.; Liu, C. Bibliometric analysis of Hericium mushrooms for medicinal and food purposes: 1992−2023. J. Future Foods 2025, 5, 317–330. [Google Scholar] [CrossRef]
- Qi, J. Hericium erinaceus: The enchanting medicinal-culinary mushroom of East Asian tradition. Integr. Med. Discov. 2024, 8, e24013. [Google Scholar] [CrossRef]
- Qi, J.; Wu, J.; Kang, S.; Gao, J.; Hirokazu, K.; Liu, H.; Liu, C. The chemical structures, biosynthesis, and biological activities of secondary metabolites from the culinary-medicinal mushrooms of the genus Hericium: A review. Chin. J. Nat. Med. 2024, 22, 676–698. [Google Scholar] [CrossRef]
- Ye, X.S.; Tian, W.J.; Wang, G.H.; Lin, K.; Zhu, S.X.; Xia, Y.Y.; Sun, B.L.; Shu, X.J.; Liu, W.; Che, H.F. The food and medicine homologous Chinese Medicine from Leguminosae species: A comprehensive review on bioactive constituents with neuroprotective effects on nervous system. Food Med. Homol. 2025, 2, 9420033. [Google Scholar] [CrossRef]

| Parameter | Placebo | Functional Congee Containing Dried Tomato Powder 200 mg per Day | Functional Congee Containing Dried Tomato Powder 400 mg per Day |
|---|---|---|---|
| Age (year) | 64.09 ± 4.00 | 64.38 ± 3.60 | 64.19 ± 3.09 |
| (p = 0.580) | (p = 0.668) | ||
| Gender (Male/Female) | (1/20) | (3/17) | (3/17) |
| Body Temperature (°C) | |||
| Baseline | 36.11 ± 0.32 | 35.99 ± 0.45 (p = 0.632) | 36.09 ± 0.37 (p = 0.922) |
| 4-week | 36.23 ± 0.50 | 36.28 ± 0.41 (p = 0.824) | 36.32 ± 0.41 (p = 0.582) |
| 8-week | 36.04 ± 0.40 | 36.23 ± 0.53 (p = 0.366) | 36.17 ± 0.37 (p = 0.648) |
| Heart Rate (beats/min) | |||
| Baseline | 70.59 ± 5.97 | 69.42 ± 7.88 (p = 0.706) | 68.33 ± 8.56 (p = 0.209) |
| 4-week | 71.95 ± 9.33 | 72.10 ± 8.06 (p = 0.834) | 70.05 ± 10.36 (p = 0.619) |
| 8-week | 71.90 ± 9.05 | 69.80 ± 9.35 (p = 0.505) | 67.95 ± 7.77 (p = 0.183) |
| Respiratory Rate (breaths/min) | |||
| Baseline | 17.18 ± 1.05 | 17.10 ± 1.14 (p = 0.815) | 17.33 ± 0.97 (p = 0.616) |
| 4-week | 17.86 ± 0.85 | 17.50 ± 1.10 (p = 0.341) | 17.60 ± 0.94 (p = 0.344) |
| 8-week | 18.38 ± 1.32 | 18.15 ±1.30 (p = 0.392) | 18.25 ± 0.97 (p = 0.560) |
| Systolic BP (mmHg) | |||
| Baseline | 126.31 ± 14.96 | 126.76 ± 8.77 (p = 0.408) | 130.00 ± 10.01 (p = 0.634) |
| 4-week | 122.38 ± 13.61 | 126.30 ± 11.33 (p = 0.441) | 121.00 ± 22.38 (p = 0.705) |
| 8-week | 126.24 ± 15.90 | 125.70 ± 11.44 (p = 0.744) | 126.50 ± 12.40 (p = 0.845) |
| Diastolic BP (mmHg) | |||
| Baseline | 70.81 ± 10.72 | 73.04 ± 6.54 (p = 0.349) | 74.52 ± 10.30 (p = 0.263) |
| 4-week | 71.95 ± 9.38 | 75.00 ± 10.29 (p = 0.473) | 72.40 ± 9.59 (p = 0.969) |
| 8-week | 74.05 ± 10.20 | 76.40 ± 8.17 (p = 0.284) | 77.45 ± 9.51 (p = 0.187) |
| Body Weight (kg) | |||
| Baseline | 57.12 ± 7.59 | 60.40 ± 7.86 (p = 0.628) | 62.90 ± 9.16 (p = 0.599) |
| 4-week | 56.89 ± 7.68 | 60.21 ± 8.00 (p = 0.124) | 60.84 ± 9.72 (p = 0.137) |
| 8-week | 56.89 ± 7.68 | 60.54 ± 7.99 (p = 0.100) | 61.41 ± 9.79 (p = 0.088) |
| Body Height (cm) | |||
| Baseline | 154.27 ± 5.12 | 156.09 ± 6.62 (p = 0.392) | 156.23 ± 6.73 (p = 0.242) |
| 4-week | 157.27 ± 5.11 | 156.24 ± 6.79 (p = 0.424) | 156.24 ± 6.73 (p = 0.242) |
| 8-week | 154.00 ± 5.08 | 156.05 ±6.79 (p = 0.345) | 155.80 ± 6.59 (p = 0.255) |
| BMI (kg/m2) | |||
| Baseline | 24.01 ± 3.08 | 24.84 ± 3.27 (p = 0.174) | 25.89 ± 4.41 (p = 0.117) |
| 4-week | 22.91 ± 5.99 | 24.79 ± 3.42 (p = 0.113) | 23.84 ± 7.11 (p = 0.444) |
| 8-week | 24.01 ± 3.26 | 24.90 ± 3.21 (p = 0.112) | 25.38 ± 4.59 (p = 0.179) |
| Event-Related Potentials | Before Consumption | After 4 Weeks of Consumption | After 8 Weeks of Consumption | ||
|---|---|---|---|---|---|
| Fz | N100 Latency (ms) | Placebo | 105.04 ± 2.73 | 107.52 ± 1.49 | 103.43 ± 2.67 |
| Functional congee containing dried tomato powder 200 mg per day | 107.33 ± 1.59 (p = 0.903) | 108.10 ± 1.41 (p = 0.865) | 106.81 ± 2.28 (p = 0.336) | ||
| Functional congee containing dried tomato powder 400 mg per day | 107.42 ± 1.54 (p = 0.789) | 105.72 ± 1.65 (p = 0.471) | 104.78 ± 2.50 (p = 0.692) | ||
| N100 Amplitude (μV) | Placebo | 5.08 ± 0.755 | 6.57 ± 1.08 | 6.31 ± 1.44 | |
| Functional congee containing dried tomato powder 200 mg per day | 7.78 ± 0.74 (p = 0.056) | 9.44 ± 1.00 * (p = 0.030) | 10.22 ± 1.71 * (p = 0.032) | ||
| Functional congee containing dried tomato powder 400 mg per day | 7.63 ± 0.88 (p = 0.071) | 8.12 ± 1.20 (p = 0.481) | 6.57 ± 1.35 (p = 0.647) | ||
| P300 Latency (ms) | Placebo | 337.54 ± 7.41 | 350.40 ± 5.94 | 352.54 ± 4.34 | |
| Functional congee containing dried tomato powder 200 mg per day | 346.42 ± 6.76 (p = 0.408) | 352.45 ± 3.84 (p = 0.568) | 358.20 ± 7.20 (p = 0.760) | ||
| Functional congee containing dried tomato powder 400 mg per day | 323.71 ± 9.96 (p = 0.422) | 343.16 ± 7.29 (p = 0.104) | 350.38 ± 4.45 (p = 0.963) | ||
| P300 Amplitude (μV) | Placebo | 9.83 ± 1.02 | 11.68 ± 1.88 | 15.68 ± 2.59 | |
| Functional congee containing dried tomato powder 200 mg per day | 9.97 ± 1.40 (p = 0.680 | 8.75 ± 1.42 (p = 0.245) | 10.29 ± 1.73 (p = 0.152) | ||
| Functional congee containing dried tomato powder 400 mg per day | 8.88 ± 1.22 (p = 0.593) | 10.74 ± 1.84 (p = 0.539) | 14.60 ± 1.79 (p = 0.913) | ||
| Cz | N100 Latency (ms) | Placebo | 108.52 ± 2.86 | 108.00 ± 1.64 | 105.93 ± 2.82 |
| Functional congee containing dried tomato powder 200 mg per day | 106.85 ± 1.49 (p = 0.174) | 108.10 ± 2.12 (p = 0.610) | 107.29 ± 1.76 (p = 0.971) | ||
| Functional congee containing dried tomato powder 400 mg per day | 106.85 ± 1.91 (p = 0.052) | 105.11 ± 2.33 (p = 0.480) | 106.61 ± 2.13 (p = 0.959) | ||
| N100 Amplitude (μV) | Placebo | 4.84 ± 0.77 | 6.56 ± 1.29 | 7.22 ± 1.18 | |
| Functional congee containing dried tomato powder 200 mg per day | 7.96 ± 1.21 (p = 0.061) | 8.87 ± 1.32 (p = 0.151) | 8.46 ± 1.13 (p = 0.471) | ||
| Functional congee containing dried tomato powder 400 mg per day | 7.84 ± 1.09 (p = 0.051) | 7.47 ± 0.93 (p = 0.190) | 6.67 ± 0.82 (p = 0.863) | ||
| P300 Latency (ms) | Placebo | 339.09 ± 8.56 | 355.70 ± 6.06 | 332.80 ± 12.34 | |
| Functional congee containing dried tomato powder 200 mg per day | 345.28 ± 7.03 (p = 0.990) | 345.25 ± 6.29 * (p = 0.036) | 350.93 ± 6.83 (p = 0.203) | ||
| Functional congee containing dried tomato powder 400 mg per day | 315.61 ± 11.49 (p = 0.054) | 333.16 ± 11.08 ** (p = 0.009) | 346.33 ± 4.28 (p = 0.704) | ||
| P300 Amplitude (μV) | Placebo | 8.49 ± 1.08 | 10.73 ± 1.47 | 13.14 ± 1.37 | |
| Functional congee containing dried tomato powder 200 mg per day | 7.82 ± 1.35 (p = 0.372) | 6.44 ± 1.48 (p = 0.123) | 9.81 ± 1.76 (p = 0.486) | ||
| Functional congee containing dried tomato powder 400 mg per day | 6.56 ± 1.10 (p = 0.110) | 9.94 ± 1.77 (p = 0.640) | 11.51 ± 2.28 (p = 0.828) | ||
| Cognitive Domains | Test Items | Treatment Group | Baseline Data | %Change in the Parameters from Baseline | |
|---|---|---|---|---|---|
| 4-Week | 8-Week | ||||
| Word Recognition | Response time | Placebo | 2081.01 ± 229.09 | −6.93 ± 12.19 | 17.41 ± 10.29 |
| Functional congee containing dried tomato powder 200 mg per day | 1625.36 ± 128.11 | −33.59 ± 7.08 (p = 0.060) | −42.76 ± 21.86 * (p = 0.035) | ||
| Functional congee containing dried tomato powder 400 mg per day | 1400.05 ± 75.37 | −15.84 ± 6.82 (p = 0.754) | −30.66 ± 3.70 (p = 0.375) | ||
| %Accuracy | Placebo | 76.06 ± 3.16 | 5.76 ± 2.47 | 6.67 ± 2.81 | |
| Functional congee containing dried tomato powder 200 mg per day | 81.42 ± 2.97 | 26.63 ± 8.69 * (p = 0.052) | 33.08 ± 10.84 * (p = 0.155) | ||
| Functional congee containing dried tomato powder 400 mg per day | 82.22 ± 3.31 | 11.56 ± 2.80 (p = 0.229) | 13.87 ± 4.30 (p = 0.457) | ||
| Placebo | 1770.77 ± 144.49 | −10.25 ± 5.92 | −24.94 ± 6.12 | ||
| Response time | Functional congee containing dried tomato powder 200 mg per day | 1493.25 ± 90.96 | −23.68 ± 3.83 (p = 0.068) | −26.00 ± 4.21 (p = 0.715) | |
| Picture Recognition | Functional congee containing dried tomato powder 400 mg per day | 1241.06 ± 69.91 | 10.59 ± 5.07 (p = 0.958) | −14.30 ± 5.52 (p = 0.220) | |
| %Accuracy | Placebo | 76.36 ± 2.85 | 11.82 ± 3.59 | 8.96 ± 4.86 | |
| Functional congee containing dried tomato powder 200 mg per day | 84.52 ± 12.73 | 7.03 ± 2.25 (p = 0.505) | 11.72 ± 6.45 (p = 0.885) | ||
| Functional congee containing dried tomato powder 400 mg per day | 81.19 ± 2.12 | 11.10 ± 2.97 (p = 0.948) | 8.25 ± 3.05 (p = 0.969) | ||
| Simple reaction | Response time | Placebo | 961.08 ± 115.64 | −10.79 ± 5.43 | −10.90 ± 6.17 |
| Functional congee containing dried tomato powder 200 mg per day | 751.94 ± 33.44 | −13.13 ± 5.55 (p = 0.754) | −15.48 ± 6.90 (p = 0.715) | ||
| Functional congee containing dried tomato powder 400 mg per day | 738.27 ± 26.75 | −7.58 ± 6.47 (p = 0.938) | −4.85 ± 4.70 (p = 0.481) | ||
| Digit vigilance | Response time | Placebo | 653.35 ± 16.11 | 6.60 ± 2.18 | 7.15 ± 4.08 |
| Functional congee containing dried tomato powder 200 mg per day | 687.62 ± 12.14 | 2.25 ± 2.13 (p = 0.090) | 12.82 ± 14.78 (p = 0.112) | ||
| Functional congee containing dried tomato powder 400 mg per day | 692.83 ± 24.04 | 23.56 ± 11.89 (p = 0.297) | 12.68 ± 8.93 (p = 0.230) | ||
| %Accuracy | Placebo | 88.75 ± 1.27 | 1.57 ± 1.57 | 0.72 ± 1.38 | |
| Functional congee containing dried tomato powder 200 mg per day | 90.04 ± 1.23 | 2.34 ± 1.34 (p = 0.552) | 2.37 ± 1.37 (p = 0.256) | ||
| Functional congee containing dried tomato powder 400 mg per day | 89.31 ± 1.12 | 87.30 ± 1.57 (p = 0.647) | 1.57 ± 1.84 (p = 0.876) | ||
| Response time | Placebo | 959.40 ± 52.72 | −1.25 ± 4.40 | −7.18 ± 5.18 | |
| Choice reaction time | Functional congee containing dried tomato powder 200 mg per day | 922.68 ± 42.30) | −9.13 ± 5.85 (p = 0.179) | −8.44 ± 3.51 (p = 0.498) | |
| Functional congee containing dried tomato powder 400 mg per day | 864.92 ± 47.51 | −9.17 ± 5.61 (p = 0.285) | −2.68 ± 2.69 (p = 0.531) | ||
| %Accuracy | Placebo | 96.64 ± 0.65 | 0.53 ± 0.52 | 0.63 ± 0.64 | |
| Functional congee containing dried tomato powder 200 mg per day | 96.95 ± 0.54 | −0.21 ± 0.91 (p = 0.256) | 0.75 ± 0.48 (p = 0.439) | ||
| Functional congee containing dried tomato powder 400 mg per day | 96.04 ± 0.68 | 0.75 ± 1.02 (p = 0.916) | 96.90 ± 0.60 (p = 0.636) | ||
| Response time | Placebo | 2174.57 ± 226.75 | −9.47 ± 8.21 | −17.70 ± 6.48 | |
| Spatial memory | Functional congee containing dried tomato powder 200 mg per day | 1709.24 ± 147.42 | −6.93 ± 12.17 (p = 0.735) | −18.69 ± 7.41 (p = 0.814) | |
| Functional congee containing dried tomato powder 400 mg per day | 1531.76 ± 68.89 | −3.83 ± 12.54 (p = 0.794) | −9.68 ± 7.27 (p = 0.309) | ||
| %Accuracy | Placebo | 71.84 ± 4.40 | 16.26 ± 6.39 | 20.05 ± 7.09 | |
| Functional congee containing dried tomato powder 200 mg per day | 78.96 ± 3.60 | 39.17 ± 9.78 (p = 0.130) | 43.16 ± 8.67 * (p = 0.049) | ||
| Functional congee containing dried tomato powder 400 mg per day | 80.86 ± 3.33 | 34.02 ± 12.45 (p = 0.657) | 43.08 ± 11.65 (p = 0.183) | ||
| Numeric working memory | Response time | Placebo | 1536.97 ± 104.48 | −12.07 ± 3.31 | −18.19 ± 3.37 |
| Functional congee containing dried tomato powder 200 mg per day | 1313.53 ± 57.73 | −15.12 ± 4.23 (p = 0.449) | −15.02 ± 4.64 (p = 0.814) | ||
| Functional congee containing dried tomato powder 400 mg per day | 1212.64 ± 42.63 | −11.00 ± 6.11 (p = 0.990) | −14.23 ± 4.58 (p = 0.335) | ||
| %Accuracy | Placebo | 86.21 ± 3.00 | 5.18 ± 5.47 | 4.44 ± 3.90 | |
| Functional congee containing dried tomato powder 200 mg per day | 88.57 ± 3.34 | 9.08 ± 5.61 (p = 0.592) | 11.08 ± 4.48 (p = 0.353) | ||
| Functional congee containing dried tomato powder 400 mg per day | 87.92 ± 2.66 | 12.36 ± 7.36 (p = 0.520) | 14.85 ± 13.87 (p = 0.824) | ||
| Parameters | Time | Placebo | Functional Congee Containing Dried Tomato Powder 200 mg per Day | Functional Congee Containing Dried Tomato Powder 400 mg per Day |
|---|---|---|---|---|
| MDA level (ng/mg.protein) | Baseline | 0.04 ± 0.01 | 0.04 ± 0.01 (p = 0.725) | 0.04 ± 0.01 (p = 0.347) |
| 4-week | 0.03 + 0.01 | 0.03 ± 0.01 (p = 0.704) | 0.03 ± 0.01 (p = 0.804) | |
| 8-week | 0.03 ± 0.01 | 0.03 ± 0.01 (p = 0.497) | 0.03 ± 0.01 (p = 0.969) | |
| SOD activity (U/mg.protein) | Baseline | 1.60 ± 0.86 | 1.67 ± 0.91 (p = 0.917) | 1.99 ± 0.48 (p = 0.159) |
| 4-week | 1.57 ± 1.22 | 2.14 ± 1.68 (p = 0.686) | 1.76 ± 1.68 (p = 0.262) | |
| 8-week | 1.97 ± 1.33 | 2.23 ± 0.41 (p = 0.735) | 2.37 ± 0.41 (p = 0.876) | |
| CAT activity (U/mg.protein) | Baseline | 1.71 ± 0.51 | 1.59 ± 0.41 (p = 0.620) | 1.47 ± 0.41 (p = 0.112) |
| 4-week | 1.50 ± 0.41 | 1.85 ± 0.67 (p = 0.106) | 1.69 ± 0.66 (p = 0.566) | |
| 8-week | 2.00 ± 0.71 | 2.06 ± 0.75 (p = 0.794) | 1.83 ± 0.74 (p = 0.657) | |
| GSH-Px activity (U/mg.protein) | Baseline | 3.66 ± 1.48 | 3.25 ± 1.43 (p = 0.657) | 3.51 ± 1.94 (p = 0.735) |
| 4-week | 3.33 ± 0.98 | 3.49 ± 1.13 (p = 0.584) | 3.70 ± 1.44 (p = 0.159) | |
| 8-week | 4.37 ± 1.50 | 4.30 ± 1.23 (p = 1.000) | 4.65 ± 1.18 (p = 0.657) |
| Parameters | Time | Placebo | Functional Congee Containing Dried Tomato Powder 200 mg per Day | Functional Congee Containing Dried Tomato Powder 400 mg per Day |
|---|---|---|---|---|
| AchE | Baseline | 8.91 ± 2.14 | 8.47 ± 1.94 (p = 0.896) | 8.72 ± 2.21 (p = 0.754) |
| (nmol/mg protein) | 4-week | 8.26 ± 2.13 | 9.20 ± 3.44 (p = 0.584) | 9.16 ± 2.75 (p = 0.241) |
| 8-week | 9.16 ± 2.13 | 9.46 ± 2.94 (p = 0.917) | 8.73 ± 2.30 (p = 0.434) | |
| MAO-A | Baseline | 0.49 ± 0.15 | 0.46 ± 0.21 (p = 0.862) | 0.46 ± 0.35 (p = 0.807) |
| (umol/h/mg protein) | 4-week | 0.48 ± 0.15 | 0.56 ± 0.23 (p = 0.335) | 0.52 ± 0.56 (p = 0.057) |
| 8-week | 0.65 ± 0.25 | 0.47 ± 0.32 * (p = 0.024) | 0.47 ± 0.20 * (p = 0.032) | |
| MAO-B | Baseline | 0.62 ± 0.30 | 0.56 ± 0.33 (p = 0.322) | 0.50 ± 0.22 (p = 0.251) |
| (umol/h/mg protein) | 4-week | 0.61 ± 0.27 | 0.60 ± 0.27 (p = 0.549) | 0.64 ± 0.30 (p = 0.958) |
| 8-week | 0.96 ± 0.22 | 0.97 ± 0.33 (p = 0.566) | 0.94 ± 0.29 (p = 0.506) | |
| Alpha-synuclein (pg/mL) | Baseline | 35.77 ± 8.12 | 35.43 ± 6.21 (p = 0.896) | 36.89 ± 7.79 (p = 0.814) |
| 4-week | 50.69 ± 11.54 | 44.82 ± 6.21 * (p = 0.048) | 43.63 ± 11.22 * (p = 0.015) | |
| 8-week | 53.23 ± 10.82 | 52.41 ± 11.98 (p = 0.597) | 48.82 ± 11.06 * (p = 0.011) | |
| PARK7 (ng/mL) | Baseline | 1.12 ± 0.21 | 1.11 ± 0.20 (p = 0.958) | 1.14 ± 0.21 (p = 0.774) |
| 4-week | 1.10 ± 0.19 | 1.13 ± 0.21 (p = 0.938) | 1.07 ± 0.20 (p = 0.382) | |
| 8-week | 1.06 ± 0.93 | 0.89 ± 0.22 (p = 0.735) | 0.82 ± 0.20 (p = 0.449) |
| Groups | Total Count of Lactobacillusspp. (Mean of CFU/mL) | Total Count of Bifidobacteriumspp. (Mean of CFU/mL) | ||
|---|---|---|---|---|
| Baseline | 8-Week | Baseline | 8-Week | |
| Placebo | 1.44 × 108 ± 1.04 × 108 | 2.82 × 107 ± 2.23 × 106 (p = 0.310) | 1.35 × 106 ± 4.43 × 104 | 5.54 × 107 ± 19.00 × 107 |
| Functional congee containing dried tomato powder 200 mg | 4.50 × 107 ± 3.21 × 107 | 1.74 × 109 ± 1.49 × 109 (p = 0.291) | 2.32 × 106 ± 7.92 × 104 | 1.16 × 106 ± 5.19 × 106 |
| Functional congee containing dried tomato powder 400 mg | 3.65 × 107 ± 9.48 × 106 | 3.54 × 108 ± 6.17 × 107 ## (p = 0.009) | 2.32 × 106 ± 2.54 × 104 | 6.30 × 105 ± 28.17 × 105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wattanathorn, J.; Muchimapura, S.; Thukham-mee, W.; Tong-un, T. Congee Containing Carotenoids-Enriched Functional Ingredient from Tomato Improves Cognition, Serum α-Synuclein, Monoaminergic Function, and Gut-Brain Axis Functions in the Elderly Volunteers. Life 2025, 15, 1093. https://doi.org/10.3390/life15071093
Wattanathorn J, Muchimapura S, Thukham-mee W, Tong-un T. Congee Containing Carotenoids-Enriched Functional Ingredient from Tomato Improves Cognition, Serum α-Synuclein, Monoaminergic Function, and Gut-Brain Axis Functions in the Elderly Volunteers. Life. 2025; 15(7):1093. https://doi.org/10.3390/life15071093
Chicago/Turabian StyleWattanathorn, Jintanaporn, Supaporn Muchimapura, Wipawee Thukham-mee, and Terdthai Tong-un. 2025. "Congee Containing Carotenoids-Enriched Functional Ingredient from Tomato Improves Cognition, Serum α-Synuclein, Monoaminergic Function, and Gut-Brain Axis Functions in the Elderly Volunteers" Life 15, no. 7: 1093. https://doi.org/10.3390/life15071093
APA StyleWattanathorn, J., Muchimapura, S., Thukham-mee, W., & Tong-un, T. (2025). Congee Containing Carotenoids-Enriched Functional Ingredient from Tomato Improves Cognition, Serum α-Synuclein, Monoaminergic Function, and Gut-Brain Axis Functions in the Elderly Volunteers. Life, 15(7), 1093. https://doi.org/10.3390/life15071093






