The Enigmatic Schizoglyphid Mite Oriboglyphus maorianus gen. and sp. n. and Its Implications for Astigmatid Life Cycle Evolution †
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Taxonomy
Key to Genera and Species of Schizoglyphidae *
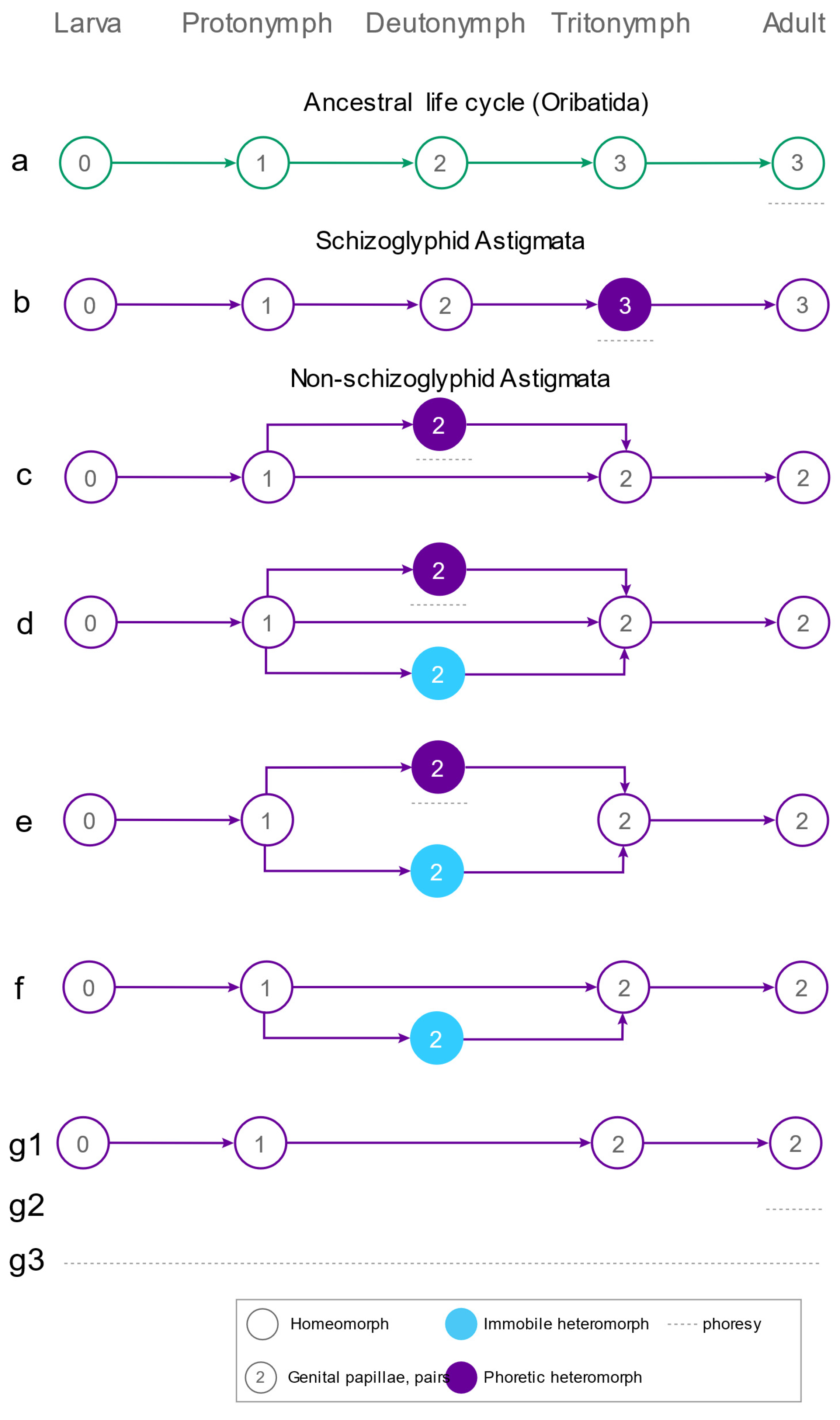
3.2. Phoretic Associations of Oribatida
3.3. Diversity of Phoresy-Related Life Cycles of Astigmata
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahunka, S. Schizoglyphidae fam. n. and new taxa of Acaridae and Anoetidae (Acari: Acarida). Acta Zool. Acad. Sci. Hung. 1978, 24, 107–131. [Google Scholar]
- Sendi, H.; Klimov, P.B.; Kolesnikov, V.B.; Káčerová, J.; Bonino, E.; Azar, D.; Robin, N. The oldest continuous association between astigmatid mites and termites preserved in Cretaceous amber reveals the evolutionary significance of phoresy. BMC Ecol. Evol. 2025, 25, 16. [Google Scholar] [CrossRef] [PubMed]
- Farish, D.J.; Axtell, R.C. Phoresy redefined and examined in Macrocheles muscaedomesticae (Acarina: Macrochelidae). Acarologia 1971, 13, 16–29. [Google Scholar]
- Greenberg, B. Mite orientation and survival on flies. Nature 1961, 190, 107–108. [Google Scholar] [CrossRef]
- Greenberg, B.; Carpenter, P.D. Factors in phoretic association of a mite and fly. Science 1960, 132, 738–739. [Google Scholar] [CrossRef]
- Houck, M.A.; OConnor, B.M. Ecological and evolutionary significance of phoresy in the Astigmata. Annu. Rev. Entomol. 1991, 36, 611–636. [Google Scholar] [CrossRef]
- OConnor, B.M. Evolutionary ecology of astigmatid mites. Annu. Rev. Entomol. 1982, 27, 385–409. [Google Scholar] [CrossRef]
- Moser, J.C.; Cross, E.A. Phoretomorph: A new phoretic phase unique to Pyemotidae (Acarina, Tarsonemoidea). Ann. Entomol. Soc. Am. 1975, 68, 820–822. [Google Scholar] [CrossRef]
- Seeman, O.D.; Walter, D.E. Phoresy and mites: More than just a free ride. Annu. Rev. Entomol. 2023, 68, 69–88. [Google Scholar] [CrossRef]
- Kuo, J.S.; Nesbitt, H.H.J. Internal morphology of the hypopus of Caloglyphus mycophagus (Megnin) (Acarina: Acaridae). Acarologia 1971, 13, 156–170. [Google Scholar]
- Wallace, D.R.J. Observations on hypopus development in the Acarina. J. Insect Physiol. 1960, 5, 216–229. [Google Scholar] [CrossRef]
- Vijayambika, V.; John, P.A. Internal morphology of the hypopus of Lardoglyphus konoi, a tyroglyphid pest on dried stored fish. Acarologia 1973, 15, 342–348. [Google Scholar] [PubMed]
- Houck, M.A.; Cohen, A.C. The potential role of phoresy in the evolution of parasitism: Radiolabelling (tritium) evidence from an astigmatid mite. Exp. Appl. Acarol. 1995, 19, 677–694. [Google Scholar] [CrossRef]
- Alberti, G.; Kanarek, G.; Dabert, J. Unusual way of feeding by the deutonymph of Neottialges evansi (Actinotrichida, Astigmata, Hypoderatidae), a subcutaneous parasite of cormorants, revealed by fine structural analyses. J. Morphol. 2016, 277, 1368–1389. [Google Scholar] [CrossRef]
- Norton, R.A. Evolutionary aspects of oribatid mite life histories and consequences for the origin of the Astigmata. In Mites: Ecological and Evolutionary Analyses of Life-History Patterns; Houck, M.A., Ed.; Chapman & Hall: New York, NY, USA; London, UK, 1994; pp. 99–135. [Google Scholar]
- Klimov, P.B.; Vorontsov, D.D.; Azar, D.; Sidorchuk, E.A.; Braig, H.R.; Khaustov, A.A.; Tolstikov, A.V. A transitional fossil mite (Astigmata: Levantoglyphidae fam. n.) from the early Cretaceous suggests gradual evolution of phoresy-related metamorphosis. Sci. Rep. 2021, 11, 15113. [Google Scholar] [CrossRef]
- OConnor, B.M. Cohort Astigmatina. In A Manual of Acarology, 3rd ed.; Krantz, G.W., Walter, D.E., Eds.; Texas Tech University Press: Lubbock, TX, USA, 2009; pp. 565–657. [Google Scholar]
- Hughes, M.; Gerber, S.; Wills, M.A. Clades reach highest morphological disparity early in their evolution. Proc. Natl. Acad. Sci. USA 2013, 110, 13875–13879. [Google Scholar] [CrossRef]
- Ruta, M.; Wagner, P.J.; Coates, M.I. Evolutionary patterns in early tetrapods. I. Rapid initial diversification followed by decrease in rates of character change. Proc. R. Soc. B-Biol. Sci. 2006, 273, 2107–2111. [Google Scholar] [CrossRef]
- Erwin, D.H. Disparity: Morphological pattern and developmental context. Palaeontology 2007, 50, 57–73. [Google Scholar] [CrossRef]
- Foote, M. The evolution of morphological diversity. Annu. Rev. Ecol. Syst. 1997, 28, 129–152. [Google Scholar] [CrossRef]
- Gould, S.J. The Disparity of the Burgess Shale Arthropod Fauna and the Limits of Cladistic-Analysis-Why We Must Strive to Quantify Morphospace. Paleobiology 1991, 17, 411–423. [Google Scholar] [CrossRef]
- Gould, S.J.; Gilinsky, N.L.; German, R.Z. Asymmetry of Lineages and the Direction of Evolutionary Time. Science 1987, 236, 1437–1441. [Google Scholar] [CrossRef]
- Schluter, D. The Ecology of Adaptive Radiation; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Simpson, G.G. Tempo and Mode in Evolution. Trans. N. Y. Acad. Sci. 1945, 8, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Etienne, R.S.; Haegeman, B. A conceptual and statistical framework for adaptive radiations with a key role for diversity dependence. Am. Nat. 2012, 180, E75–E89. [Google Scholar] [CrossRef] [PubMed]
- Dumont, E.R.; Dávalos, L.M.; Goldberg, A.; Santana, S.E.; Rex, K.; Voigt, C.C. Morphological innovation, diversification and invasion of a new adaptive zone. Proc. R. Soc. B-Biol. Sci. 2012, 279, 1797–1805. [Google Scholar] [CrossRef] [PubMed]
- Arthur, W.; Farrow, M. The pattern of variation in centipede segment number as an example of developmental constraint in evolution. J. Theor. Biol. 1999, 200, 183–191. [Google Scholar] [CrossRef]
- Losos, J.; Mahler, D.L. Adaptive Radiation: The Interaction of Ecological Opportunity, Adaptation, and Speciation; Sinauer: Sunderland, MA, USA, 2010. [Google Scholar]
- Griffiths, D.A.; Atyeo, W.T.; Norton, R.A.; Lynch, C.A. The idiosomal chaetotaxy of astigmatid mites. J. Zool. 1990, 220, 1–32. [Google Scholar] [CrossRef]
- Norton, R.A. Morphological evidence for the evolutionary origin of Astigmata (Acari: Acariformes). Exp. Appl. Acarol. 1998, 22, 559–594. [Google Scholar] [CrossRef]
- Grandjean, F. L’infracapitulum et la manducation chez les Oribates et d’autres Acariens. Ann. Des Sci. Nat. Zool. (11e Série) 1957, 19, 234–281. [Google Scholar]
- Grandjean, F. Au sujet de l’organe de Claparède, des eupathidies multiples et des taenidies mandibulaires chez les Acariens actinochitineux. Arch. Sci. Phys. Nat. 5ème Période 1946, 28, 63–87. [Google Scholar]
- Grandjean, F. La chaetotaxie des pattes chez les Acaridiae. Bull. Société Zool. Fr. 1939, 64, 50–60. [Google Scholar]
- Hammen, L.v.d. An Introduction to Comparative. Arachnology; SPB Academic Publishing bv: Hague, The Netherlands, 1989. [Google Scholar]
- Klimov, P.B.; OConnor, B.M.; Chetverikov, P.E.; Bolton, S.J.; Pepato, A.R.; Mortazavi, A.L.; Tolstikov, A.V.; Bauchan, G.R.; Ochoa, R. Comprehensive phylogeny of acariform mites (Acariformes) provides insights on the origin of the four-legged mites (Eriophyoidea), a long branch. Mol. Phylogenet. Evol. 2018, 119, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Dabert, M.; Witaliński, W.; Kazmierski, A.; Olszanowski, Z.; Dabert, J. Molecular phylogeny of acariform mites (Acari, Arachnida): Strong conflict between phylogenetic signal and long-branch attraction artifacts. Mol. Phylogenet. Evol. 2010, 56, 222–241. [Google Scholar] [CrossRef] [PubMed]
- Klimov, P.B.; OConnor, B.M. Morphology, Evolution, and Host Associations of Bee-Associated Mites of the Family Chaetodactylidae (Acari: Astigmata), with a Monographic Revision of North American Taxa; MP199; Miscellaneous Publications Museum of Zoology University of Michigan: Ann Arbor, MI, USA, 2008; pp. 1–243. [Google Scholar]
- Norton, R.A. Observations on phoresy by oribatid mites (Acari: Oribatei). Int. J. Acarol. 1980, 6, 121–130. [Google Scholar] [CrossRef]
- Ermilov, S.G.; OConnor, B.M. New species of Monoschelobates Balogh et Mahunka 1969 and Multoribates Hammer 1961 (Acari, Oribatida, Scheloribatidae), phoretic on passalid beetles from the Neotropical region. Zool. Zhurnal 2021, 100, 618–626. [Google Scholar] [CrossRef]
- Ermilov, S.G.; Oconnor, B.M. New Perscheloribates species (Acari, Oribatida, Scheloribatidae) phoretic on beetles (Insecta, Coleoptera). Acarologia 2020, 60, 289–300. [Google Scholar] [CrossRef]
- Ermilov, S.G.; Frolov, A.V. New data on oribatid mites (Acari, Oribatida) phoretic on passalid beetles (Coleoptera, Passalidae) from the Afrotropical and Oriental regions, with descriptions of three new species from Congo, Gab on and Ghana. Syst. Appl. Acarol. 2021, 26, 769–787. [Google Scholar]
- Ermilov, S.G.; Frolov, A.V. New and interesting oribatid mites (Acari, Oribatida) phoretic on Aceraius grandis (Coleoptera, Passalidae) from Vietnam. Syst. Appl. Acarol. 2019, 24, 945–961. [Google Scholar] [CrossRef]
- Ermilov, S.G. Oribatid mites (Acari: Oribatida) phoretic on passalid beetles (Coleoptera: Passalidae), with description of a new species from Indonesia. Ecol. Montenegrina 2019, 22, 90–96. [Google Scholar] [CrossRef]
- Pernek, M.; Hrasovec, B.; Matošević, D.; Pilaš, I.; Kirisits, T.; Moser, J. Phoretic mites of three bark beetles (Pityokteines spp.) on Silver fir. J. Pest Sci. 2008, 81, 35–42. [Google Scholar] [CrossRef]
- Pernek, M.; Wirth, S.F.; Blomquist, S.R.; Avtzis, D.N.; Moser, J.C. New associations of phoretic mites on Pityokteines curvidens (Coleoptera, Curculionidae, Scolytinae). Cent. Eur. J. Biol. 2012, 7, 63–68. [Google Scholar] [CrossRef]
- Knee, W. A new Paraleius species (Acari, Oribatida, Scheloribatidae) associated with bark beetles (Curculionidae, Scolytinae) in Canada. ZooKeys 2017, 667, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Khaustov, A.A.; Klimov, P.B.; Trach, V.A.; Bobylev, A.N.; Salavatulin, V.M.; Khaustov, V.A.; Tolstikov, A.V. Review of mites (Acari) associated with the European spruce bark beetle, Ips typographus (Coleoptera: Curculionidae: Scolytinae) in Asian Russia. Acarina 2018, 26, 3–79. [Google Scholar] [CrossRef]
- Kunst, M. Euscheloribates samšiňáki n. g., n. sp., eine neue moosmilbe aus der Tschechoslowakei (Acarina, Oribatei). Cas. Ceskoslovenské Spol. Entomol. 1958, 55, 67–70. [Google Scholar]
- Woolley, T.A. A new and phoretic oribatid mite (Acarina: Cryptostigmata: Licnodamaeidae). Proc. Entomol. Soc. Wash. 1969, 71, 476–481. [Google Scholar]
- Ermilov, S.G.; Abramov, V.V. Eremella ryabinini (Acari, Oribatida, Eremellidae), a new oribatid mite species phoretic on Amphotis marginata (Coleoptera, Nitidulidae) from Russia. Persian J. Acarol. 2023, 12, 189–197. [Google Scholar]
- Beaty, L.; Esser, H.; Miranda, R.; Norton, R. First report of phoresy by an oribatid mite (Trhypochthoniidae: Archegozetes magnus) on a frog (Leptodactylidae: Engystomops pustulosus). Int. J. Acarol. 2013, 39, 325–326. [Google Scholar] [CrossRef]
- Krivolutsky, D.A.; Lebedeva, N.V. Oribatid mites (Oribatei) in bird feathers: Passeriformes. Acta Zool. Litu. 2004, 14, 19–38. [Google Scholar] [CrossRef]
- Lebedeva, N.V. Oribatid mites transported by birds to polar islands: A review. Berichte Polar Meeresforsch. 2012, 640, 152–161. [Google Scholar]
- Krivolutsky, D.A.; Lebedeva, N.V. Oribatid mites (Oribatei, Acariformes) in bird feathers: Non-passerines. Acta Zool. Litu. 2004, 14, 26–47. [Google Scholar] [CrossRef]
- Marshall, V.G.; Reeves, R.M.; Norton, R.A. Catalog of the Oribatida (Acari) of continental United States and Canada. In Memoirs of the Entomological Society of Canada; Entomological Society of Canada: Winnipeg, MB, Canada, 1987; pp. R5–418. [Google Scholar]
- Lindquist, E.E. Associations between mites and other arthropods in forest floor habitats. Can. Entomol. 1975, 107, 425–437. [Google Scholar] [CrossRef]
- Camerik, A.M.; de Lillo, E.; Lalkhan, C. The neotype of Pediculaster mesembrinae (Canestrini, 1881) (Acari: Siteroptidae) and the description of all life stages. Int. J. Acarol. 2006, 32, 45–67. [Google Scholar] [CrossRef]
- Cross, E.A.; Moser, J.C.; Rack, G. Some new forms of Pyemotes (Acarina: Pyemotidae) from forest insects, with remarks on polymorphism. Int. J. Acarol. 1981, 7, 179–196. [Google Scholar] [CrossRef]
- Ebermann, E. Records of polymorphism in the mite family Scutacaridae (Acari, Tarsonemina). Acarologia 1991, 32, 119–138. [Google Scholar]
- Norton, R.A. An example of phoretomorphs in the mite family Scutacaridae. J. Ga. Entomol. Soc. 1977, 12, 185–186. [Google Scholar]
- Baker, E.W.; Roubik, D.W.; Delfinado-Baker, M. The developmental stages and dimorphic males of Chaetodactylus panamensis, n. sp. (Acari: Chaetodactylidae) associated with solitary bee (Apoidea: Anthophoridae). Int. J. Acarol. 1987, 13, 65–73. [Google Scholar] [CrossRef]
- Lombert, H.A.P.M.; OConnor, B.M.; Lukoschus, F.S.; Whitaker, J.O., Jr. Ontogeny, systematics and ecology of Sennertia (Amsennertia) americana Delfinado & Baker, 1976 (Acari: Chaetodactylidae) from the nest of the carpenter bee, Xylocopa virginica (Hymenoptera: Anthophoridae). Int. J. Acarol. 1987, 13, 113–129. [Google Scholar]
- Bongers, M.G.H.; OConnor, B.M.; Lukoschus, F.S. Morphology and ontogeny of histiostomatid mites (Acari: Asigmata) associated with cattle dung in the Netherlands. Zool. Meded. 1985, 223, 1–56. [Google Scholar]
- OConnor, B.M. Generic relationships in the Chaetodactylidae (Acari: Astigmata) with description of a new genus. Acarologia 1993, 34, 345–362. [Google Scholar]
- Fan, Q.-H.; Zhang, Z.-Q. Revision of Rhizoglyphus Claparède (Acari: Acaridae) of Australasia and Oceania; Systematic & Applied Acarology Society: London, UK, 2004. [Google Scholar]
- Wurst, E. Investigations on the anatomy and the behaviour of the fur mite Listrophorus leuckarti (Acari: Listrophoridae). Stuttg. Beiträge Naturkunde. Ser. A (Biol.) 1993, 53, 1–68. [Google Scholar]
- Klompen, J.S.H.; Lukoschus, F.S.; O’Connor, B.M. Ontogeny, life history and sex ratio evolution in Ensliniella kostylevi (Acari: Winterschmidtiidae). J. Zool. 1987, 213, 591–607. [Google Scholar] [CrossRef]
- Wurst, E.; Havelka, P. Redescription and life history of Tytodectes strigis (Acari: Hypoderatidae), a parasite of the barn owl Tyto alba (Aves: Strigidae). Stuttg. Beiträge Naturkunde. Ser. A (Biol.) 1997, 554, 1–39. [Google Scholar]
- Fashing, N.J. Life-history patterns of astigmatid inhabitants of water-filled treeholes. In Mites: Ecological and Evolutionary Analyses of Life-History Patterns; Houck, M.A., Ed.; Chapman & Hall: New York, NY, USA; London, UK, 1994; pp. 160–185. [Google Scholar]
- Fashing, N.J. A new subfamily of Acaridae, the Naiadacarinae, from water-filled treeholes (Acarina: Acaridae). Acarologia 1974, 16, 166–181. [Google Scholar]
- Fashing, N.J. Nepenthacarus, a new genus of Histiostomatidae (Acari: Astigmata) inhabiting the pitchers of Nepenthes mirabilis (Lour.) Druce in Far North Queensland, Australia. Aust. J. Entomol. 2002, 41, 7–17. [Google Scholar] [CrossRef]
- Fashing, N.J. Deutonymphal dimorphism in the genus Hericia (Astigmata: Algophagidae). In Modern Acarology. Volume II: Proceedings of the 8 International Congress of Acarology Held in České Budějovice, Czechoslovakia, 6–11 August 1990; Dusbábek, F., Buvka, V., Eds.; SPB Academic Publishing: The Hague, The Netherlands, 1990; pp. 287–291. [Google Scholar]
- Knülle, W. Interaction between genetic and inductive factors controlling the expression of dispersal and dormancy morphs in dimorphic astigmatic mites. Evolution 2003, 57, 828–838. [Google Scholar]
- Lukoschus, F.S.; Scheperboer, G.; Fain, A.; Nadchatram, M. Life cycle of Alabidopus asiaticus sp. nov. (Acarina: Astigmata: Glycyphagidae) and hypopus of Alabidopus malaysiensis sp. nov. ex. Rattus spp. from Malaysia. Int. J. Acarol. 1981, 7, 161–177. [Google Scholar] [CrossRef]
- Wurst, E.; Pfister, T. On the biology of Baloghella melis Mahunka, 1963 (Acari: Acaridida: Glycyphagidae). Bonn. Zool. Beiträge 1990, 41, 157–162. [Google Scholar]
- Baker, E.W. Natural history of Plummers Island, Maryland. XV. Descriptions of the stages of Chaetodactylus krombeini, new species, a mite associated with the bee, Osmia lignaria Say (Acarina: Chaetodactylidae). Proc. Biol. Soc. Wash. 1962, 75, 227–236. [Google Scholar]
- Van Asselt, L. Observations on the life cycle of Chaetodactylus osmiae (Dufour, 1839) (Acari: Chaetodactylidae) parasitic on the solitary bee, Osmia rufa (L.), 1758 (Insecta: Hymenoptera) in Belgium. Int. J. Acarol. 2000, 26, 221–228. [Google Scholar] [CrossRef]
- Wurst, E.; Kovac, D. Description and biology of the mite Tensiostoma veliaphilum n. gen. n. sp. from the water surface of bamboo phytotelmata in Southeast Asia (Arachnida, Acari, Astigmata, Histiostomatidae). Senckenb. Biol. 2003, 82, 63–98. [Google Scholar]
- Griffiths, D.A. A further systematic study of the genus Acarus, L., 1758 (Acaridae, Acarina), with a key to species. Bull. Br. Mus. (Nat. Hist.) Zool. 1970, 19, 85–118. [Google Scholar]
- Hughes, A.M. The Mites of Stored Food and Houses, 2nd ed.; Her Majesty’s Stationery Office: London, UK, 1976; p. 400. [Google Scholar]
- Griffiths, D.A. A revision of the genus Acarus, L. 1758 (Acaridae, Acarina). Bull. Br. Mus. (Nat. Hist.) Zool. 1964, 11, 413–464. [Google Scholar]
- Fain, A.; Coineau, Y.; Andre, H.M. Description de deux especes nouvelles d’astigmates sabulicoles (Acari). Acarologia 1993, 34, 149–158. [Google Scholar]
- Fain, A. Notes on the Hyadesiidae Halbert, 1915 and Algophagidae Fain, 1974, nov. tax. (Acari, Astigmata), with a redescription of Hyadesia curassaviensis Viets, 1936 and H. sellai Viets, 1937. Acarologia 1981, 22, 47–61. [Google Scholar]
- Fan, Q.-H.; Zhang, Z.-Q. Tyrophagus (Acari: Astigmata: Acaridae). Fauna of New Zealand = Ko te Aitanga Pepeke o Aotearoa (No. 56); Manaaki Whenua Press: Lincoln, New Zealand, 2007; p. 291. [Google Scholar]
- Klimov, P.B.; Hubert, J.; Erban, T.; Perotti, M.A.; Braig, H.R.; Flynt, A.; He, Q.; Cui, Y. Genomic and metagenomic analyses of the domestic mite Tyrophagus putrescentiae identify it as a widespread environmental contaminant and a host of a basal, mite-specific Wolbachia lineage (supergroup Q). Int. J. Parasitol. 2024, 54, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Gaud, J.; Atyeo, W.T. Feather mites of the World (Acarina, Astigmata): The supraspecific taxa. Part 1. Text. K. Mus. Voor Midden Afr./Tervuren Belg. Ann. Zool. Wet. 1996, 277, 1–193. [Google Scholar]
- Atyeo, W.T.; Braasch, N.L. The feather mite genus Proctophyllodes (Sarcoptiformes: Proctophyllodidae). Bull. Univ. Neb. State Mus. 1966, 5, 1–354. [Google Scholar]
- Klompen, J.S.H. Phylogenetic relationships in the mite family Sarcoptidae (Acari: Astigmata). Misc. Publ. Mus. Zool. Univ. Mich. 1992, 180, 1–154. [Google Scholar]
- Yunker, C.E. New genera and species of Ewingidae (Acari:Sarcoptiformes) from Pagurids (Crustacea) with notes on Ewingia coenobitae Pearse, 1929. Rev. Zool. Bot. Afr. 1970, 81, 237–254. [Google Scholar]
- Fain, A.; Yunker, C.E.; van Goethem, J.; Johnston, D.E. Notes on the Ewingiidae (Acari, Astigmata) living in association with pagurids and fresh-water crabs (Crustacea) with description of a new genus and a new species. Bull. L’institut R. Sci. Nat. Belg. Entomol. 1982, 54, 1–7. [Google Scholar]
- OConnor, B.M. Life-history modifications in astigmatid mites. In Mites: Ecological and Evolutionary Studies of Life-History Patterns. Chapman & Hall, New York; Houck, M.A., Ed.; Chapman & Hall: New York, NY, USA, 1994; pp. 136–159. [Google Scholar]
- Fain, A. A Review of the Family Epidermoptidae Trouessart Parasitic on the Skin of Birds (Acarina: Sarcoptiformes) Part 1 & 2; Paleis der Academien: Bruxelles, Belgium, 1965; Volume 27, pp. 1–176, 171–144. [Google Scholar]
- Evans, G.O.; Fain, A.; Bafort, J. Découverte du cycle évolutif du genre Myialges avec description d’une espèce nouvelle (Myialgidae: Sarcoptiformes). Bull. Ann. Soc. R. D’entomologie Belg. 1963, 99, 486–500. [Google Scholar]
- Klimov, P.B.; OConnor, B.M. Is permanent parasitism reversible?-Critical evidence from early evolution of house dust mites. Syst. Biol. 2013, 62, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Pachl, P.; Domes, K.; Schulz, G.; Norton, R.A.; Scheu, S.; Schaefer, I.; Maraun, M. Convergent evolution of defense mechanisms in oribatid mites (Acari, Oribatida) shows no ‘ghosts of predation past’. Mol. Phylogenet. Evol. 2012, 65, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Sanders, F.H.; Norton, R.A. Anatomy and function of the ptychoid defensive mechanism in the mite Euphthiracarus cooki (Acari: Oribatida). J. Morphol. 2004, 259, 119–154. [Google Scholar] [CrossRef] [PubMed]
- Ermilov, S.G.; Hugo-Coetzee, E.; Khaustov, A.A.; Khaustov, V.A. New faunistic and taxonomic data on oribatid mites (Acari, Oribatida) inhabiting soil and termite nests in the Golden Gate Highlands National Park (eastern South Africa). Int. J. Acarol. 2021, 47, 152–158. [Google Scholar] [CrossRef]
- Lo, N.; Tokuda, G.; Watanabe, H.; Rose, H.; Slaytor, M.; Maekawa, K.; Bandi, C.; Noda, H. Evidence from multiple gene sequences indicates that termites evolved from wood-feeding cockroaches. Curr. Biol. 2000, 10, 801–804. [Google Scholar] [CrossRef]
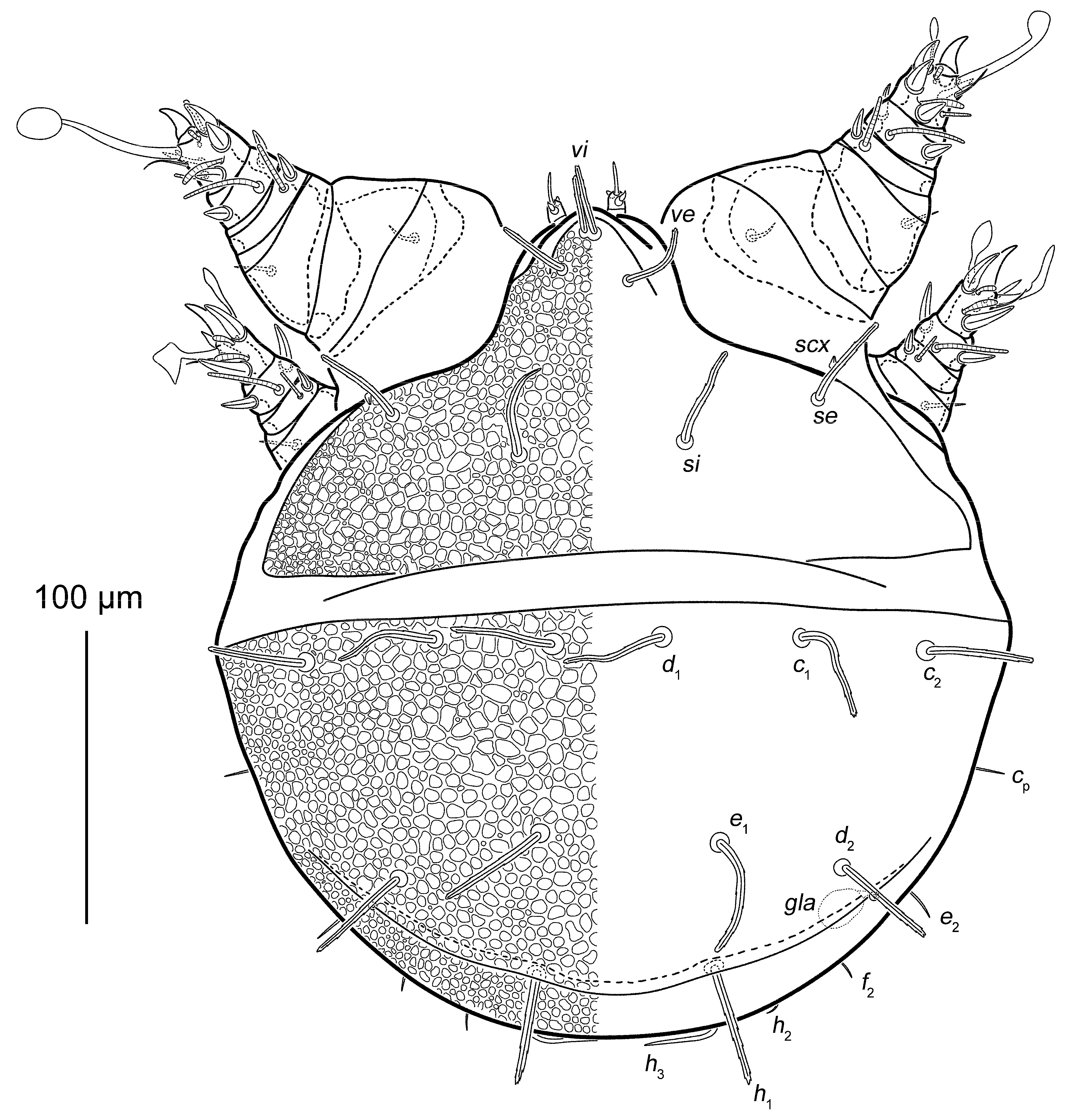
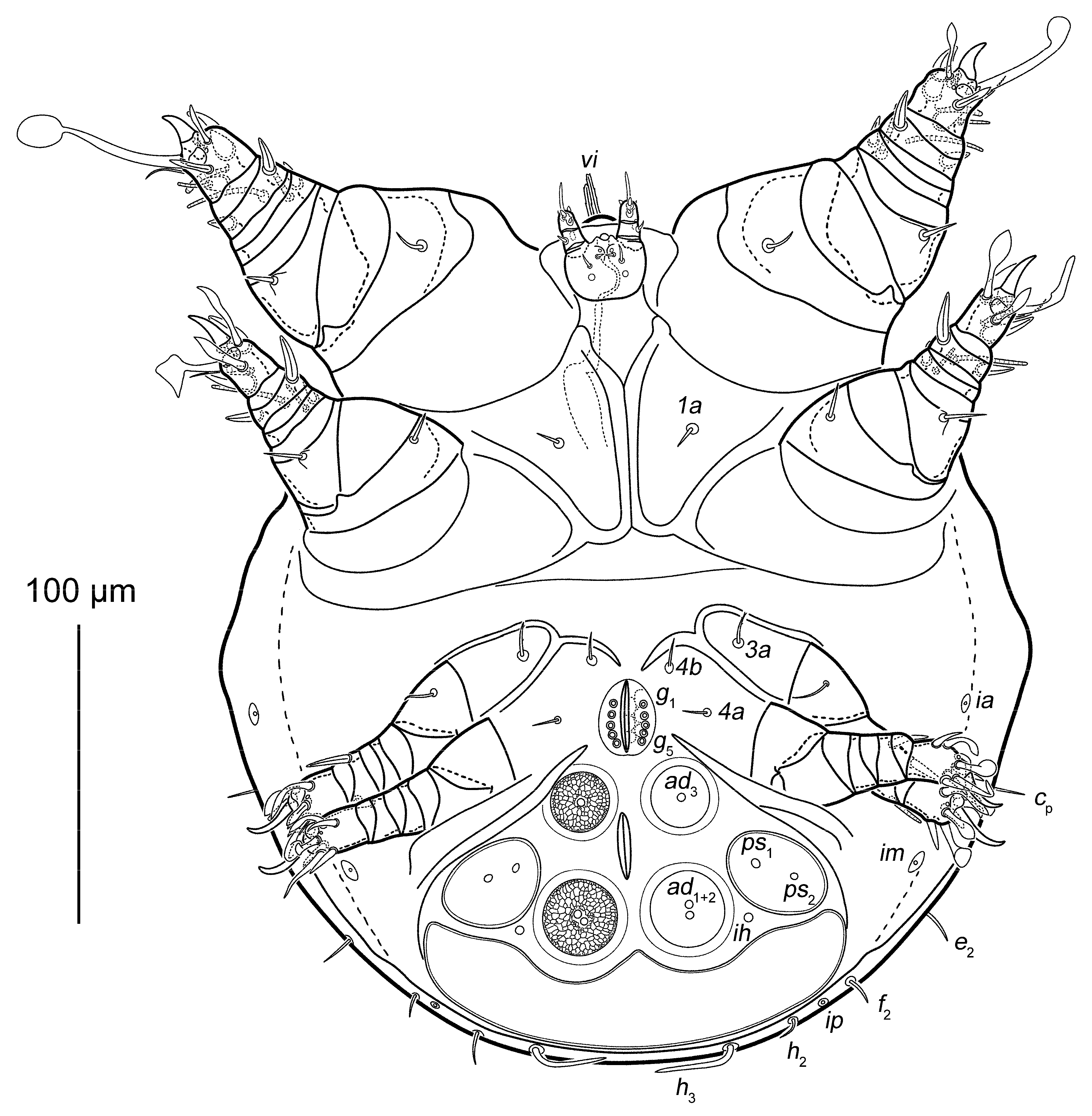

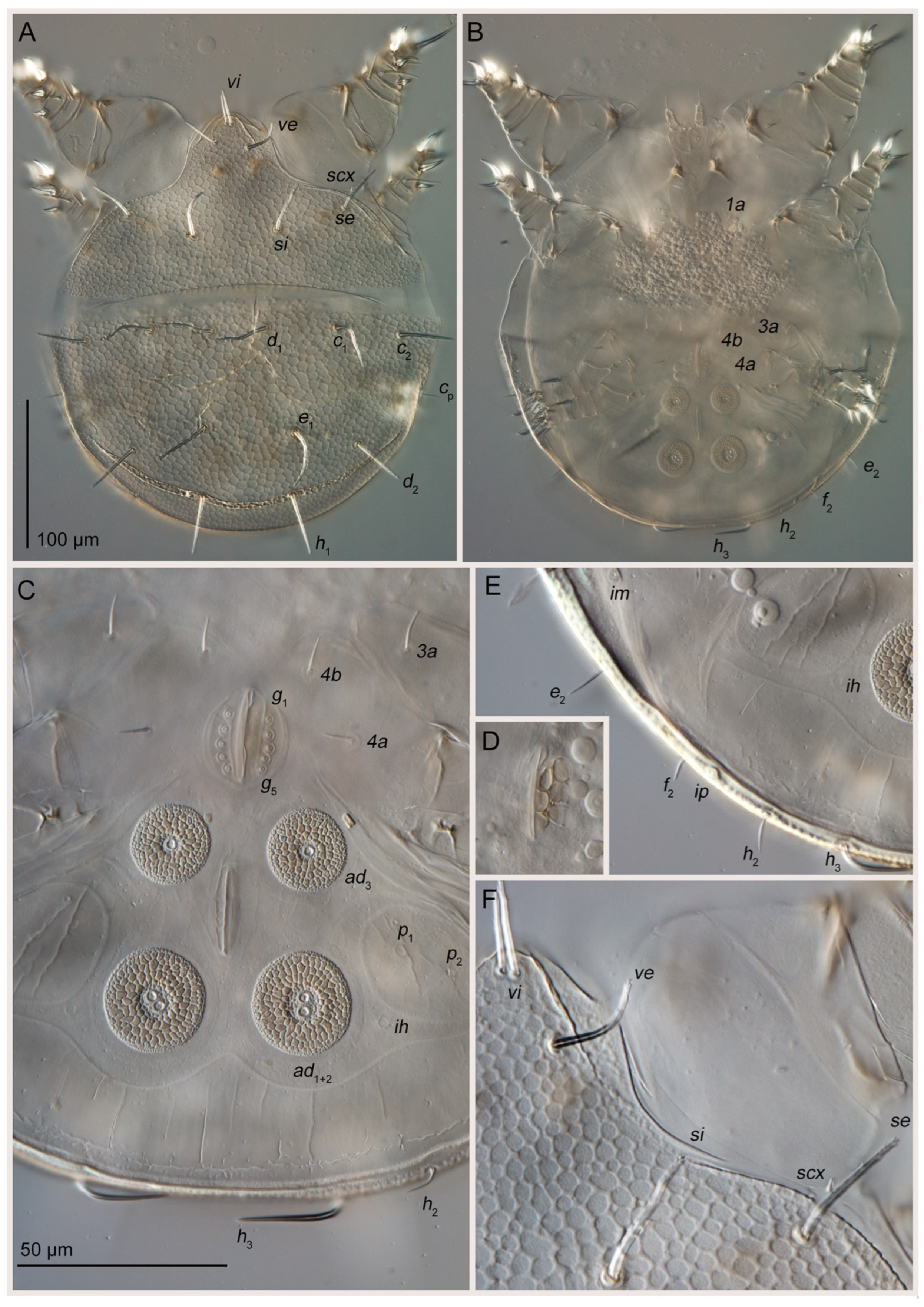
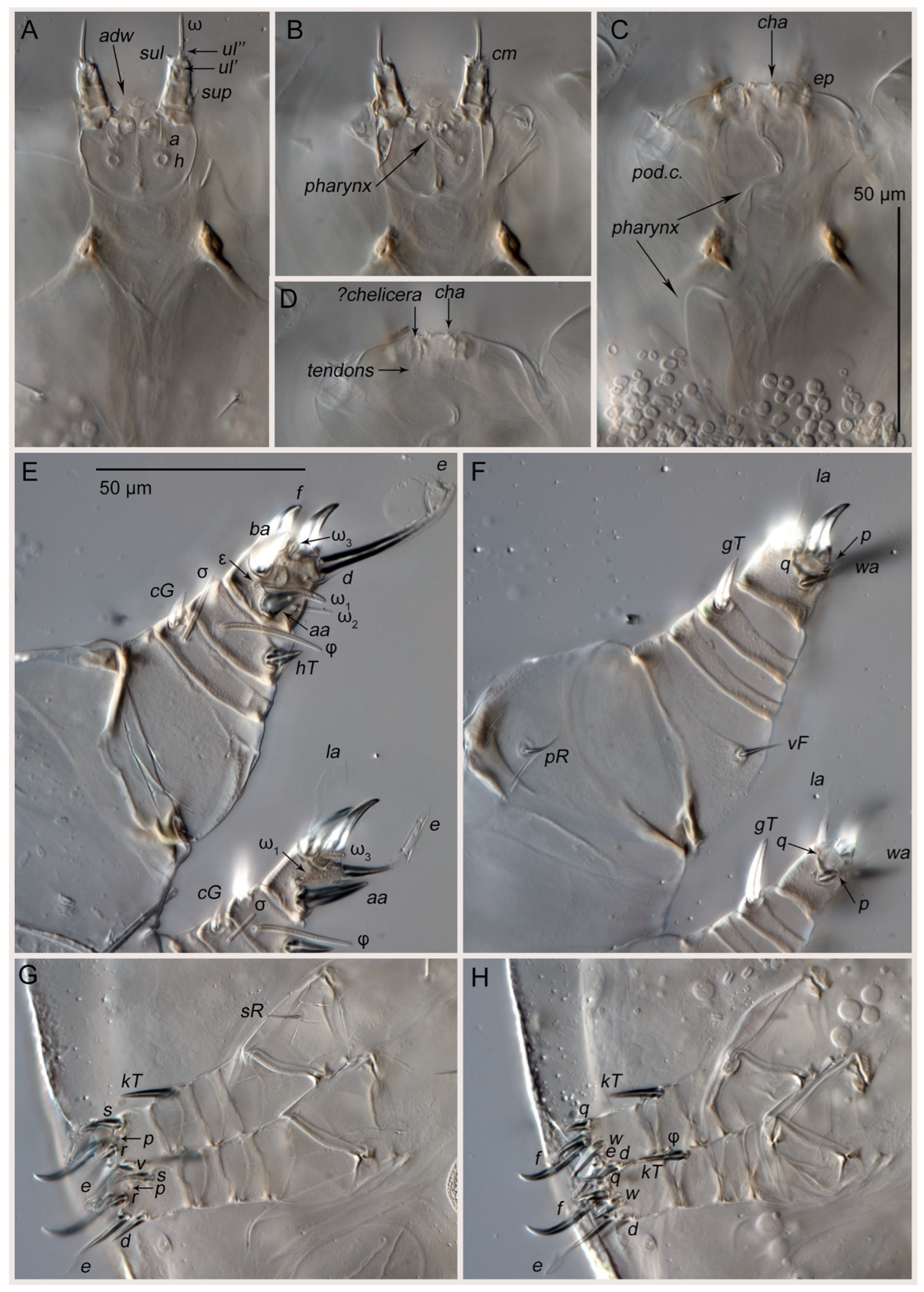
| Leg | Tr | Fe | Ge | Ti | Ta |
|---|---|---|---|---|---|
| I | Pr | vF | cG, σ | gT, hT, φ | aa, ba, f, e, d, la, wa, q, p, ω1, ω2, ω3, ε |
| II | Pr | vF | cG, σ | gT, hT, φ | aa, f, e, d, la, wa, q, p, ω1, ω3 |
| III | sR | - | - | kT, φ | d, e, f, r, w, s, q, p |
| IV | - | - | - | kT | d, e, f, r, w, s, v, q, p |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimov, P.B.; Kolesnikov, V.B.; Shaw, M.; Fan, Q.-H.; Zhang, Z.-Q.; OConnor, B. The Enigmatic Schizoglyphid Mite Oriboglyphus maorianus gen. and sp. n. and Its Implications for Astigmatid Life Cycle Evolution. Life 2025, 15, 1085. https://doi.org/10.3390/life15071085
Klimov PB, Kolesnikov VB, Shaw M, Fan Q-H, Zhang Z-Q, OConnor B. The Enigmatic Schizoglyphid Mite Oriboglyphus maorianus gen. and sp. n. and Its Implications for Astigmatid Life Cycle Evolution. Life. 2025; 15(7):1085. https://doi.org/10.3390/life15071085
Chicago/Turabian StyleKlimov, Pavel B., Vasiliy B. Kolesnikov, Matt Shaw, Qing-Hai Fan, Zhi-Qiang Zhang, and Barry OConnor. 2025. "The Enigmatic Schizoglyphid Mite Oriboglyphus maorianus gen. and sp. n. and Its Implications for Astigmatid Life Cycle Evolution" Life 15, no. 7: 1085. https://doi.org/10.3390/life15071085
APA StyleKlimov, P. B., Kolesnikov, V. B., Shaw, M., Fan, Q.-H., Zhang, Z.-Q., & OConnor, B. (2025). The Enigmatic Schizoglyphid Mite Oriboglyphus maorianus gen. and sp. n. and Its Implications for Astigmatid Life Cycle Evolution. Life, 15(7), 1085. https://doi.org/10.3390/life15071085






