Methadone-Induced Toxicity—An Unexpected Challenge for the Brain and Heart in ICU Settings: Case Report and Review of the Literature
Abstract
1. Introduction
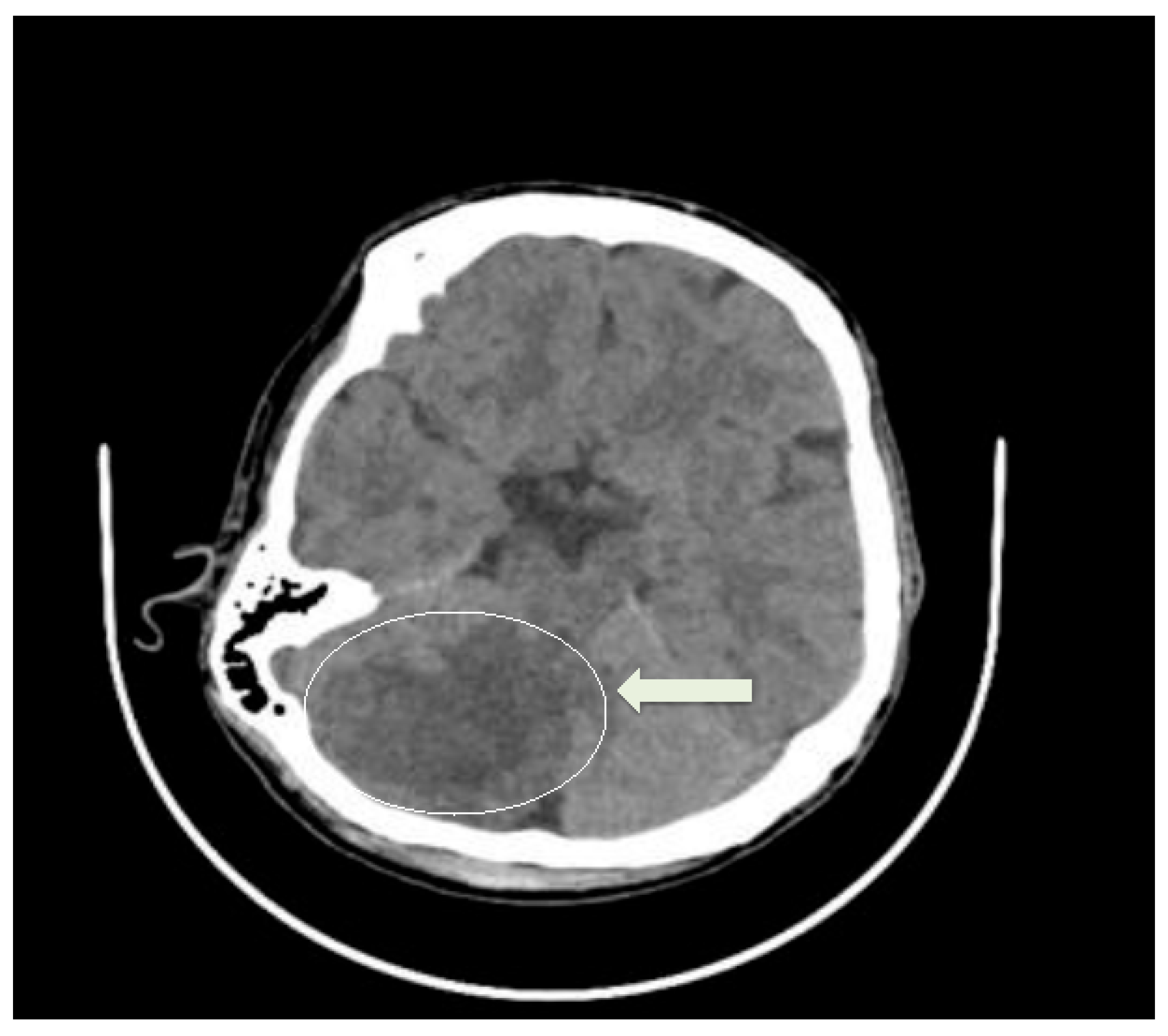
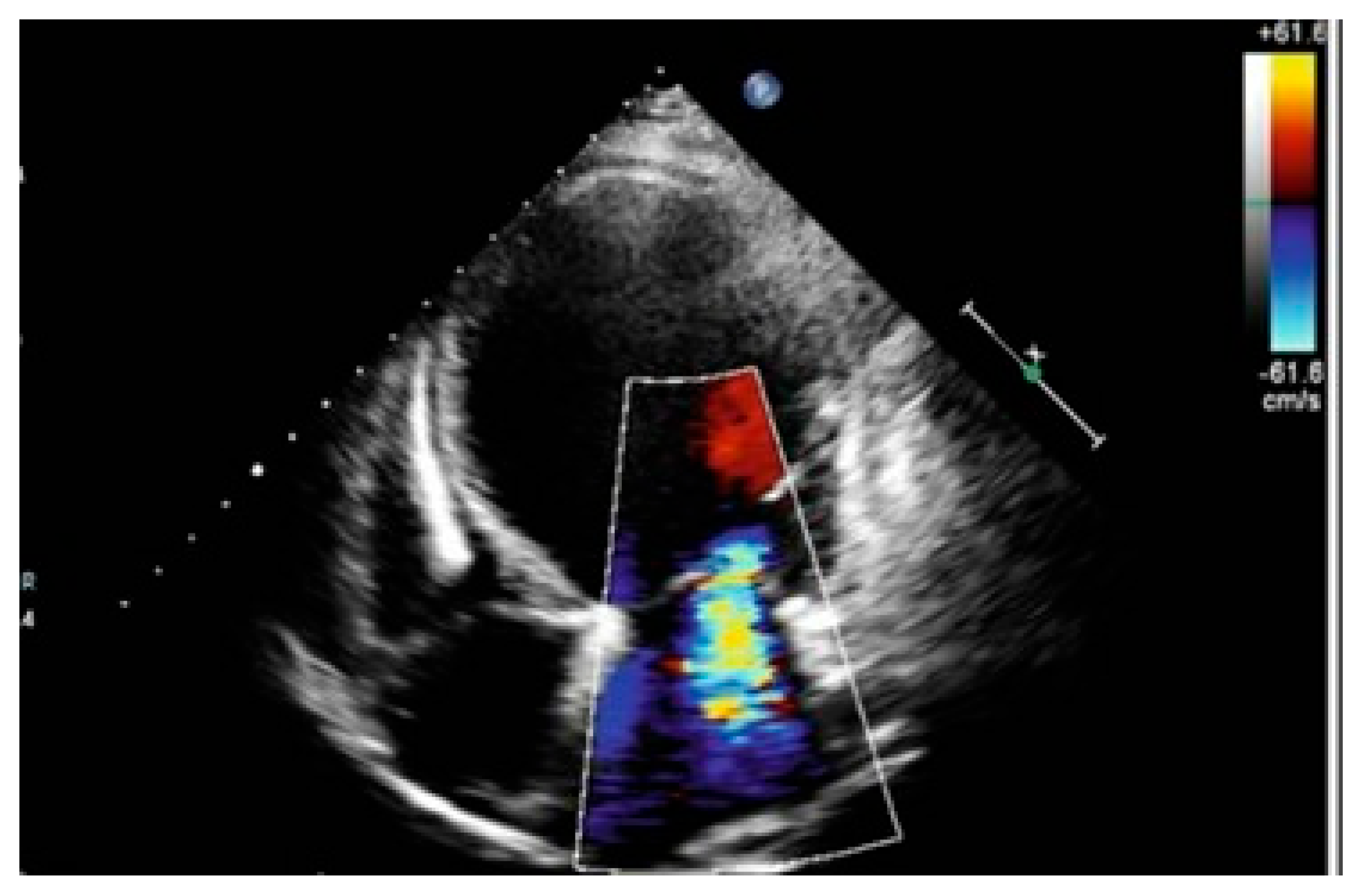
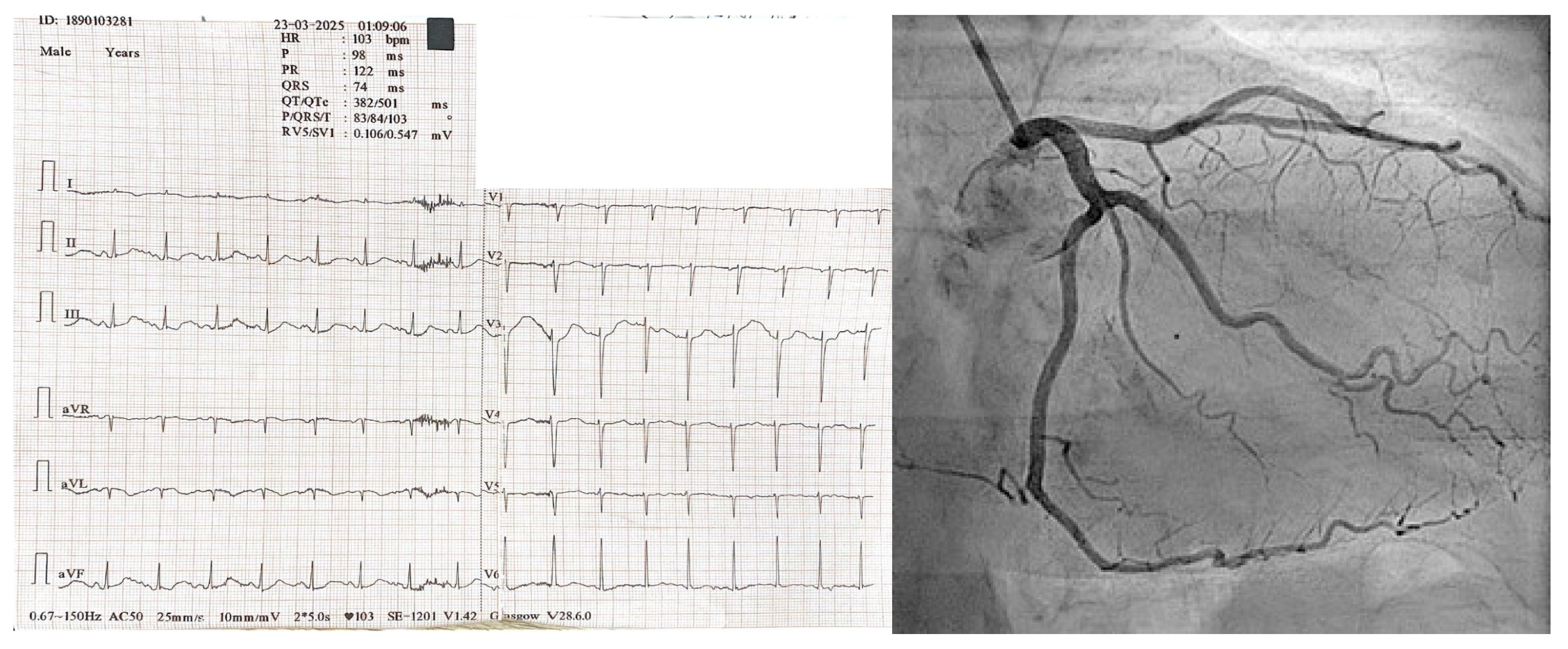
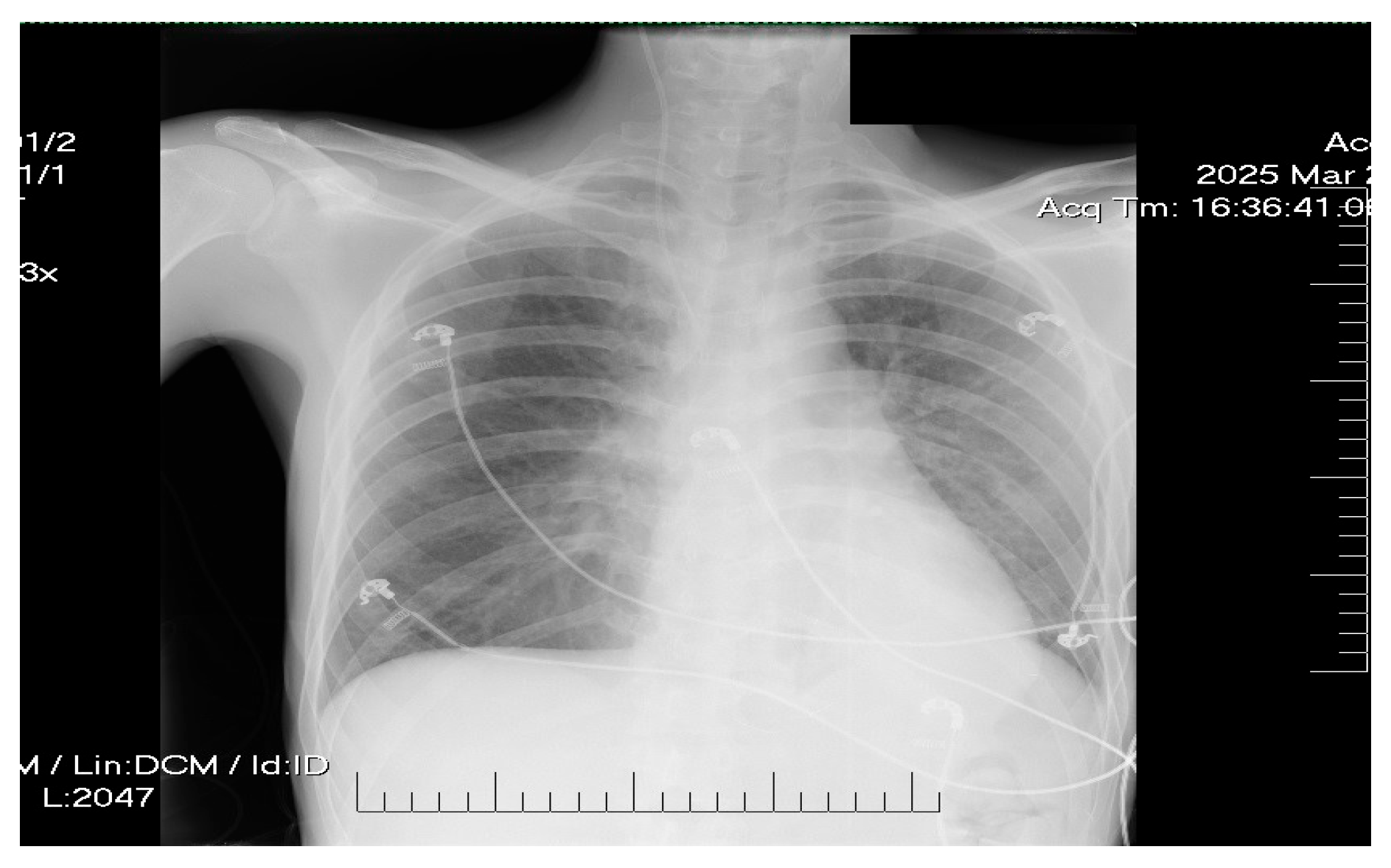
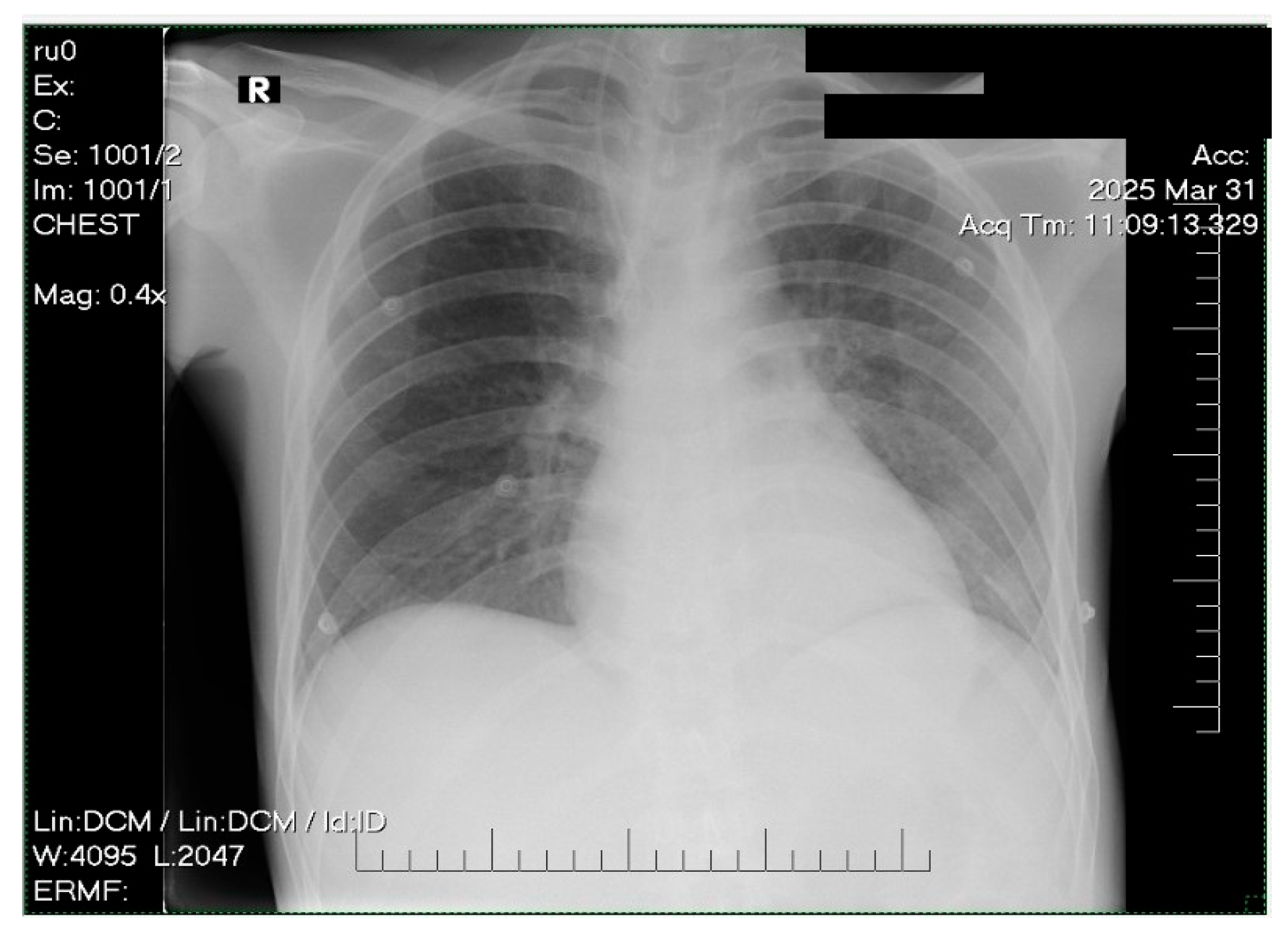
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Raji, M.A.; Priyadarshni, S.; Yu, X.; Digbeu, B.; Kuo, Y.-F. Association of Medication-Assisted Therapy with New Onset of Cardiac Arrhythmia in Patients Diagnosed with Opioid Use Disorders. Am. J. Med. 2022, 135, 864–870.e3. [Google Scholar] [CrossRef]
- Zünkler, B.J.; Wos-Maganga, M. Comparison of the Effects of Methadone and Heroin on Human Ether-à-Go-Go-Related Gene Channels. Cardiovasc. Toxicol. 2010, 10, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.V.; Ferdinandy, P.; Liaudet, L.; Pacher, P. Drug-Induced Mitochondrial Dysfunction and Cardiotoxicity: Mechanisms and Potential Role of Methadone-Triggered Mitochondrial Impairment in Cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1109–H1125. [Google Scholar] [CrossRef]
- Olson, P.C.; Agarwal, V.; Lafferty, J.C.; Bekheit, S. Takotsubo Cardiomyopathy Precipitated by Opiate Withdrawal. Heart Lung J. Cardiopulm. Acute Care 2018, 47, 73–75. [Google Scholar] [CrossRef]
- Krantz, M.J.; Palmer, R.B.; Haigney, M.C.P. Cardiovascular Complications of Opioid Use: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 205–223. [Google Scholar] [CrossRef]
- Boom, M.; Niesters, M.; Sarton, E.; Aarts, L.; Smith, T.W.; Dahan, A. Non-Analgesic Effects of Opioids: Opioid-Induced Respiratory Depression. Curr. Pharm. Des. 2012, 18, 5994–6004. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Lerman, A.; Rihal, C.S. Apical Ballooning Syndrome (Tako-Tsubo or Stress Cardiomyopathy): A Mimic of Acute Myocardial Infarction. Am. Heart J. 2008, 155, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Ashburn, M.A. Cardiac Effects of Opioid Therapy. Pain Med. 2015, 16, S27–S31. [Google Scholar] [CrossRef]
- Daugherty, L.E. Extracorporeal Membrane Oxygenation as Rescue Therapy for Methadone-Induced Pulmonary Edema. Pediatr. Emerg. Care 2011, 27, 633–634. [Google Scholar] [CrossRef]
- Corré, J.; Pillot, J.; Hilbert, G. Methadone-Induced Toxic Brain Damage. Case Rep. Radiol. 2013, 2013, 602981. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-Y.; Chen, L.-Y.; Liu, H.-C.; Wu, C.-S.; Yang, S.-Y.; Pan, C.-H.; Tsai, S.-Y.; Chen, C.-C.; Kuo, C.-J. Antipsychotic Medications and Stroke in Schizophrenia: A Case-Crossover Study. PLoS ONE 2017, 12, e0179424. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-Y.; Choi, N.-K.; Jung, S.-Y.; Lee, J.; Kwon, J.S.; Park, B.-J. Risk of Ischemic Stroke with the Use of Risperidone, Quetiapine and Olanzapine in Elderly Patients: A Population-Based, Case-Crossover Study. J. Psychopharmacol. Oxf. Engl. 2013, 27, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Gasimova, U.; Afzal, K.M.; Acharya, A.B. Neurological Manifestations of Chronic Methadone Maintenance Therapy: A Case Report and Literature Review. Cureus 2022, 14, e29534. [Google Scholar] [CrossRef]
- Shu, Y.; Ding, Y.; Liu, L.; Zhang, Q. Cardiac adverse events associated with quetiapine: Disproportionality analysis of FDA adverse event reporting system. CNS Neurosci. Ther. 2023, 29, 2705–2716. [Google Scholar] [CrossRef]
- Li, W.; Li, Q.; Wang, Y.; Zhu, J.; Ye, J.; Yan, X.; Li, Y.; Chen, J.; Liu, J.; Li, Z.; et al. Methadone-Induced Damage to White Matter Integrity in Methadone Maintenance Patients: A Longitudinal Self-Control DTI Study. Sci. Rep. 2016, 6, 19662. [Google Scholar] [CrossRef]
- Cobilinschi, C.; Mirea, L.; Andrei, C.-A.; Ungureanu, R.; Cotae, A.-M.; Avram, O.; Isac, S.; Grințescu, I.M.; Țincu, R. Biodetoxification Using Intravenous Lipid Emulsion, a Rescue Therapy in Life-Threatening Quetiapine and Venlafaxine Poisoning: A Case Report. Toxics 2023, 11, 917. [Google Scholar] [CrossRef]
- Spadotto, V.; Zorzi, A.; Elmaghawry, M.; Meggiolaro, M.; Pittoni, G.M. Heart Failure Due to “Stress Cardiomyopathy”: A Severe Manifestation of the Opioid Withdrawal Syndrome. Eur. Heart J. Acute Cardiovasc. Care 2013, 2, 84–87. [Google Scholar] [CrossRef]
- Perez-Alvarez, S.; Cuenca-Lopez, M.D.; de Mera, R.M.M.-F.; Puerta, E.; Karachitos, A.; Bednarczyk, P.; Kmita, H.; Aguirre, N.; Galindo, M.F.; Jordán, J. Methadone Induces Necrotic-like Cell Death in SH-SY5Y Cells by an Impairment of Mitochondrial ATP Synthesis. Biochim. Biophys. Acta 2010, 1802, 1036–1047. [Google Scholar] [CrossRef]
- Mihalcea, L.; Sebastian, I.; Simion-Cotorogea, M.; Klimko, A.; Droc, G. Reverse Takotsubo Cardiomyopathy after Orthotopic Liver Transplantation. A Case Report. J. Crit. Care Med. Univ. Med. Si Farm. Din Targu-Mures 2022, 8, 117–122. [Google Scholar] [CrossRef]
- Taha, M.B.; Dasa, O.; Al-Ani, M.; Taha, O.B.; Radhakrishnan, N.S.; Ahmed, M.M. Acute Right Ventricular Failure: A Novel Presentation of Methadone-Induced Cardiotoxicity. BMJ Case Rep. 2020, 13, e237168. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, K.; Shojaie, M.; Pourdavood, A.H.; Khajouei, M. Stress Cardiomyopathy (Takotsubo Syndrome) Following Accidental Methadone Poisoning; Report of Two Pediatric Cases. Arch. Acad. Emerg. Med. 2019, 7, e22. [Google Scholar] [PubMed]
- Isac, S.; Panaitescu, A.M.; Iesanu, M.I.; Zeca, V.; Cucu, N.; Zagrean, L.; Peltecu, G.; Zagrean, A.-M. Maternal Citicoline-Supplemented Diet Improves the Response of the Immature Hippocampus to Perinatal Asphyxia in Rats. Neonatology 2020, 117, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, P.; Kuo, Y.-F.; Digbeu, B.D.E.; Harmouch, W.; Mai, S.; Raji, M. The Impact of Medication-Assisted Treatment for Opioid Use Disorder on Congestive Heart Failure Outcomes. Am. Heart J. Plus Cardiol. Res. Pract. 2024, 46, 100456. [Google Scholar] [CrossRef]
- Substance Abuse and Mental Health Services Administration (SAMHSA). Federal Guidelines for Opioid Treatment Programs; HHS Publication No. (SMA) PEP21-FEDGUIDEOTP; SAMHSA: Rockville, MD, USA, 2021.
- World Health Organization. Guidelines for the Psychosocially Assisted Pharmacological Treatment of Opioid Dependence; WHO Press: Geneva, Switzerland, 2009. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristina, B.G.; Isac, S.; Teodorescu, G.-D.; Isac, T.; Martac, C.; Cobilinschi, C.; Pavel, B.; Andreescu, C.V.; Droc, G. Methadone-Induced Toxicity—An Unexpected Challenge for the Brain and Heart in ICU Settings: Case Report and Review of the Literature. Life 2025, 15, 1084. https://doi.org/10.3390/life15071084
Cristina BG, Isac S, Teodorescu G-D, Isac T, Martac C, Cobilinschi C, Pavel B, Andreescu CV, Droc G. Methadone-Induced Toxicity—An Unexpected Challenge for the Brain and Heart in ICU Settings: Case Report and Review of the Literature. Life. 2025; 15(7):1084. https://doi.org/10.3390/life15071084
Chicago/Turabian StyleCristina, Buzatu Georgiana, Sebastian Isac, Geani-Danut Teodorescu, Teodora Isac, Cristina Martac, Cristian Cobilinschi, Bogdan Pavel, Cristina Veronica Andreescu, and Gabriela Droc. 2025. "Methadone-Induced Toxicity—An Unexpected Challenge for the Brain and Heart in ICU Settings: Case Report and Review of the Literature" Life 15, no. 7: 1084. https://doi.org/10.3390/life15071084
APA StyleCristina, B. G., Isac, S., Teodorescu, G.-D., Isac, T., Martac, C., Cobilinschi, C., Pavel, B., Andreescu, C. V., & Droc, G. (2025). Methadone-Induced Toxicity—An Unexpected Challenge for the Brain and Heart in ICU Settings: Case Report and Review of the Literature. Life, 15(7), 1084. https://doi.org/10.3390/life15071084








