The Relationship Between Tramadol-Induced Oxidative Testis Injury and Reproductive Function Disorder and Adenosine Triphosphate
Abstract
1. Introduction
2. Material and Methods
2.1. Animals
2.2. Chemical Substances
2.3. Experimental Groups
2.4. Experimental Procedure
2.5. Specimen Preparation
2.6. MDA, GSH, SOD, and CAT Analyses in Testicular Tissues
2.7. TNF-α, IL-1β, and IL-6 Analyses in Testicular Tissues
2.8. Histopathological Examination
2.9. Statistical Analyses
3. Results
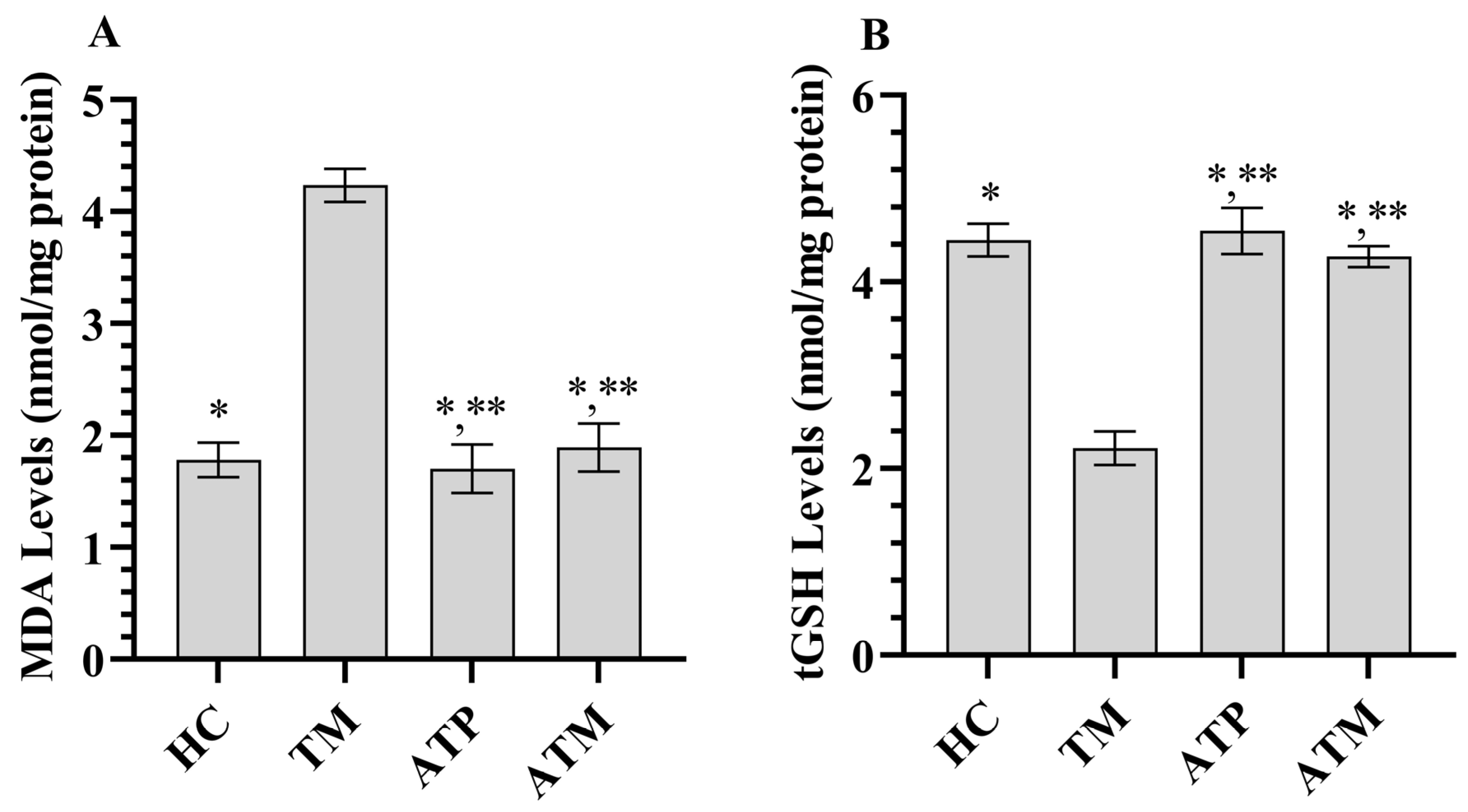
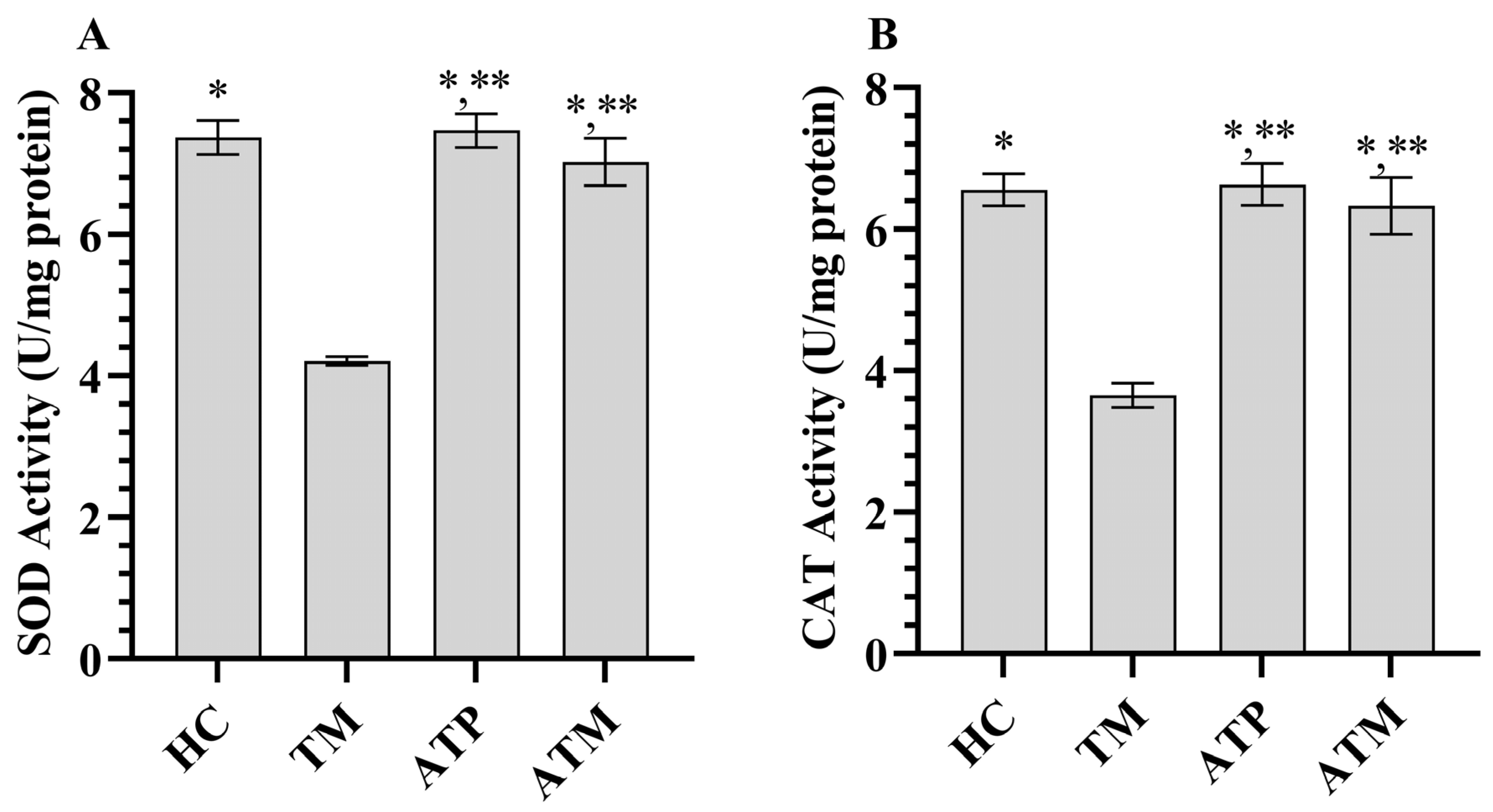
3.1. Testicular Tissue MDA and GSH Analysis Results
3.2. Testicular Tissue IL-1β, IL-6, and TNF-α Analysis Results
3.3. Reproduction Test Results
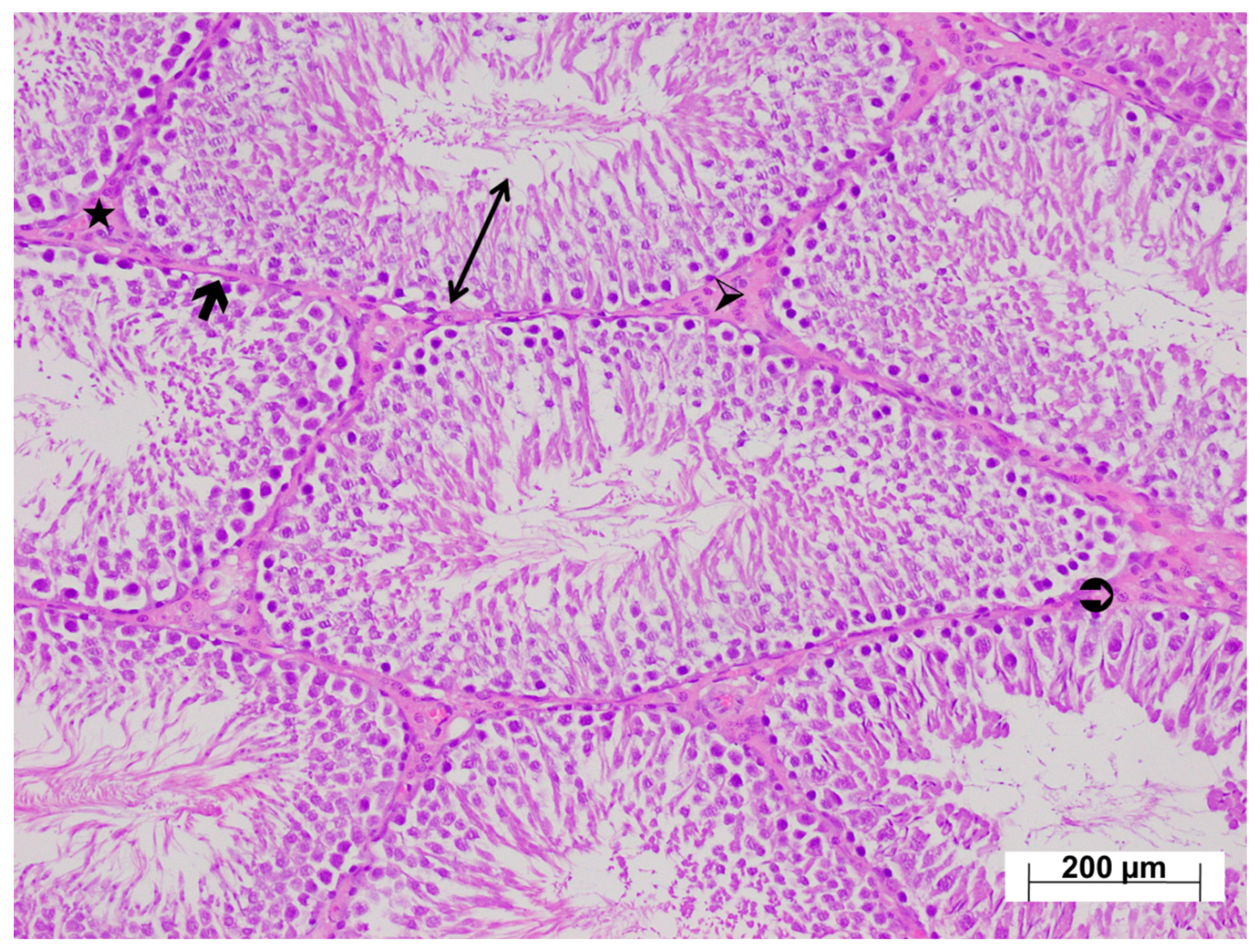
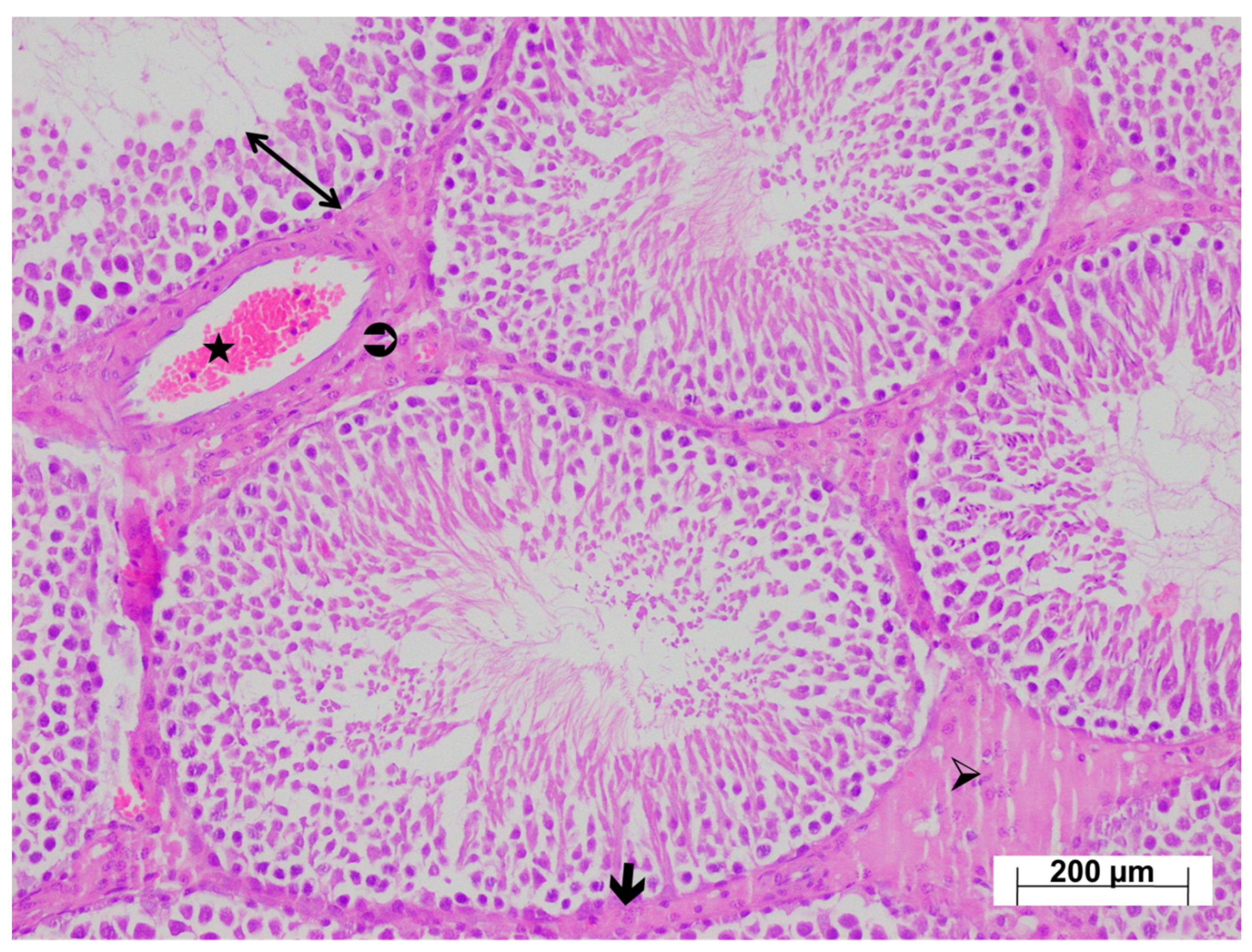
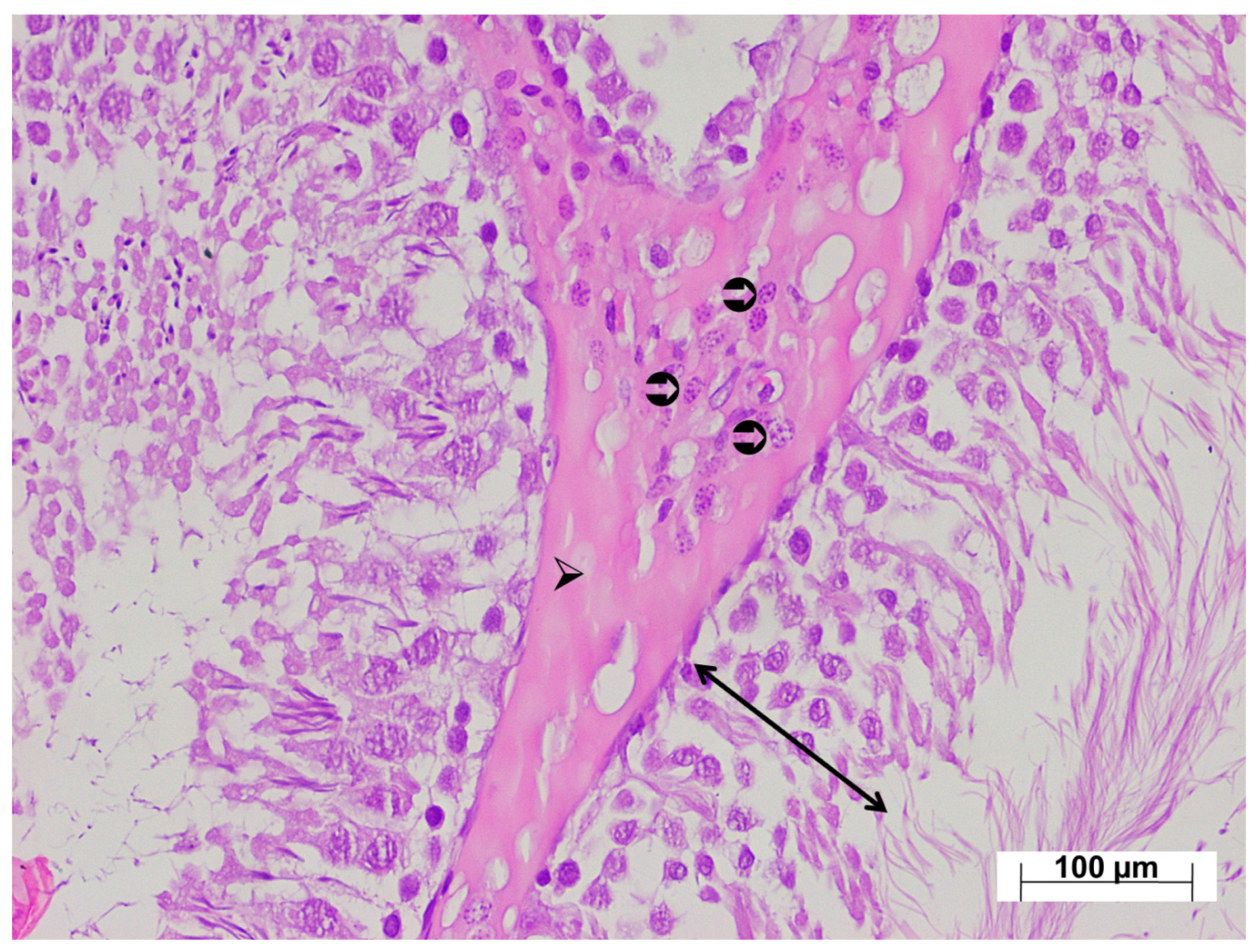
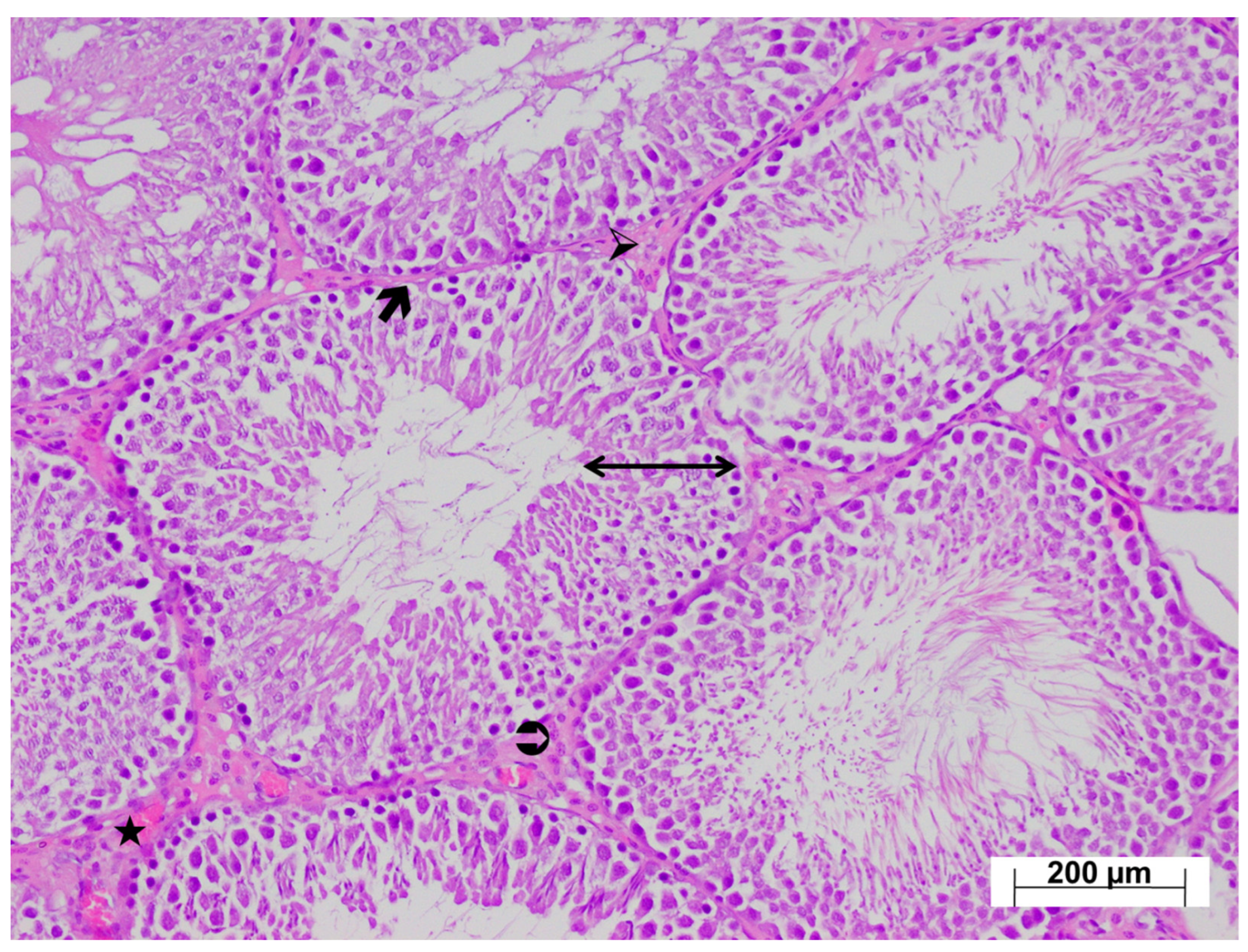
3.4. Histopathological Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pilia, E.; Finco, G. Tramadol antimicrobial activity in peripheral nerve blocks. Is it a real possibility? J. Clin. Anesth. 2024, 92, 111316. [Google Scholar] [CrossRef] [PubMed]
- Grond, S.; Sablotzki, A. Clinical Pharmacology of Tramadol. Clin. Pharmacokinet. 2004, 43, 879–923. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, F.A. Tramadol for the treatment of premature ejaculation. Eur. Urol. 2012, 61, 744–745. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.; Barbosa, J.; Leal, S.; Afonso, L.P.; Lobo, J.; Moreira, R.; Queirós, O.; Carvalho, F.; Dinis-Oliveira, R.J. Effective analgesic doses of tramadol or tapentadol induce brain, lung and heart toxicity in Wistar rats. Toxicology 2017, 385, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Koohsari, M.; Ahangar, N.; Mohammadi, E.; Shaki, F. Ameliorative Effect of Melatonin Against Reproductive Toxicity of Tramadol in Rats via the Regulation of Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis-related Gene Expression Signaling Pathway. Addict. Health 2020, 12, 118–129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El-Ghawet, H.A. Effects of tramadol on the reproductive function of Wistar albino rats. Eur. J. Exp. Biol. 2015, 5, 56–64. [Google Scholar]
- Adelakun, S.A.; Ukwenya, V.O.; Akintunde, O.W. Vitamin B12 ameliorates tramadol-induced oxidative stress, endocrine imbalance, apoptosis and NO/iNOS/NF-κB expression in Sprague Dawley rats through regulatory mechanism in the pituitary-gonadal axis. Tissue Cell 2022, 74, 101697. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, K.; Manthari, R.K.; Najibi, A.; Jia, Z.; Ommati, M.M.; Heidari, R. Mitochondrial dysfunction and oxidative stress are involved in the mechanism of tramadol-induced renal injury. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100049. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Faria, J.; Barbosa, J.; Queirós, O.; Moreira, R.; Carvalho, F.; Dinis-Oliveira, R.J. Comparative study of the neurotoxicological effects of tramadol and tapentadol in SH-SY5Y cells. Toxicology 2016, 359–360, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Elmorsy, E.M.; Al Doghaither, H.A.; Al-Ghafari, A.B. Bioenergetics disruption, oxidative stress, and inflammation as underlying mechanisms of tramadol-induced nephrotoxicity. J. Biochem. Mol. Toxicol. 2024, 38, e23777. [Google Scholar] [CrossRef]
- Gholami, M.; Ghelichkhani, Z.; Aghakhani, R.; Klionsky, D.J.; Motaghinejad, O.; Motaghinejad, M.; Koohi, M.K.; Hassan, J. Minocycline Acts as a Neuroprotective Agent Against Tramadol-Induced Neurodegeneration: Behavioral and Molecular Evidence. Int. J. Prev. Med. 2024, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.; Grider, M.H. Physiology, Adenosine Triphosphate. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar] [PubMed]
- Bonora, M.; Patergnani, S.; Rimessi, A.; De Marchi, E.; Suski, J.M.; Bononi, A.; Giorgi, C.; Marchi, S.; Missiroli, S.; Poletti, F.; et al. ATP synthesis and storage. Purinergic Signal. 2012, 8, 343–357. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saquet, A.A.; Streif, J.; Bangerth, F. Changes in ATP, ADP and pyridine nucleotide levels related to the incidence of physiological disorders in ‘Conference’ pears and ‘Jonagold’ apples during controlled atmosphere storage. J. Hortic. Sci. Biotechnol. 2000, 75, 243–249. [Google Scholar] [CrossRef]
- Yi, C.; Jiang, Y.; Shi, J.; Qu, H.; Xue, S.; Duan, X.; Shi, J.; Prasad, N.K. ATP-regulation of antioxidant properties and phenolics in litchi fruit during browning and pathogen infection process. Food Chem. 2010, 118, 42–47. [Google Scholar] [CrossRef]
- Zhu, Z.; Kawai, T.; Umehara, T.; Hoque, S.M.; Zeng, W.; Shimada, M. Negative effects of ROS generated during linear sperm motility on gene expression and ATP generation in boar sperm mitochondria. Free Radic. Biol. Med. 2019, 141, 159–171. [Google Scholar] [CrossRef]
- Ozer, M.; Ince, S.; Altuner, D.; Suleyman, Z.; Cicek, B.; Gulaboglu, M.; Mokhtare, B.; Gursul, C.; Suleyman, H. Protective Effect of Adenosine Triphosphate Against 5-Fluorouracil-Induced Oxidative Ovarian Damage In Vivo. Asian Pac. J. Cancer Prev. 2023, 24, 1007–1013. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ali, H.A.; Afifi, M.; Saber, T.M.; Makki, A.A.; Keshta, A.T.; Baeshen, M.; Al-Farga, A. Neurotoxic, hepatotoxic and nephrotoxic effects of tramadol administration in rats. J. Mol. Neurosci. 2020, 70, 1934–1942. [Google Scholar] [CrossRef]
- Goth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef]
- Cosentino, M.J.; Nishida, M.; Rabinowitz, R.; Cockett, A.T. Histological changes occurring in the contralateral testes of prepubertal rats subjected to various durations of unilateral spermatic cord torsion. J. Urol. 1985, 133, 906–911. [Google Scholar] [CrossRef]
- Keskin, E.; Erdogan, A.; Suleyman, H.; Yazici, G.N.; Sunar, M.; Gul, M.A. Effect of sunitinib on testicular oxidative and proinflammatory damage induced by ischemia–reperfusion in rats. Rev. Int. De Andrología 2022, 20, S17–S23. [Google Scholar] [CrossRef]
- Yeter, B.; Suleyman, Z.; Bulut, S.; Cicek, B.; Coban, T.A.; Demir, O.; Suleyman, H. Effect of adenosine triphosphate on methylphenidate-induced oxidative and inflammatory kidney damage in rats. Drug Chem. Toxicol. 2025, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kisaoglu, A.; Borekci, B.; Yapca, O.E.; Bilen, H.; Suleyman, H. Tissue damage and oxidant/antioxidant balance. Eurasian J. Med. 2013, 45, 47–49. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bedir, F.; Kocaturk, H.; Ozgeris, F.B.; Yazici, G.N.; Suleyman, Z.; Suleyman, H. The effect of taxifolin on experimental testicular ischemia reperfusion injury in rats. A biochemical and histopathological analysis. Rev. Int. Androl. 2022, 20, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Süleyman, H.; Özçiçek, A. Molecular Mechanism of Ischemia Reperfusion Injury. Arch. Basic Clin. Res. 2020, 2, 25–27. [Google Scholar] [CrossRef]
- Yapanoglu, T.; Ozkaya, F.; Yilmaz, A.H.; Mammadov, R.; Cimen, F.K.; Hirik, E.; Altuner, D. Effect of etoricoxib on experimental oxidative testicular ischemia-reperfusion damage in rats induced with torsion-detorsion. Korean J. Physiol. Pharmacol. 2017, 21, 457–464. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anwar, M.M.; Laila, I.M.I. Protective and restorative potency of diosmin natural flavonoid compound against tramadol-induced testicular damage and infertility in male rats. Nat. Prod. Res. 2023, 37, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Irato, P.; Santovito, G. Enzymatic and Non-Enzymatic Molecules with Antioxidant Function. Antioxidants 2021, 10, 579. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khorsandi, L.; Varaa, N.; Dadfar, R.; Vastegani, S.M.; Yousef, A.F.; Ahangarpour, A.; Keshavarz-Zarjani, A. The protective effect of ozone on the mice testicular damage induced by methotrexate. JBRA Assist. Reprod. 2024, 28, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P.; Slama, P.; Roychoudhury, S. Oxidative Stress, Testicular Inflammatory Pathways, and Male Reproduction. Int. J. Mol. Sci. 2021, 22, 10043. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, D.; Panda, S.K.; Jena, G.R.; Sethy, K.; Mishra, S.K.; Swain, B.K.; Naik, P.K.; Beura, C.K.; Behera, R. Alternations of Fertility Parameters by Graded Dose of Inorganic Arsenic in Adult Male White Pekin Ducks. Biol. Trace Elem. Res. 2023, 201, 5358–5367. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Li, L.; Liu, T.; Zhang, R.; Yang, P.; Wang, X.; Dai, L. Mechanisms of isoniazid and rifampicin-induced liver injury and the effects of natural medicinal ingredients: A review. Front. Pharmacol. 2022, 13, 1037814. [Google Scholar] [CrossRef] [PubMed]
- Walenta, L.; Fleck, D.; Fröhlich, T.; von Eysmondt, H.; Arnold, G.J.; Spehr, J.; Schwarzer, J.U.; Köhn, F.M.; Spehr, M.; Mayerhofer, A. ATP-mediated Events in Peritubular Cells Contribute to Sterile Testicular Inflammation. Sci. Rep. 2018, 8, 1431. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khodamoradi, K.; Amini-Khoei, H.; Khosravizadeh, Z.; Hosseini, S.R.; Dehpour, A.R.; Hassanzadeh, G. Oxidative stress, inflammatory reactions and apoptosis mediated the negative effect of chronic stress induced by maternal separation on the reproductive system in male mice. Reprod. Biol. 2019, 19, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Abd, H.H.; Ahmed, H.A.; Mutar, T.F. Moringa oleifera leaves extract modulates toxicity, sperms alterations, oxidative stress, and testicular damage induced by tramadol in male rats. Toxicol. Res. 2020, 9, 101–106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Minisy, F.M.; Shawki, H.H.; El Omri, A.; Massoud, A.A.; Omara, E.A.; Metwally, F.G.; Badawy, M.A.; Hassan, N.A.; Hassan, N.S.; Oishi, H. Pomegranate Seeds Extract Possesses a Protective Effect against Tramadol-Induced Testicular Toxicity in Experimental Rats. Biomed. Res. Int. 2020, 2020, 2732958. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hassan, M.H.; Saadeldin, A.A.; Alsagheer, G.; Desoky, T.; Hasan, A.S. Biochemical and Pharmacological Assessments of Tramadol Abuse on Human Male Fertility: Relation to Seminal Plasma 8-Hydroxyguanosine and Zinc. Indian J. Clin. Biochem. 2024, 39, 489–505. [Google Scholar] [CrossRef]
- Marinaro, C.; Lettieri, G.; Chianese, T.; Bianchi, A.R.; Zarrelli, A.; Palatucci, D.; Scudiero, R.; Rosati, L.; De Maio, A.; Piscopo, M. Exploring the Molecular and Toxicological Mechanism Associated with Interactions Between Heavy Metals and the Reproductive System of Mytilus galloprovincialis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2024, 275, 109778. [Google Scholar] [CrossRef]


| Biochemical Parameters | HC | TM | ATP | ATM | F (3, 20) | p Values |
|---|---|---|---|---|---|---|
| Mean ± Standard Deviation | ||||||
| MDA | 1.78 ± 0.15 * | 4.23 ± 0.15 | 1.70 ± 0.22 *,** | 1.89 ± 0.22 *,** | 258.099 | <0.001 |
| tGSH | 4.45 ± 0.18 * | 2.22 ± 0.18 | 4.56 ± 0.25 *,** | 4.27 ± 0.11 *,** | 215.054 | <0.001 |
| SOD | 7.37 ± 0.24 * | 4.21 ± 0.06 | 7.47 ± 0.24 *,** | 7.03 ± 0.33 *,** | 252.414 | <0.001 |
| CAT | 6.56 ± 0.23 * | 3.65 ± 0.17 | 6.63 ± 0.30 *,** | 6.33 ± 0.40 *,** | 149.396 | <0.001 |
| TNF-α | 2.78 ± 0.09 * | 4.69 ± 0.13 | 2.75 ± 0.06 *,** | 2.85 ± 0.16 *,** | 395.830 | <0.001 |
| IL-1β | 3.08 ± 0.28 * | 5.83 ± 0.11 | 3.12 ± 0.17 *,** | 4.17 ± 0.11 * | 298.600 | <0.001 |
| IL-6 | 2.38 ± 0.19 * | 4.43 ± 0.17 | 2.32 ± 0.18 *,** | 2.91 ± 0.17 * | 192.944 | <0.001 |
| Groups | Number of Animals | Developing Infertility | % | Not Developing Infertility | % |
|---|---|---|---|---|---|
| HC | 6 | 0 | 0 | 6 | 100 |
| TM | 6 | 4 | 66.7 | 2 | 33.3 |
| ATP | 6 | 0 | 0 | 6 | 100 |
| ATM | 6 | 1 | 16.7 | 5 | 83.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bedir, F.; Kocatürk, H.; Altay, M.S.; Mammadov, R.; Süleyman, B.; Coban, T.A.; Yazici, G.N.; Bulut, S.; Süleyman, H. The Relationship Between Tramadol-Induced Oxidative Testis Injury and Reproductive Function Disorder and Adenosine Triphosphate. Life 2025, 15, 1078. https://doi.org/10.3390/life15071078
Bedir F, Kocatürk H, Altay MS, Mammadov R, Süleyman B, Coban TA, Yazici GN, Bulut S, Süleyman H. The Relationship Between Tramadol-Induced Oxidative Testis Injury and Reproductive Function Disorder and Adenosine Triphosphate. Life. 2025; 15(7):1078. https://doi.org/10.3390/life15071078
Chicago/Turabian StyleBedir, Fevzi, Hüseyin Kocatürk, Mehmet Sefa Altay, Renad Mammadov, Bahadır Süleyman, Taha Abdulkadir Coban, Gülce Naz Yazici, Seval Bulut, and Halis Süleyman. 2025. "The Relationship Between Tramadol-Induced Oxidative Testis Injury and Reproductive Function Disorder and Adenosine Triphosphate" Life 15, no. 7: 1078. https://doi.org/10.3390/life15071078
APA StyleBedir, F., Kocatürk, H., Altay, M. S., Mammadov, R., Süleyman, B., Coban, T. A., Yazici, G. N., Bulut, S., & Süleyman, H. (2025). The Relationship Between Tramadol-Induced Oxidative Testis Injury and Reproductive Function Disorder and Adenosine Triphosphate. Life, 15(7), 1078. https://doi.org/10.3390/life15071078








