Enhancing Bone–Cartilage Interface Healing in Osteochondral Autograft Transplantation: Effects of BMAC Augmentation and Rehabilitation Protocols
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Procedure Steps
2.2. Participants
2.3. Surgical Treatment and BMAC Augmentation
2.4. Postoperative Rehabilitation
2.5. Imaging Assessment and Use of the MOCART 2.0 Score
- -
- Complete or minor hypertrophy (100–150% fill): 20 pts; major hypertrophy ≥150% or 75–99% fill: 15 pts; 50–74%: 10 pts; 25–49%: 5 pts; and <25% or delamination: 0 pts.
- -
- Complete integration: 15 pts; split-like defect ≤2 mm: 10 pts; defect >2 mm but <50%: 5 pts; and ≥50%: 0 pts.
- -
- Surface intact: 10 pts; irregular <50%: 5 pts; and irregular ≥50%: 0 pts.
- -
- Structure homogeneous: 10 pts; inhomogeneous: 0 pts.
- -
- Signal normal: 15 pts; minor abnormal: 10 pts; and severe abnormal: 0 pts.
- -
- No bony defect/overgrowth: 10 pts; minor (<thickness or <50%): 5 pts; and major (≥thickness or ≥50%): 0 pts.
- -
- No subchondral change: 20 pts; minor edema <50%: 15 pts; severe edema ≥50%: 10 pts; and cyst ≥5 mm or osteonecrosis: 0 pts.
- -
- TE: 25–35 ms for PDw sequences, 10–15 ms for T1w.
- -
- TR: 3000–4000 ms for PDw, 500–800 ms for T1w.
- -
- Section thickness: 1.5–3 mm, with minimum intersectional spacing (gap 0–10%).
- -
- Slices: 25–48.
- -
- FOV: 140–160 mm, with a minimum acquisition matrix of 384 × 384.
- -
- Orientation: sagittal, supplemented by transverse sequences.
2.6. Statistical Analysis
3. Results
Graft Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hangody, L.; Dobos, J.; Baló, E.; Pánics, G.; Hangody, L.R.; Berkes, I. Clinical experiences with autologous osteochondral mosaicplasty in an athletic population: A 17-year prospective multicenter study. Am. J. Sports Med. 2010, 38, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Hangody, L.; Vásárhelyi, G.; Hangody, L.R.; Sükösd, Z.; Tibay, G.; Bartha, L.; Bodó, G. Autologous osteochondral grafting—technique and long-term results. Injury 2008, 39 (Suppl. S1), 32–39. [Google Scholar] [CrossRef] [PubMed]
- Hangody, L.; Fules, P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: Ten years of experimental and clinical experience. J. Bone Jt. Surg. Am. 2003, 85, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Hangody, L.; Kish, G.; Karpati, Z. Arthroscopic autogenous osteochondral mosaicplasty for the treatment of femoral condylar articular defect. Knee Surg. Sports Traumatol. Arthros. 1997, 5, 262–267. [Google Scholar] [CrossRef]
- Pearce, S.G.; Hurtig, M.B.; Clarnette, R.; Kalra, M.; Cowan, B.; Miniaci, A. An investigation of 2 techniques for optimizing joint surface congruency using multiple osteochondral autografts. Arthroscopy 2001, 17, 50–55. [Google Scholar] [CrossRef]
- Solheim, E.; Hegna, J.; Inderhaug, E. Long-term survival after microfracture and mosaicplasty for knee articular cartilage repair: A comparative study between two treatments cohorts. Cartilage 2020, 11, 71–76. [Google Scholar] [CrossRef]
- Solheim, E.; Hegna, J.; Strand, T.; Harlem, T.; Inderhaug, E. Randomized study of longterm (15–17 years) outcome after microfracture versus mosaicplasty in knee articular cartilage defects. Am. J. Sports Med. 2018, 46, 826–831. [Google Scholar] [CrossRef]
- Lane, J.G.; Massie, J.B.; Ball, S.T.; Amiel, M.E.; Chen, A.C.; Bae, W.C.; Sah, R.L.; Amiel, D. Follow-up of osteochondral plug transfers in a goat model: A 6-month study. Am. J. Sports Med. 2004, 32, 1440–1450. [Google Scholar] [CrossRef]
- Kusano, T.; Jakob, R.P.; Gautier, E.; Magnussen, R.A.; Hoogewoud, H.; Jacobi, M. Treatment of isolated chondral and osteochondral defects in the knee by autologous matrix-induced chondrogenesis (AMIC). Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 2109–2115. [Google Scholar] [CrossRef]
- Patil, S.; Butcher, W.; D’Lima, D.D.; Steklov, N.; Bugbee, W.D.; Hoenecke, H.R. Effect of osteochondral graft insertion forces on chondrocyte viability. Am. J. Sports Med. 2008, 36, 1726–1732. [Google Scholar] [CrossRef]
- Brittberg, M.; Winalski, C.S. Evaluation of cartilage injuries and repair. J. Bone Jt. Surg. Am. 2003, 85 (Suppl. S2), 58–69. [Google Scholar] [CrossRef] [PubMed]
- Miniaci, A.; Tytherleigh-Strong, G. Fixation of unstable osteochondritis dissecans lesions of the knee using arthroscopic autogenous osteochondral grafting (mosaicplasty). Arthroscopy 2007, 23, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Bedi, A.; Feeley, B.T.; Williams, R.J. Management of Articular Cartilage Defects of the Knee. J. Bone Jt. Surg. 2010, 92, 994–1009. [Google Scholar] [CrossRef] [PubMed]
- Emre, T.Y.; Ege, T.; Kose, O.; Tekdos Demircioglu, D.; Seyhan, B.; Uzun, M. Factors affecting the outcome of osteochondral autografting (mosaicplasty) in articular cartilage defects of the knee joint: Retrospective analysis of 152 cases. Arch. Orthop. Trauma. Surg. 2013, 133, 531–536. [Google Scholar] [CrossRef]
- Keszég, M.; Hangody, L.; Egyed, Z.; Tóth, G.; Pánics, G. Long-term (10–25 years) outcomes of knee osteochondral autologous transplantation in soccer players. J. Cartil. Jt. Preserv. 2022, 2, 100062. [Google Scholar] [CrossRef]
- Matricali, G.A.; Dereymaeker, G.P.; Luytenm, F.P. Donor site morbidity after articular cartilage repair procedures: A review. Acta Orthop. Belg. 2010, 76, 669–674. [Google Scholar]
- Andrade, R.; Vasta, S.; Pereira, R.; Pereira, H.; Papalia, R.; Karahan, M.; Oliveira, J.M.; Reis, R.L.; Espregueira-Mendes, J. Knee donor-site morbidity after mosaicplasty—A systematic review. J. Exp. Orthop. 2016, 3, 31. [Google Scholar] [CrossRef]
- Bexkens, R.; Ogink, P.T.; Doornberg, J.N.; Kerkhoffs, G.M.M.J.; Eygendaal, D.; Oh, L.S.; Bekerom, M.P.J.v.D. Donor-site morbidity after osteochondral autologous transplantation for osteochondritis dissecans of the capitellum: A systematic review and meta-analysis. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 2237–2246. [Google Scholar] [CrossRef]
- Ferreira, C.; Vuurberg, G.; Oliveira, J.M.; Espregueira-Mendes, J.; Pereira, H.; Reis, R.L.; Ripoll, P.L. Good clinical outcome after osteochondral autologous transplantation surgery for osteochondral lesions of the talus but at the cost of a high rate of complications: A systematic review. J. ISAKOS 2016, 1, 184–191. [Google Scholar] [CrossRef]
- Çakar, A.; Aktaş, M.A.; Atıcı, A. Predictors of Donor Site Morbidity Following Osteochondral Graft Harvesting from the Healthy Knee using Lysholm Knee Score. Istanb. Med. J. 2025, 26, 73–80. [Google Scholar] [CrossRef]
- Gherghel, R.; Onu, I.; Iordan, D.A.; Antohe, B.A.; Rezus, I.-I.; Alexa, O.; Macovei, L.A.; Rezus, E. A New Approach to Postoperative Rehabilitation following Mosaicplasty and Bone Marrow Aspiration Concentrate (BMAC) Augmentation. Biomedicines 2024, 12, 1164. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, M.M.; Raudner, M.; Marlovits, S.; Bohndorf, K.; Weber, M.; Zalaudek, M.; Röhrich, S.; Szomolanyi, P.; Filardo, G.; Windhager, R.; et al. The MOCART (Magnetic Resonance Observation of Cartilage Repair Tissue) 2.0 Knee Score and Atlas. Cartilage 2021, 13 (Suppl. S1), 571S–587S. [Google Scholar] [CrossRef] [PubMed]
- Zaffagnini, S.; Boffa, A.; Andriolo, L.; Reale, D.; Busacca, M.; Di Martino, A.; Filardo, G. Mosaicplasty versus Matrix-Assisted Autologous Chondrocyte Transplantation for Knee Cartilage Defects: A Long-Term Clinical and Imaging Evaluation. Appl. Sci. 2020, 10, 4615. [Google Scholar] [CrossRef]
- Quarch, V.M.; Enderle, E.; Lotz, J.; Frosch, K.H. Fate of large donor site defects in osteochondral transfer procedures in the knee joint with and without TruFit plugs. Arch. Orthop. Trauma. Surg. 2014, 134, 657–666. [Google Scholar] [CrossRef]
- Guo, X.; Ma, Y.; Min, Y.; Sun, J.; Shi, X.; Gao, G.; Sun, L.; Wang, J. Progress and prospect of technical and regulatory challenges on tissue-engineered cartilage as therapeutic combination product. Bioact Mater. 2022, 20, 501–518. [Google Scholar] [CrossRef]
- Branam, G.M.; Saber, A.Y. Osteochondral Autograft Transplantation. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560655/ (accessed on 23 March 2025).
- Solheim, E.; Hegna, J.; Inderhaug, E. Clinical outcome after mosaicplasty of knee articular cartilage defects of patellofemoral joint versus tibiofemoral joint. J. Orthop. 2019, 18, 36–40. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef]
- Kim, G.B.; Kim, J.-D.; Choi, Y.; Choi, C.H.; Lee, G.W. Intra-Articular Bone Marrow Aspirate Concentrate Injection in Patients with Knee Osteoarthritis. Appl. Sci. 2020, 10, 5945. [Google Scholar] [CrossRef]
- Keeling, L.E.; Belk, J.W.; Kraeutler, M.J.; Kallner, A.C.; Lindsay, A.; McCarty, E.C.; Postma, W.F. Bone Marrow Aspirate Concentrate for the Treatment of Knee Osteoarthritis: A Systematic Review. Am. J. Sports Med. 2022, 50, 2315–2323. [Google Scholar] [CrossRef]
- Skowroński, J.; Skowroński, R.; Rutka, M. Large cartilage lesions of the knee treated with bone marrow concentrate and collagen membrane–results. Ortop. Traumatol. Rehabil. 2013, 15, 69–76. [Google Scholar]
- Krych, A.J.; Nawabim, D.H.; Farshad-Amacker, N.A.; Jones, K.J.; Maak, T.G.; Potter, H.G.; Williams, R.J., III. Bone marrow concentrate improves early cartilage phase maturation of a scaffold plug in the knee: A comparative magnetic resonance imaging analysis to platelet-rich plasma and control. Am. J. Sports Med. 2016, 44, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lin, K.M.; Burge, A.J.; Balazs, G.C.; Williams, R.J., 3rd. Bone Marrow Aspirate Concentrate Does Not Improve Osseous Integration of Osteochondral Allografts for the Treatment of Chondral Defects in the Knee at 6 and 12 Months: A Comparative Magnetic Resonance Imaging Analysis. Am. J. Sports Med. 2019, 47, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, H.; Xiang, D.; Shao, J.; Fu, Q.; Han, Y.; Zhu, J.; Chen, Y.; Qian, Q. The clinical efficacy of arthroscopic therapy with knee infrapatellar fat pad cell concentrates in treating knee cartilage lesion: A prospective, randomized, and controlled study. J. Orthop. Surg. Res. 2021, 16, 87. [Google Scholar] [CrossRef]
- Johnson, M. Transcutaneous Electrical Nerve Stimulation: Mechanisms, Clinical Application and Evidence. Rev. Pain. 2007, 1, 7–11. [Google Scholar] [CrossRef]
- Zhou, Z.; Zheng, J.; Meng, X.; Wang, F. Effects of Electrical Stimulation on Articular Cartilage Regeneration with a Focus on Piezoelectric Biomaterials for Articular Cartilage Tissue Repair and Engineering. Int. J. Mol. Sci. 2023, 24, 1836. [Google Scholar] [CrossRef]
- do Carmo Almeida, T.C.; Dos Santos Figueiredo, F.W.; Barbosa Filho, V.C.; de Abreu, L.C.; Fonseca, F.L.A.; Adami, F. Effects of Transcutaneous Electrical Nerve Stimulation on Proinflammatory Cytokines: Systematic Review and Meta-Analysis. Mediat. Inflamm. 2018, 2018, 1094352. [Google Scholar] [CrossRef]
- Cherian, J.J.; Harrison, P.E.; Benjamin, S.A.; Bhave, A.; Harwin, S.F.; Mont, M.A. Do the Effects of Transcutaneous Electrical Nerve Stimulation on Knee Osteoarthritis Pain and Function Last? J. Knee Surg. 2016, 29, 497–501. [Google Scholar] [CrossRef]
- Deep Oscillation Can Help Fast and Effectively. Pain, Swelling, Edema or Wound Healing Problems? Available online: https://physiomed.de/en/tiefenoszillation/ (accessed on 4 June 2025).
- Hausmann, M.; Ober, J.; Lepley, A.S. The Effectiveness of Deep Oscillation Therapy on Reducing Swelling and Pain in Athletes with Acute Lateral Ankle Sprains. J. Sport. Rehabil. 2019, 28, 902–905. [Google Scholar] [CrossRef]
- Vladeva, E.; Mihaylova, M.; Panayotovam, L. Deep Oscillations- Reducing Edema and Improving Kinesiology in the Early Stages after Knee Joint Arthroplasty. J. IMAB 2021, 2, 3577–3581. [Google Scholar] [CrossRef]
- Watson, T. Ultrasound in contemporary physiotherapy practice. Ultrasonics 2008, 48, 321–329. [Google Scholar] [CrossRef]
- Watson, T. Electrotherapy and Tissue Repair. Sport. Med. 2006, 29, 7–13. [Google Scholar]
- Schumann, D.; Kujat, R.; Zellner, J.; Angele, M.K.; Nerlich, M.; Mayr, E.; Angele, P. Treatment of Human Mesenchymal Stem Cells with Pulsed Low Intensity Ultrasound Enhances the Chondrogenic Phenotype In Vitro. Biorheology 2006, 43, 431–443. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Perrucini, P.D.; Poli-Frederico, R.C.; de Almeida Pires-Oliveira, D.A.; Dragonetti Bertin, L.; Beltrão Pires, F.; Shimoya-Bittencourt, W.; Santos, V.M.; Coelho, J.M.; de Oliveira, R.F. Anti-Inflammatory and Healing Effects of Pulsed Ultrasound Therapy on Fibroblasts. Am. J. Phys. Med. Rehabil. 2020, 99, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, P.B.; Nelson, C.T.; Cyrus, J.W.; Goldman, A.H.; Patel, N.K. The Role of Cryotherapy After Total Knee Arthroplasty: A Systematic Review. J. Arthroplast. 2023, 38, 950–956. [Google Scholar] [CrossRef]
- van Ooij, B.; Wiegerinck, J.I.; Wegener, J.T.; Van Dijk, C.N.; Schafroth, M. UCryotherapy after Total Knee Arthroplasty provides faster recovery and better ranges of motion in short term follow up. Results of a prospective comparative study. Acta Orthop. Belg. 2020, 86, 463–469. [Google Scholar]
- Matei, D.; Luca, C.; Onu, I.; Matei, P.; Iordan, D.A.; Buculei, I. Effects of Exercise Training on the Autonomic Nervous System with a Focus on Anti-Inflammatory and Antioxidants Effects. Antioxidants 2022, 11, 350. [Google Scholar] [CrossRef]
- Onu, I.; Iordan, D.A.; Codreanu, C.M.; Matei, D.; Galaction, A.I. Anti-inflammatory effects of exercise training. A systematic review. Balneo Res. J. 2021, 12, 418–425. [Google Scholar] [CrossRef]
- MOSAICPLASTY and OATS SURGERY. Here Are Guidelines that Will Help you in Preparing for Cartilage Transfer Surgery. Available online: https://www.massgeneral.org/assets/mgh/pdf/orthopaedics/sports-medicine/physical-therapy/rehabilitation-protocol-for-mosaicplasty-and-oats-knee-surgery.pdf (accessed on 4 June 2025).
- Onu, A.; Trofin, D.M.; Tutu, A.; Onu, I.; Galaction, A.I.; Sardaru, D.P.; Trofin, D.; Onita, C.A.; Iordan, D.A.; Matei, D.V. Integrative Strategies for Preventing and Managing Metabolic Syndrome: The Impact of Exercise and Diet on Oxidative Stress Reduction-A Review. Life 2025, 15, 757. [Google Scholar] [CrossRef]
| Group | Treatment | Rehabilitation Protocol | Patients (n) |
|---|---|---|---|
| Group 1 | OAT with BMAC augmentation | Two-phase, 12 weeks: Phase I (0–6 weeks): non-load-bearing physiotherapy exercises, TENS, ultrasound, Deep Oscillation Phase II (6–12 weeks): partial weight-bearing, electrostimulation, quadriceps toning | 9 |
| Group 2 | OAT without BMAC | Two-phase, 12 weeks: same protocol as Group 1 without biological augmentation | 11 |
| Group 3 | OAT without BMAC | Single-phase (Phase I), 6 weeks: non-load-bearing physiotherapy exercises, TENS, ultrasound, Deep Oscillation, without progression to loading or electrostimulation | 9 |
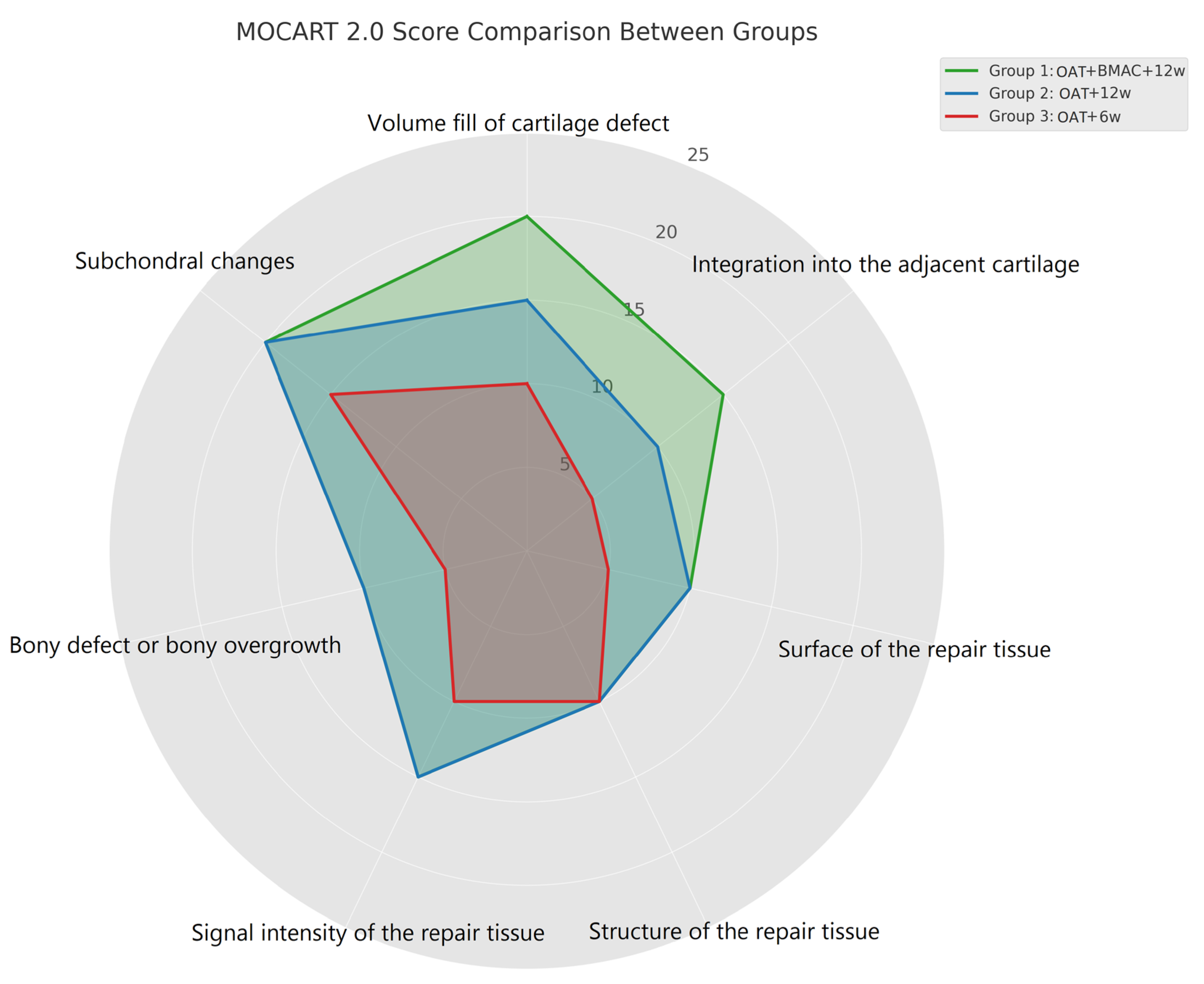
| MOCART 2.0: Cartilage Repair Tissue Assessment (Knee) | Points | Group 1 OAT + BMAC + 12w (n = 9) | Group 2 OAT + 12w (n = 11) | Group 3 OAT + 6w (n = 9) | |
|---|---|---|---|---|---|
| Volume fill of cartilage defect | Complete filling or minor hypertrophy | 20 | 5 | 4 | 1 |
| Major hypertrophy (≥150%) or 75% to 99% filling | 15 | 2 | 4 | 3 | |
| 50% to 74% filling | 10 | 2 | 2 | 3 | |
| 25% to 49% filling | 5 | 0 | 1 | 2 | |
| <25% filling or complete delamination | 0 | 0 | 0 | 0 | |
| Integration into the adjacent cartilage | Complete 15p | 15 | 5 | 5 | 2 |
| Split-like defect ≤2 mm | 10 | 3 | 5 | 3 | |
| Defect >2 mm but <50% of repair tissue length | 5 | 1 | 1 | 4 | |
| Defect ≥50% of repair tissue length | 0 | 0 | 0 | 0 | |
| Surface of the repair tissue | Intact | 10 | 6 | 6 | 3 |
| Damaged: <50% of the repair tissue diameter | 5 | 3 | 4 | 3 | |
| Damaged: ≥50% of the repair tissue diameter | 0 | 0 | 1 | 3 | |
| Structure of the repair tissue | Homogeneous 10p | 9 | 9 | 6 | |
| Inhomogeneous 0p | 0 | 2 | 3 | ||
| Signal intensity of the repair tissue | Normal | 15 | 7 | 7 | 5 |
| Minor abnormal: minor hyperintense/minor hypointense | 10 | 2 | 4 | 3 | |
| Severely abnormal/fluid-like | 0 | 0 | 0 | 1 | |
| Bony defect or bony overgrowth | No bony defect or overgrowth | 10 | 5 | 7 | 4 |
| Bony defect: depth < thickness of adjacent cartilage or overgrowth <50% of adjacent cartilage | 5 | 4 | 3 | 3 | |
| Bony defect: depth ≥ thickness of adjacent cartilage or overgrowth ≥50% of adjacent cartilage | 0 | 1 | 2 | ||
| Subchondral changes | No subchondral changes | 20 | 8 | 8 | 4 |
| Minor edema-like marrow signal: maximum diameter <50% of the repair tissue diameter | 15 | 1 | 3 | 3 | |
| Severe edema-like marrow signal: maximum diameter ≥50% of the repair tissue diameter | 10 | 0 | 0 | 2 | |
| Subchondral cysts ≥5 mm or osteonecrosis-like signal | 0 | 0 | 0 | 0 | |
| Total score | 96.1 | 80.2 | 71.7 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gherghel, R.; Onu, I.; Onu, A.; Rezus, I.-I.; Alexa, O.; Iordan, D.A.; Macovei, L.A.; Rezus, E. Enhancing Bone–Cartilage Interface Healing in Osteochondral Autograft Transplantation: Effects of BMAC Augmentation and Rehabilitation Protocols. Life 2025, 15, 1066. https://doi.org/10.3390/life15071066
Gherghel R, Onu I, Onu A, Rezus I-I, Alexa O, Iordan DA, Macovei LA, Rezus E. Enhancing Bone–Cartilage Interface Healing in Osteochondral Autograft Transplantation: Effects of BMAC Augmentation and Rehabilitation Protocols. Life. 2025; 15(7):1066. https://doi.org/10.3390/life15071066
Chicago/Turabian StyleGherghel, Robert, Ilie Onu, Ana Onu, Ioana-Irina Rezus, Ovidiu Alexa, Daniel Andrei Iordan, Luana Andreea Macovei, and Elena Rezus. 2025. "Enhancing Bone–Cartilage Interface Healing in Osteochondral Autograft Transplantation: Effects of BMAC Augmentation and Rehabilitation Protocols" Life 15, no. 7: 1066. https://doi.org/10.3390/life15071066
APA StyleGherghel, R., Onu, I., Onu, A., Rezus, I.-I., Alexa, O., Iordan, D. A., Macovei, L. A., & Rezus, E. (2025). Enhancing Bone–Cartilage Interface Healing in Osteochondral Autograft Transplantation: Effects of BMAC Augmentation and Rehabilitation Protocols. Life, 15(7), 1066. https://doi.org/10.3390/life15071066








