New Contributions to Deepen the Quality-Based Safety Assessment in the Consumption of Edible Nasturtium Flowers—The Role of Volatilome
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Samples and Sample Treatment
2.3. Total Phenolic Content
2.4. Determination of Antioxidant Properties from Nasturtium Flower Extract
2.4.1. DPPH Assay
2.4.2. ABTS Assay
2.5. HS-SPME Extraction
2.6. GC-qMS Conditions
2.7. Statistical Analysis
3. Results and Discussion
3.1. Volatilomic Fingerprint of Nasturtium
3.2. Odor of Some Identified VOMs and Their Potential Bioactive Effects
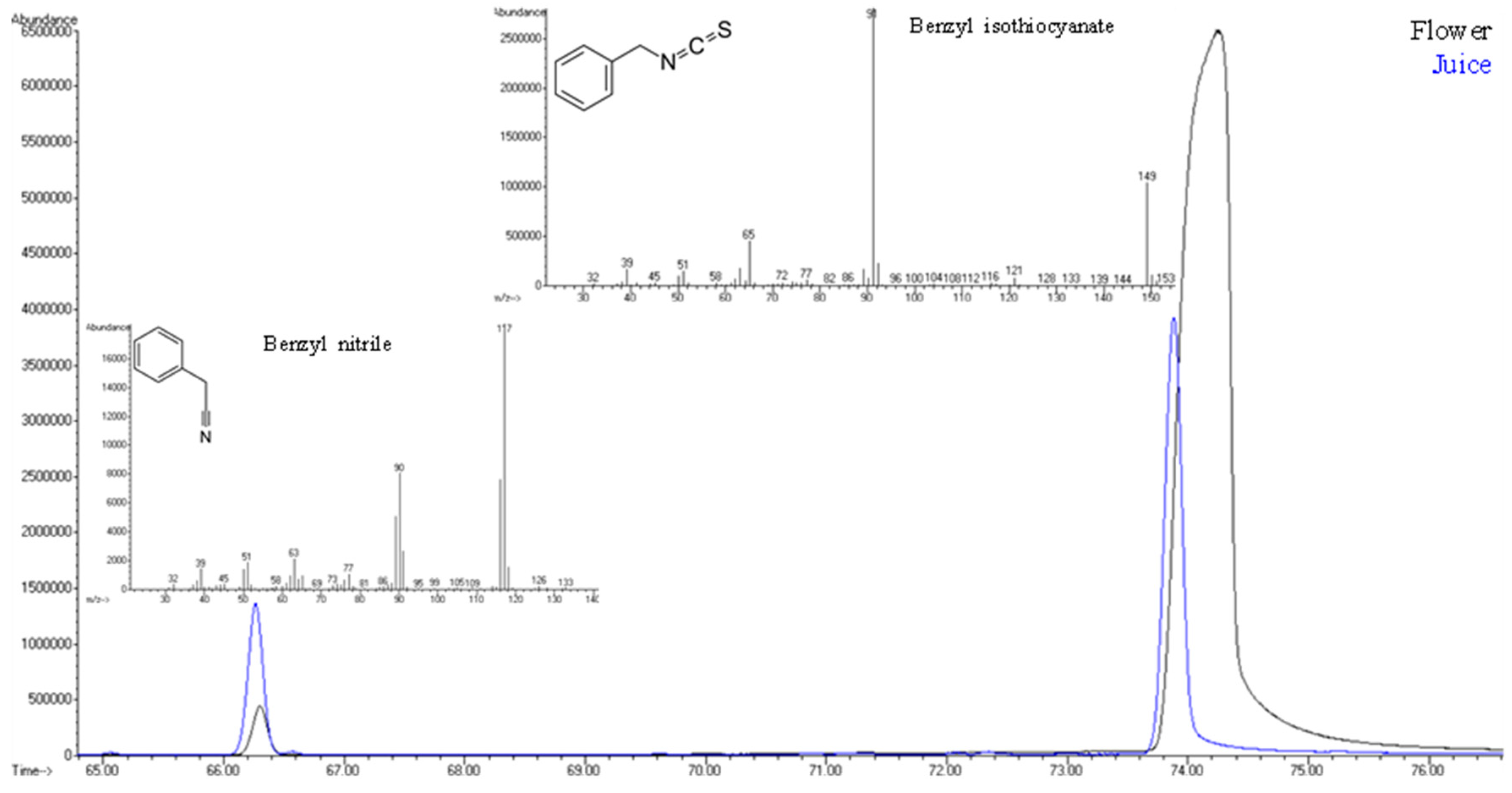
3.3. Bridging Qualitative Findings with Potential Health Risks
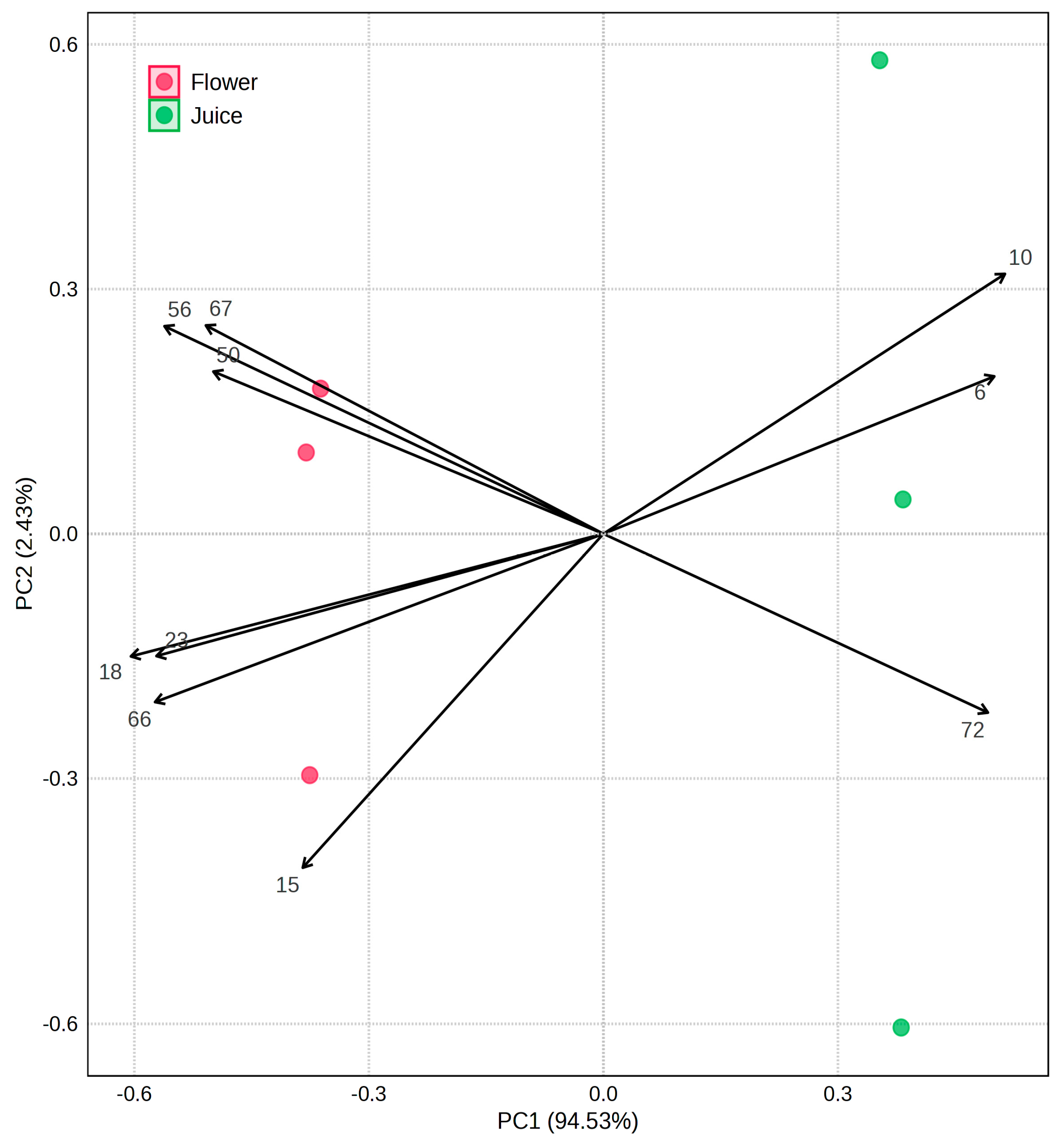
3.4. Multivariate Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jakubczyk, K.; Janda, K.; Watychowicz, K.; Łukasiak, J.; Wolska, J. Garden Nasturtium (Tropaeolum majus L.)—A Source of Mineral Elements and Bioactive Compounds. Rocz. Panstw. Zakl. Hig. 2018, 69, 119–126. [Google Scholar] [PubMed]
- Olayanju, J.B.; Bozic, D.; Naidoo, U.; Sadik, O.A. A Comparative Review of Key Isothiocyanates and Their Health Benefits. Nutrients 2024, 16, 757. [Google Scholar] [CrossRef]
- Lin, J.F.; Tsai, T.F.; Yang, S.C.; Lin, Y.C.; Chen, H.E.; Chou, K.Y.; Hwang, T.I.S. Benzyl Isothiocyanate Induces Reactive Oxygen Species-Initiated Autophagy and Apoptosis in Human Prostate Cancer Cells. Oncotarget 2017, 8, 20220–20234. [Google Scholar] [CrossRef] [PubMed]
- Kupke, F.; Herz, C.; Hanschen, F.S.; Platz, S.; Odongo, G.A.; Helmig, S.; Bartolomé Rodríguez, M.M.; Schreiner, M.; Rohn, S.; Lamy, E. Cytotoxic and Genotoxic Potential of Food-Borne Nitriles in a Liver in vitro Model. Sci. Rep. 2016, 6, 37631. [Google Scholar] [CrossRef]
- More, S.J.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.I.; Hernández-Jerez, A.F.; Hougaard Bennekou, S.; Koutsoumanis, K.P.; Machera, K.; Naegeli, H.; et al. Guidance on the Use of the Threshold of Toxicological Concern Approach in Food Safety Assessment. EFSA J. 2019, 17, e05708. [Google Scholar] [CrossRef]
- Official Journal of the European Union European; European Union. Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods; Official Journal of the European Union: Luxembourg, 2006; pp. 9–25, 30/12. [Google Scholar]
- Faloni de Andrade, S.; da Graça Lopes Serrador, M.; Pereira da Silva, A.; Giacomelli Tavares, R.; Monteiro Rodrigues, L.; Costa, M.d.C. Pilot Study of Unconventional Food Plant (UFP): Adherence to Nasturtium (Tropaeolum majus L.) in the Diet and Monitoring of Biometric and Clinical Indicators. J. Biomed. Biopharm. Res. 2022, 19, 299–313. [Google Scholar] [CrossRef]
- Figueira, J.A.; Porto-Figueira, P.; Berenguer, C.; Pereira, J.A.M.; Câmara, J.S. Evaluation of the Health-Promoting Properties of Selected Fruits. Molecules 2021, 26, 4202. [Google Scholar] [CrossRef] [PubMed]
- Izcara, S.; Perestrelo, R.; Morante-Zarcero, S.; Sierra, I.; Câmara, J.S. Volatilomic Fingerprinting from Edible Flowers. Unravelling Some Impact Compounds behind Its Attractiveness. Food Biosci. 2022, 50, 102188. [Google Scholar] [CrossRef]
- van Den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas—Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- El-Sayed, A.M. The Pherobase: Database of Insect Pheromones and Semiochemicals. Available online: http://www.pherobase.com (accessed on 14 February 2025).
- Acree, T.E.; Arn, H. Flavornet. Available online: https://www.flavornet.org/d_kovats_db5.html (accessed on 15 June 2025).
- PubChem. National Library of Medicine, National Center for Biotechnology Information. Available online: https://pubchem.ncbi.nlm.nih.gov/docs/compounds (accessed on 15 June 2025).
- Pang, Z.; Chong, J.; Zhou, G.; De Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the Gap between Raw Spectra and Functional Insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Fukalova Fukalova, T.; Moreno-Peris, E.; García-Martínez, M.D.; Raigón Jiménez, M.D. Assessment of the Volatile Profiles and Identification of Differentiating Aromas of Wild Undervalued Plants. Front. Nutr. 2022, 9, 912680. [Google Scholar] [CrossRef] [PubMed]
- Vrca, I.; Jug, B.; Fredotović, Ž.; Vuko, E.; Brkan, V.; Šestić, L.; Juretić, L.; Dunkić, V.; Nazlić, M.; Ramić, D.; et al. Significant Benefits of Environmentally Friendly Hydrosols from Tropaeolum majus L. Seeds with Multiple Biological Activities. Plants 2023, 12, 3897. [Google Scholar] [CrossRef]
- Aguiar, J.; Gonçalves, J.L.; Alves, V.L.; Câmara, J.S. Relationship between Volatile Composition and Bioactive Potential of Vegetables and Fruits of Regular Consumption—An Integrative Approach. Molecules 2021, 26, 3653. [Google Scholar] [CrossRef]
- Li, S.-F.; Zhang, S.-B.; Lv, Y.-Y.; Zhai, H.-C.; Li, N.; Hu, Y.-S.; Cai, J.-P. Metabolomic Analyses Revealed Multifaceted Effects of Hexanal on Aspergillus Flavus Growth. Appl. Microbiol. Biotechnol. 2021, 105, 3745–3757. [Google Scholar] [CrossRef]
- Lin, H.; Li, Z.; Sun, Y.; Zhang, Y.; Wang, S.; Zhang, Q.; Cai, T.; Xiang, W.; Zeng, C.; Tang, J. D-Limonene: Promising and Sustainable Natural Bioactive Compound. Appl. Sci. 2024, 14, 4605. [Google Scholar] [CrossRef]
- Dinh, T.N.; Parat, M.-O.; Ong, Y.S.; Khaw, K.Y. Anticancer Activities of Dietary Benzyl Isothiocyanate: A Comprehensive Review. Pharmacol. Res. 2021, 169, 105666. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Khan, A.L.; Ali, L.; Khan, A.R.; Waqas, M.; Hussain, J.; Lee, I.-J.; Shin, J.-H. Benzaldehyde as an Insecticidal, Antimicrobial, and Antioxidant Compound Produced by Photorhabdus temperata M1021. J. Microbiol. 2015, 53, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Zieniuk, B.; Jasińska, K.; Wierzchowska, K.; Fabiszewska, A. Enzymatic Synthesis of Flavours and Fragrances, Antioxidants and Antimicrobials on the Example of Benzyl Alcohol and Its Selected Derivatives. In Proceedings of the 3rd International Electronic Conference on Foods: Food, Microbiome, and Health—A Celebration of the 10th Anniversary of Foods’ Impact on Our Wellbeing, Online, 1–15 October 2022; Volume 18, p. 2. [Google Scholar] [CrossRef]
- Xie, B.; Nagalingam, A.; Kuppusamy, P.; Muniraj, N.; Langford, P.; Győrffy, B.; Saxena, N.K.; Sharma, D. Benzyl Isothiocyanate Potentiates P53 Signaling and Antitumor Effects against Breast Cancer through Activation of P53-LKB1 and P73-LKB1 Axes. Sci. Rep. 2017, 7, 40070. [Google Scholar] [CrossRef]
- Sofrata, A.; Santangelo, E.M.; Azeem, M.; Borg-Karlson, A.-K.; Gustafsson, A.; Pütsep, K. Benzyl Isothiocyanate, a Major Component from the Roots of Salvadora Persica Is Highly Active against Gram-Negative Bacteria. PLoS ONE 2011, 6, e23045. [Google Scholar] [CrossRef]
- Izcara, S.; Perestrelo, R.; Morante-Zarcero, S.; Câmara, J.S.; Sierra, I. High Throughput Analytical Approach Based on ΜQuEChERS Combined with UHPLC-PDA for Analysis of Bioactive Secondary Metabolites in Edible Flowers. Food Chem. 2022, 393, 133371. [Google Scholar] [CrossRef]
- Okulicz, M.; Hertig, I. Benzyl Isothiocyanate Disturbs Lipid Metabolism in Rats in a Way Independent of Its Thyroid Impact Following in Vivo Long-Term Treatment and in Vitro Adipocytes Studies. J. Physiol. Biochem. 2013, 69, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Neska, J.; Swoboda, P.; Przybyszewska, M.; Kotlarz, A.; Bolla, N.; Miłoszewska, J.; Grygorowicz, M.; Kutner, A.; Markowicz, S. The Effect of Analogues of 1α,25-Dihydroxyvitamin D2 on the Regrowth and Gene Expression of Human Colon Cancer Cells Refractory to 5-Fluorouracil. Int. J. Mol. Sci. 2016, 17, 903. [Google Scholar] [CrossRef] [PubMed]
- Toxin and Toxin Target Database (T3DB). Available online: http://www.t3db.ca/ (accessed on 15 June 2025).
- Api, A.M.; Belsito, D.; Botelho, D.; Bruze, M.; Burton, G.A.; Buschmann, J.; Cancellieri, M.A.; Dagli, M.L.; Date, M.; Dekant, W.; et al. RIFM Fragrance Ingredient Safety Assessment, Benzonitrile, CAS Registry Number 100-47-0. Food Chem. Toxicol. 2021, 153, 112303. [Google Scholar] [CrossRef] [PubMed]
- More, S.J.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.I.; Hernández-Jerez, A.F.; Bennekou, S.H.; Koutsoumanis, K.; Lambré, C.; Machera, K.; et al. Guidance on the Use of the Benchmark Dose Approach in Risk Assessment. EFSA J. 2022, 20, e07584. [Google Scholar] [CrossRef]
- Kassie, F.; Pool-Zobel, B.; Parzefall, W.; Knasmuller, S. Genotoxic Effects of Benzyl Isothiocyanate, a Natural Chemopreventive Agent. Mutagenesis 1999, 14, 595–604. [Google Scholar] [CrossRef]
- Cox, L.A.; Ketelslegers, H.B.; Lewis, R.J. The Shape of Low-Concentration Dose–Response Functions for Benzene: Implications for Human Health Risk Assessment. Crit. Rev. Toxicol. 2021, 51, 95–116. [Google Scholar] [CrossRef]
- More, S.J.; Benford, D.; Hougaard Bennekou, S.; Bampidis, V.; Bragard, C.; Halldorsson, T.I.; Hernández-Jerez, A.F.; Koutsoumanis, K.; Lambré, C.; Machera, K.; et al. Guidance on Risk–Benefit Assessment of Foods. EFSA J. 2024, 22, e8875. [Google Scholar] [CrossRef]

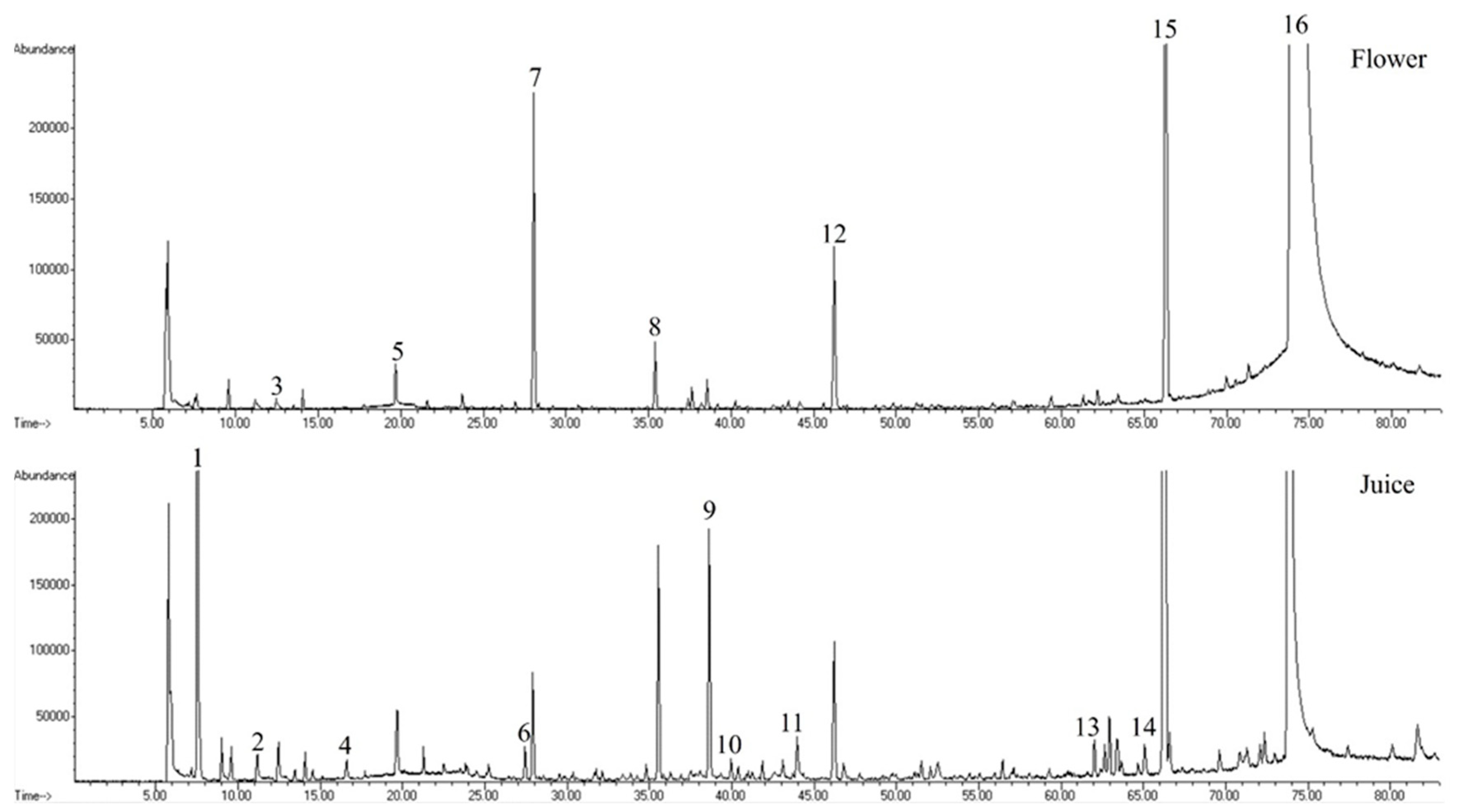
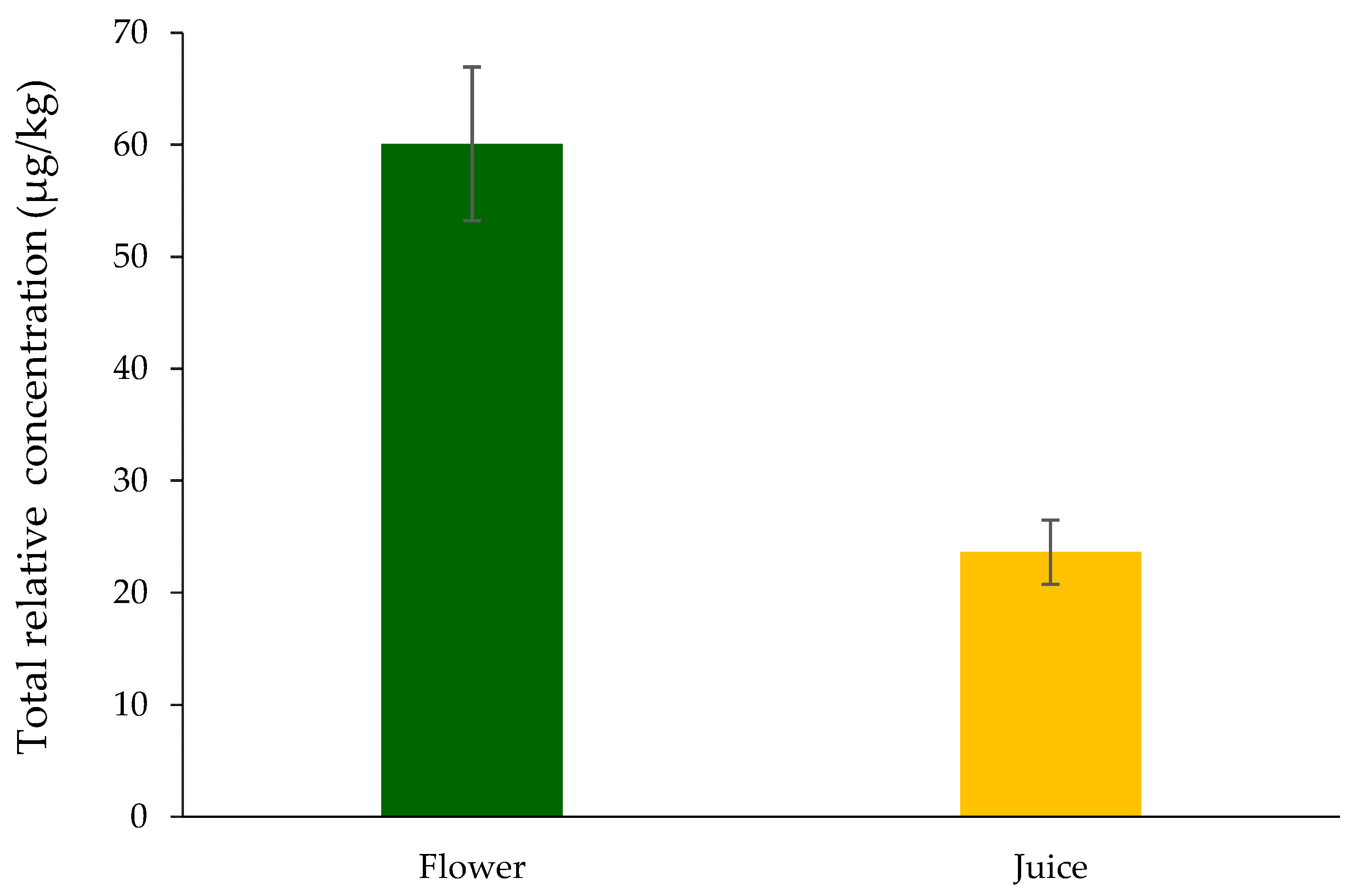
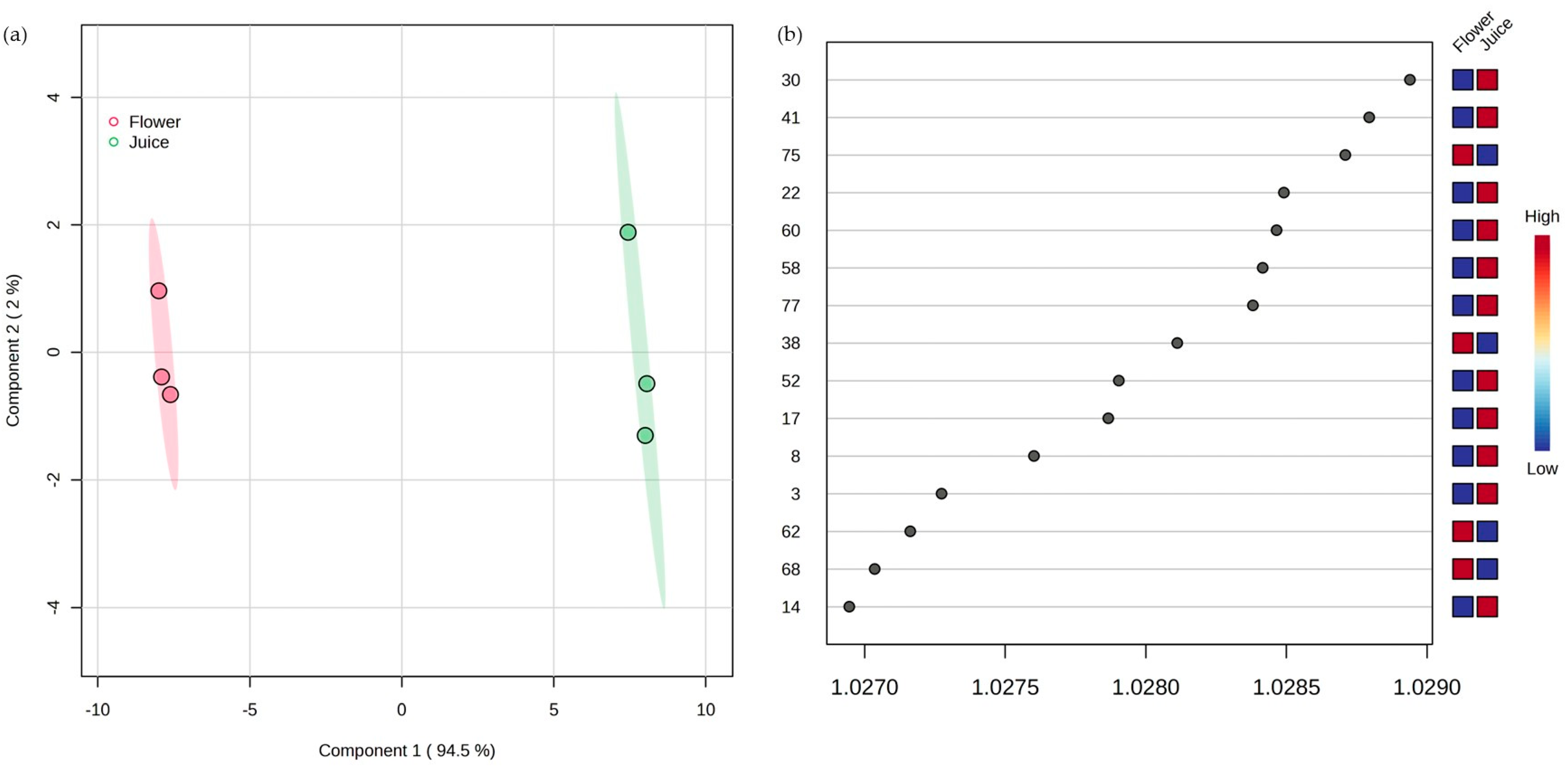
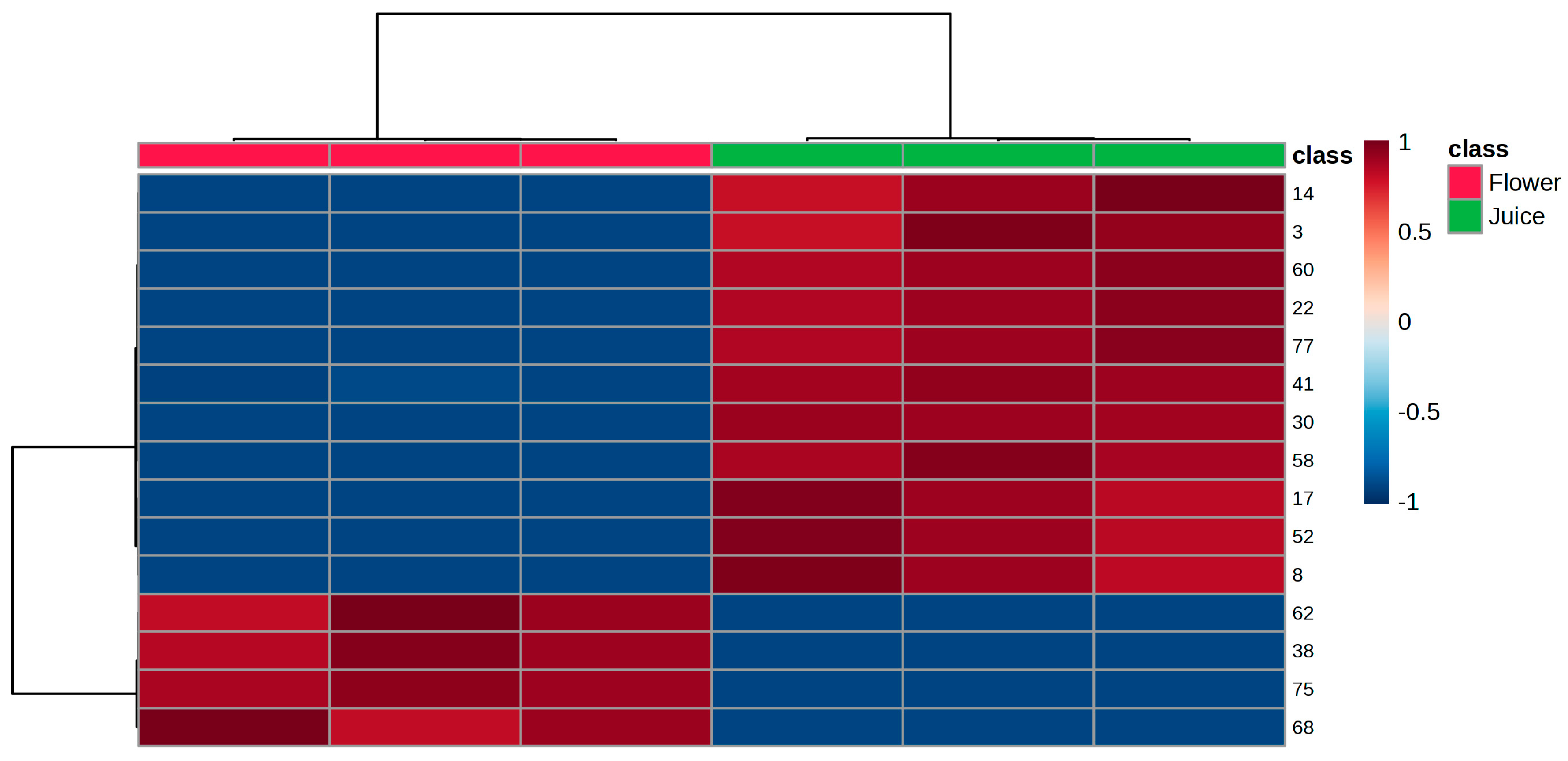
| RT (min) | Peak nº | KI Cal a | KI Lit b | VOMs | Chemical Family | Relative Concentration (µg/kg) (RSD) | %RPA (RSD) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flower | Juice | Flower | Juice | ||||||||||
| 7.17 | 1 | <800 | <800 | Acetaldehyde | Aldehyde | - | 0.011 | (10) | - | 0.048 | (12) | ||
| 7.77 | 2 | <800 | <800 | Dimethyl sulfide | Sulfur | - | 0.003 | (18) | - | 0.011 | (24) | ||
| 8.98 | 3 | 837 | 837 | 2-Methylpropanal | Aldehyde | - | 0.005 | (8) | - | 0.021 | (10) | ||
| 9.31 | 4 | 853 | 850 | Methyl acetate | Ester | - | 0.006 | (24) | - | 0.026 | (25) | ||
| 10.32 | 5 | 900 | 901 | Tetrahydrofuran | Furan | 0.002 | (11) | - | 0.004 | (1) | - | ||
| 10.96 | 6 | 919 | 921 | Ethyl acetate | Ester | 0.003 | (23) | 0.004 | (7) | 0.005 | (33) | 0.015 | (14) |
| 13.51 | 7 | 933 | 923 | 2-Ethyl furan | Furan | 0.004 | (13) | 0.018 | (15) | 0.007 | (22) | 0.077 | (3) |
| 14.55 | 8 | 948 | 956 | 3-Pentanone | Ketone | - | 0.019 | (5) | - | 0.083 | (10) | ||
| 16.6 | 9 | 998 | 991 | 1-Penten-3-one | Ketone | - | 0.037 | (17) | - | 0.159 | (18) | ||
| 17.78 | 10 | 1025 | 1030 | Toluene | H | 0.007 | (6) | 0.009 | (15) | 0.011 | (17) | 0.037 | (21) |
| 19.68 | 11 | 1065 | 1067 | Hexanal | Aldehyde | 0.064 | (6) | 0.132 | (20) | 0.107 | (6) | 0.553 | (8) |
| 22.73 | 12 | 1125 | 1125 | (E)-2-pentenal | Aldehyde | 0.003 | (3) | 0.019 | (15) | 0.005 | (8) | 0.082 | (10) |
| 23.72 | 13 | 1145 | 1158 | β-Myrcene | Terpenoid | 0.024 | (20) | - | 0.041 | (31) | - | ||
| 23.74 | 14 | 1146 | 1151 | 4-Methyl-3-heptanone | Ketone | - | 0.013 | (9) | - | 0.054 | (15) | ||
| 26.11 | 15 | 1188 | 1198 | Limonene | Terpenoid | 0.007 | (20) | 0.005 | (15) | 0.012 | (28) | 0.020 | (17) |
| 26.94 | 16 | 1203 | 1204 | (Z)-2-hexenal | Aldehyde | 0.012 | (2) | 0.006 | (7) | 0.02 | (13) | 0.024 | (11) |
| 27.03 | 17 | 1205 | 1214 | 2-Pentyl furan | Furan | - | 0.051 | (5) | - | 0.217 | (10) | ||
| 27.90 | 18 | 1222 | 1225 | (E)-2-hexenal | Aldehyde | 0.639 | (16) | 0.205 | (13) | 1.093 | (24) | 0.876 | (14) |
| 28.38 | 19 | 1232 | 1236 | Ethyl hexanoate | Ester | 0.009 | (15) | - | 0.016 | (24) | - | ||
| 29.19 | 20 | 1247 | 1235 | Methyl isothiocyanate | I | 0.008 | (17) | - | 0.014 | (26) | - | ||
| 29.31 | 21 | 1249 | 1240 | 3-Octanone | Ketone | - | 0.011 | (15) | - | 0.047 | (11) | ||
| 29.70 | 22 | 1256 | 1262 | Styrene | H | - | 0.009 | (5) | - | 0.039 | (12) | ||
| 30.76 | 23 | 1276 | 1275 | Hexyl acetate | Ester | 0.007 | (17) | 0.004 | (7) | 0.012 | (28) | 0.017 | (11) |
| 31.41 | 24 | 1287 | 1278 | Octanal | Aldehyde | - | 0.012 | (8) | - | 0.049 | (11) | ||
| 32.43 | 25 | 1306 | 1310 | 1-Octen-3-one | Ketone | - | 0.017 | (15) | - | 0.071 | (16) | ||
| 33.95 | 26 | 1336 | 1312 | 3-Heptanol | Alcohol | - | 0.015 | (15) | - | 0.066 | (17) | ||
| 34.22 | 27 | 1342 | 1338 | (E)-2-heptenal | Aldehyde | - | 0.010 | (11) | - | 0.043 | (4) | ||
| 34.92 | 28 | 1355 | 1365 | 6-Methyl 5-hepten-2-one | Ketone | 0.006 | (21) | 0.027 | (12) | 0.01 | (28) | 0.117 | (14) |
| 35.38 | 29 | 1364 | 1357 | 1-Hexanol | Alcohol | 0.107 | (2) | 0.396 | (4) | 0.181 | (11) | 1.696 | (10) |
| 36.36 | 30 | 1383 | 1374 | (E)-3-hexen-1-ol | Alcohol | - | 0.012 | (1) | - | 0.053 | (10) | ||
| 37.41 | 31 | 1383 | 1389 | (Z)-3-hexen-1-ol | Alcohol | 0.022 | (22) | - | 0.039 | (33) | - | ||
| 38.19 | 32 | 1419 | 1419 | Nonanal | Aldehyde | 0.018 | (17) | 0.142 | (3) | 0.031 | (28) | 0.610 | (11) |
| 38.60 | 33 | 1428 | 1420 | (E)-2-hexen-1-ol | Alcohol | 0.045 | (4) | 0.507 | (15) | 0.075 | (8) | 2.163 | (16) |
| 39.20 | 34 | 1441 | - | 4-Methyl-2,4-hexadiene | H | 0.011 | (19) | - | 0.02 | (30) | - | ||
| 40.23 | 35 | 1463 | 1457 | Ethyl octanoate | Ester | 0.014 | (9) | 0.028 | (4) | 0.023 | (20) | 0.120 | (12) |
| 40.35 | 36 | 1465 | 1467 | (E)-2-octenal | Aldehyde | - | 0.029 | (18) | - | 0.124 | (16) | ||
| 40.52 | 37 | 1469 | 1474 | Decanal | Aldehyde | 0.006 | (22) | - | 0.011 | (32) | - | ||
| 40.98 | 38 | 1478 | 1462 | Dimethyl styrene | H | 0.008 | (8) | - | 0.013 | (19) | - | ||
| 42.56 | 39 | 1493 | 1498 | Acetic acid | Acid | - | 0.031 | (17) | 0.015 | (6) | - | ||
| 42.58 | 40 | 1495 | 1497 | (E,E)-2,4-heptadienal | Aldehyde | 0.009 | (18) | - | - | 0.133 | (18) | ||
| 43.12 | 41 | 1511 | 1503 | 2-Ethyl-1-hexanol | Alcohol | 0.008 | (4) | 0.027 | (3) | 0.014 | (15) | 0.114 | (10) |
| 43.49 | 42 | 1519 | 1546 | β-Cubebene | Terpene | 0.014 | (12) | - | 0.024 | (23) | - | ||
| 44.15 | 43 | 1524 | 1519 | (E)-2-nonenal | Aldehyde | 0.017 | (4) | 0.025 | (4) | 0.029 | (15) | 0.108 | (10) |
| 45.58 | 44 | 1532 | 1526 | β-Bourbonene | Terpene | 0.013 | (14) | - | 0.022 | (25) | - | ||
| 46.25 | 45 | 1548 | 1550 | Benzaldehyde | Aldehyde | 0.342 | (6) | 0.286 | (2) | 0.579 | (17) | 1.23 | (12) |
| 46.80 | 46 | 1552 | 1567 | 1-Octanol | Alcohol | 0.007 | (20) | 0.033 | (17) | 0.012 | (31) | 0.138 | (15) |
| 49.64 | 47 | 1567 | 1577 | Undecanal | Aldehyde | - | 0.007 | (11) | - | 0.031 | (5) | ||
| 49.83 | 48 | 1571 | 1581 | Hexyl hexanoate | Ester | 0.014 | (16) | - | 0.024 | (27) | - | ||
| 50.32 | 49 | 1582 | 1573 | β-Cedrene | Terpene | 0.006 | (13) | - | 0.011 | (18) | - | ||
| 51.19 | 50 | 1601 | 1610 | Ethyl decanoate | Ester | 0.012 | (7) | 0.009 | (13) | 0.02 | (19) | 0.038 | (11) |
| 51.26 | 51 | 1603 | 1602 | Methyl benzoate | Ester | - | 0.012 | (10) | - | 0.052 | (5) | ||
| 51.49 | 52 | 1609 | 1626 | Menthol | Terpene | - | 0.033 | (4) | - | 0.143 | (10) | ||
| 51.96 | 53 | 1621 | 1630 | (E)-2-decenal | Aldehyde | - | 0.026 | (13) | - | 0.108 | (9) | ||
| 52.07 | 54 | 1624 | 1624 | 1-Nonanol | Alcohol | - | 0.060 | (14) | - | 0.254 | (12) | ||
| 52.12 | 55 | 1625 | - | Cycloheptane | H | 0.010 | (16) | - | 0.018 | (27) | - | ||
| 52.54 | 56 | 1635 | 1631 | Benzeneacetaldehyde | Aldehyde | 0.008 | (1) | 0.005 | (14) | 0.014 | (12) | 0.023 | (21) |
| 53.49 | 57 | 1658 | 1653 | Ethyl benzoate | Ester | - | 0.011 | (9) | - | 0.045 | (12) | ||
| 55.03 | 58 | 1695 | 1695 | Dodecanal | Aldehyde | - | 0.013 | (5) | - | 0.054 | (7) | ||
| 55.84 | 59 | 1713 | 1706 | 2,6-Dichloroanisole | Ethers | 0.019 | (16) | - | 0.033 | (27) | - | ||
| 55.96 | 60 | 1716 | 1716 | 1,2-Dimethoxy benzene | H | - | 0.012 | (5) | - | 0.051 | (13) | ||
| 57.18 | 61 | 1744 | 1740 | 1-Dodecanol | Alcohol | 0.011 | (12) | 0.006 | (10) | 0.018 | (21) | 0.024 | (9) |
| 57.88 | 62 | 1760 | 1763 | Naphthalene | H | 0.005 | 10 | - | 0.008 | (22) | - | ||
| 58.09 | 63 | 1764 | 1766 | (E,Z)-2,4-decadienal | Aldehyde | - | 0.008 | (9) | - | 0.036 | (20) | ||
| 59.39 | 64 | 1793 | 1798 | Methyl 2-hydroxybenzoate | Ester | 0.007 | (13) | 0.016 | (1) | 0.011 | (25) | 0.068 | (10) |
| 60.36 | 65 | 1811 | 1819 | (E,E)-2,4-decadienal | Aldehyde | - | 0.009 | (8) | - | 0.038 | (18) | ||
| 61.33 | 66 | 1837 | 1837 | Ethyl dodecanoate | Ester | 0.009 | (10) | 0.005 | (11) | 0.016 | (10) | 0.021 | (13) |
| 61.44 | 67 | 1840 | 1849 | Hexanoic acid | Acid | 0.015 | (19) | 0.009 | (11) | 0.025 | (23) | 0.038 | (14) |
| 62.17 | 68 | 1859 | 1859 | Geranylacetone | Terpenes | 0.026 | (4) | - | 0.044 | (7) | - | ||
| 63.38 | 69 | 1890 | 1889 | Benzyl alcohol | Alcohol | 0.022 | (5) | 0.067 | (6) | 0.037 | (6) | 0.288 | (14) |
| 65.07 | 70 | 1907 | 1908 | Phenylethyl alcohol | Alcohol | 0.008 | (14) | 0.086 | (18) | 0.014 | (25) | 0.363 | (13) |
| 66.26 | 71 | 1914 | - | Benzonitrile | Nitrile | 1.312 | (3) | 5.082 | (18) | 2.202 | (8) | 21.35 | (6) |
| 68.91 | 72 | 1929 | 1938 | Methyl tetradecanoate | Ester | 0.007 | (8) | 0.010 | (14) | 0.012 | (4) | 0.041 | (9) |
| 69.99 | 73 | 1934 | 1933 | Tetradecanal | Aldehyde | 0.026 | (13) | - | 0.043 | (1) | - | ||
| 70.95 | 74 | 2039 | 2050 | Octanoic acid | Acid | - | 0.062 | (19) | - | 0.261 | (11) | ||
| 71.29 | 75 | 2041 | 2046 | 3-Phenyl-2-propenal | Aldehyde | 0.035 | (6) | - | 0.060 | (17) | - | ||
| 74.06 | 76 | 2155 | 2143 | Benzyl isothiocyanate | I | 57.115 | (12) | 15.842 | (12) | 94.95 | (1) | 67.08 | (2) |
| 77.41 | 77 | 2171 | 2191 | Methyl hexadecanoate | Ester | - | 0.029 | (5) | - | 0.126 | (13) | ||
| 80.15 | 78 | 2284 | 2294 | Decanoic acid | Acid | - | 0.054 | (16) | - | 0.229 | (15) | ||
| Peak nº | VOMs | Flower | Juice | Odor | Bioactive Effect |
|---|---|---|---|---|---|
| 6 | Ethyl acetate | x | x | Fruity, sweet, solvent | Antimicrobial |
| 11 | Hexanal | x | x | Fresh, green grass, leaf | Antimicrobial, antifungal |
| 13 | β-Myrcene | x | Citrus, fruit, wood | Analgesic, anti-inflammatory, antibiotic, anticancer, antioxidant | |
| 15 | Limonene | x | x | Citrus, fruit, wood | Antimutagenic, antitumor, antioxidant, antimicrobial, antiproliferative, chemoprotective, anthelmintic, insecticidal |
| 18 | (E)-2-hexenal | x | x | Fresh, green | Antimicrobial, antioxidant, cytotoxic |
| 23 | Hexyl acetate | x | x | Acid, citrus, fruit, green, herbal, rubber, spice, tobacco | Antimicrobial |
| 28 | 6-Methyl 5-hepten-2-one | x | x | Citrus, fatty, green | Antimicrobial, insecticidal |
| 29 | 1-Hexanol | x | x | Floral, sweet | Antifungal |
| 32 | Nonanal | x | x | Aldehydic, citrus, waxy | Antimicrobial, anticancer, cytotoxic |
| 33 | (E)-2-hexen-1-ol | x | x | Fresh, green, grass, leaf | Antimicrobial |
| 41 | 2-Ethyl-1-hexanol | x | x | Citrus, fresh, floral, oil, sweet | Antimicrobial, cytotoxic |
| 45 | Benzaldehyde | x | x | Bitter almond | Antitumor, antioxidant, antimicrobial, cytotoxic |
| 46 | 1-Octanol | x | x | Citrus, fatty, pungent | Antifungal |
| 69 | Benzyl alcohol | x | x | Blackberry, floral, fruit | Antimicrobial, antiparasitic |
| 70 | Phenylethyl alcohol | x | x | Floral, herbal, honey, pollen, rose, spice, sweet | Antimicrobial, antioxidant, antienzymatic |
| 71 | Benzonitrile | x | x | Bitter almond, sweet | Antimicrobial |
| 76 | Benzyl isothiocyanate | x | x | Cabbage, radish, vegetative | Anticancer, antibacterial, antifungal, anti-inflammatory, antioxidant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perestrelo, R.; da Graça Lopes, M.; da Silva, A.P.; Costa, M.d.C.; Câmara, J.S. New Contributions to Deepen the Quality-Based Safety Assessment in the Consumption of Edible Nasturtium Flowers—The Role of Volatilome. Life 2025, 15, 1053. https://doi.org/10.3390/life15071053
Perestrelo R, da Graça Lopes M, da Silva AP, Costa MdC, Câmara JS. New Contributions to Deepen the Quality-Based Safety Assessment in the Consumption of Edible Nasturtium Flowers—The Role of Volatilome. Life. 2025; 15(7):1053. https://doi.org/10.3390/life15071053
Chicago/Turabian StylePerestrelo, Rosa, Maria da Graça Lopes, Alda Pereira da Silva, Maria do Céu Costa, and José S. Câmara. 2025. "New Contributions to Deepen the Quality-Based Safety Assessment in the Consumption of Edible Nasturtium Flowers—The Role of Volatilome" Life 15, no. 7: 1053. https://doi.org/10.3390/life15071053
APA StylePerestrelo, R., da Graça Lopes, M., da Silva, A. P., Costa, M. d. C., & Câmara, J. S. (2025). New Contributions to Deepen the Quality-Based Safety Assessment in the Consumption of Edible Nasturtium Flowers—The Role of Volatilome. Life, 15(7), 1053. https://doi.org/10.3390/life15071053









