Longitudinal Myocardial Deformation as an Emerging Biomarker for Post-Traumatic Cardiac Dysfunction
Abstract
1. Introduction
2. Pathophysiology of Post-Traumatic Cardiac Dysfunction
3. Limitations of Traditional Diagnostic Methods in Trauma-Related Cardiac Dysfunction
| Diagnostic Modality | Clinical Role | Limitations in Trauma Context | Emerging Enhancements/Solutions | Ref. |
|---|---|---|---|---|
| Electrocardiography (ECG) | Rapid bedside assessment; identifies arrhythmias, ischemia, and conduction abnormalities. | Low sensitivity for subtle myocardial injury. Non-specific changes such as transient ST-segment or T-wave abnormalities. May be normal despite significant dysfunction. | AI-enhanced ECG interpretation. Combined use with biomarkers and imaging for improved diagnostic yield. | [58,59] |
| Conventional Echocardiography | Assesses systolic function, wall motion, and pericardial effusion. Detects cardiac tamponade and contusion. | Left ventricular ejection fraction (LVEF) may be preserved despite dysfunction. Poor sensitivity to early or subclinical myocardial changes. Limited insight into myocardial mechanics. | Speckle-tracking echocardiography (STE) and global longitudinal strain (GLS) for early detection of myocardial dysfunction. | [60,61] |
| Cardiac Biomarkers (e.g., Troponin, hs-Tn) | Detects myocardial cell injury. Commonly used to evaluate myocardial infarction or contusion. | Elevated levels may result from non-cardiac trauma-related stress (inflammation, hypoxia). May be normal in cases of functional rather than structural injury. Lack of anatomical or functional context. | Multiparametric biomarker panels including IL-6, TNF-α, MPO, and ICAM-1. Use in combination with imaging and clinical scoring systems. | [62,63] |
| Natriuretic Peptides (BNP, NT-proBNP) | Reflects ventricular wall stress. Useful in evaluating potential heart failure. | Elevated in non-cardiac conditions such as renal dysfunction, sepsis, or volume overload. Low specificity for direct cardiac injury. | Adjunctive role in multimodal risk stratification. Should not be used as standalone diagnostics. | [64,65] |
| Computed Tomography (CT Angiography) | Visualizes vascular trauma, aortic injury, and pericardial effusion. | Limited capability in assessing myocardial function. Exposure to radiation. Not suitable for dynamic or functional cardiac evaluation. | Used primarily for vascular and structural assessment. Limited utility in evaluating myocardial performance. | [66,67] |
| Cardiac Magnetic Resonance Imaging (MRI) | High-resolution myocardial tissue characterization. Differentiates fibrosis, oedema, and inflammation. | Limited availability and high cost. Long scan times. Not practical in acute trauma settings. | Selective use in stable or subacute trauma patients. Valuable for follow-up and definitive tissue-level assessment. | [68,69] |
| Overall Strategy and Future Direction | Foundational role in initial cardiac assessment and Triage. | No single modality adequately captures structural, functional, and molecular abnormalities. Risk of delayed or missed diagnosis of subclinical dysfunction. | Multimodal diagnostic strategies integrating ECG, strain imaging, biomarkers, and MRI. Use of AI for risk stratification and predictive analytics. Personalized diagnostics based on trauma severity comorbidities and resource availability. | [70,71] |
4. Longitudinal Myocardial Deformation: A Sensitive Marker for Post-Traumatic Cardiac Dysfunction
| Component | Description | Clinical Relevance | Advantages Over Traditional Methods | Limitations/Challenges | Future Perspectives | Ref. |
|---|---|---|---|---|---|---|
| Global Longitudinal Strain (GLS) | Quantitative measure of myocardial fiber shortening during systole, assessed via speckle-tracking echocardiography (STE) | Detects subclinical dysfunction in trauma patients, even with normal LVEF | Higher sensitivity to early dysfunction; fiber-level analysis | Requires advanced echocardiographic equipment and trained operators | Standardization and integration into trauma protocols | [83,84] |
| Mechanisms of Dysfunction | Includes direct injury, inflammation, oxidative stress, neuro- hormonal imbalance | Identifies myocardial injury even without visible structural damage | Can localize injury patterns undetectable by ECG or LVEF | Cannot differentiate exact ethology (e.g., contusion vs. inflammation) without adjunct biomarkers | Combined use with cardiac biomarkers and imaging (e.g., MRI) | [85,86] |
| Early Detection | Identifies early myocardial impairment prior to LVEF drop | Enables prompt intervention and monitoring | Better than conventional echocardiography for initial trauma screening | Limited access in emergency trauma settings | Implementation of point-of-care STE platforms | [87,88] |
| Risk Stratification | Differentiates high- from low-risk patients based on GLS thresholds | Guides intensity of monitoring and therapy | Provides prognostic information beyond ECG or biomarkers | No universally accepted GLS cut-offs in trauma | Development of trauma-specific GLS algorithms | [89,90] |
| Prognostic Value | Reduced GLS predicts worse outcomes (HF, arrhythmias, mortality) | Long-term risk assessment and patient counseling | Tracks recovery or deterioration longitudinally | Long-term data still limited in trauma populations | Prospective multicenter cohort validation | [91,92] |
| Therapeutic Monitoring | Monitors response to cardioprotective (e.g., beta-blockers) or anti-inflammatory therapies | Tailors treatment to myocardial recovery trajectory | Non-invasive and repeatable compared to cardiac MRI | Requires repeat echocardiographic access | Integration with biomarker panels and AI for monitoring | [93,94] |
| Versus LVEF | GLS detects dysfunction with preserved LVEF | Identifies hidden myocardial damage early | Adds value where LVEF is insensitive | Requires familiarity with strain analysis | Promote educational training for clinicians | [95,96] |
| Versus Biomarkers (e.g., Troponin, BNP) | GLS reflects mechanical function, while biomarkers reflect biochemical stress | Useful where biomarker levels are normal despite dysfunction | More specific to mechanical impairment | Biomarkers remain useful for systemic context | Combine GLS with inflammatory and oxidative stress markers | [97,98] |
5. Clinical Applications of Longitudinal Myocardial Deformation in Trauma Care
| Clinical Application | Description | Key Benefits | Challenges | Ref. |
|---|---|---|---|---|
| Early Detection of Cardiac Injury | Detects subclinical myocardial dysfunction in blunt chest trauma, even when ECG and LVEF are normal. | Enables early diagnosis and intervention for myocardial contusion or trauma-induced contractility impairment. | May be overlooked in patients without visible trauma; requires high-resolution imaging and trained operators. | [110,111] |
| Assessment During Systemic Inflammatory Response | Evaluates myocardial dysfunction linked to inflammation and oxidative stress post-trauma (e.g., IL-6, TNF-α). | Provides insight into inflammation-induced cardiac injury and helps guide anti-inflammatory treatment. | Requires biomarker correlation; inflammation-related changes may be transient or confounded by other injuries. | [112,113] |
| Risk Stratification and Prognostication | Identifies high-risk patients with reduced GLS for future cardiac complications, even if asymptomatic. | Supports targeted monitoring and early therapeutic strategies to prevent heart failure and arrhythmias. | No established trauma-specific GLS thresholds; prognostic implications need further validation. | [114,115] |
| Guiding Therapeutic Interventions | Informs timely initiation of cardioprotective therapies (e.g., beta-blockers, ACE inhibitors) and fluid optimization. | Improves outcomes by tailoring treatments to individual myocardial function. | Requires dynamic monitoring and clinical interpretation; risk of over-treatment in ambiguous cases. | [116] |
| Long-Term Cardiac Monitoring | Tracks myocardial recovery and remodeling in trauma survivors through serial GLS measurements. | Enables personalized follow-up and long-term care planning. | Long-term access to imaging may be limited; adherence to follow-up may be poor. | [117] |
| Integration with Advanced Imaging Modalities | Combines with cardiac MRI/CT to assess structural damage (e.g., fibrosis, oedema, scarring). | Enhances diagnostic accuracy and understanding of myocardial pathology. | MRI/CT access may be limited; high cost and patient instability may preclude use in acute trauma. | [118,119] |
| AI and Machine Learning Integration | Enhances GLS analysis and risk prediction through automated algorithms and data-driven models. | Improves consistency, speed, and predictive power of strain interpretation. | Requires robust datasets; AI systems must be validated for trauma populations. | [120] |
| Implementation in Clinical Practice | Offers a non-invasive, sensitive tool for routine trauma cardiac assessment. | Facilitates evidence-based, personalized trauma cardiology care. | Requires specialized echocardiography equipment and training; vendor variability in GLS measurements. | [38] |
| Future Research and Validation | Needed for establishing trauma-specific GLS reference values and studying utility in subgroups (e.g., TBI, hemorrhagic shock). | Expands clinical relevance and optimizes use in diverse trauma settings. | Limited large-scale prospective studies; subgroup-specific data currently sparse. | [121] |
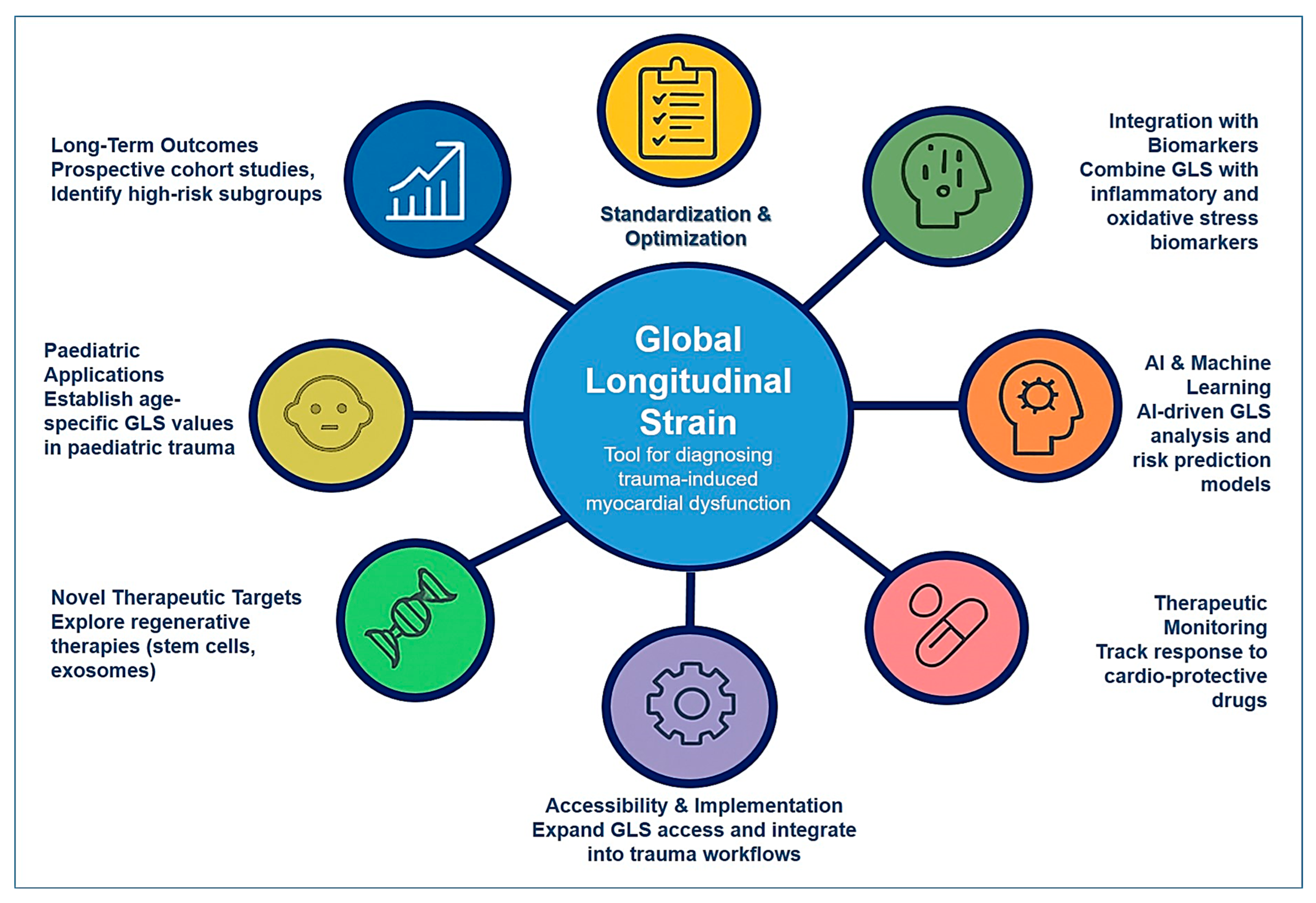
6. Future Directions and Research Opportunities
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weber, B.; Lackner, I.; Gebhard, F.; Miclau, T.; Kalbitz, M. Trauma, a Matter of the Heart-Molecular Mechanism of Post-Traumatic Cardiac Dysfunction. Int. J. Mol. Sci. 2021, 22, 737. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.D.; Ohe, N.T.; Bader, Y.; Afify, N.; Al-Homedi, Z.; Alwedami, S.M.; O’Sullivan, S.; Campos, L.A.; Baltatu, O.C. Heart Rate Variability Indices as Possible Biomarkers for the Severity of Post-traumatic Stress Disorder Following Pregnancy Loss. Front. Psychiatry 2022, 12, 700920. [Google Scholar]
- Weber, B.; Lackner, I.; Braun, C.K.; Kalbitz, M.; Huber-Lang, M.; Pressmar, J. Laboratory Markers in the Management of Pediatric Polytrauma: Current Role and Areas of Future Research. Front. Pediatr. 2021, 9, 622753. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Huang, J.L.; Fang, Y.; Du, H.L.; Chen, Y.L.; Zhao, S.Q. Molecular biomarkers of blunt cardiac injury: Recent advances and future perspectives. Expert Rev. Mol. Diagn. 2024, 24, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Kalil, R.; Saretta, R.; Franci, A.; Baracioli, L.M.; Galas, F.R.B.G.; Gil, J.S.; Ferino, A.; Giacovone, C.; Oliveira, I.; Souza, J.; et al. Post-COVID-19 Cardiopulmonary Symptoms: Predictors and Imaging Features in Patients after Hospital Discharge. Arq. Bras. Cardiol. 2023, 120, e20220642. [Google Scholar]
- Omar, T.; İnci, K.; Oflu, Y.; Dilek, M.; Çelik, Z.B.; Kına, S.; İliş, D.; Bucak, H.M. The predictive value of left ventricular global longitudinal strain in normotensive critically ill septic patients. Crit. Care Sci. 2023, 35, 187–195. [Google Scholar] [CrossRef]
- Lima, M.R.; Silva, D. Septic cardiomyopathy: A narrative review. Rev. Port. Cardiol. 2023, 42, 471–481. [Google Scholar] [CrossRef]
- Cho, S.K.S.; Darby, J.R.T.; Williams, G.K.; Holman, S.L.; Rai, A.; Van Amerom, J.F.P.; Fan, C.P.; Macgowan, C.K.; Selvanayagam, J.B.; Morrison, J.L.; et al. Post-Myocardial Infarction Remodeling and Hyperkinetic Remote Myocardium in Sheep Measured by Cardiac MRI Feature Tracking. J. Magn. Reson. Imaging 2025, 61, 1323–1335. [Google Scholar] [CrossRef]
- Villar-Calle, P.; Kochav, J.D.; Vadaketh, K.; Chiu, C.; Tak, K.; Agoglia, H.; Liberman, N.; Nguyen, K.L.; Vizcarra-Tellez, A.; Wu, A.L.; et al. Tissue-Based Predictors of Impaired Right Ventricular Strain in Coronary Artery Disease: A Multicenter Stress Perfusion Study. Circ.-Cardiovasc. Imaging 2024, 17, e016852. [Google Scholar] [CrossRef]
- Shvets, D.A.; Povetkin, S. Limitations of Diagnosis of Ischemic Left Ventricular Dysfunction Using the Values of Strain, Twist and Untwist in Patients with Myocardial Infarction of Various Localization. Kardiologiya 2024, 64, 55–62. [Google Scholar] [CrossRef]
- Roger, G.; Denormandie, P.; Gobe, T.; Azzolina, D.; Pham, T.; Chantalat, C.; Cuveillier, D.; Bouchachi, A.; Jourdain, P.; Lai, C.; et al. Left ventricular global longitudinal strain and acute myocardial injury in patients with sickle cell disease admitted to the intensive care unit for vaso-occlusive crisis. Br. J. Haematol. 2024, 204, 2007–2015. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.T.; Liu, H.L.; Song, Y.; Huang, Y.L.; Zhang, C.Q. Quantitative assessment of left ventricular myocardial work in patients with different types of atrial fibrillation by non-invasive pressure-strain loop technique. Echocardiography 2024, 41, e15801. [Google Scholar] [CrossRef]
- Tolu-Akinnawo, O.Z.; Ezekwueme, F.; Omolayo, O.; Batheja, S.; Awoyemi, T. Advancements in Artificial Intelligence in Noninvasive Cardiac Imaging: A Comprehensive Review. Clin. Cardiol. 2025, 48, e70087. [Google Scholar] [CrossRef] [PubMed]
- Udoy, I.A.; Hassan, O. AI-Driven Technology in Heart Failure Detection and Diagnosis: A Review of the Advancement in Personalized Healthcare. Symmetry 2025, 17, 469. [Google Scholar] [CrossRef]
- Geneş, M.; Çelik, M. Evaluation of Left Ventricular Systolic Functions of Patients with Exaggerated High Blood Pressure Response to Treadmill Exercise Test with Two-Dimensional Longitudinal Strain Imaging. Anatol. J. Cardiol. 2024, 29, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Baykiz, D.; Govdeli, E.A.; Ozer, P.K.; Karaayvaz, E.B.; Catma, Y.; Medetalibeyoglu, A.; Cagatay, A.; Umman, B.; Tukek, T.; Bugra, Z. Evaluation the relationship of left ventricular global longitudinal strain and laboratory parameters in discharged patients with COVID-19: A follow-up study. Int. J. Cardiovasc. Imaging 2021, 37, 2451–2464. [Google Scholar] [CrossRef]
- Biffl, W.L.; Fawley, J.A.; Mohan, R.C. Diagnosis and management of blunt cardiac injury: What you need to know. J. Trauma Acute Care Surg. 2024, 96, 685–693. [Google Scholar] [CrossRef]
- Akbar, M.; Raju, V.S.; Setia, P.; Chaturvedi, K.; Rao, M. Blunt chest trauma-induced myocardial infarction: A case of sudden death by homicide. Egypt. J. Forensic Sci. 2024, 14, 4. [Google Scholar] [CrossRef]
- Bekbossynova, M.; Mukarov, M.; Kanabekova, P.; Shaktybek, Z.; Sugralimova, M.; Batpen, A.; Kozhakhmetova, A.; Sholdanova, Z.; Zhanbolat, A. Biochemical markers of myocardial contusion after blunt chest trauma. Eur. J. Trauma Emerg. Surg. 2025, 51, 189. [Google Scholar] [CrossRef]
- Zhou, W.M.; Wang, N.; Dong, S.; Huan, Z.R.; Sui, L.J.; Ge, X. PRG4 mitigates hemorrhagic shock-induced cardiac injury by inhibiting mitochondrial dysregulation, oxidative stress and NLRP3-mediated pyroptosis. Int. Immunopharmacol. 2024, 137, 112507. [Google Scholar] [CrossRef]
- Li, A.L.; Lian, L.; Chen, X.N.; Cai, W.H.; Fan, X.B.; Fan, Y.J.; Li, T.T.; Xie, Y.Y.; Zhang, J.P. The role of mitochondria in myocardial damage caused by energy metabolism disorders: From mechanisms to therapeutics. Free. Radic. Biol. Med. 2023, 208, 236–251. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Carleton, R.N.; Neary, J.P. Cardiac function and posttraumatic stress disorder: A review of the literature and case report. Health Promot. Chronic Dis. Prev. Can. 2023, 43, 472–480. [Google Scholar] [CrossRef]
- Ma, D.S. Effects of Trauma-Related Shock on Myocardial Function in the Early Period Using Transthoracic Echocardiography. J. Trauma Inj. 2021, 34, 119–125. [Google Scholar] [CrossRef]
- Nair, L.; Winkle, B.; Senanayake, E. Managing blunt cardiac injury. J. Cardiothorac. Surg. 2023, 18, 71. [Google Scholar] [CrossRef] [PubMed]
- Kyriazidis, I.P.; Jakob, D.A.; Vargas, J.A.H.; Franco, O.H.; Degiannis, E.; Dorn, P.; Pouwels, S.; Patel, B.; Johnson, I.; Houdlen, C.J.; et al. Accuracy of diagnostic tests in cardiac injury after blunt chest trauma: A systematic review and meta-analysis. World J. Emerg. Surg. 2023, 18, 36. [Google Scholar] [CrossRef]
- Li, R.; Ye, J.J.; Gan, L.; Zhang, M.; Sun, D.; Li, Y.; Wang, T.; Chang, P. Traumatic inflammatory response: Pathophysiological role and clinical value of cytokines. Eur. J. Trauma Emerg. Surg. 2023, 50, 1313–1330. [Google Scholar] [CrossRef]
- Katkenov, N.; Mukhatayev, Z.; Kozhakhmetov, S.; Sailybayeva, A.; Bekbossynova, M.; Kushugulova, A. Systematic Review on the Role of IL-6 and IL-1β in Cardiovascular Diseases. J. Cardiovasc. Dev. Dis. 2024, 11, 206. [Google Scholar] [CrossRef]
- Dhalla, N.S.; Ostadal, P.; Tappia, P.S. Involvement of Oxidative Stress and Antioxidants in Modification of Cardiac Dysfunction Due to Ischemia–Reperfusion Injury. Antioxidants 2025, 14, 340. [Google Scholar] [CrossRef]
- Lin, W.; Chen, H.; Chen, X.; Guo, C. The Roles of Neutrophil-Derived Myeloperoxidase (MPO) in Diseases: The New Progress. Antioxidants 2024, 13, 132. [Google Scholar] [CrossRef]
- Suh, J.I.; da Roza, D.L.; Cadamuro, F.M.; Malbouisson, L.M.S.; Sanches, T.R.; Andrade, L. Catecholamine concentration as a predictor of mortality in emergency surgical patients. Int. J. Emerg. Med. 2024, 17, 95. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Briasoulis, A.; Sarafidis, P.; Magouliotis, D.; Athanasiou, T.; Paraskevaidis, I.; Skoularigis, J.; Xanthopoulos, A. The Sympathetic Nervous System in Hypertensive Heart Failure with Preserved LVEF. J. Clin. Med. 2023, 12, 6486. [Google Scholar] [CrossRef]
- Omerovic, E.; Citro, R.; Bossone, E.; Redfors, B.; Backs, J.; Bruns, B.; Ciccarelli, M.; Couch, L.S.; Dawson, D.; Grassi, G.; et al. Pathophysiology of Takotsubo syndrome—A joint scientific statement from the Heart Failure Association Takotsubo Syndrome Study Group and Myocardial Function Working Group of the European Society of Cardiology—Part 1: Overview and the central role for catecholamines and sympathetic nervous system. Eur. J. Heart Fail. 2022, 24, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xiong, T.; Yang, Y.; Zuo, B.; Chen, X.; Wang, D. Metabolic remodeling in takotsubo syndrome. Front. Cardiovasc. Med. 2022, 9, 1060070. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Kaur, R.; Kumari, P.; Pasricha, C.; Singh, R. ICAM-1 and VCAM-1: Gatekeepers in various inflammatory and cardiovascular disorders. Clin. Chim. Acta 2023, 548, 117487. [Google Scholar] [CrossRef] [PubMed]
- Kanuparthy, M.; Manthana, R.; Kaushik, H.; Xiang, K.; Hamze, J.; Marimekala, D.; Feng, J.; Sellke, F.W. Microvascular Dysfunction Following Cardioplegic Arrest and Cardiopulmonary Bypass: Impacts of Diabetes and Hypertension. Biomedicines 2025, 13, 409. [Google Scholar] [CrossRef]
- BaniHani, H.A.; Khaled, L.H.; Al Sharaa, N.M.; Al Saleh, R.A.; Bin Ghalaita, A.K.; Bin Sulaiman, A.S.; Holeihel, A. Causes, Diagnosis, Treatment, and Prognosis of Cardiac Fibrosis: A Systematic Review. Cureus 2025, 17, e81264. [Google Scholar] [CrossRef]
- Wang, B.X. Diagnosis and Management of Hypertensive Heart Disease: Incorporating 2023 European Society of Hypertension and 2024 European Society of Cardiology Guideline Updates. J. Cardiovasc. Dev. Dis. 2025, 12, 46. [Google Scholar] [CrossRef]
- Smiseth, O.A.; Rider, O.; Cvijic, M.; Valkovič, L.; Remme, E.W.; Voigt, J.-U. Myocardial Strain Imaging. JACC Cardiovasc. Imaging 2025, 18, 340–381. [Google Scholar] [CrossRef]
- Alexeeva, E.; Shingarova, M.; Dvoryakovskaya, T.; Lomakina, O.; Fetisova, A.; Isaeva, K.; Chomakhidze, A.; Chibisova, K.; Krekhova, E.; Kozodaeva, A.; et al. Safety and efficacy of canakinumab treatment for undifferentiated autoinflammatory diseases: The data of a retrospective cohort two-centered study. Front. Med. 2023, 10. [Google Scholar] [CrossRef]
- Kozlov, A.V.; Javadov, S.; Sommer, N. Cellular ROS and Antioxidants: Physiological and Pathological Role. Antioxidants 2024, 13, 602. [Google Scholar] [CrossRef]
- Johri, N.; Matreja, P.S.; Maurya, A.; Varshney, S.; Smritigandha. Role of β-blockers in Preventing Heart Failure and Major AdverseCardiac Events Post Myocardial Infarction. Curr. Cardiol. Rev. 2023, 19, 24–31. [Google Scholar] [CrossRef]
- Tanisha; Amudha, C.; Raake, M.; Samuel, D.; Aggarwal, S.; Bashir, Z.M.D.; Marole, K.K.; Maryam, I.; Nazir, Z. Diagnostic Modalities in Heart Failure: A Narrative Review. Cureus 2024, 16, e67432. [Google Scholar] [CrossRef]
- El-Andari, R.; O’Brien, D.; Bozso, S.J.; Nagendran, J. Blunt cardiac trauma: A narrative review. Mediastinum 2021, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Latif, R.K.; Clifford, S.P.; Ghafghazi, S.; Phipps, Z.; Chen, J.J.; Sangroula, D.; Khan, A.Z.; Saleem, J.; Farah, I.; Huang, J.; et al. Echocardiography and Management for Cardiac Trauma. J. Cardiothorac. Vasc. Anesth. 2022, 36, 3265–3277. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.; Ring, L.; Oxborough, D.; Harkness, A.; Bennett, S.; Rana, B.; Sutaria, N.; Lo Giudice, F.; Shun-Shin, M.; Paton, M.; et al. The assessment of left ventricular diastolic function: Guidance and recommendations from the British Society of Echocardiography. Echo Res. Pract. 2024, 11, 16. [Google Scholar] [CrossRef]
- Yeong, C.C.; Harrop, D.L.; Ng, A.C.T.; Wang, W.Y.S. Global longitudinal strain manually measured from mid-myocardial lengths is a reliable alternative to speckle tracking global longitudinal strain. J. Cardiovasc. Imaging 2024, 32, 35. [Google Scholar] [CrossRef] [PubMed]
- Le, T.-T.; Huang, W.; Singh, G.K.; Toh, D.-F.; Ewe, S.H.; Tang, H.C.; Loo, G.; Bryant, J.A.; Ang, B.; Tay, E.L.-W.; et al. Echocardiographic Global Longitudinal Strain Is Associated with Myocardial Fibrosis and Predicts Outcomes in Aortic Stenosis. Front. Cardiovasc. Med. 2021, 8, 750016. [Google Scholar] [CrossRef]
- Batchelor, R.J.; Dipnall, J.F.; Read, D.; Cameron, P.; Fitzgerald, M.; Stub, D.; Lefkovits, J. Prevalence and clinical outcomes of acute myocardial infarction in patients presenting with major trauma. Injury 2025, 56, 111996. [Google Scholar] [CrossRef]
- Loutati, R.; Bruoha, S.; Taha, L.; Karmi, M.; Perel, N.; Maller, T.; Sabouret, P.; Galli, M.; Zoccai, G.B.; De Rosa, S.; et al. Association between peak troponin level and prognosis among patients admitted to intensive cardiovascular care unit. Int. J. Cardiol. 2024, 417, 132556. [Google Scholar] [CrossRef]
- Desai, R.; Damarlapally, N.; Bareja, S.; Arote, V.; SuryaVasudevan, S.; Mehta, K.; Ashfaque, M.; Jayachandran, Y.; Sampath, S.; Behera, A.; et al. A systematic review and meta-analysis evaluating the association of high sensitivity troponin levels with outcomes in patients with stable coronary artery disease. Curr. Med. Res. Opin. 2024, 40, 1685–1695. [Google Scholar] [CrossRef]
- Desaegher, A.; Marin, V.; Beauvieux, M.C.; Colombiès, B.; Lauga, M.; Alloug, S.; Kalkan, S.; Castaing-Mouhica, G.; Lacape, G.; Rucheton, B.; et al. Exploring strategies to rapidly identify false positives in high-sensitivity cardiac troponin I assay: A prospective study. Clin. Chim. Acta 2025, 565, 119996. [Google Scholar] [CrossRef]
- Ludwikowska, K.M.; Tokarczyk, M.; Paleczny, B.; Tracewski, P.; Szenborn, L.; Kusa, J. Clinical Significance of B-Type Natriuretic Peptide and N-Terminal Pro-B-Type Natriuretic Peptide in Pediatric Patients: Insights into Their Utility in the Presence or Absence of Pre-Existing Heart Conditions. Int. J. Mol. Sci. 2024, 25, 8781. [Google Scholar] [CrossRef] [PubMed]
- Kryczka, K.E.; Demkow, M.; Dzielinska, Z. Biomarkers in Peripartum Cardiomyopathy-What We Know and What Is Still to Be Found. Biomolecules 2024, 14, 103. [Google Scholar] [CrossRef]
- Abraham, M.K.; Madanan, A.S.; Varghese, S.; Shkhair, A.I.; Indongo, G.; Rajeevan, G.; Kala, A.B.; George, S. Luminescence “Turn-On” Sensing of Brain Natriuretic Peptide (BNP)—Dilated Cardiomyopathy Biomarker Based on the MoS Nanosheet Quenched Terbium Citrate Complex. ACS Appl. Bio Mater. 2024, 7, 6044–6054. [Google Scholar] [CrossRef]
- Song, J.L.; Fan, B.; Qiu, L.Q.; Li, Q.; Chen, G.Y. Brain natriuretic peptide as a predictive marker of mortality in sepsis: An updated systematic review and meta-analysis. BMC Anesthesiol. 2024, 24, 276. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, T.; Isayama, K.; Yamamoto, Y.; Teramoto, A. Cardiopulmonary haemodynamic changes after severe head injury. Br. J. Neurosurg. 2004, 18, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Dujardin, K.S.; McCully, R.B.; Wijdicks, E.F.M.; Tazelaar, H.D.; Seward, J.B.; McGregor, C.G.A.; Olson, L.J. Myocardial dysfunction associated with brain death: Clinical, echocardiographic, and pathologic features. J. Heart Lung Transpl. 2001, 20, 350–357. [Google Scholar] [CrossRef]
- Zafeiri, M.; Knott, K.; Lampejo, T. Acute myocarditis: An overview of pathogenesis, diagnosis and management. Panminerva Med. 2024, 66, 174–187. [Google Scholar] [CrossRef]
- Abohelwa, M.; Mohamed, A.A.; Del-Rio-Pertuz, G.; Elgwairi, E.; Nguyen, T.H.; Elmassry, M.; Parmar, K.; Rao, S.J.A.; Patel, B.; Hamous, K.; et al. Cardiac Muscle Injury and Echocardiographic Plus Electrocardiographic Findings in Patients with 2019 Novel Coronavirus (COVID-19): A Retrospective Cohort Study. Cjc Open 2024, 6, 108–117. [Google Scholar] [CrossRef]
- Xourgia, E.; Brignoli, K.; Linder, O.; Neagoe, A.M.; Capek, L.; Bruno, J.; Strickler, E.; Bakula, A.; Pavlicek-Bahlo, M.; Fürholz, M.; et al. Speckle-tracking echocardiography of left and right ventricle and acute cellular rejection in orthotropic heart transplantation: A systematic review and meta-analysis. Int. J. Cardiovasc. Imaging 2025, 41, 669–679. [Google Scholar] [CrossRef]
- Prajapati, R.; Qin, T.T.; Connelly, K.A.; Merdad, A.; Chow, C.M.; Leong-Poi, H.; Ong, G. Echocardiographic Assessment of Cardiac Remodeling According to Obesity Class. Am. J. Cardiol. 2025, 236, 34–41. [Google Scholar] [CrossRef]
- Christenson, R.H. Cardiac Markers: A Chest Pain Center Focus. In Short Stay Management of Chest Pain; Humana Press: Totowa, NJ, USA, 2009; pp. 91–113. [Google Scholar] [CrossRef]
- Ahmed, N.A.; Redwan, F.N.; Jahjah, A.S.; Al-Shehabi, Z.A. Evaluating high-sensitivity cardiac troponin I for early detection of treatment-related cardiotoxicity in HER2-positive breast cancer patients. Ann. Med. Surg. 2025, 87, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Nishi, H.; Nangaku, M.; Sofue, T.; Kagimura, T.; Narita, I. N-terminal pro-brain natriuretic peptide and cardiorenal outcome in patients with anaemia in chronic kidney disease. Esc Heart Fail. 2025, 12, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Ari, M.; Cevval, Z.K.; Usul, E.; Ari, E.; Kahveci, U. Role of Cardiac Biomarkers and Tomographic Right Ventricular Dysfunction Findings in the Treatment of Pulmonary Thromboembolism. Eurasian J. Emerg. Med. 2024, 23, 224–230. [Google Scholar] [CrossRef]
- Martinez-Lucio, T.S.; Mendoza-Ibañez, O.I.; Liu, W.L.; Mostafapour, S.; Li, Z.K.; Providência, L.; de Souza, G.S.; Mohr, P.; Dobrolinska, M.M.; van Leer, B.; et al. Long Axial Field of View PET/CT: Technical Aspects in Cardiovascular Diseases. Semin. Nucl. Med. 2025, 55, 52–66. [Google Scholar] [CrossRef]
- Tore, D.; Faletti, R.; Palmisano, A.; Salto, S.; Rocco, K.; Santonocito, A.; Gaetani, C.; Biondo, A.; Bozzo, E.; Giorgino, F.; et al. Cardiac computed tomography with late contrast enhancement: A review. Heliyon 2024, 10, e32436. [Google Scholar] [CrossRef]
- Fu, Q.H.; Tong, L.; Zhang, H.Z.; Xu, H. Multimodal Imaging Diagnosis of Apical Ventricular Aneurysm with Thrombosis Resulting From Blunt Myocardial Injury: A Case Report. J. Clin. Ultrasound 2025, early view. [Google Scholar] [CrossRef]
- Mortezaeian, H.; Tabib, A.; Pouraliakbar, H.; Anafje, M.; Ebrahimi, P.; Soltani, P. Ventricular Septal Defect and Mitral Regurgitation Due to Penetrating Cardiac Trauma; a Case Report and Review of Literature. Arch. Acad. Emerg. Med. 2024, 12, e25. [Google Scholar]
- Rivera Boadla, M.E.; Sharma, N.R.; Varghese, J.; Lamichhane, S.; Khan, M.H.; Gulati, A.; Khurana, S.; Tan, S.; Sharma, A. Multimodal Cardiac Imaging Revisited by Artificial Intelligence: An Innovative Way of Assessment or Just an Aid? Cureus 2024, 16, e64272. [Google Scholar] [CrossRef]
- Shaikh, M.F.W.; Mama, M.S.; Proddaturi, S.H.; Vidal, J.; Gnanasekaran, P.; Kumar, M.S.; Clarke, C.J.; Reddy, K.S.; Bello, H.M.; Raquib, N.; et al. The Role of Artificial Intelligence in the Prediction, Diagnosis, and Management of Cardiovascular Diseases: A Narrative Review. Cureus 2025, 17, e81332. [Google Scholar] [CrossRef]
- Qamar, S.R.; Wu, Y.H.; Nicolaou, S.; Murray, N. State of the Art Imaging Review of Blunt and Penetrating Cardiac Trauma. Can. Assoc. Radiol. J. 2020, 71, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Qamar, S.R.; Murray, N.; Nicolaou, S. Imaging of Cardiac Trauma. Radiol. Clin. N. Am. 2019, 57, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Lange, T.; Schuster, A. Quantification of Myocardial Deformation Applying CMR-Feature-Tracking—All About the Left Ventricle? Curr. Heart Fail. Rep. 2021, 18, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Leitman, M.; Tyomkin, V. Evaluating Right Ventricular Function Using Longitudinal Displacement. Medicina 2025, 61, 446. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Zhang, J.; Xie, Y.; Wang, W.; Zhang, Y.; Xie, M.; Zhang, L. Speckle-Tracking Echocardiography in Right Ventricular Function of Clinically Well Patients with Heart Transplantation. Diagnostics 2024, 14, 1305. [Google Scholar] [CrossRef]
- Pambianchi, G.; Marchitelli, L.; Cundari, G.; Ruoli, L.; Conia, L.; Catalano, C.; Galea, N. Takotsubo syndrome: Left atrial and ventricular myocardial strain impairment in the subacute and convalescent phases assessed by CMR. Eur. Radiol. Exp. 2024, 8, 34. [Google Scholar] [CrossRef]
- Makeev, M.I.; Saidova, M.A.; Safiullina, A.A.; Komlev, A.E.; Kuchin, I.V.; Kantemirova, M.M.; Imaev, T.E. Prediction of Cardiovascular Events and Structural and Functional Remodeling of the Heart in Patients with Severe Mitral Regurgitation of Various Genesis Underwent Transcatheter Mitral Valve Repair “Edge-to-Edge”. Kardiologiya 2024, 64, 3–15. [Google Scholar] [CrossRef]
- Liu, S.Q.; Laghzali, O.; Shalikar, S.; Rusu, M.C.; Carrier, L.; Niendorf, T.; Ku, M.C. Cardiac MRI Strain as an Early Indicator of Myocardial Dysfunction in Hypertrophic Cardiomyopathy. Int. J. Mol. Sci. 2025, 26, 1407. [Google Scholar] [CrossRef]
- Neveu, A.; Aghezzaf, S.; Oger, E.; L’official, G.; Curtis, E.; Galli, E.; Montaigne, D.; Coisne, A.; Donal, E. Primary mitral regurgitation: Toward a better quantification on left ventricular consequences. Clin. Cardiol. 2024, 47, e24190. [Google Scholar] [CrossRef]
- Slawinski, G.; Hawryszko, M.; Lizewska-Springer, A.; Nabialek-Trojanowska, I.; Lewicka, E. Global Longitudinal Strain in Cardio-Oncology: A Review. Cancers 2023, 15, 986. [Google Scholar] [CrossRef]
- Myers, S.; Gupta, D.K.; Izzy, M. The clinical relevance of the new criteria for cirrhotic cardiomyopathy and future directions. Liver Transplant. 2025, 31, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, G.; Carerj, S.; Di Bella, G.; Manganaro, R.; Pizzino, F.; Restelli, D.; Pelaggi, G.; Lofrumento, F.; Licordari, R.; Taverna, G.; et al. Clinical Applications of Myocardial Work in Echocardiography: A Comprehensive Review. J. Cardiovasc. Echogr. 2024, 34, 99–113. [Google Scholar] [CrossRef]
- Paelinck, B.P.; Bondue, A.; Robyns, T.; Eyskens, F. Left ventricular hypertrophy: Do not forget Fabry disease. Diagnostic work-up and differential diagnosis. Acta Cardiol. 2024, 79, 642–649. [Google Scholar] [CrossRef]
- Nemes, A. Cardiac Mechanics and Valvular and Vascular Abnormalities in Hypereosinophilic Syndrome. J. Clin. Med. 2024, 13, 1403. [Google Scholar] [CrossRef] [PubMed]
- Tsampasian, V.; Victor, K.; Bhattacharyya, S.; Oxborough, D.; Ring, L.M. Echocardiographic assessment of aortic regurgitation: A narrative review. Echo Res. Pract. 2024, 11, 1. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Fagiani, V.; Nicolosi, G.L.; Lombardo, M. Echocardiographic assessment of left ventricular mechanics in individuals with mitral valve prolapse: A systematic review and meta-analysis. Int. J. Cardiovasc. Imaging 2024, 40, 1617–1629. [Google Scholar] [CrossRef]
- Martini, L.; Lisi, M.; Pastore, M.C.; Righini, F.M.; Rubboli, A.; Henein, M.Y.; Cameli, M. The Role of Speckle Tracking Echocardiography in the Evaluation of Advanced-Heart-Failure Patients. J. Clin. Med. 2024, 13, 4037. [Google Scholar] [CrossRef] [PubMed]
- Kashlan, B.; Kinno, M.; Syed, M. Perioperative myocardial injury and infarction after noncardiac surgery: A review of pathophysiology, diagnosis, and management. Front. Cardiovasc. Med. 2024, 11, 1323425. [Google Scholar] [CrossRef]
- Eichhorn, C.; Koeckerling, D.; Reddy, R.K.; Ardissino, M.; Rogowski, M.; Coles, B.; Hunziker, L.; Greulich, S.; Shiri, I.; Frey, N.; et al. Risk Stratification in Nonischemic Dilated Cardiomyopathy Using CMR Imaging A Systematic Review and Meta-Analysis. JAMA-J. Am. Med. Assoc. 2024, 332, 1535–1550. [Google Scholar] [CrossRef]
- Saito, N.; Kato, S.; Azuma, M.; Horita, N.; Utsunomiya, D. Prognostic impact of MRI-derived feature tracking myocardial strain in patients with non-ischaemic dilated cardiomyopathy: A systematic review and meta-analysis. Clin. Radiol. 2024, 79, e702–e714. [Google Scholar] [CrossRef]
- He, X.G.; Li, Y.X.; Wang, Y.; Tian, W.; Li, Z.F.; Ge, L.; Wang, G.; Chen, Z.X. Prognostic Value of CT-Derived Myocardial Biomarkers: Extracellular Volume Fraction and Strain in Patients with Severe Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-analysis. Acad. Radiol. 2024, 31, 4352–4364. [Google Scholar] [CrossRef] [PubMed]
- Bright, C.; Rizvi, A.; Ezekwueme, F.; Schiff, M.; Kliner, J.; Hindes, M.; Thorn, K.; Kowalski, V.; Hovanec, P.; Draxinger, A.; et al. Impact of guideline directed medical therapy on myocardial function in adults with congenital heart disease. Int. J. Cardiol. 2024, 414, 132413. [Google Scholar] [CrossRef] [PubMed]
- Bohbot, Y.; Pezel, T.; Demirkiran, A.; Androulakis, E.; Houshmand, G.; Szabo, L.; Manka, R.; Botezatu, S.B.; Rodríguez-Palomares, J.F.; Biering-Sorensen, T.; et al. European Association of Cardiovascular Imaging survey on cardiovascular multimodality imaging in acute myocarditis. Eur. Heart J.-Cardiovasc. Imaging 2024, 25, 892–900. [Google Scholar] [CrossRef]
- Ilkhomova, L.T.; Bekmetova, F.M.; Fozilov, K.G.; Tursunova, N.B.; Abdullaeva, S.Y.; Khotamova, M.; Doniyorov, S.N.; Usmonova, N.A.; Bekmetova, S.I. Left Ventricular Mechanical Dispersion in the Development of Ventricular Arrhythmia in Patients after Q-Wave Myocardial Infarction. Int. J. Biomed. 2024, 14, 558–562. [Google Scholar] [CrossRef]
- Gao, X.; Xiao, W.W.; Ji, L.Q.; Li, H.Y.; Zou, A.L.Z.; Zhang, X.R.; Miao, Z.M.; Yang, S.Y.; Yu, S.M. Assessment of Left Ventricular Functional Impairment in Patients with Chronic Kidney Disease Using Three-Dimensional Speckle Tracking Imaging. Echocardiography 2024, 41, e15928. [Google Scholar] [CrossRef]
- Carrizales-Sepúlveda, E.; Ordaz-Farías, A.; Vera-Pineda, R.; Rodríguez-Gutierrez, R.; Flores-Ramírez, R. Comprehensive echocardiographic and biomarker assessment of patients with diabetic ketoacidosis. Cardiovasc. Diabetol. 2024, 23, 385. [Google Scholar] [CrossRef]
- Netea, S.A.; Biesbroek, G.; Groenink, M.; Planken, R.N.; de Winter, R.J.; Blom, N.A.; Kuijpers, T.W.; Kuipers, I.M. Long-term global longitudinal strain abnormalities in paediatric patients after multisystem inflammatory syndrome in children correlate with cardiac troponin T: A single-centre cohort study. Cardiol. Young 2024, 34, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Sartorio, A.; Dal Pont, C.; Romano, S. Standard and New Echocardio Techniques, Such as Global Longitudinal Strain, to Monitor the Impact of Diets on Cardiovascular Diseases and Heart Function. Nutrients 2024, 16, 1471. [Google Scholar] [CrossRef]
- Anwar, A.M. Potential Diagnostic and Prognostic Values of Left Atrial Strain in Valvular Heart Disease. J. Cardiovasc. Echogr. 2024, 34, 41–49. [Google Scholar] [CrossRef]
- Matsubara, D.; Chang, J.; Kauffman, H.L.; Wang, Y.; Nadaraj, S.; Patel, C.; Paridon, S.M.; Fogel, M.A.; Quartermain, M.D.; Banerjee, A. Longitudinal Assessment of Cardiac Outcomes of Multisystem Inflammatory Syndrome in Children Associated with COVID-19 Infections. J. Am. Heart Assoc. 2022, 11, e023251. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Abbate, A.; Boutjdir, M.; Capecchi, P.L. Fir(e)ing the Rhythm. JACC Basic Transl. Sci. 2023, 8, 728–750. [Google Scholar] [CrossRef]
- Kadoglou, N.P.E.; Dimopoulou, A.; Korakianitis, I.; Parperis, K. Advanced Parameters of Myocardial Strain and Cardiac Biomarkers Indicate Subclinical Systolic Myocardial Dysfunction in Patients with Systemic Lupus Erythematous. Biomedicines 2024, 12, 2638. [Google Scholar] [CrossRef] [PubMed]
- Jain, H.; Shahzad, M.; Usman, M.; Kc, A.; Singh, J.; Jain, J.; Odat, R.M.; Goyal, A.; Ahmed, F.; Ahmed, R. Detection of Myocardial Deformation Patterns and Prognostic Value of Routine Echocardiographic Parameters in Patients with Cardiac Sarcoidosis Versus Extracardiac Sarcoidosis: Systematic Review and Meta-Analysis. Diagnostics 2025, 15, 518. [Google Scholar] [CrossRef]
- Strauss, M.H.; Hall, A.S.; Narkiewicz, K. The Combination of Beta-Blockers and ACE Inhibitors Across the Spectrum of Cardiovascular Diseases. Cardiovasc. Drugs Ther. 2021, 37, 757–770. [Google Scholar] [CrossRef]
- Lange, T.; Gertz, R.J.; Schulz, A.; Backhaus, S.J.; Evertz, R.; Kowallick, J.T.; Hasenfuß, G.; Desch, S.; Thiele, H.; Stiermaier, T.; et al. Impact of myocardial deformation on risk prediction in patients following acute myocardial infarction. Front. Cardiovasc. Med. 2023, 10, 1199936. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, M.; Weilbacher, F.; Katzenschlager, S.; Weigand, M.A.; Popp, E. Severe trauma associated cardiac failure. Scand. J. Trauma Resusc. Emerg. Med. 2024, 32, 4. [Google Scholar] [CrossRef]
- Lisi, C.; Moser, L.J.; Mergen, V.; Klambauer, K.; Uçar, E.; Eberhard, M.; Alkadhi, H. Advanced myocardial characterization and function with cardiac CT. Int. J. Cardiovasc. Imaging 2024. [Google Scholar] [CrossRef] [PubMed]
- Pezzini, S.; Daus, F.; Galli, G.; Farina, A.; Fragasso, G.; Spoladore, R. Cardiac Magnetic Resonance in Heart Failure: Diagnostic and Prognostic Assessments. J. Cardiovasc. Dev. Dis. 2025, 12, 200. [Google Scholar] [CrossRef]
- Ghita-Pettigrew, M.; Edgar, K.S.; Kuburas, R.; Brown, K.H.; Walls, G.M.; Facchi, C.; Grieve, D.J.; Watson, C.J.; McWilliam, A.; van Herk, M.; et al. Dose-dependent changes in cardiac function, strain and remodelling in a preclinical model of heart base irradiation. Radiother. Oncol. 2024, 193, 110113. [Google Scholar] [CrossRef]
- Vago, H.; Szabo, L.; Dohy, Z.; Czimbalmos, C.; Toth, A.; Suhai, F.I.; Barczi, G.; Gyarmathy, V.A.; Becker, D.; Merkely, B. Early cardiac magnetic resonance imaging in troponin-positive acute chest pain and non-obstructed coronary arteries. Heart 2020, 106, 992–1000. [Google Scholar] [CrossRef]
- Triantafyllou, C.; Nikolaou, M.; Ikonomidis, I.; Bamias, G.; Kouretas, D.; Andreadou, I.; Tsoumani, M.; Thymis, J.; Papaconstantinou, I. Effects of Anti-Inflammatory Treatment and Surgical Intervention on Endothelial Glycocalyx, Peripheral and Coronary Microcirculatory Function and Myocardial Deformation in Inflammatory Bowel Disease Patients: A Two-Arms Two-Stage Clinical Trial. Diagnostics 2021, 11, 993. [Google Scholar] [CrossRef]
- Sun, L.J.; Qiao, W.; Xiao, Y.J.; Ren, W.D. Layer-specific strain for assessing the effect of naringin on systolic myocardial dysfunction induced by sepsis and its underlying mechanisms. J. Int. Med. Res. 2021, 49, 300060520986369. [Google Scholar] [CrossRef]
- Wazzan, A.A.; Taconne, M.; Rolle, V.L.; Forsaa, M.I.; Haugaa, K.H.; Galli, E.; Hernandez, A.; Edvardsen, T.; Donal, E. Risk profiles for ventricular arrhythmias in hypertrophic cardiomyopathy through clustering analysis including left ventricular strain. Int. J. Cardiol. 2024, 409, 132167. [Google Scholar] [CrossRef]
- Ghanbari, F.; Cirillo, J.; Rodriguez, J.; Yue, J.; Morales, M.A.; Kramer, D.B.; Manning, W.J.; Nezafat, R.; Ngo, L.H. MRI Assessment of Myocardial Deformation for Risk Stratification of Major Arrhythmic Events in Patients with Non-Ischemic Cardiomyopathy Eligible for Primary Prevention Implantable Cardioverter Defibrillators. J. Magn. Reson. Imaging 2024, 60, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Yurdam, F.S.; Gurses, E. Myocardial bridge and beta blockers: Effect on left ventricular strain parameters. Acta Cardiol. 2023, 78, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, G.; Iannone, F.P.; Di Lorenzo, A.; Testa, C.; Ciccarelli, M.; Venturini, E.; Cesaro, A.; Pacileo, M.; Tagliamonte, E.; D’Andrea, A.; et al. Potential Role of Global Longitudinal Strain in Cardiac and Oncological Patients Undergoing Cardio-Oncology Rehabilitation (CORE). Clin. Pract. 2023, 13, 384–397. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, L.; Zhang, Z.; Lin, Y.; Ji, M.; He, Q.; Xie, M.; Li, Y. Clinical Utility of Strain Imaging in Assessment of Myocardial Fibrosis. J. Clin. Med. 2023, 12, 743. [Google Scholar] [CrossRef]
- Li, R.; Xu, X.-X.; Li, C.-P.; Yang, H.-F.; Wang, L.-L.; Yang, F.; He, W.-F.; Yang, Y.-X.; Zheng, Y.-C.; Feng, X.-Y. Utilization of Cardiac Magnetic Resonance Imaging for Assessing Myocardial Fibrosis in Prognosis Evaluation and Risk Stratification of Patients with Dilated Cardiomyopathy. Rev. Cardiovasc. Med. 2025, 26, 25654. [Google Scholar] [CrossRef]
- Kwan, A.C.; Chang, E.W.; Jain, I.; Theurer, J.; Tang, X.; Francisco, N.; Haddad, F.; Liang, D.; Fábián, A.; Ferencz, A.; et al. Deep Learning-Derived Myocardial Strain. JACC Cardiovasc. Imaging 2024, 17, 715–725. [Google Scholar] [CrossRef]
- Kitov, S.; Vladimirova-Kitova, L. A review of alternative measurements in strain imaging for ventricular arrhythmia prediction. Folia Medica 2024, 66, 599–607. [Google Scholar] [CrossRef]
- Negishi, T.; Negishi, K.; Thavendiranathan, P.; Cho, G.Y.; Popescu, B.A.; Vinereanu, D.; Kurosawa, K.; Penicka, M.; Marwick, T.H.; Investigators, S. Effect of Experience and Training on the Concordance and Precision of Strain Measurements. JACC Cardiovasc. Imaging 2017, 10, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Nicolosi, G.L.; Granato, A.; Bonanomi, A.; Rigamonti, E.; Lombardo, M. Influence of chest wall conformation on reproducibility of main echocardiographic indices of left ventricular systolic function. Minerva Cardiol. Angiol. 2024, 72, 111–124. [Google Scholar] [CrossRef]
- Sade, L.E.; Joshi, S.S.; Cameli, M.; Cosyns, B.; Delgado, V.; Donal, E.; Edvardsen, T.; Carvalho, R.F.; Manka, R.; Podlesnikar, T.; et al. Current clinical use of speckle-tracking strain imaging: Insights from a worldwide survey from the European Association of Cardiovascular Imaging (EACVI). Eur. Heart J. Cardiovasc. Imaging 2023, 24, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Ruiz, M.F.; Ruiz-Rivera, A.; Soriano-Ursúa, M.A.; Martinez-Hernandez, C.; Manuel-Apolinar, L.; Castillo-Hernandez, C.; Guevara-Balcazar, G.; Farfán-García, E.D.; Mejia-Ruiz, A.; Rubio-Gayosso, I.; et al. Global longitudinal strain is superior to ejection fraction for detecting myocardial dysfunction in end-stage renal disease with hyperparathyroidism. World J. Cardiol. 2022, 14, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xie, M.; Zhang, L.; Zhang, Y.; Zhang, P.; Chen, X.; Ji, M.; Gao, L.; He, Q.; Wu, Z.; et al. Prognostic Value of LV Global Longitudinal Strain by 2D and 3D Speckle-Tracking Echocardiography in Patients with HFpEF. Circ. Cardiovasc. Imaging 2025, 18, e016975. [Google Scholar] [CrossRef]
- Tylutka, A.; Walas, Ł.; Zembron-Lacny, A. Level of IL-6, TNF, and IL-1β and age-related diseases: A systematic review and meta-analysis. Front. Immunol. 2024, 15, 1330386. [Google Scholar] [CrossRef]
- Doolub, G.; Khurshid, S.; Theriault-Lauzier, P.; Nolin Lapalme, A.; Tastet, O.; So, D.; Labrecque Langlais, E.; Cobin, D.; Avram, R. Revolutionising Acute Cardiac Care with Artificial Intelligence: Opportunities and Challenges. Can. J. Cardiol. 2024, 40, 1813–1827. [Google Scholar] [CrossRef]
- Maturi, B.; Dulal, S.; Sayana, S.B.; Ibrahim, A.; Ramakrishna, M.; Chinta, V.; Sharma, A.; Ravipati, H. Revolutionizing Cardiology: The Role of Artificial Intelligence in Echocardiography. J. Clin. Med. 2025, 14, 625. [Google Scholar] [CrossRef]
- Chowdhury, M.A.; Rizk, R.; Chiu, C.; Zhang, J.J.; Scholl, J.L.; Bosch, T.J.; Singh, A.; Baugh, L.A.; McGough, J.S.; Santosh, K.C.; et al. The Heart of Transformation: Exploring Artificial Intelligence in Cardiovascular Disease. Biomedicines 2025, 13, 427. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Yu, Y.; Li, C.; Zhang, C. Advances in the study of exosomes derived from mesenchymal stem cells and cardiac cells for the treatment of myocardial infarction. Cell Commun. Signal. 2023, 21, 202. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekbossynova, M.; Saliev, T.; Mukarov, M.; Sugralimova, M.; Batpen, A.; Kozhakhmetova, A.; Sholdanova, Z. Longitudinal Myocardial Deformation as an Emerging Biomarker for Post-Traumatic Cardiac Dysfunction. Life 2025, 15, 1052. https://doi.org/10.3390/life15071052
Bekbossynova M, Saliev T, Mukarov M, Sugralimova M, Batpen A, Kozhakhmetova A, Sholdanova Z. Longitudinal Myocardial Deformation as an Emerging Biomarker for Post-Traumatic Cardiac Dysfunction. Life. 2025; 15(7):1052. https://doi.org/10.3390/life15071052
Chicago/Turabian StyleBekbossynova, Makhabbat, Timur Saliev, Murat Mukarov, Madina Sugralimova, Arman Batpen, Anar Kozhakhmetova, and Zhumagul Sholdanova. 2025. "Longitudinal Myocardial Deformation as an Emerging Biomarker for Post-Traumatic Cardiac Dysfunction" Life 15, no. 7: 1052. https://doi.org/10.3390/life15071052
APA StyleBekbossynova, M., Saliev, T., Mukarov, M., Sugralimova, M., Batpen, A., Kozhakhmetova, A., & Sholdanova, Z. (2025). Longitudinal Myocardial Deformation as an Emerging Biomarker for Post-Traumatic Cardiac Dysfunction. Life, 15(7), 1052. https://doi.org/10.3390/life15071052





