The Siderophore Phymabactin Facilitates the Growth of the Legume Symbiont Paraburkholderia phymatum in Aluminium-Rich Martian Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Media, and Cultivation
2.2. Preparation of Linear Gradient Plates
2.3. Sample Preparation for Siderophore Screening Analysis
2.4. UHPLC-MS Method
3. Results
3.1. The Siderophore Phymabactin Is Important for the Growth of P. phymatum in Martian Soil and in Aluminium-Rich Medium

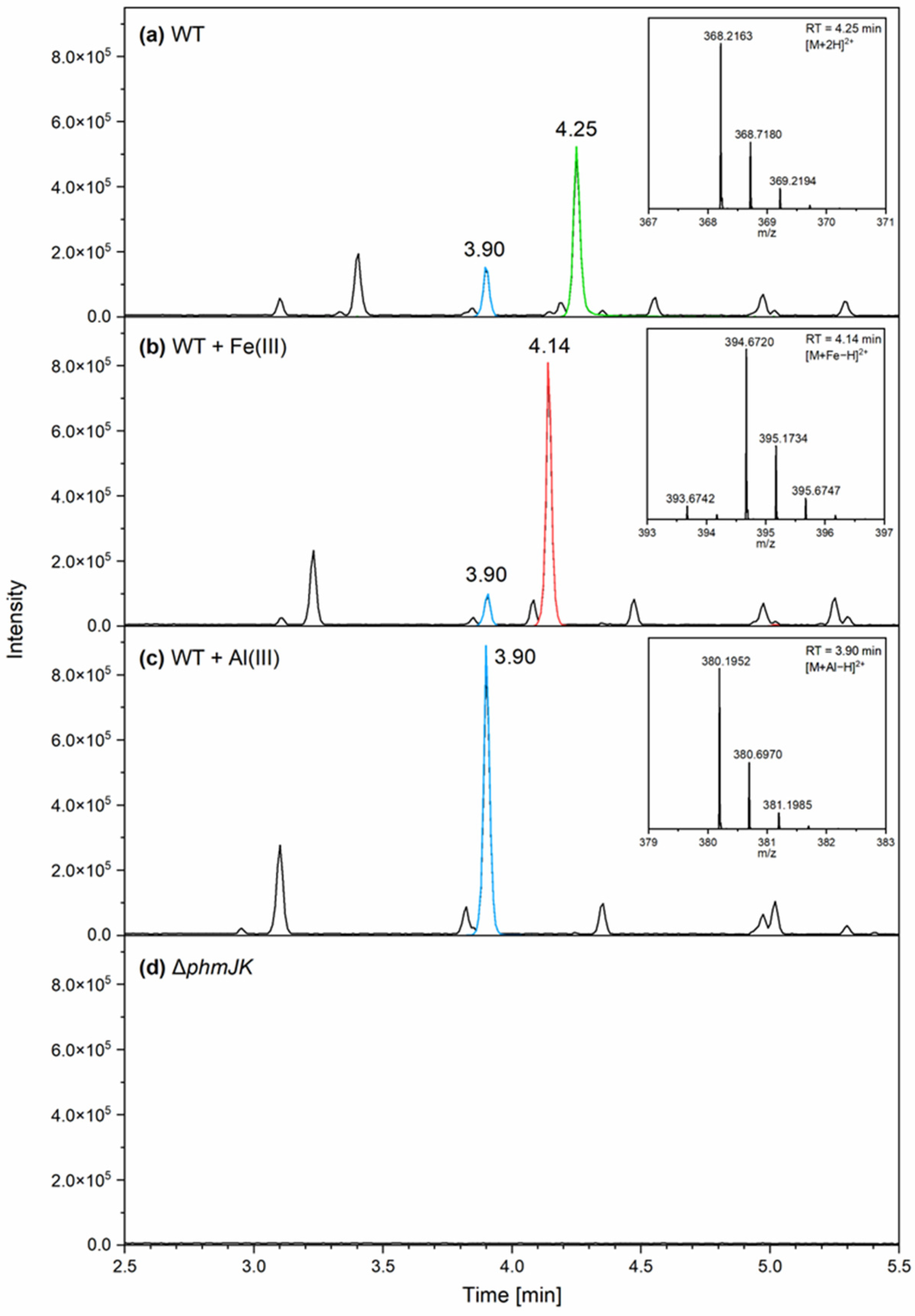
3.2. Phymabactin Chelates Fe(III) and Al(III)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MMS-2 | enhanced Mojave Mars Simulant 2 |
| UHPLC-MS | Ultra-high performance liquid chromatography–mass spectrometry |
| wt% | Percent per weight |
References
- Nguyen, M.; Knowling, M.; Tran, N.N.; Burgess, A.; Fisk, I.; Watt, M.; Escribà-Gelonch, M.; This, H.; Culton, J.; Hessel, V. Space farming: Horticulture systems on spacecraft and outlook to planetary space exploration. Plant Physiol. Biochem. 2023, 194, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Iovane, M.; Izzo, L.G.; Romano, L.E.; Aronne, G. Simulated microgravity affects directional growth of pollen tubes in candidate space crops. Front. Plant Sci. 2023, 14, 1186967. [Google Scholar] [CrossRef]
- Smith, S.M.; Lane, H.W.; Zwart, S.R. Spaceflight Metabolism and Nutritional Support. In Principles of Clinical Medicine for Space Flight; Springer: New York, NY, USA, 2020; pp. 413–439. [Google Scholar] [CrossRef]
- Odeh, R.; Guy, C.L. Gardening for Therapeutic People-Plant Interactions during Long-Duration Space Missions. Open Agric. 2017, 2, 1–13. [Google Scholar] [CrossRef]
- Nasseri, A.T.; Rasoul-Ami, S.; Morowvat, M.H.; Ghasemi, Y. Single Cell Protein: Production and Process. Am. J. Food Technol. 2011, 6, 103–116. [Google Scholar] [CrossRef]
- Li, Y.P.; Ahmadi, F.; Kariman, K.; Lackner, M. Recent advances and challenges in single cell protein (SCP) technologies for food and feed production. NPJ Sci. Food 2024, 8, 66. [Google Scholar] [CrossRef]
- Tei, F.; de Neve, S.; de Haan, J.; Kristensen, H.L. Nitrogen management of vegetable crops. Agric. Water Manag. 2020, 240, 106316. [Google Scholar] [CrossRef]
- Oyetunji, O.; Bolan, N.; Hancock, G. A comprehensive review on enhancing nutrient use efficiency and productivity of broadacre (arable) crops with the combined utilization of compost and fertilizers. J. Environ. Manag. 2022, 317, 115395. [Google Scholar] [CrossRef]
- Lindström, K.; Mousavi, S.A. Effectiveness of nitrogen fixation in rhizobia. Microb. Biotechnol. 2020, 13, 1314–1335. [Google Scholar] [CrossRef]
- Masson-Boivin, C.; Sachs, J.L. Symbiotic nitrogen fixation by rhizobia-the roots of a success story. Curr. Opin. Plant Biol. 2018, 44, 7–15. [Google Scholar] [CrossRef]
- Bellés-Sancho, P.; Beukes, C.; James, E.K.; Pessi, G. Nitrogen-Fixing Symbiotic Paraburkholderia Species: Current Knowledge and Future Perspectives. Nitrogen 2023, 4, 135–158. [Google Scholar] [CrossRef]
- Liu, X.; Hu, B.; Chu, C. Nitrogen assimilation in plants: Current status and future prospects. J. Genet. Genom. 2022, 49, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Vargas, L.K.; Volpiano, C.G.; Lisboa, B.B.; Giongo, A.; Beneduzi, A.; Passaglia, L.M.P. Potential of Rhizobia as Plant Growth-Promoting Rhizobacteria; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 153–174. [Google Scholar] [CrossRef]
- Bellés-Sancho, P.; Liu, Y.; Heiniger, B.; von Salis, E.; Eberl, L.; Ahrens, C.H.; Zamboni, N.; Bailly, A.; Pessi, G. A novel function of the key nitrogen-fixation activator NifA in beta-rhizobia: Repression of bacterial auxin synthesis during symbiosis. Front. Plant Sci. 2022, 13, 991548. [Google Scholar] [CrossRef]
- Bellés-Sancho, P.; Lardi, M.; Liu, Y.; Hug, S.; Pinto-Carbó, M.A.; Zamboni, N.; Pessi, G. Paraburkholderia phymatum Homocitrate Synthase NifV Plays a Key Role for Nitrogenase Activity during Symbiosis with Papilionoids and in Free-Living Growth Conditions. Cells 2021, 10, 952. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.J.; Mathesius, U. Phytohormone regulation of legume-rhizobia interactions. J. Chem. Ecol. 2014, 40, 770–790. [Google Scholar] [CrossRef]
- Zhao, C.Z.; Huang, J.; Gyaneshwar, P.; Zhao, D. Rhizobium sp. IRBG74 Alters Arabidopsis Root Development by Affecting Auxin Signaling. Front. Microbiol. 2017, 8, 2556. [Google Scholar] [CrossRef]
- Li, B.; Dong, C.; Chu, Z.; Zhang, W.; Wang, M.; Liu, H.; Xie, B. Synthesis, characterization and application of ion exchange resin as a slow-release fertilizer for wheat cultivation in space. Acta Astronaut. 2016, 127, 579–586. [Google Scholar] [CrossRef]
- Hug, S.; Liu, Y.; Heiniger, B.; Bailly, A.; Ahrens, C.H.; Eberl, L.; Pessi, G. Differential Expression of Paraburkholderia phymatum Type VI Secretion Systems (T6SS) Suggests a Role of T6SS-b in Early Symbiotic Interaction. Front. Plant Sci. 2021, 12, 699590. [Google Scholar] [CrossRef]
- Hug, S.; Heiniger, B.; Bolli, K.; Paszti, S.; Eberl, L.; Ahrens, C.H.; Pessi, G. Paraburkholderia sabiae Uses One Type VI Secretion System (T6SS-1) as a Powerful Weapon against Notorious Plant Pathogens. Microbiol. Spectr. 2023, 11, e0162223. [Google Scholar] [CrossRef]
- Geetha, S.J.; Joshi, S.J. Engineering rhizobial bioinoculants: A strategy to improve iron nutrition. Sci. World J. 2013, 2013, 315890. [Google Scholar] [CrossRef]
- Huo, Y.; Kang, J.P.; Ahn, J.C.; Kim, Y.J.; Piao, C.H.; Yang, D.U.; Yang, D.C. Siderophore-producing rhizobacteria reduce heavy metal-induced oxidative stress in Panax ginseng Meyer. J. Ginseng Res. 2021, 45, 218–227. [Google Scholar] [CrossRef]
- Fahde, S.; Boughribil, S.; Sijilmassi, B.; Amri, A. Rhizobia: A Promising Source of Plant Growth-Promoting Molecules and Their Non-Legume Interactions: Examining Applications and Mechanisms. Agriculture 2023, 13, 1279. [Google Scholar] [CrossRef]
- Schalk, I.J.; Hannauer, M.; Braud, A. New roles for bacterial siderophores in metal transport and tolerance. Environ. Microbiol. 2011, 13, 2844–2854. [Google Scholar] [CrossRef] [PubMed]
- Bellés-Sancho, P.; Lardi, M.; Liu, Y.; Eberl, L.; Zamboni, N.; Bailly, A.; Pessi, G. Metabolomics and Dual RNA-Sequencing on Root Nodules Revealed New Cellular Functions Controlled by Paraburkholderia phymatum NifA. Metabolites 2021, 11, 455. [Google Scholar] [CrossRef] [PubMed]
- de Campos, S.B.; Lardi, M.; Gandolfi, A.; Eberl, L.; Pessi, G. Mutations in Two Paraburkholderia phymatum Type VI Secretion Systems Cause Reduced Fitness in Interbacterial Competition. Front. Microbiol. 2017, 8, 2473. [Google Scholar] [CrossRef] [PubMed]
- Lardi, M.; de Campos, S.B.; Purtschert, G.; Eberl, L.; Pessi, G. Competition Experiments for Legume Infection Identify Burkholderia phymatum as a Highly Competitive β-Rhizobium. Front. Microbiol. 2017, 8, 1527. [Google Scholar] [CrossRef]
- Lardi, M.; Pessi, G. Functional Genomics Approaches to Studying Symbioses between Legumes and Nitrogen-Fixing Rhizobia. High Throughput 2018, 7, 15. [Google Scholar] [CrossRef]
- Liu, X.; You, S.; Liu, H.; Yuan, B.; Wang, H.; James, E.K.; Wang, F.; Cao, W.; Liu, Z.K. Diversity and Geographic Distribution of Microsymbionts Associated With Invasive Mimosa Species in Southern China. Front. Microbiol. 2020, 11, 563389. [Google Scholar] [CrossRef]
- Kunito, T.; Owaki, M.; Ihyo, Y.; Sumi, H.; Toda, H.; Fukuda, D.; Park, H.-D. Genera Burkholderia and Lipomyces are predominant aluminum-resistant microorganisms isolated from acidic forest soils using cycloheximide-amended growth media. Ann. Microbiol. 2012, 62, 1339–1344. [Google Scholar] [CrossRef]
- Golaz, D.; Papenfuhs, C.K.; Bellés-Sancho, P.; Eberl, L.; Egli, M.; Pessi, G. RNA-seq analysis in simulated microgravity unveils down-regulation of the beta-rhizobial siderophore phymabactin. NPJ Microgravity 2024, 10, 44. [Google Scholar] [CrossRef]
- Peters, G.H.; Abbey, W.; Bearman, G.H.; Mungas, G.S.; Smith, J.A.; Anderson, R.C.; Douglas, S.; Beegle, L.W. Mojave Mars simulant—Characterization of a new geologic Mars analog. Icarus 2008, 197, 470–479. [Google Scholar] [CrossRef]
- Lehner, B.A.E.; Haenggi, C.N.; Schleppi, J.; Brouns, S.J.J.; Cowley, A. Bacterial modification of lunar and Martian regolith for plant growth in life support systems. In Proceedings of the 69th International Astronautical Congress, IAC-18, A1, 7, 7, x42645, Bremen, Germany, 1–5 October 2018. [Google Scholar]
- Yan, L.; Riaz, M.; Li, S.; Cheng, J.; Jiang, C. Harnessing the power of exogenous factors to enhance plant resistance to aluminum toxicity; a critical review. Plant Physiol. Biochem. 2023, 203, 108064. [Google Scholar] [CrossRef]
- Peys, A.; Isteri, V.; Yliniemi, J.; Yorkshire, A.S.; Lemougna, P.N.; Utton, C.; Provis, J.L.; Snellings, R.; Hanein, T. Sustainable iron-rich cements: Raw material sources and binder types. Cem. Concr. Res. 2022, 157, 106834. [Google Scholar] [CrossRef]
- Clark, D.J.; Maaløe, O. DNA replication and the division cycle in Escherichia coli. J. Mol. Biol. 1967, 23, 99–112. [Google Scholar] [CrossRef]
- Szybalski, W. Gradient plates for the study of microbial resistance to antibiotics. Bacteriol. Proc. 1952, 36. [Google Scholar]
- Rehm, K.; Vollenweider, V.; Gu, S.; Friman, V.-P.; Kümmerli, R.; Wei, Z.; Bigler, L. Chryseochelins-structural characterization of novel citrate-based siderophores produced by plant protecting Chryseobacterium spp. Metallomics 2023, 15, mfad008. [Google Scholar] [CrossRef]
- Stephan, H.; Freund, S.; Beck, W.; Jung, G.; Meyer, J.M.; Winkelmann, G. Ornibactins--a new family of siderophores from Pseudomonas. Biometals 1993, 6, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Rehm, K.; Vollenweider, V.; Kümmerli, R.; Bigler, L. A comprehensive method to elucidate pyoverdines produced by fluorescent Pseudomonas spp. by UHPLC-HR-MS/MS. Anal. Bioanal. Chem. 2022, 414, 2671–2685. [Google Scholar] [CrossRef]
- Moulin, L.; Klonowska, A.; Caroline, B.; Booth, K.; Vriezen, J.A.C.; Melkonian, R.; James, E.K.; Young, J.P.W.; Bena, G.; Hauser, L.; et al. Complete Genome sequence of Burkholderia phymatum STM815(T), a broad host range and efficient nitrogen-fixing symbiont of Mimosa species. Stand. Genom. Sci. 2014, 9, 763–774. [Google Scholar] [CrossRef]
- Costa, N.; Bonetto, A.; Ferretti, P.; Casarotto, B.; Massironi, M.; Altieri, F.; Nava, J.; Favero, M. Analytical data on three Martian simulants. Data Brief 2024, 57, 111099. [Google Scholar] [CrossRef]
- Cannon, K.M.; Britt, D.T.; Smith, T.M.; Fritsche, R.F.; Batcheldor, D. Mars global simulant MGS-1: A Rocknest-based open standard for basaltic martian regolith simulants. Icarus 2019, 317, 470–478. [Google Scholar] [CrossRef]
- Achilles, C.N.; Downs, R.T.; Ming, D.W.; Rampe, E.B.; Morris, R.V.; Treiman, A.H.; Morrison, S.M.; Blake, D.F.; Vaniman, D.T.; Ewing, R.C.; et al. Mineralogy of an active eolian sediment from the Namib dune, Gale crater, Mars. JGR Planets 2017, 122, 2344–2361. [Google Scholar] [CrossRef]
- Yaroshevsky, A.A. Abundances of chemical elements in the Earth’s crust. Geochem. Int. 2006, 44, 48–55. [Google Scholar] [CrossRef]
- Agnoli, K.; Lowe, C.A.; Farmer, K.L.; Husnain, S.I.; Thomas, M.S. The ornibactin biosynthesis and transport genes of Burkholderia cenocepacia are regulated by an extracytoplasmic function sigma factor which is a part of the Fur regulon. J. Bacteriol. 2006, 188, 3631–3644. [Google Scholar] [CrossRef]
- Mathew, A.; Jenul, C.; Carlier, A.L.; Eberl, L. The role of siderophores in metal homeostasis of members of the genus Burkholderia. Environ. Microbiol. Rep. 2016, 8, 103–109. [Google Scholar] [CrossRef]
- Botté, A.; Zaidi, M.; Guery, J.; Fichet, D.; Leignel, V. Aluminium in aquatic environments: Abundance and ecotoxicological impacts. Aquat. Ecol. 2022, 56, 751–773. [Google Scholar] [CrossRef]
- Bhuvaneshwari, M.; Bairoliya, S.; Parashar, A.; Chandrasekaran, N.; Mukherjee, A. Differential toxicity of Al2O3 particles on Gram-positive and Gram-negative sediment bacterial isolates from freshwater. Environ. Sci. Pollut. Res. Int. 2016, 23, 12095–12106. [Google Scholar] [CrossRef]
- Luo, M.; Zang, R.; Wang, X.; Chen, Z.; Song, X.; Ju, J.; Huang, H. Natural Hydroxamate-Containing Siderophore Acremonpeptides A-D and an Aluminum Complex of Acremonpeptide D from the Marine-Derived Acremonium persicinum SCSIO 115. J. Nat. Prod. 2019, 82, 2594–2600. [Google Scholar] [CrossRef]
- Roy, N.; Chakrabartty, P.K. Effect of aluminum on the production of siderophore by Rhizobium sp. (Cicer arietinum). Curr. Microbiol. 2000, 41, 5–10. [Google Scholar] [CrossRef]
- Rouws, L.; Barauna, A.; Beukes, C.; Rouws, J.R.; de Faria, S.M.; Gross, E.; dos Reis, F.B.; Simon, M.F.; Maluk, M.; Odee, D.W.; et al. Soil characteristics drive contrasting patterns of association between symbiotic rhizobia of endemic and widespread Mimosa species in Brazil. Appl. Soil Ecol. 2024, 204, 105741. [Google Scholar] [CrossRef]
- Tripathi, M.; Munot, H.P.; Shouche, Y.; Meyer, J.M.; Goel, R. Isolation and functional characterization of siderophore-producing lead- and cadmium-resistant Pseudomonas putida KNP9. Curr. Microbiol. 2005, 50, 233–237. [Google Scholar] [CrossRef]
- Tripathi, M.; Kumar, S.; Makarana, G.; Goel, R. Metal-Tolerant Bioinoculant Pseudomonas putida KNP9 Mediated Enhancement of Soybean Growth under Heavy Metal Stress Suitable for Biofuel Production at the Metal-Contaminated Site. Energies 2023, 16, 4508. [Google Scholar] [CrossRef]
- Raihan, M.R.H.; Rahman, M.; Mahmud, N.U.; Adak, M.K.; Islam, T.; Fujita, M.; Hasanuzzaman, M. Application of Rhizobacteria, Paraburkholderia fungorum and Delftia sp. Confer Cadmium Tolerance in Rapeseed (Brassica campestris) through Modulating Antioxidant Defense and Glyoxalase Systems. Plants 2022, 11, 2738. [Google Scholar] [CrossRef] [PubMed]
- Moulin, L.; Munive, A.; Dreyfus, B.; Boivin-Masson, C. Nodulation of legumes by members of the beta-subclass of Proteobacteria. Nature 2001, 411, 948–950. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golaz, D.; Bürgi, L.; Egli, M.; Bigler, L.; Pessi, G. The Siderophore Phymabactin Facilitates the Growth of the Legume Symbiont Paraburkholderia phymatum in Aluminium-Rich Martian Soil. Life 2025, 15, 1044. https://doi.org/10.3390/life15071044
Golaz D, Bürgi L, Egli M, Bigler L, Pessi G. The Siderophore Phymabactin Facilitates the Growth of the Legume Symbiont Paraburkholderia phymatum in Aluminium-Rich Martian Soil. Life. 2025; 15(7):1044. https://doi.org/10.3390/life15071044
Chicago/Turabian StyleGolaz, Daphné, Luca Bürgi, Marcel Egli, Laurent Bigler, and Gabriella Pessi. 2025. "The Siderophore Phymabactin Facilitates the Growth of the Legume Symbiont Paraburkholderia phymatum in Aluminium-Rich Martian Soil" Life 15, no. 7: 1044. https://doi.org/10.3390/life15071044
APA StyleGolaz, D., Bürgi, L., Egli, M., Bigler, L., & Pessi, G. (2025). The Siderophore Phymabactin Facilitates the Growth of the Legume Symbiont Paraburkholderia phymatum in Aluminium-Rich Martian Soil. Life, 15(7), 1044. https://doi.org/10.3390/life15071044








