The ADVanced Organ Support (ADVOS) Hemodialysis System in Postoperative Cardiogenic Shock and Multiple Organ Failure: First Results in Cardiac Surgery Patients
Abstract
1. Introduction
2. Materials and Methods
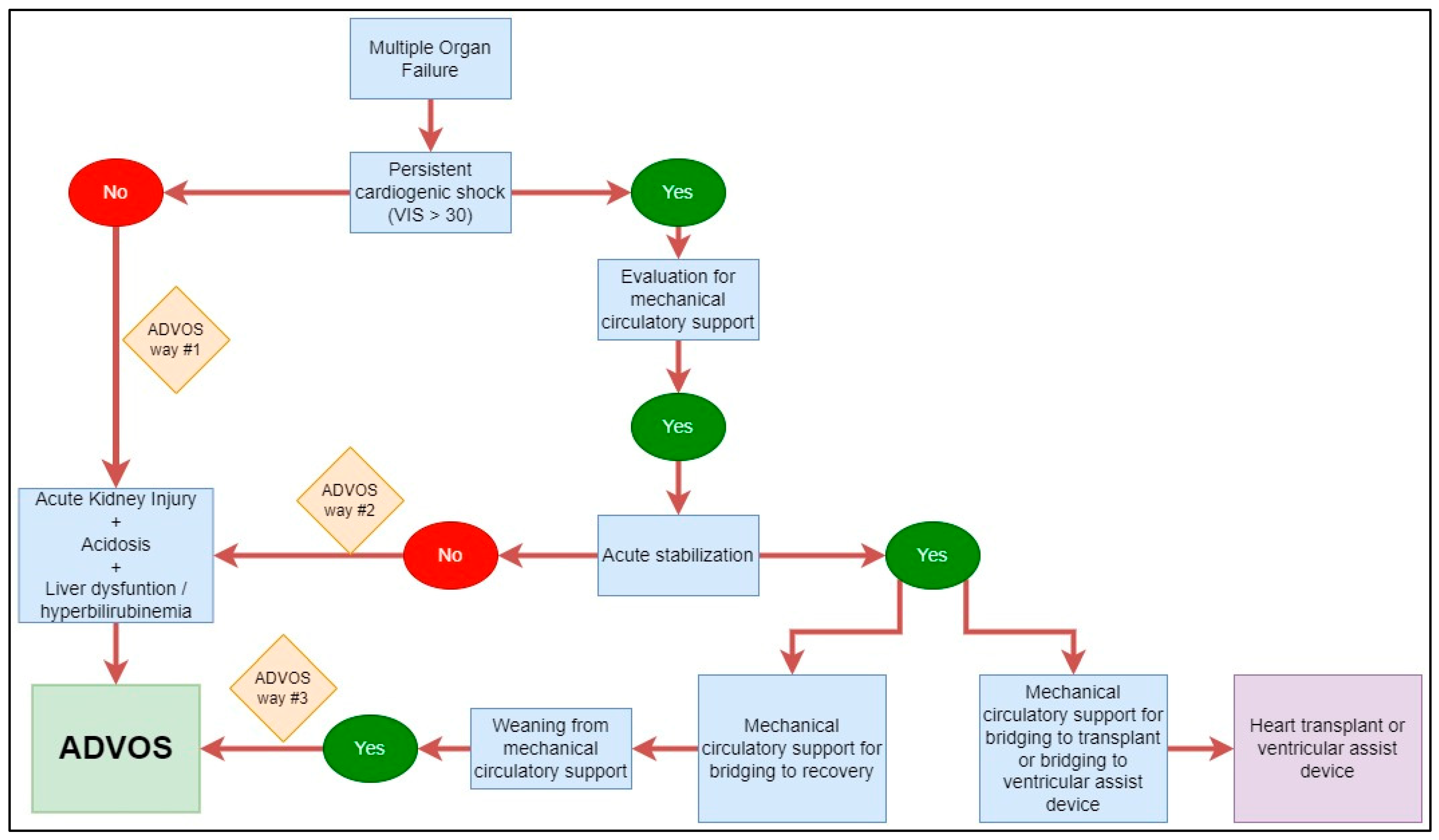
2.1. Patients and Study Design
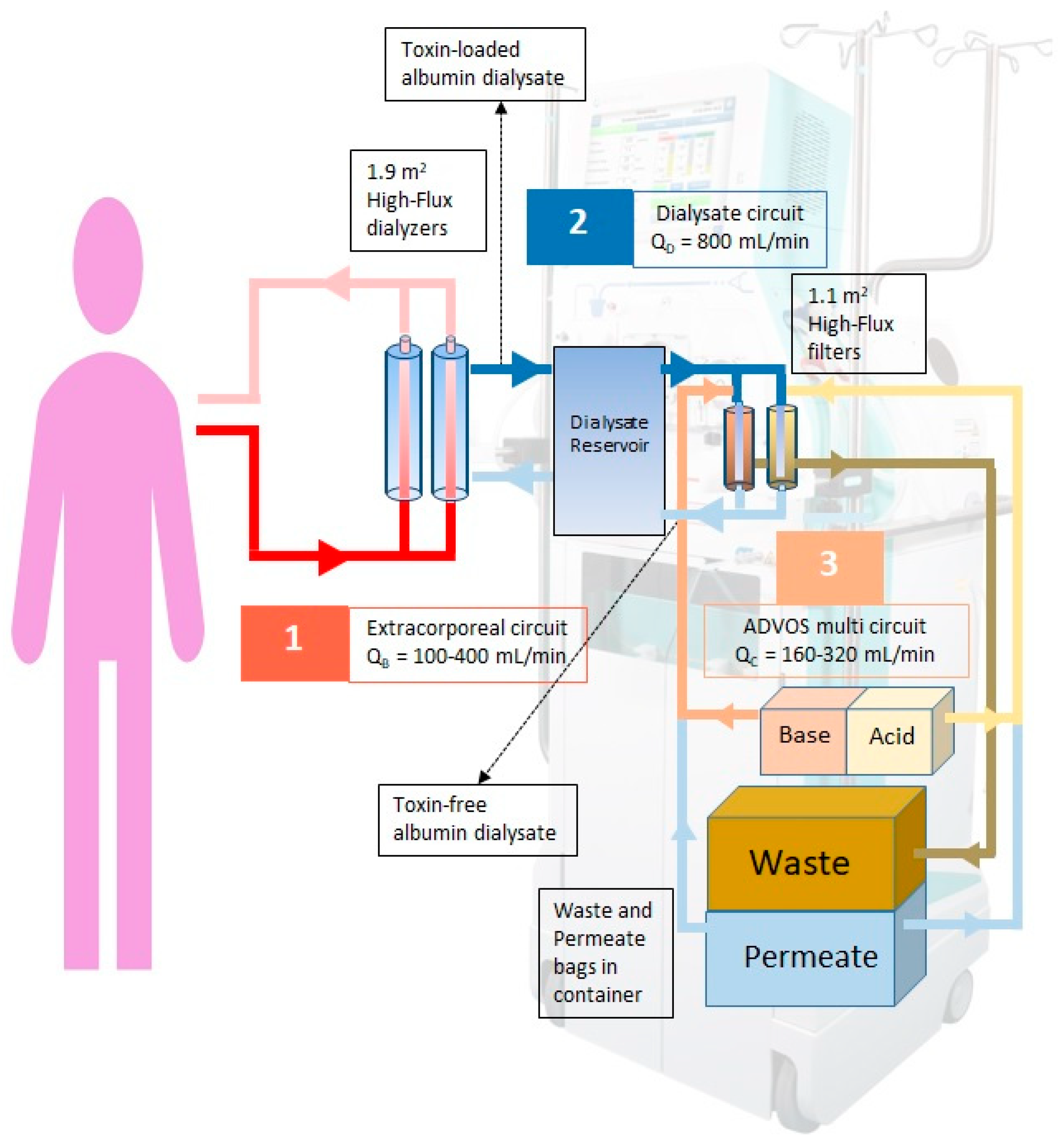
2.2. ADVOS Hemodialysis System
2.3. Intervention
2.4. Data Collection and Analysis
2.4.1. Vasoactive Inotropic Score (VIS)
2.4.2. Standardized Mortality Ratio (SMR)
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Extracorporeal Treatment Settings
3.3. Performance and Outcome
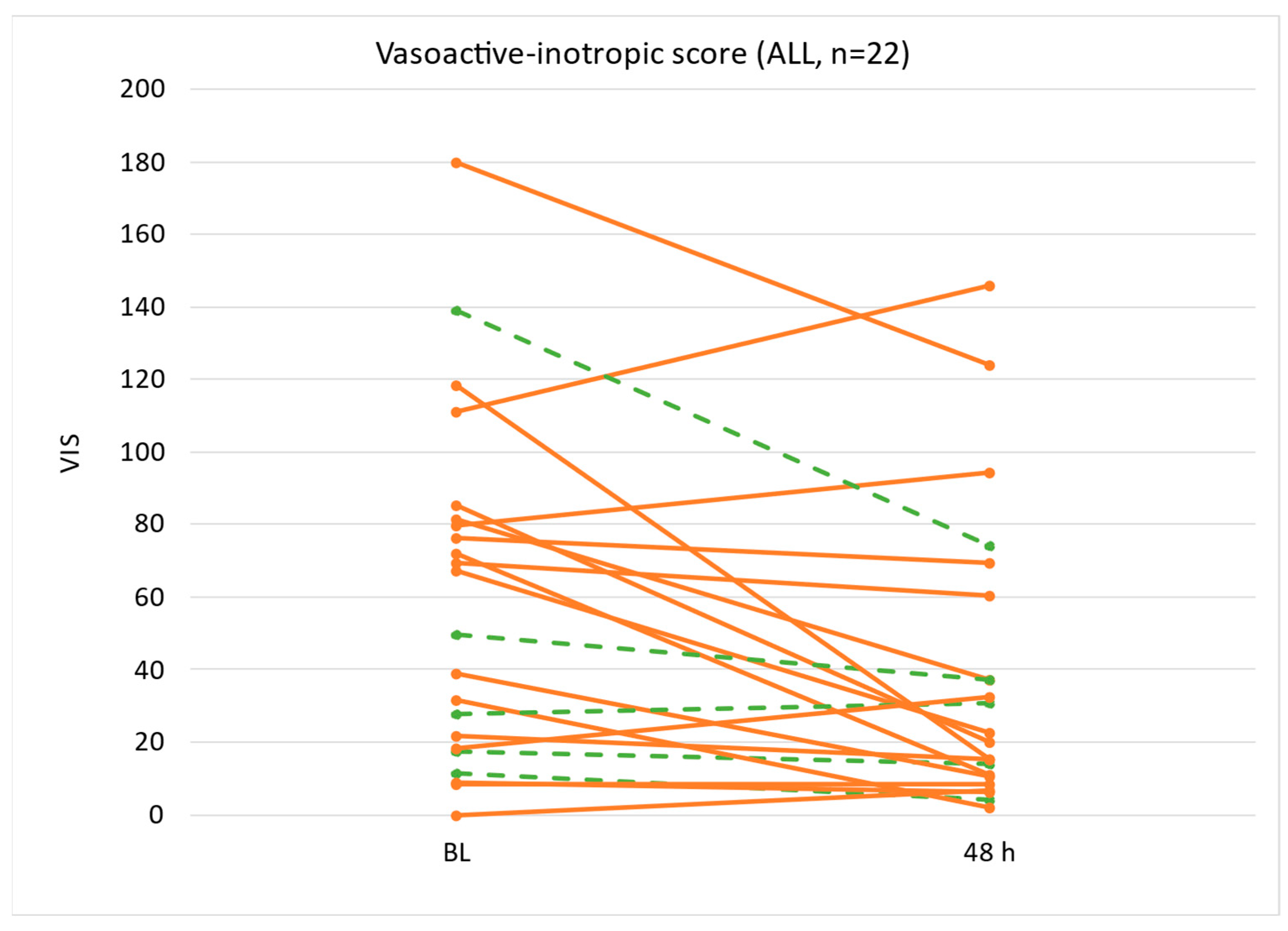
3.3.1. Vasoactive Inotropic Score
3.3.2. Change of Treatment Performance Parameters After Two Consecutive ADVOS Sessions
3.3.3. Standardized Mortality Ratio
4. Discussion
4.1. Interpretation and Generalizability
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laghlam, D.; Benghanem, S.; Ortuno, S.; Bouabdallaoui, N.; Manzo-Silberman, S.; Hamzaoui, O.; Aissaoui, N. Management of cardiogenic shock: A narrative review. Ann. Intensive Care 2024, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Jentzer, J.C.; Wiley, B.; Bennett, C.; Murphree, D.H.; Keegan, M.T.; Gajic, O.; Kashani, K.B.; Barsness, G.W. Early noncardiovascular organ failure and mortality in the cardiac intensive care unit. Clin. Cardiol. 2020, 43, 516–523. [Google Scholar] [CrossRef]
- Holland, E.M.; Moss, T.J. Acute Noncardiovascular Illness in the Cardiac Intensive Care Unit. J. Am. Coll. Cardiol. 2017, 69, 1999–2007. [Google Scholar] [CrossRef]
- Cheng, X.-F.; Wang, K.; Zhang, H.-T.; Zhang, H.; Jiang, X.-Y.; Lu, L.-C.; Chen, C.; Cheng, Y.-Q.; Wang, D.-J.; Li, K. Risk factors for postoperative myocardial injury-related cardiogenic shock in patients undergoing cardiac surgery. J. Cardiothorac. Surg. 2023, 18, 220. [Google Scholar] [CrossRef]
- van Diepen, S.; Katz, J.N.; Albert, N.M.; Henry, T.D.; Jacobs, A.K.; Kapur, N.K.; Kilic, A.; Menon, V.; Ohman, E.M.; Sweitzer, N.K.; et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement from the American Heart Association. Circulation 2017, 136, e232–e268. [Google Scholar] [CrossRef]
- Chioncel, O.; Parissis, J.; Mebazaa, A.; Thiele, H.; Desch, S.; Bauersachs, J.; Harjola, V.-P.; Antohi, E.-L.; Arrigo, M.; Ben Gal, T.; et al. Epidemiology, pathophysiology and contemporary management of cardiogenic shock—A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 1315–1341. [Google Scholar] [CrossRef] [PubMed]
- Jentzer, J.C.; Pöss, J.; Schaubroeck, H.; Morrow, D.A.; Hollenberg, S.M.; Mebazaa, A. Advances in the Management of Cardiogenic Shock. Crit. Care Med. 2023, 51, 1222–1233. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, A.-K.; Riebandt, J.; Bernardi, M.H.; Distelmaier, K.; Goliasch, G.; Zimpfer, D.; Laufer, G.; Wiedemann, D. Fate of patients weaned from post-cardiotomy extracorporeal life support. Eur. J. Cardiothorac. Surg. 2022, 61, 1178–1185. [Google Scholar] [CrossRef]
- Sasmita, B.R.; Wang, C.; Xie, S. Vasopressors and inotropes in cardiogenic shock patients: An analysis of the MIMIC-IV database. Front. Cardiovasc. Med. 2023, 10, 1300839. [Google Scholar] [CrossRef]
- Acharya, M.; Berger, R.; Popov, A.-F. The role of the ADVanced Organ Support (ADVOS) system in critically ill patients with multiple organ failure. Artif. Organs 2022, 46, 735–746. [Google Scholar] [CrossRef]
- Allescher, J.; Rasch, S.; Wiessner, J.R.; Perez Ruiz de Garibay, A.; Huberle, C.; Hesse, F.; Schulz, D.; Schmid, R.M.; Huber, W.; Lahmer, T. Extracorporeal carbon dioxide Removal (ECCO(2) R) with the Advanced Organ Support (ADVOS) system in critically ill COVID-19 patients. Artif. Organs 2021, 45, 1522–1532. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, V.; Weber, T.; Roedl, K.; Motaabbed, J.; Tariparast, A.; Jarczak, D.; de Garibay, A.P.R.; Kluwe, J.; Boenisch, O.; Herkner, H.; et al. Advanced organ support (ADVOS) in the critically ill: First clinical experience in patients with multiple organ failure. Ann. Intensive Care 2020, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Sommerfeld, O.; Neumann, C.; Becker, J.; von Loeffelholz, C.; Roth, J.; Kortgen, A.; Bauer, M.; Sponholz, C. Extracorporeal albumin dialysis in critically ill patients with liver failure: Comparison of four different devices-A retrospective analysis. Int. J. Artif. Organs 2023, 46, 481–491. [Google Scholar] [CrossRef]
- Belletti, A.; Lerose, C.C.; Zangrillo, A.; Landoni, G. Vasoactive-Inotropic Score: Evolution, Clinical Utility, and Pitfalls. J. Cardiothorac. Vasc. Anesth. 2021, 35, 3067–3077. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.L.; Bota, D.P.; Bross, A.; Melot, C.; Vincent, J.L. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001, 286, 1754–1758. [Google Scholar] [CrossRef]
- Alviar, C.L.; Miller, P.E.; McAreavey, D.; Katz, J.N.; Lee, B.; Moriyama, B.; Soble, J.; van Diepen, S.; Solomon, M.A.; Morrow, D.A. Positive Pressure Ventilation in the Cardiac Intensive Care Unit. J. Am. Coll. Cardiol. 2018, 72, 1532–1553. [Google Scholar] [CrossRef]
- Tavazzi, G.; Rossello, X.; Grand, J.; Gierlotka, M.; Sionis, A.; Ahrens, I.; Hassager, C.; Price, S. Epidemiology, monitoring, and treatment strategy in cardiogenic shock. A multinational cross-sectional survey of ESC-acute cardiovascular care association research section. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 706–711. [Google Scholar] [CrossRef]
- Henry, T.D.; Tomey, M.I.; Tamis-Holland, J.E.; Thiele, H.; Rao, S.V.; Menon, V.; Klein, D.G.; Naka, Y.; Piña, I.L.; Kapur, N.K.; et al. Invasive Management of Acute Myocardial Infarction Complicated by Cardiogenic Shock: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e815–e829. [Google Scholar] [CrossRef]
- Shankar, A.; Gurumurthy, G.; Sridharan, L.; Gupta, D.; Nicholson, W.J.; Jaber, W.A.; Vallabhajosyula, S. A Clinical Update on Vasoactive Medication in the Management of Cardiogenic Shock. Clin. Med. Insights Cardiol. 2022, 16, 11795468221075064. [Google Scholar] [CrossRef]
- Thiele, H.; Zeymer, U.; Akin, I.; Behnes, M.; Rassaf, T.; Mahabadi, A.A.; Lehmann, R.; Eitel, I.; Graf, T.; Seidler, T.; et al. Extracorporeal Life Support in Infarct-Related Cardiogenic Shock. N. Engl. J. Med. 2023, 389, 1286–1297. [Google Scholar] [CrossRef]
- Møller, J.E.; Engstrøm, T.; Jensen, L.O.; Eiskjær, H.; Mangner, N.; Polzin, A.; Schulze, P.C.; Skurk, C.; Nordbeck, P.; Clemmensen, P.; et al. Microaxial Flow Pump or Standard Care in Infarct-Related Cardiogenic Shock. N. Engl. J. Med. 2024, 390, 1382–1393. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, A.M.; Copeland, H.; Deswal, A.; Gluck, J.; Givertz, M.M. The International Society for Heart and Lung Transplantation/Heart Failure Society of America Guideline on Acute Mechanical Circulatory Support. J. Heart Lung Transplant. 2023, 42, e1–e64. [Google Scholar] [CrossRef] [PubMed]
- Sommer, W.; Arif, R.; Kremer, J.; Al Maisary, S.; Verch, M.; Tochtermann, U.; Karck, M.; Meyer, A.L.; Warnecke, G. Temporary circulatory support with surgically implanted microaxial pumps in postcardiotomy cardiogenic shock following coronary artery bypass surgery. JTCVS Open 2023, 15, 252–260. [Google Scholar] [CrossRef]
- Marini, M.; Belleggia, S.; Brugiatelli, L.; Francioni, M.; Battistoni, I.; Shkoza, M.; Pongetti, G.; Angelini, L.; Belfioretti, L.; Matassini, M.V. The golden hour in shock management: Do a lot, do it quickly. Eur. Heart J. Suppl. 2024, 26, i78–i83. [Google Scholar] [CrossRef]
- Nersesian, G.; Ott, S.; Fardman, A.; Lanmueller, P.; Lewin, D.; Bernhardt, A.; Emrich, F.; Faerber, G.; Szabó, G.; Oezkur, M.; et al. Temporary Mechanical Circulatory Support in Cardiogenic Shock Patients after Cardiac Procedures: Selection Algorithm and Weaning Strategies. Life 2023, 13, 2045. [Google Scholar] [CrossRef]
- Fuhrmann, V.; Kneidinger, N.; Herkner, H.; Heinz, G.; Nikfardjam, M.; Bojic, A.; Schellongowski, P.; Angermayr, B.; Kitzberger, R.; Warszawska, J.; et al. Hypoxic hepatitis: Underlying conditions and risk factors for mortality in critically ill patients. Intensive Care Med. 2009, 35, 1397–1405. [Google Scholar] [CrossRef]
- Abadeer, A.I.; Kurlansky, P.; Chiuzan, C.; Truby, L.; Radhakrishnan, J.; Garan, R.; Topkara, V.; Yuzefpolskaya, M.; Colombo, P.; Takeda, K.; et al. Importance of stratifying acute kidney injury in cardiogenic shock resuscitated with mechanical circulatory support therapy. J. Thorac. Cardiovasc. Surg. 2017, 154, 856–864. [Google Scholar] [CrossRef]
- Perez Ruiz de Garibay, A.; Kortgen, A.; Leonhardt, J.; Zipprich, A.; Bauer, M. Critical care hepatology: Definitions, incidence, prognosis and role of liver failure in critically ill patients. Crit. Care 2022, 26, 289. [Google Scholar] [CrossRef]
- Tu, G.-W.; Xu, J.-R.; Liu, L.; Zhu, D.-M.; Yang, X.-M.; Wang, C.-S.; Ma, G.-G.; Luo, Z.; Ding, X.-Q. Preemptive renal replacement therapy in post-cardiotomy cardiogenic shock patients: A historically controlled cohort study. Ann. Transl. Med. 2019, 7, 534. [Google Scholar] [CrossRef]
- Jentzer, J.C.; Kashani, K.B.; Wiley, B.M.; Patel, P.C.; Baran, D.A.; Barsness, G.W.; Henry, T.D.; van Diepen, S. Laboratory Markers of Acidosis and Mortality in Cardiogenic Shock: Developing a Definition of Hemometabolic Shock. Shock 2022, 57, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, F.G.; Kellum, J.A.; Park, M.; Ranzani, O.T.; Barbeiro, H.V.; de Souza, H.P.; da Cruz Neto, L.M.; da Silva, F.P. Relationship between acid-base status and inflammation in the critically ill. Crit. Care 2014, 18, R154. [Google Scholar] [CrossRef]
- Kellum, J.A.; Song, M.; Li, J. Science review: Extracellular acidosis and the immune response: Clinical and physiologic implications. Crit. Care 2004, 8, 331–336. [Google Scholar] [CrossRef][Green Version]
- Stringer, W.; Wasserman, K.; Casaburi, R.; Porszasz, J.; Maehara, K.; French, W. Lactic acidosis as a facilitator of oxyhemoglobin dissociation during exercise. J. Appl. Physiol. 1994, 76, 1462–1467. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, P.; Brimioulle, S.; Leeman, M.; Hallemans, R.; Melot, C.; Naeije, R. Enhancement of hypoxic pulmonary vasoconstriction by metabolic acidosis in dogs. Anesthesiology 1990, 73, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Farber, M.O.; Szwed, J.J.; Dowell, A.R.; Strawbridge, R.A. The acute effects of respiratory and metabolic acidosis on renal function in the dog. Clin. Sci. Mol. Med. 1976, 50, 165–169. [Google Scholar] [CrossRef]
- Tournadre, J.P.; Allaouchiche, B.; Malbert, C.H.; Chassard, D. Metabolic acidosis and respiratory acidosis impair gastro-pyloric motility in anesthetized pigs. Anesth. Analg. 2000, 90, 74–79. [Google Scholar] [CrossRef]
- Engstrom, M.; Schott, U.; Romner, B.; Reinstrup, P. Acidosis impairs the coagulation: A thromboelastographic study. J. Trauma. 2006, 61, 624–628. [Google Scholar] [CrossRef]
- Mitchell, J.H.; Wildenthal, K.; Johnson, R.L., Jr. The effects of acid-base disturbances on cardiovascular and pulmonary function. Kidney Int. 1972, 1, 375–389. [Google Scholar] [CrossRef]
- Jentzer, J.C.; Schrage, B.; Patel, P.C.; Kashani, K.B.; Barsness, G.W.; Holmes, D.R., Jr.; Blankenberg, S.; Kirchhof, P.; Westermann, D. Association Between the Acidemia, Lactic Acidosis, and Shock Severity with Outcomes in Patients with Cardiogenic Shock. J. Am. Heart Assoc. 2022, 11, e024932. [Google Scholar] [CrossRef]
- Perez Ruiz de Garibay, A.; Kellum, J.A.; Honigschnabel, J.; Kreymann, B. Respiratory and metabolic acidosis correction with the ADVanced Organ Support system. Intensive Care Med. Exp. 2019, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Skelton, L.A.; Boron, W.F.; Zhou, Y. Acid-base transport by the renal proximal tubule. J. Nephrol. 2010, 23, S4–S18. [Google Scholar]
- Dorman, P.J.; Sullivan, W.J.; Pitts, R.F. The renal response to acute respiratory acidosis. J. Clin. Investig. 1954, 33, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.R.; Sacha, G.L.; Siuba, M.T.; Lam, S.W.; Reddy, A.J.; Duggal, A.; Vachharajani, V. Association of Arterial pH With Hemodynamic Response to Vasopressin in Patients with Septic Shock: An Observational Cohort Study. Crit. Care Explor. 2022, 4, e0634. [Google Scholar] [CrossRef]
- Campbell, G.S.; Houle, D.B.; Crisp, N.W., Jr.; Weil, M.H.; Brown, E.B., Jr. Depressed response to intravenous sympathicomimetic agents in humans during acidosis. Dis. Chest 1958, 33, 18–22. [Google Scholar] [CrossRef]
- Weil, M.H.; Houle, D.B.; Brown, E.B., Jr.; Campbell, G.S.; Heath, C. Vasopressor agents; influence of acidosis on cardiac and vascular responsiveness. Calif. Med. 1958, 88, 437–440. [Google Scholar] [PubMed]
- Wang, B.C.; Sundet, W.D.; Goetz, K.L. Vasopressin in plasma and cerebrospinal fluid of dogs during hypoxia or acidosis. Am. J. Physiol. 1984, 247, E449–E455. [Google Scholar] [CrossRef]
- Makhoul, M.; Heuts, S.; Mansouri, A.; Taccone, F.S.; Obeid, A.; Mirko, B.; Broman, L.M.; Malfertheiner, M.V.; Meani, P.; Raffa, G.M.; et al. Understanding the “Extracorporeal Membrane Oxygenation Gap” in Veno-Arterial Configuration for Adult Patients: Timing and Causes of Death. Artif. Organs 2021, 45, 1155–1167. [Google Scholar] [CrossRef]
- Jentzer, J.C.; van Diepen, S. The SCAI Shock Classification Has a New Home: The Cardiac Surgery Intensive Care Unit. J. Am. Coll. Cardiol. 2023, 82, 1707–1710. [Google Scholar] [CrossRef]
- Roeschl, T.; Hinrichs, N.; Hommel, M.; Pfahringer, B.; Balzer, F.; Falk, V.; O‘Brien, B.; Ott, S.C.; Potapov, E.; Schoenrath, F.; et al. Systematic Assessment of Shock Severity in Postoperative Cardiac Surgery Patients. J. Am. Coll. Cardiol. 2023, 82, 1691–1706. [Google Scholar] [CrossRef]
- Fuhrmann, V.; Perez Ruiz de Garibay, A.; Faltlhauser, A.; Tyczynski, B.; Jarczak, D.; Lutz, J.; Weinmann-Menke, J.; Kribben, A.; Kluge, S. Registry on extracorporeal multiple organ support with the advanced organ support (ADVOS) system: 2-year interim analysis. Medicine 2021, 100, e24653. [Google Scholar] [CrossRef] [PubMed]
- Falkensteiner, C.; Kortgen, A.; Leonhardt, J.; Bauer, M.; Sponholz, C. Comparison of albumin dialysis devices molecular adsorbent recirculating system and ADVanced Organ Support system in critically ill patients with liver failure—A retrospective analysis. Ther. Apher. Dial. 2020, 25, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Kaps, L.; Ahlbrand, C.J.; Gadban, R.; Nagel, M.; Labenz, C.; Klimpke, P.; Holtz, S.; Boedecker, S.; Michel, M.; Kremer, W.M.; et al. Applicability and safety of discontinuous ADVanced Organ Support (ADVOS) in the treatment of patients with acute-on-chronic liver failure (ACLF) outside of intensive care. PLoS ONE 2021, 16, e0249342. [Google Scholar] [CrossRef] [PubMed]


| Parameters | ALL (n = 22) |
|---|---|
| Age (years) | 59 (51–65) |
| Sex (male, %) | 77% |
| Body height | 175 (172–178) |
| Body weight | 88 (72–100) |
| Vasoactive and supportive substances (%) | 95% |
| VIS | 59 (19–81) |
| Mechanical ventilation (%) | 95% |
| BIPAP | 59% |
| ASB | 36% |
| NIV CPAP | 5% |
| Acidosis (%) | 64% |
| Secondary acquired liver injury/failure | 95% |
| CLIF-C ACLF score | 57 (54–59) |
| n.d. | 5% |
| ACLF grade 1 | 0% |
| ACLF grade 2 | 27% |
| ACLF grade 3 | 68% |
| MELD score | 27 (25–30) |
| Glasgow coma score | 4 (4–4) |
| SOFA score | 15 (14–18) |
| SOFA cardiac | 4 (4–4) |
| SOFA respiratory | 2 (1–2) |
| SOFA liver | 2 (1–3) |
| SOFA kidney | 3 (2–4) |
| SOFA coagulation | 2 (1–2) |
| SOFA GCS | 4 (4–4) |
| Interventions | ALL (n = 22) |
|---|---|
| Prior emergency surgery | 9% |
| Prior AMI (<48 h) | 0% |
| CS prior surgery | 5% |
| CPR prior surgery | 0% |
| ECLS w/o surgery | 5% |
| Durable MCS | 5% |
| LVAD | 5% |
| LVAD/RVAD | 0% |
| TAH | 0% |
| BiVAD | 0% |
| Post HTx | 5% |
| Impella | 18% |
| Combination procedure prior surgery | 9% |
| CABG | 0% |
| AVR | 18% |
| MVR | 9% |
| Aortic surgery | 5% |
| Acute Infective Endocarditis | 5% |
| Acute Type A Aortic Dissection | 0% |
| COPD | 0% |
| Pulmonary Hypertension | 5% |
| HFrEF | 5% |
| HFmrEF | 0% |
| HFpEF | 0% |
| Moderately/severely reduced right ventricular function | 18% |
| Chronic neuropathology | 5% |
| Chronic renal insufficiency | 5% |
| Acute kidney injury | 64% |
| Chronic liver insufficiency | 0% |
| ADVOS | ALL (n = 22) | |
|---|---|---|
| Total number of treatment sessions | 110 | |
| Treatment session/patient | 4 (3–4) | |
| Median treatment duration (h) | 23 (16–24) | |
| Median blood flow (mL/min) | 150 (150–150) | |
| Median concentrate flow (mL/min) | 160 (160–160) | |
| Median dialysate pH value | 7.8 (7.6–8.0) | |
| Maximal dialysate pH value | 7.8 (7.8–8.5) | |
| Median ultrafiltration volume (mL) | 2072 (754–3438) | |
| CiCa anticoagulation (% of treatments) | 98% | |
| Additional anticoagulation in patient (e.g., heparin) (% of treatments) | 75% | |
| Total number of treatment abortions (n, %) | 4 (4%) | |
| ECLS | ALL (n = 22) | |
| Time | BL | 48 h |
| Number of patients on ECLS | 10 | 7 |
| Median blood flow (L/min) | 2.6 (1.8–3.9) | 4.2 (3.1–4.3) |
| Median sweep gas flow (L/min) | 4.0 (2.6–4.0) | 3.0 (2.4–4.4) |
| Impella | ALL (n =22) | |
| Time | BL | 48 h |
| Number of patients on Impella | 8 | 8 |
| Median blood flow (L/min) | 3.4 (3.1–4.1) | 3.1 (2.5–4.4) |
| Parameter | ALL (n = 22) | ||||
|---|---|---|---|---|---|
| BL | 24 h | 48 h | Diff. (BL vs. 48 h) | Sig. | |
| Noradrenaline highest dose (µg/kg/min) | 0.470 (0.125–0.755) | 0.225 (0.108–0.488) | 0.180 (0.071–0.435) | −0.199 (−0.056–−0.242) | 0.009 |
| Vasoactive inotropic score | 59 (19–81) | 31 (17–58) | 21 (11–55) | −21 (−7–−36) | 0.007 |
| Parameter | ALL (n = 22) | |||
|---|---|---|---|---|
| BL | 48 h | Diff. | Sig. | |
| Bilirubin (mg/dL) | 5.0 (1.8–9.0) | 6.6 (2.4–10.1) | 0.8 (2.3–−0.6) | 0.241 |
| Creatinine (mg/dL) | 1.32 (0.99–2.30) | 1.04 (0.70–1.48) | −0.5 (−0.2–−0.9) | 0.002 |
| BUN (mg/dL) | 12.3 (7.7–16.1) | 6.3 (4.7–9.6) | −6.3 (−3.2–−9.5) | 0.000 |
| IL-6 (ng/L) | 213 (94–647) | 136 (105–365) | −394 (92–−879) | 0.105 |
| Procalcitonin (ng/mL) | 1.3 (0.6–5.6) | 3.3 (1.2–6.7) | 1.5 (6.1–−3.1) | 0.489 |
| Lactate (ng/mL) | 2.3 (1.7–4.7) | 1.8 (1.2–2.2) | −1.7 (0.2–−3.5) | 0.077 |
| Blood pH | 7.33 (7.29–7.40) | 7.44 (7.38–7.44) | 0.08 (0.13–0.04) | 0.001 |
| Bicarbonate (mmol/L) | 22.6 (20.5–24.5) | 26.8 (25.5–27.8) | 4.2 (6.2–2.3) | 0.000 |
| Base excess (mmol/L) | −3.2 (−5.0-1.5) | 2.4 (0.6–3.1) | 5.5 (7.9–3.1) | 0.000 |
| Na+ (mmol/L) | 141 (133–145) | 138 (136–140) | −1.0 (1.8–−3.7) | 0.473 |
| Cl− (mmol/L) | 105 (101–110) | 105 (103–108) | −1.1 (1.9–−4.1) | 0.462 |
| Phosphate (mmol/L) | 1.54 (1.03–2.06) | 1.07 (0.84–1.53) | −0.40 (−0.10–−0.69) | 0.010 |
| RBCs (/pL) | 3.0 (2.8–3.3) | 2.9 (2.7–3.1) | −0.1 (0.0–−0.3) | 0.067 |
| WBCs (/nL) | 10.9 (8.5–16.8) | 11.9 (9.5–16.0) | −0.2 (2.8–−3.3) | 0.886 |
| Platelets (/nL) | 107 (87–150) | 101 (80–122) | −24 (8–−55) | 0.132 |
| FiO2 | 0.50 (0.45–0.69) | 0.40 (0.31–0.49) | 0.13 (0.05–0.20) | 0.002 |
| PEEP (mbar) | 12 (11–14) | 10 (10–12) | 1.3 (0.3–2.4) | 0.017 |
| Tidal Volume (mL/kg) | 5.6 (4.4–6.2) | 5.3 (4.7–6.5) | −0.21 (−0.7–0.3) | 0.388 |
| Driving Pressure (mbar) | 12 (10–15) | 12 (10–16) | −0.2 (−1.8–1.4) | 0.761 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walter, V.; Hinrichs, E.; Alloush, T.; de Garibay, A.P.R.; Warnecke, G.; Sommer, W.; Gravert, H.; Grothusen, C.; Becker, J.; Thiem, A.; et al. The ADVanced Organ Support (ADVOS) Hemodialysis System in Postoperative Cardiogenic Shock and Multiple Organ Failure: First Results in Cardiac Surgery Patients. Life 2025, 15, 1042. https://doi.org/10.3390/life15071042
Walter V, Hinrichs E, Alloush T, de Garibay APR, Warnecke G, Sommer W, Gravert H, Grothusen C, Becker J, Thiem A, et al. The ADVanced Organ Support (ADVOS) Hemodialysis System in Postoperative Cardiogenic Shock and Multiple Organ Failure: First Results in Cardiac Surgery Patients. Life. 2025; 15(7):1042. https://doi.org/10.3390/life15071042
Chicago/Turabian StyleWalter, Veronika, Ekaterina Hinrichs, Tarek Alloush, Aritz Perez Ruiz de Garibay, Gregor Warnecke, Wiebke Sommer, Hanna Gravert, Christina Grothusen, Janine Becker, Alexander Thiem, and et al. 2025. "The ADVanced Organ Support (ADVOS) Hemodialysis System in Postoperative Cardiogenic Shock and Multiple Organ Failure: First Results in Cardiac Surgery Patients" Life 15, no. 7: 1042. https://doi.org/10.3390/life15071042
APA StyleWalter, V., Hinrichs, E., Alloush, T., de Garibay, A. P. R., Warnecke, G., Sommer, W., Gravert, H., Grothusen, C., Becker, J., Thiem, A., & Panholzer, B. (2025). The ADVanced Organ Support (ADVOS) Hemodialysis System in Postoperative Cardiogenic Shock and Multiple Organ Failure: First Results in Cardiac Surgery Patients. Life, 15(7), 1042. https://doi.org/10.3390/life15071042






