The Effect of the Number of Adjuvant Chemotherapy Cycles Following Cancer Surgery on Taste Alteration, Energy Intake, and Life Quality: 6-Month Follow-Up Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection Process
2.2. Taste Alteration
2.3. Quality of Life
2.4. Energy Requirement and Energy Intake at T1
2.5. Statistical Analysis
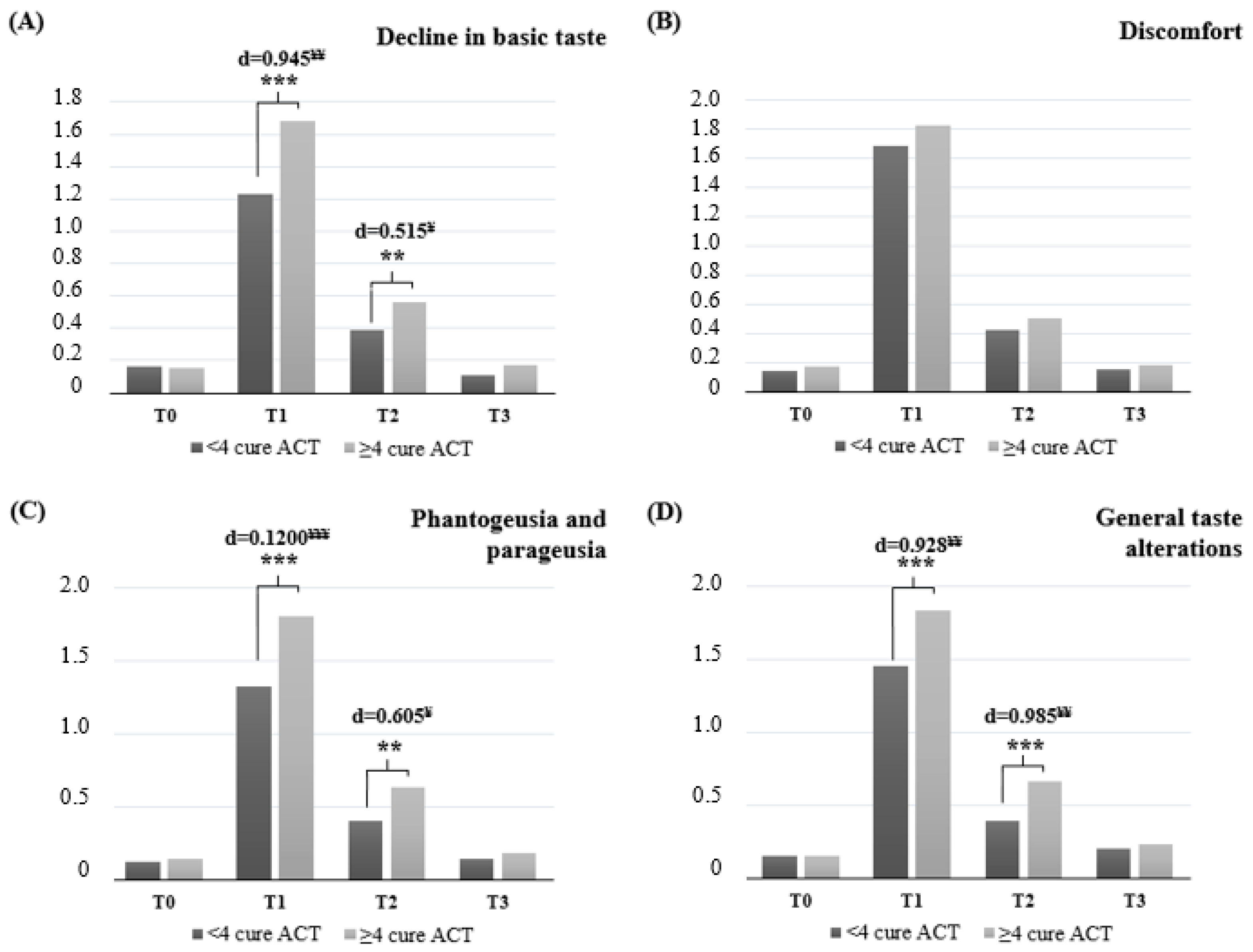
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACT | Adjuvant chemotherapy |
| TAs | Taste alterations |
| QoL | Quality of life |
| BeBis | Nutrition Information System |
| CiTAS | Chemotherapy-induced Taste Alteration Scale |
| FACT-G | Functional Assessment of Cancer Therapy |
| PWB | Phsical well-being |
| SWB | Social and family well-being |
| EWB | Emotional well-being |
| FWB | Functional well-being |
| BMR | Basal metabolic rate |
| PAL | Physical activity level |
References
- Liu, H.; Ma, X.; Sun, C.; Wu, M.; Xu, Z.; Zhou, S.; Yao, N.; Liu, S.; Qin, X.; Han, Z. Concurrent chemoradiotherapy followed by adjuvant chemotherapy versus concurrent chemoradiotherapy alone in locally advanced cervical cancer: A systematic review and meta-analysis. Front. Oncol. 2022, 12, 997030. [Google Scholar] [CrossRef]
- Knight, K.R.G.; Kraemer, D.F.; Neuwelt, E.A. Ototoxicity in children receiving platinum chemotherapy: Underestimating a commonly occurring toxicity that may influence academic and social development. J. Clin. Oncol. 2005, 23, 8588–8596. [Google Scholar] [CrossRef]
- Quasthoff, S.; Hartung, H.P. Chemotherapy-induced peripheral neuropathy. J. Neurol. 2002, 249, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Zabernigg, A.; Gamper, E.M.; Giesinger, J.M.; Rumpold, G.; Kemmler, G.; Gattringer, K.; Sperner-Unterweger, B.; Holzner, B. Taste alterations in cancer patients receiving chemotherapy: A neglected side effect? Oncologist 2010, 15, 913–920. [Google Scholar] [CrossRef]
- Jensen, S.B.; Mouridsen, H.T.; Bergmann, O.J.; Reibel, J.; Brünner, N.; Nauntofte, B. Oral mucosal lesions, microbial changes, and taste disturbances induced by adjuvant chemotherapy in breast cancer patients. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2008, 106, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Lindley, C.; McCune, J.S.; Thomason, T.E.; Lauder, D.; Sauls, A.; Adkins, S.; Sawyer, M.T. Perception of Chemotherapy Side Effects Cancer versus Noncancer Patients. Cancer Pract. 1999, 7, 59–65. [Google Scholar] [CrossRef]
- Bernhardson, B.M.; Tishelman, C.; Rutqvist, L.E. Self-reported taste and smell changes during cancer chemotherapy. Support. Care Cancer 2008, 16, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Rehwaldt, M.; Wickham, R.; Purl, S.; Tariman, J.; Blendowski, C.; Shott, S.; Lappe, M. Self-care strategies to cope with taste changes after chemotherapy. Oncol. Nurs. Forum. 2009, 36, E47–E56. [Google Scholar] [CrossRef]
- Ravasco, P.; Monteiro-Grillo, I.; Marques Vidal, P.; Camilo, M.E. Impact of nutrition on outcome: A prospective randomized controlled trial in patients with head and neck cancer undergoing radiotherapy. Head. Neck 2005, 27, 659–668. [Google Scholar] [CrossRef]
- Boltong, A.; Keast, R.; Aranda, S. Talking about taste: How do oncology clinicians discuss and document taste problems? Cancer Forum 2011, 35, 81–87. Available online: https://search.informit.org/doi/abs/10.3316/INFORMIT.266241821732306 (accessed on 15 May 2024).
- Brewin, T. Can a tumour cause the same appetite perversion or taste change as a pregnancy? Lancet 1980, 316, 907–908. [Google Scholar] [CrossRef] [PubMed]
- DeWys, W.D. Changes in taste sensation and feeding behaviour in cancer patients: A review. J. Hum. Nutr. 1978, 32, 447–453. [Google Scholar] [PubMed]
- Ripamonti, C.; Zecca, E.; Brunelli, C.; Fulfaro, F.; Villa, S.; Balzarini, A.; Bombardieri, E.; De Conno, F. A randomized, controlled clinical trial to evaluate the effects of zinc sulfate on cancer patients with taste alterations caused by head and neck irradiation. Cancer 1998, 82, 1938–1945. [Google Scholar] [CrossRef]
- Hong, J.H.; Omur-Ozbek, P.; Stanek, B.T.; Dietrich, A.M.; Duncan, S.E.; Lee, Y.W.; Lesser, G. Taste and odor abnormalities in cancer patients. J. Support. Oncol. 2009, 7, 58–65. Available online: https://www.researchgate.net/publication/24395309 (accessed on 9 June 2024).
- Comeau, T.B.; Epstein, J.B.; Migas, C. Taste and smell dysfunction in patients receiving chemotherapy: A review of current knowledge. Support. Care Cancer 2001, 9, 575–580. [Google Scholar] [CrossRef]
- Schiffman, S.S. Critical illness and changes in sensory perception. Proc. Nutr. Soc. 2007, 66, 331–345. [Google Scholar] [CrossRef]
- Doty, R.L.; Shah, M.; Bromley, S.M. Drug-Induced Taste Disorders. Drug Saf. 2008, 31, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, S.S.; Sattely-Miller, E.A.; Taylor, E.L.; Graham, B.G.; Landerman, L.R.; Zervakis, J.; Campagna, L.K.; Cohen, H.J.; Blackwell, S.; Garst, J.L. Combination of flavor enhancement and chemosensory education improves nutritional status in older cancer patients. J. Nutr. Health Aging 2007, 11, 439. Available online: https://www.proquest.com/scholarly-journals/combination-flavor-enhancement-chemosensory/docview/222241178/se-2 (accessed on 22 June 2024).
- Schiffman, S.S.; Graham, B.G. Taste and smell perception affect appetite and immunity in the elderly. Eur. J. Clin. Nutr. 2000, 54, 54–63. [Google Scholar] [CrossRef]
- Bernhardson, B.M. 5 themes described the experiences of patients with chemotherapy induced oral mucositis. Evid. Based Nurs. 2003, 6, 62. [Google Scholar] [CrossRef]
- Bernhardson, B.M.; Tishelman, C.; Rutqvist, L.E. Chemosensory changes experienced by patients undergoing cancer chemotherapy: A qualitative interview study. J. Pain. Symptom Manag. 2007, 34, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Strasser, F.; Demmer, R.; Böhme, C.; Schmitz, S.F.H.; Thuerlimann, B.; Cerny, T.; Gillessen, S. Prevention of docetaxel-or paclitaxel-associated taste alterations in cancer patients with oral glutamine: A randomized, placebo-controlled, double-blind study. Oncologist 2008, 13, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Ahn, M.S.; Choi, Y.W.; Kang, S.Y.; Choi, J.H.; Lee, H.W.; Park, M.; Kim, H. Analysis of treatment outcomes according to the cycles of adjuvant chemotherapy in gastric cancer: A retrospective nationwide cohort study. BMC Cancer 2022, 22, 948. [Google Scholar] [CrossRef]
- Jeong, S.H.; Yoo, M.W.; Son, Y.G.; Oh, S.J.; Kim, J.H.; Kim, H.I.; Park, J.-M.; Hur, H.; Jee, Y.S.; Hwang, S.-H.; et al. Appropriate Number of Adjuvant Chemotherapy Cycles for Patients with Stage 2 or 3 Gastric Cancer After Curative Gastrectomy: A Multicenter Cohort Study. Ann. Surg. Oncol. 2021, 28, 4458–4470. [Google Scholar] [CrossRef]
- Kano, T.; Kanda, K. Development and validation of a chemotherapy-induced taste alteration scale. Oncol. Nurs. Forum 2013, 40, E79–E85. Available online: https://researchmap.jp/read0037630/published_papers/12234650/attachment_file.pdf (accessed on 3 July 2024). [CrossRef] [PubMed]
- Sozeri, E.; Kutluturkan, S. The Validity and Reliability of Turkish Version of the Chemotherapy-induced Taste Alteration Scale (CiTAS). Clin. Nurs. Res. 2018, 27, 235–249. [Google Scholar] [CrossRef]
- Cella, D.F.; Tulsky, D.S.; Gray, G.; Sarafian, B.; Linn, E.; Bonomi, A.; Silberman, M.; Yellen, S.B.; Winicour, P.; Brannon, J. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J. Clin. Oncol. 1993, 11, 570–579. [Google Scholar] [CrossRef]
- Ay, S.; Parvizi, M. The functional assessment of cancer therapy general FACT-G scale reliability and validity of a Turkish version. Celal Bayar Üniversitesi Sağlık Bilim. Enstitüsü Derg. 2021, 8, 198–203. [Google Scholar] [CrossRef]
- FAO/WHO/UNU Expert Consultation. Nutrition Technical Report Series 1. Human Energy Requirements. Report of a Joint FAO/WHO/UNU Expert Consultation; FAO: Rome, Italy, 2001; pp. 17–24. [Google Scholar]
- Harris, J.A.; Benedict, F.G. A Biometric Study of Basal Metabolism in Man; Carnegie institution of Washington: Washington, DC, USA, 1919. [Google Scholar]
- Jané, M.; Xiao, Q.; Yeung, S.; Azevedo, F.; Ben-Shachar, M.S.; Caldwell, A.R.; Cousineau, D.; Dunleavy, D.J.; Elsherif, M.; Harlow, T.J.; et al. Guide to Effect Sizes and Confidence Intervals. 2024. Available online: https://osf.io/download/87vke/ (accessed on 17 December 2024).
- Ponticelli, E.; Clari, M.; Frigerio, S.; De Clemente, A.; Bergese, I.; Scavino, E.; Bernardini, A.; Sacerdote, C. Dysgeusia and health-related quality of life of cancer patients receiving chemotherapy: A cross-sectional study. Eur. J. Cancer Care 2017, 26, e12633. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, N.; Zhang, Y.; Yan, J.; Chen, L.; He, H.; Sun, S.; Zhang, Y.; Zhang, M. Prevalence and risk factors of chemotherapy-induced taste alterations among cancer patients: A systematic review and meta-analysis. Eur. J. Oncol. Nurs. 2024, 74, 102735. [Google Scholar] [CrossRef]
- Al-Amouri, F.M.; Badrasawi, M. Taste alteration and its relationship with nutritional status among cancer patients receiving chemotherapy, cross-sectional study. PLoS ONE 2024, 19, e0302990. [Google Scholar] [CrossRef] [PubMed]
- Kiss, N.; Symons, K.; Hewitt, J.; Davis, H.; Ting, C.; Lee, A.; Boltong, A.; Tucker, R.M.; Tan, S.-Y. Taste function in adults undergoing cancer radiotherapy or chemotherapy, and implications for nutrition management: A systematic review. J. Acad. Nutr. Diet. 2021, 121, 278–304. [Google Scholar] [CrossRef]
- Spinelli, S.; Mini, E.; Monteleone, E.; Angiolini, C.; Roviello, G. Altertaste: Improving Food Pleasure and Intake of Oncology Patients Receiving Chemotherapy. Future Oncol. 2021, 17, 2573–2579. [Google Scholar] [CrossRef]
- Drareni, K.; Bensafi, M.; Giboreau, A.; Dougkas, A. Chemotherapy-induced taste and smell changes influence food perception in cancer patients. Support. Care Cancer 2021, 29, 2125–2132. [Google Scholar] [CrossRef]
- Turcott, J.G.; Juárez-Hernández, E.; De La Torre-Vallejo, M.; Sánchez-Lara, K.; Luvian-Morales, J.; Arrieta, O. Value: Changes in the Detection and Recognition Thresholds of Three Basic Tastes in Lung Cancer Patients Receiving Cisplatin and Paclitaxel and Its Association with Nutritional and Quality of Life Parameters. Nutr. Cancer 2016, 68, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Lara, K.; Sosa-Sánchez, R.; Green-Renner, D.; Rodríguez, C.; Laviano, A.; Motola-Kuba, D.; Arrieta, O. Influence of taste disorders on dietary behaviors in cancer patients under chemotherapy. Nutr. J. 2010, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, S.; Hummel, T.; Böhner, C.; Berktold, S.; Hundt, W.; Kriner, M.; Heinrich, P.; Sommer, H.; Hanusch, C.; Prechtl, A.; et al. Qualitative and Quantitative Assessment of Taste and Smell Changes in Patients Undergoing Chemotherapy for Breast Cancer or Gynecologic Malignancies. J. Clin. Oncol. 2009, 27, 1899–1905. [Google Scholar] [CrossRef]
- Pedersini, R.; di Mauro, P.; Zamparini, M.; Bosio, S.; Zanini, B.; Amoroso, V.; Turla, A.; Monteverdi, S.; Zanini, A.; Laini, L.; et al. Taste Alterations Do Not Affect Change in Food Habits and Body Weight in Breast Cancer Patients. In Vivo 2022, 36, 1860–1867. [Google Scholar] [CrossRef]
- Larsen, A.K.; Thomsen, C.; Sanden, M.; Skadhauge, L.B.; Anker, C.B.; Mortensen, M.N.; Bredie, W.L.P. Taste alterations and oral discomfort in patients receiving chemotherapy. Support. Care Cancer 2021, 29, 7431–7439. [Google Scholar] [CrossRef]
- Silva, I.M.; Donaduzzi, L.C.; Perini, C.C.; Couto, S.A.; Werneck, R.I.; de Araújo, M.R.; Kurahashi, M.; Johann, A.C.; Azevedo-Alanis, L.R.; Vieira, A.R.; et al. Association of xerostomia and taste alterations of patients receiving antineoplastic chemotherapy: A cause for nutritional concern. Clin. Nutr. ESPEN 2021, 43, 532–535. [Google Scholar] [CrossRef]
- Arikan, F.; Ergen, M.; Ozturk, E.; Kutluturkan, S. Taste alteration in cancer patients receiving chemotherapy: A cross-sectional study. Turk. J. Oncol. 2019, 34, 222–230. [Google Scholar] [CrossRef]
- Vrignaud, P.; Semiond, D.; Benning, V.; Beys, E.; Bouchard, H.; Gupta, S. Preclinical profile of cabazitaxel. Drug Des. Devel Ther. 2014, 8, 1851–1867. [Google Scholar] [CrossRef]
- Murtaza, B.; Hichami, A.; Khan, A.S.; Ghiringhelli, F.; Khan, N.A. Alteration in taste perception in cancer: Causes and strategies of treatment. Front. Physiol. 2017, 8, 134. [Google Scholar] [CrossRef]
- Kaizu, M.; Komatsu, H.; Yamauchi, H.; Yamauchi, T.; Sumitani, M.; Doorenbos, A.Z. Characteristics of taste alterations in people receiving taxane-based chemotherapy and their association with appetite, weight, and quality of life. Support. Care Cancer 2021, 29, 5103–5114. [Google Scholar] [CrossRef]
- Pellegrini, M.; Merlo, F.D.; Agnello, E.; Monge, T.; Devecchi, A.; Casalone, V.; Montemurro, F.; Ghigo, E.; Sapino, A.; Bo, S. Dysgeusia in Patients with Breast Cancer Treated with Chemotherapy—A Narrative Review. Nutrients 2023, 15, 226. [Google Scholar] [CrossRef] [PubMed]
- Malta, C.E.N.; De Lima Martins, J.O.; Carlos, A.C.A.M.; Freitas, M.O.; Magalhães, I.A.; De Vasconcelos, H.C.A.; Silva-Fernandes, I.J.d.L.; Silva, P.G.d.B. Risk factors for dysgeusia during chemotherapy for solid tumors: A retrospective cross-sectional study. Support. Care Cancer 2022, 30, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Boltong, A.; Aranda, S.; Keast, R.; Wynne, R.; Francis, P.A.; Chirgwin, J.; Gough, K. A prospective cohort study of the effects of adjuvant breast cancer chemotherapy on taste function, food liking, appetite and associated nutritional outcomes. PLoS ONE 2014, 9, e103512. [Google Scholar] [CrossRef]
- Celik Aysegul, R.N.; Duzgun Gonul, R.N. The factors influencing the taste alterations in patients receiving chemotherapy. Int. J. Caring Sci. 2019, 12, 1684–1690. [Google Scholar]
- Denda, Y.; Niikura, N.; Satoh-Kuriwada, S.; Yokoyama, K.; Terao, M.; Morioka, T.; Tsuda, B.; Okamura, T.; Ota, Y.; Tokuda, Y.; et al. Taste alterations in patients with breast cancer following chemotherapy: A cohort study. Breast Cancer 2020, 27, 954–962. [Google Scholar] [CrossRef]
- Bleumer, T.; Abel, J.; Böhmerle, W.; Schröder, S.; Yap, S.A.; Schaeper, N.D.E.; Hummel, T.; Stintzing, S.; Stephan, L.U.; Pelzer, U. Smell and Taste Alterations in Patients Receiving Curative or Palliative Chemotherapy—The CONKO 021—ChemTox Trial. Cancers 2024, 16, 2495. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, A.; Rudzki, G.; Lewandowski, T.; Próchnicki, M.; Rudzki, S.; Laskowska, B.; Brudniak, J. Quality of life of cancer patients treated with chemotherapy. Int. J. Environ. Res. Public Health 2020, 17, 6938. [Google Scholar] [CrossRef] [PubMed]
- Dehkordi, A.; Heydarnejad, M.S.; Fatehi, D. Quality of life in cancer patients undergoing chemotherapy. Oman Med. J. 2009, 24, 204. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.R.; Chen, M.D.; Fowler, J.M.; Carson, L.F.; Twiggs, L.B. The Effect of Prolonged Cycles of Chemotherapy on Quality of Life in Gynaecologic Cancer Patients. J. Obstet. Gynaecol. 1997, 23, 197–203. [Google Scholar] [CrossRef]
- Cheng, K.K.F.; Lim, E.Y.T.; Kanesvaran, R. Quality of life of elderly patients with solid tumours undergoing adjuvant cancer therapy: A systematic review. BMJ Open 2018, 8, e018101. [Google Scholar] [CrossRef] [PubMed]
- Mols, F.; Vingerhoets, A.J.; Coebergh, J.W.; van de Poll-Franse, L.V. Quality of life among long-term breast cancer survivors: A systematic review. Eur. J. Cancer 2005, 41, 2613–2619. [Google Scholar] [CrossRef]
- Bezjak, A.; Lee, C.W.; Ding, K.; Brundage, M.; Winton, T.; Graham, B.; Whitehead, M.; Johnson, D.H.; Livingston, R.B.; Seymour, L.; et al. Quality-of-Life Outcomes for Adjuvant Chemotherapy in Early-Stage Non–Small-Cell Lung Cancer: Results From a Randomized Trial. J. Clin. Oncol. 2008, 26, 5052–5059. [Google Scholar] [CrossRef]
- Mebis, J.; Censabella, S.; Engels, S.; Van Marsenille, C.; Orye, G.; Marquette, S.; Vansteelant, L.; Luyten, D.; Maes, A.; Noé, L.; et al. Quality of Life, Fatigue, and Subjective Cognitive Functioning Immediately and 6 Months After Adjuvant Chemotherapy in Breast Cancer Patients. Uhasselt. 2019. Available online: https://documentserver.uhasselt.be/handle/1942/29208 (accessed on 15 February 2025).
- Ulibarri-Ochoa, A.; Ruiz-de-Alegría, B.; López-Vivanco, G.; García-Vivar, C.; Iraurgi, I. Differences in quality of life and emotional well-being in breast, colon, and lung cancer patients during outpatient adjuvant chemotherapy: A longitudinal study. Cancer Nurs. 2023, 46, E99–E109. [Google Scholar] [CrossRef]
| Characteristics | Participants | p | |
|---|---|---|---|
| <4 ACT Cycle (n = 46) | ≥4 ACT Cycle (n = 41) | ||
| Median age, years (range) | 56.5 (26–71) | 60.7 (23–68) | 0.254 |
| BMI (kg/m2) (X ± SD) | 25.90 ± 6.85 | 24.90 ± 8.39 | 0.465 |
| Gender, n (%) | |||
| Female | 24 (52.2) | 23 (56.1) | 0.978 |
| Male | 22 (47.8) | 18 (43.9) | 0.220 |
| Cancer type, n (%) | |||
| Breast cancer | 12 (26.2) | 15 (36.6) | 0.960 |
| Lung cancer | 9 (19.6) | 5 (12.2) | 0.452 |
| Stomach cancer | 6 (13.0) | 8 (19.5) | 0.982 |
| Colorectal cancer | 11 (23.9) | 9 (22.0) | 0.957 |
| Pancreatic cancer | 6 (13.0) | 4 (9.7) | 0.726 |
| Lymphoma | 2 (4.3) | 0 (0.0) | 0.024 * |
| Chemotherapy regimen, n (%) | |||
| Anthracycline-based | 7 | 9 | 0.683 |
| Taxane-based | 12 | 12 | 1.000 |
| Antracycline and taxane-based | 27 | 20 | 0.790 |
| Participants | |||
|---|---|---|---|
| <4 ACT Cycle (n = 46) | ≥4 ACT Cycle (n = 41) | p | |
| Recent weight loss, n (%) | |||
| Moderate (<5%) | 34 (73.9) | 26 (63.4) | 0.044 * |
| Moderate to severe (5–10%) | 7 (15.2) | 3 (7.3) | |
| Severe (>10%) | 5 (10.9) a | 12 (29.3) b | |
| Meeting energy requirements, n (%) | |||
| Hypometabolic (<90% of predicted TEE) | 9 (19.5) a | 20 (48.8) b | 0.029 * |
| Normometabolic (90–110% of predicted TEE) | 28 (61.0) | 18 (43.9) | |
| Hypermetabolic (>110% of predicted TEE) | 9 (19.5) | 3 (7.3) | |
| CiTAS Scores (X ± SD) | ||||
|---|---|---|---|---|
| Decline in Basic Taste | Discomfort | Phantogeusia and Parageusia | General Taste Alterations | |
| BMI (kg/m2) | ||||
| <18 | 1.00 ± 0.00 | 1.67 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 |
| 18–24.99 | 1.40 ± 0.75 | 1.73 ± 0.76 | 1.55 ± 0.94 | 1.74 ± 0.93 |
| 25–29.99 | 1.48 ± 0.74 | 1.79 ± 0.73 | 1.58 ± 0.90 | 1.59 ± 0.79 |
| >30 | 1.51 ± 0.76 | 1.60 ± 0.60 | 1.52 ± 1.12 | 1.43 ± 0.73 |
| p = 0.673 | p = 0.569 | p = 0.827 | p = 0.667 | |
| Age groups (years) | ||||
| 18–40 | 1.54 ± 0.79 a& | 1.58 ± 0.50 | 1.23 ± 0.45 | 1.55 ± 0.98 |
| 41–50 | 1.37 ± 0.72 b& | 1.78 ± 0.83 | 1.46 ± 0.83 | 1.71 ± 0.98 |
| 51–60 | 1.40 ± 0.62 | 1.59 ± 0.68 | 1.46 ± 0.93 | 1.46 ± 0.68 |
| 61 and over | 1.48 ± 0.83 | 1.91 ± 0.78 | 1.75 ± 1.02 | 1.77 ± 0.88 |
| p = 0.894 | p = 0.433 | p = 0.311 | p = 0.798 | |
| Gender | ||||
| Male | 1.37 ± 0.65 | 1.80 ± 0.79 | 1.50 ± 0.86 | 1.63 ± 0.78 |
| Female | 1.51 ± 0.81 | 1.72 ± 0.69 | 1.60 ± 0.97 | 1.65 ± 0.91 |
| p = 0.632 | p = 0.901 | p = 0.357 | p = 0.704 | |
| Cancer type | ||||
| Breast cancer | 1.51 ± 0.85 | 1.69 ± 0.78 | 1.64 ± 0.90 | 1.80 ± 0.97 a& |
| Lung cancer | 1.29 ± 0.62 | 1.67 ± 0.55 | 1.10 ± 0.28 | 1.27 ± 0.68 |
| Stomach cancer | 1.40 ± 0.63 | 1.93 ± 0.83 a& | 1.69 ± 1.07 | 1.66 ± 0.76 |
| Colorectal cancer | 1.58 ± 0.82 | 1.94 ± 0.82 a& | 1.87 ± 1.16 | 1.94 ± 0.85 a& |
| Pancreatic cancer | 1.36 ± 0.67 | 1.60 ± 0.53 | 1.20 ± 0.63 | 1.25 ± 0.63 |
| Lymphoma | 1.00 ± 0.00 | 1.08 ± 0.12 b& | 1.33 ± 0.47 | 1.00 ± 0.00 b& |
| p = 0.802 | p = 0.605 | p = 0.069 | p = 0.028 * | |
| Chemotherapy regimen | ||||
| Anthracycline-based | 1.31 ± 0.58 | 1.61 ± 0,65 | 1.34 ± 0.56 | 1.40 ± 0.30 |
| Taxane-based | 1.46 ± 0.25 | 1.77 ± 0.67 | 1.51 ± 0.24 | 1.61 ± 0.17 |
| Antracycline and taxane-based | 1.50 ± 0.44 | 1.53 ± 0.52 | 1.64 ± 0.40 | 1.57 ± 0.05 |
| p = 0.242 | p = 0.125 | p = 0.106 | p = 0.128 | |
| FACT-G | Treatment | Mean (SD) | η2 | |||
|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | |||
| Physical well-being | <4 cycle | 21.4 (5.2) a | 22.8 (4.8) | 23.8 (4.4) b | 23.6 (4.1) b | 0.256 & |
| ≥4 cycle | 21.0 (5.4) a | 18.1 (5.5) b | 20.7 (4.8) a | 22.3 (4.5) c | 0.325 & | |
| p = 0.524 | p = 0.035 * | p = 0.021 * | p = 0.085 | |||
| d = 0.500 ¥ | d = 0.516 ¥ | |||||
| Social/family well-being | <4 cycle | 22.6 (5.6) | 21.9 (3.9) | 23.2 (4.2) | 23.9 (4.6) | |
| ≥4 cycle | 22.2 (4.8) a | 19.5 (4.6) b | 21.8 (4.1) | 22.5 (3.9) a | 0.284 & | |
| p = 0.225 | p = 0.046 | p = 0.057 | p = 0.087 | |||
| Emotional well-being | <4 cycle | 16.9 (4.2) | 17.4 (5.0) | 17.8 (3.6) | 18.1 (3.9) | |
| ≥4 cycle | 17.1 (4.9) | 17.0 (4.5) | 17.5 (3.8) | 17.8 (4.2) | ||
| p = 0.076 | p = 0.150 | p = 0.236 | p = 0.145 | |||
| Functional well-being | <4 cycle | 16.3 (3.7) a | 15.8 (3.4) | 17.4 (4.1) b | 19.2 (3.9) c | 0.305 & |
| ≥4 cycle | 16.7 (4.1) a | 14.5 (3.8) b | 15.2 (3.5) | 16.1 (3.6) a | 0.228 & | |
| p = 0.574 | p = 0.010 * | p = 0.024 * | p = 0.010 * | |||
| d = 0.621 ¥ | d = 0.562 ¥ | d = 0.585 ¥ | ||||
| Total score | <4 cycle | 77.2 (15.6) a | 77.9 (12.4) | 82.2 (14.6) | 84.8 (15.1) b | 0.368 & |
| ≥4 cycle | 77.0 (13.4) a | 69.1 (12.7) b | 76.2 (15.0) a | 79.7 (14.8) a | 0.320 & | |
| p = 0.298 | p = 0.001 * | p = 0.028 * | p = 0.162 | |||
| d = 0.590 ¥ | d = 0.500 ¥ | |||||
| CiTAS scores | ||||
|---|---|---|---|---|
| Decline in Basic Taste | Discomfort | Phantogeusia and Parageusia | General Taste Alterations | |
| Recent weight loss (%BW) | 0.149 | 0.250 | 0.166 * | 0.262 * |
| Meeting energy requirements (%TER) | −0.248 * | −0.141 | −0.130 * | −0.105 * |
| FACT-G | ||||
| Physical well-being | −0.357 ** | −0.175 | −0.173 | −0.280 ** |
| Social/family well-being | −0.193 | −0.188 | −0.157 | −0.224 * |
| Emotional well-being | −0.032 | −0.052 | −0.015 | −0.050 |
| Functional well-being | −0.337 ** | −0.294 ** | −0.160 | −0.287 ** |
| Total score | −0.407 ** | −0.240 ** | −0.270 ** | −0.347 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yilmaz, C.S.; Caliskan İzgi, H.; Türker, P.F. The Effect of the Number of Adjuvant Chemotherapy Cycles Following Cancer Surgery on Taste Alteration, Energy Intake, and Life Quality: 6-Month Follow-Up Study. Life 2025, 15, 1031. https://doi.org/10.3390/life15071031
Yilmaz CS, Caliskan İzgi H, Türker PF. The Effect of the Number of Adjuvant Chemotherapy Cycles Following Cancer Surgery on Taste Alteration, Energy Intake, and Life Quality: 6-Month Follow-Up Study. Life. 2025; 15(7):1031. https://doi.org/10.3390/life15071031
Chicago/Turabian StyleYilmaz, Can Selim, Hilal Caliskan İzgi, and Perim Fatma Türker. 2025. "The Effect of the Number of Adjuvant Chemotherapy Cycles Following Cancer Surgery on Taste Alteration, Energy Intake, and Life Quality: 6-Month Follow-Up Study" Life 15, no. 7: 1031. https://doi.org/10.3390/life15071031
APA StyleYilmaz, C. S., Caliskan İzgi, H., & Türker, P. F. (2025). The Effect of the Number of Adjuvant Chemotherapy Cycles Following Cancer Surgery on Taste Alteration, Energy Intake, and Life Quality: 6-Month Follow-Up Study. Life, 15(7), 1031. https://doi.org/10.3390/life15071031








