Immediate Breast Reconstruction with Fat-Graft-Augmented ICAP Flaps
Abstract
1. Introduction
2. Patients and Methods
3. Operative Technique
4. Results
5. Clinical Examples
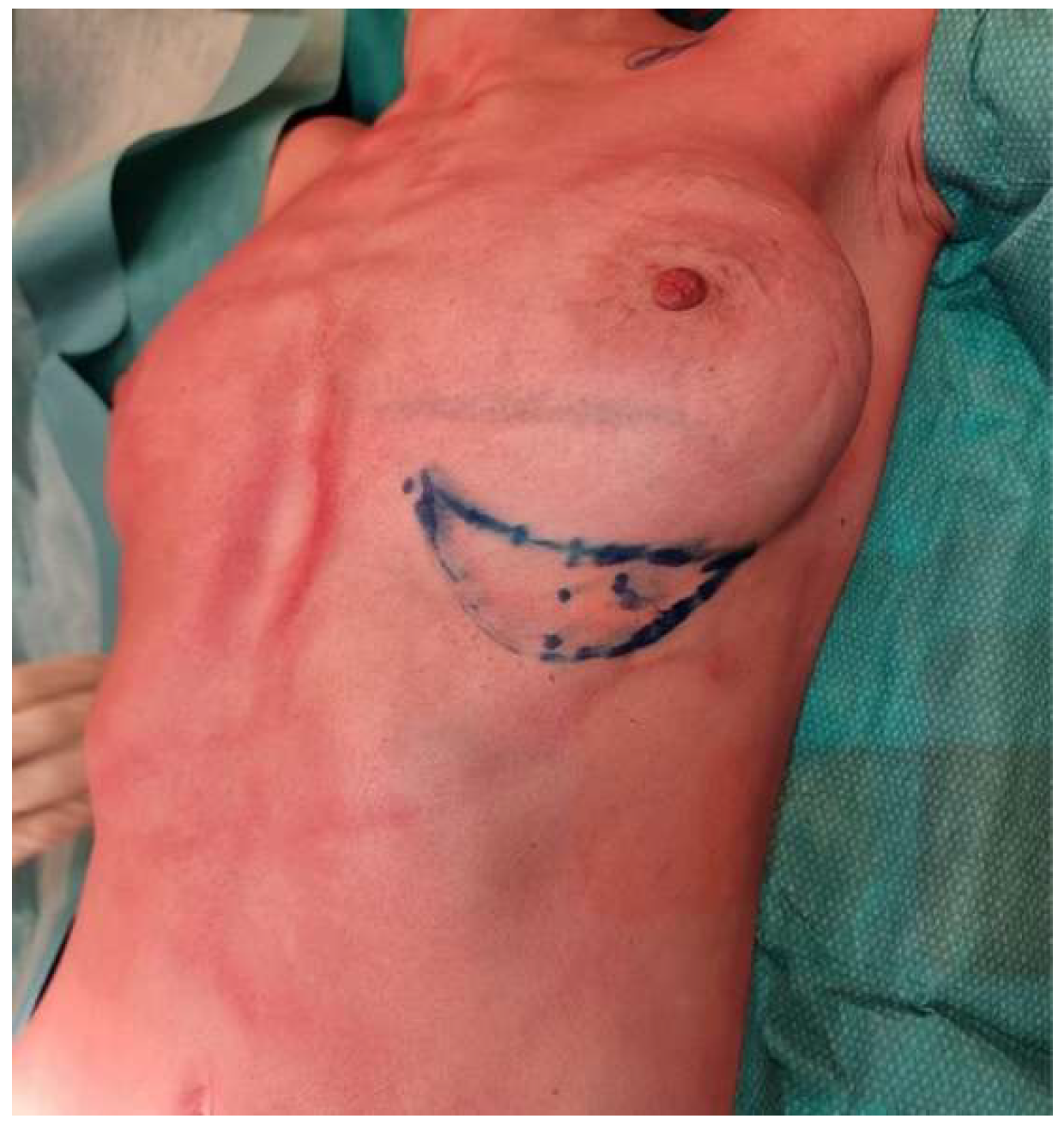
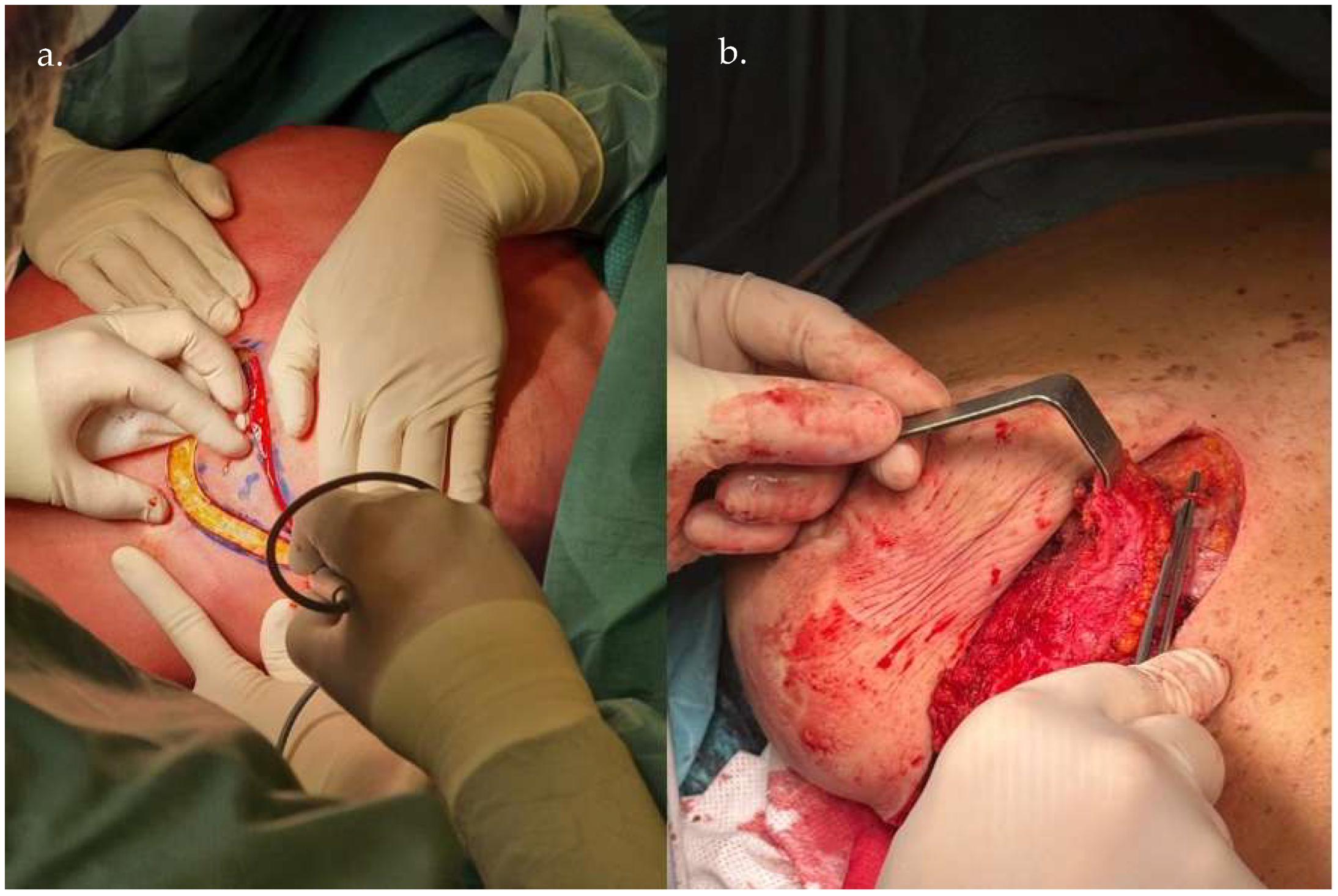
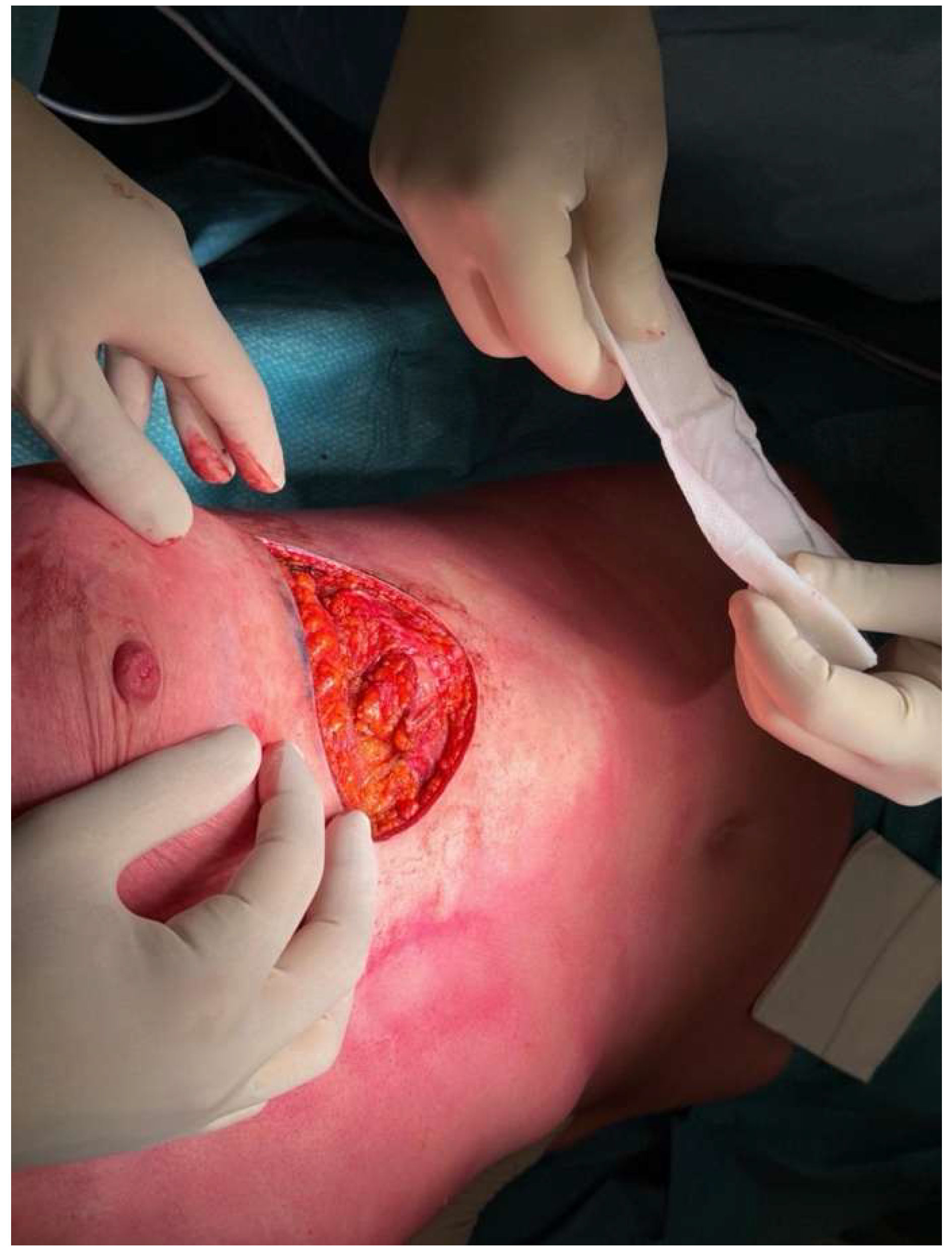
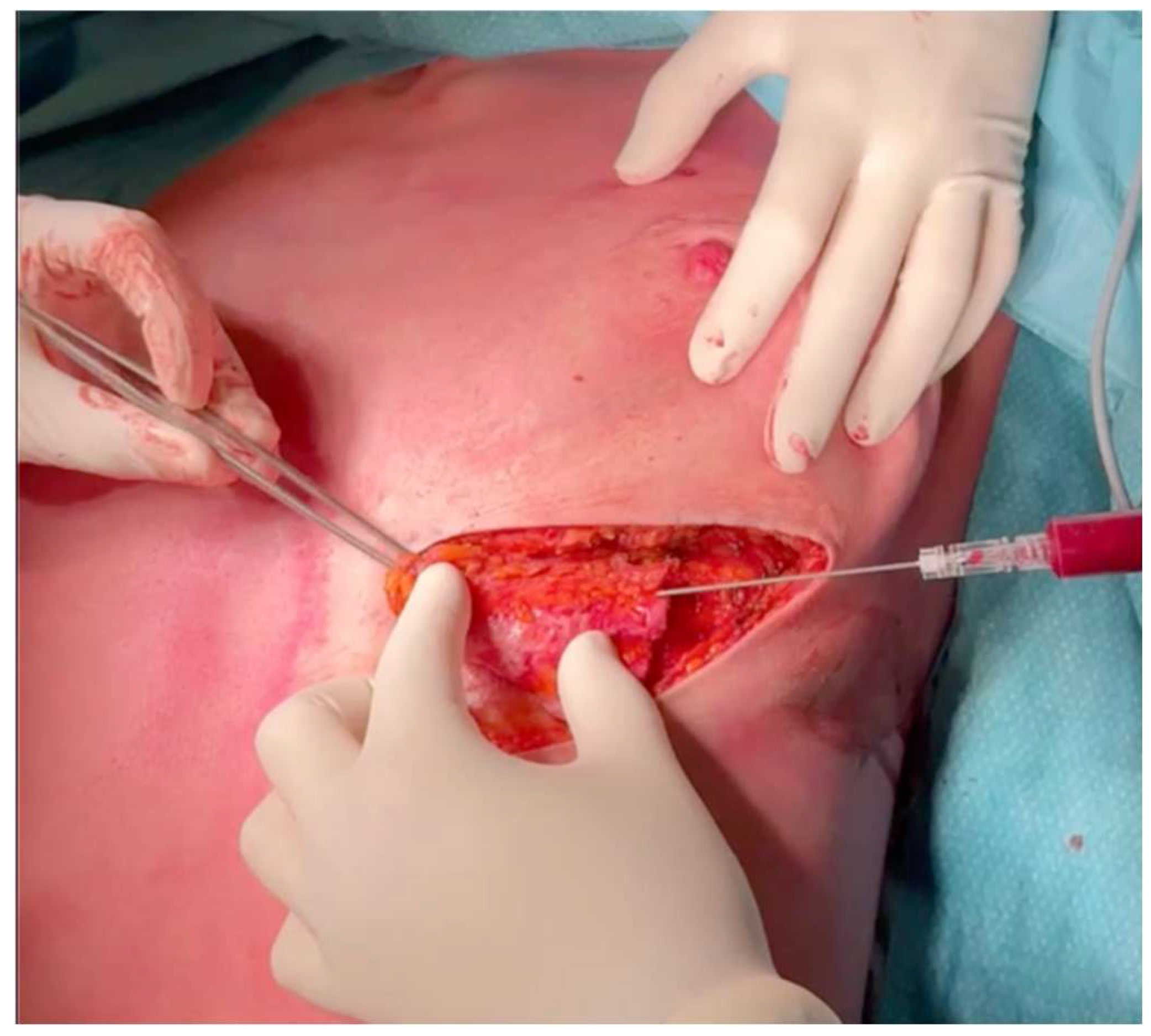
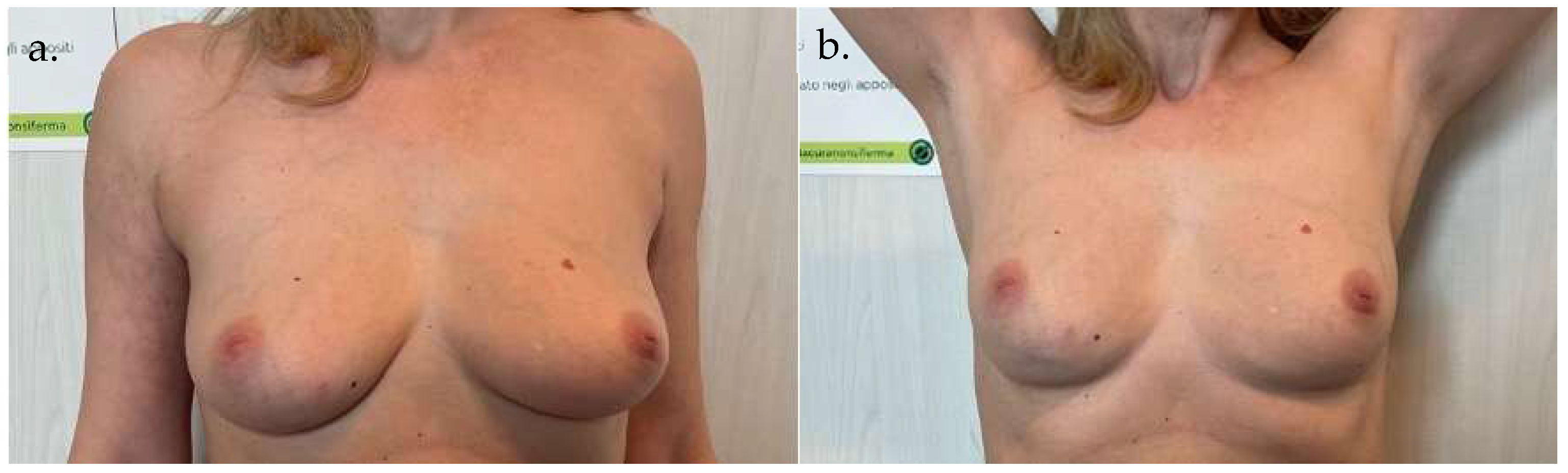
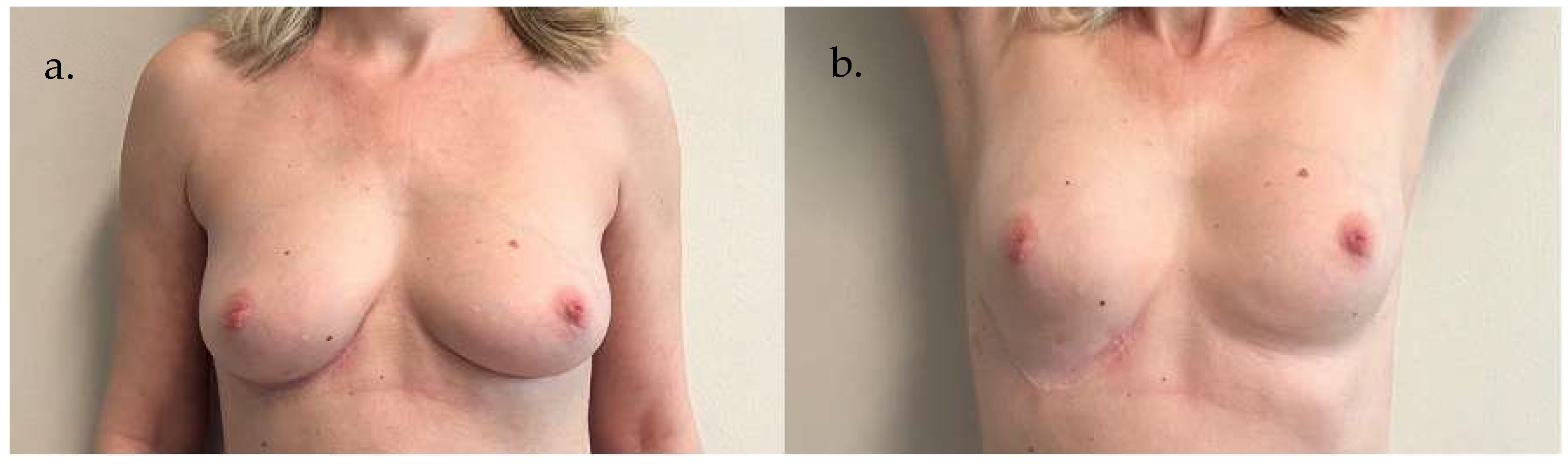
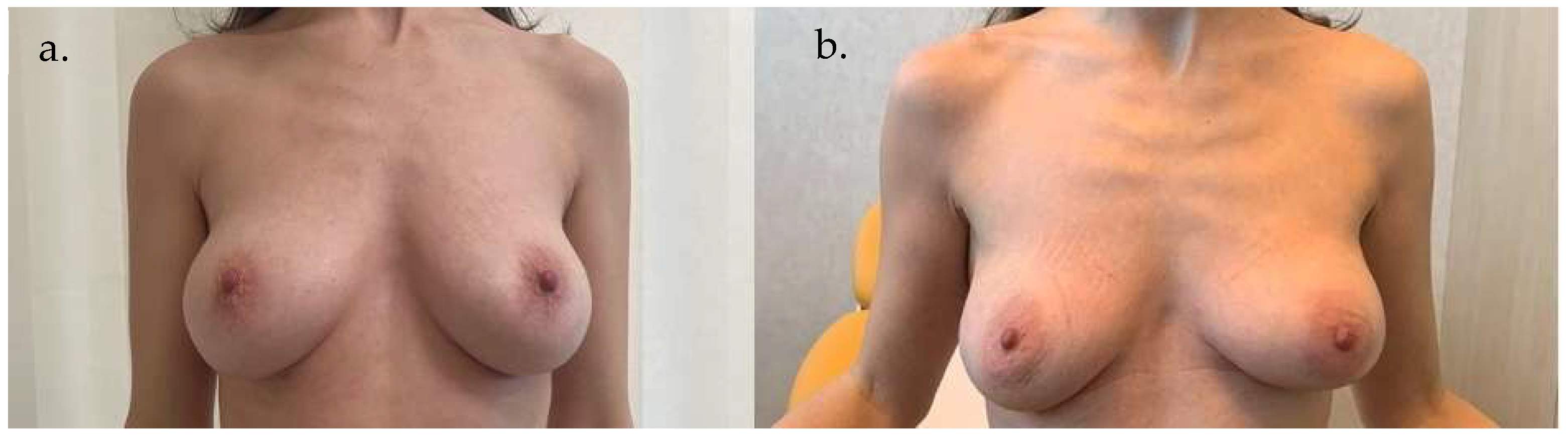
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kwei, S.; Borud, L.J.; Lee, B.T. Mastopexy with autologous augmentation after massive weight loss: The intercostal artery perforator (ICAP) flap. Ann. Plast. Surg. 2006, 57, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Badran, H.A.; El-Helaly, M.S.; Safe, I. The lateral intercostal neurovascular free flap. Plast. Reconstr. Surg. 1984, 73, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, M.; Van Landuyt, K.; de Frene, B.; Roche, N.; Blondeel, P.; Monstrey, S. The versatility of the intercostal artery perforator (ICAP) flaps. J. Plast. Reconstr. Aesthet. Surg. 2006, 59, 644–652. [Google Scholar] [CrossRef]
- Tenna, S.; Brunetti, B.; Morelli Coppola, M.; Persichetti, P. The anterior intercostal artery perforator flap: Clinical applications in partial breast reconstruction. Plast Reconstr Surg. 2017, 140, 746e–747e. [Google Scholar] [CrossRef]
- Bar-Meir, E.D.; Yueh, J.H.; Tobias, A.M.; Lee, B.T. Autologous fat grafting: A technique for stabilization of the microvascular pedicle in DIEP flap reconstruction. Microsurgery 2008, 28, 495–498. [Google Scholar] [CrossRef]
- Heine, N.; Eigenberger, A.; Brebant, V.; Hoesl, V.; Brix, E.; Prantl, L.; Kempa, S. Comparison of skin sensitivity following breast reconstruction with three different techniques: Autologous fat grafting, DIEP flap, and expander/implant. Clin. Hemorheol. Microcirc. 2022, 80, 389–397. [Google Scholar] [CrossRef]
- Laporta, R.; Longo, B.; Sorotos, M.; Pagnoni, M.; Santanelli di Pompeo, F. Breast reconstruction with delayed fat-graft-augmented DIEP flap in patients with insufficient donor-site volume. Aesthetic Plast. Surg. 2015, 39, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Angrigiani, C.; Rancati, A.O.; Masià, J.; Farhadi, J.; Khouri, K.; Acquaviva, J. Modified anterior intercostal artery perforator flap (AICAP) for autologous breast volume restoration after explantation. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 2916–2924. [Google Scholar] [CrossRef]
- Egro, F.M.; Roy, E.; Rubin, J.P.; Coleman, S.R. Evolution of the Coleman technique. Plast Reconstr Surg. 2022, 150, 329e–336e. [Google Scholar] [CrossRef]
- Regnault, P. Breast ptosis: Definition and treatment. Clin. Plast. Surg. 1976, 3, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Slavin, S.A.; Halperin, T. Reconstruction of the breast conservation deformity. Semin Plast Surg. 2004, 18, 89–94. [Google Scholar] [CrossRef]
- Clough, K.B.; Thomas, S.S.; Fitoussi, A.D.; Couturaud, B.; Reyal, F.; Falcou, M.-C. Reconstruction after conservative treatment for breast cancer: Cosmetic sequelae classification revisited. Plast Reconstr Surg. 2004, 114, 1743–1749. [Google Scholar] [CrossRef] [PubMed]
- Clough, K.B.; Kroll, S.S.; Audretsch, W. An approach to the repair of partial mastectomy defects. Plast Reconstr Surg. 1999, 104, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Esser, J. Biological or Artery Flaps of the Face; Institut Esser de Chirurgie Structive: Monaco, 1931. [Google Scholar]
- Dibbell, D.G. Use of a long island flap to bring sensation to the sacral area in young paraplegics. Plast. Reconstr. Surg. 1974, 54, 220–223. [Google Scholar] [CrossRef]
- Daniel, R.A. Intercostal neurovascular island skin flap. In Grabb’s Encyclopedia of Flaps, 2nd ed.; Strauch, B., Vasconz, L., Hall-Findlay, E., Eds.; Lippincott-Raven: Philadelphia, PA, USA, 1998; Chapter 414; Volume III, p. 1632. [Google Scholar]
- Daniel, R.K.; Williams, H.B. The free transfer of skin flaps by microvascular anastomoses: An experimental study and a reappraisal. Plast. Reconstr. Surg. 1973, 52, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, M.; Spano, A.; Van Landuyt, K.; D’hErde, K.; Blondeel, P.; Monstrey, S. The lateral intercostal artery perforators: Anatomical study and clinical application in breast surgery. Plast. Reconstr. Surg. 2008, 121, 389–396. [Google Scholar] [CrossRef]
- Munhoz, A.M.; Montag, E.; Arruda, E.; Brasil, J.A.; Aldrighi, J.M.; Gemperli, R.; Filassi, J.R.; Ferreira, M.C. Immediate conservative breast surgery reconstruction with perforator flaps: New challenges in the era of partial mastectomy reconstruction? Breast 2011, 20, 212–217. [Google Scholar] [CrossRef]
- Rosatti, F.; Melita, D.; Marchica, P.; Musmarra, I.; Ciancio, F.; DE Lorenzi, F.; Toia, F.; LA Padula, S.; Lombardo, G.A. Local flaps for partial breast reconstruction. Minerva Surg. 2025, 80, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Hakakian, C.S.; Lockhart, R.A.; Kulber, D.A.; Aronowitz, J.A. Lateral intercostal artery perforator flap in breast reconstruction: A simplified pedicle permits an expanded role. Ann Plast Surg. 2016, 76 (Suppl. S3), S184–S190. [Google Scholar] [CrossRef]
- Carrasco-López, C.; Julian Ibañez, J.F.; Vilà, J.; Tomás, M.A.L.; López, J.N.; Miguel, I.P.; Fernandez-Llamazares-Rodriguez, J.; Higueras-Suñe, C. Anterior intercostal artery perforator flap in immediate breast reconstruction: Anatomical study and clinical application. Microsurgery 2017, 37, 603–610. [Google Scholar] [CrossRef]
- Mangialardi, M.L.; Baldelli, I.; Salgarello, M.; Raposio, E.M. Breast reconstruction using the lateral thoracic, thoracodorsal, and intercostal arteries perforator flaps. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3334. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, M.; Ohno, Y.; Morioka, E.; Noguchi, M.; Nakano, Y.; Kosaka, T.; Shimada, K. A novel oncoplastic technique for breast cancer localized in the lower pole of the breast. J. Surg. Oncol. 2018, 117, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Schaverien, M.V.; Kuerer, H.M.; Caudle, A.S.; Smith, B.D.; Hwang, R.F.; Robb, G.L. Outcomes of volume replacement oncoplastic breast-conserving surgery using chest wall perforator flaps: Comparison with volume displacement oncoplastic surgery and total breast reconstruction. Plast. Reconstr. Surg. 2020, 146, 14–27. [Google Scholar] [CrossRef]
- Ho, W.; Stallard, S.; Doughty, J.; Mallon, E.; Romics, L. Oncological outcomes and complications after volume replacement oncoplastic breast conservations—The Glasgow experience. Breast Cancer 2016, 10, 223–228. [Google Scholar] [CrossRef]
- Lipman, K.; Graw, G.; Nguyen, D. Lateral intercostal artery perforator (LICAP) flap for breast volume augmentation: Applications for oncoplastic and massive weight loss surgery. JPRAS Open. 2021, 29, 123–134. [Google Scholar] [CrossRef]
- Losken, A.; Pinell, X.A.; Sikoro, K.; Yezhelyev, M.V.; Anderson, E.; Carlson, G.W. Autologous fat grafting in secondary breast reconstruction. Ann. Plast. Surg. 2011, 66, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Maione, L.; Caviggioli, F.; Vinci, V.; Lisa, A.; Barbera, F.; Siliprandi, M.; Battistini, A.; Klinger, F.; Klinger, M. Fat graft in composite breast augmentation with round implants: A new concept for breast reshaping. Aesthetic Plast. Surg. 2018, 42, 1465–1471. [Google Scholar] [CrossRef]
- Kanchwala, S.K.; Glatt, B.S.; Conant, E.F.; Bucky, L.P. Autologous fat grafting to the reconstructed breast: The management of acquired contour deformities. Plast. Reconstr. Surg. 2009, 124, 409–418. [Google Scholar] [CrossRef]
- Kaoutzanis, C.; Xin, M.; Ballard, T.N.S.; Welch, K.B.; Momoh, A.O.; Kozlow, J.H.; Brown, D.L.; Cederna, P.S.; Wilkins, E.G. Autologous fat grafting after breast reconstruction in postmastectomy patients: Complications, biopsy rates, and locoregional cancer recurrence rates. Ann. Plast. Surg. 2016, 76, 270–275. [Google Scholar] [CrossRef]
- Klinger, M.; Losurdo, A.; Lisa, A.V.E.; Morenghi, E.; Vinci, V.; Corsi, F.; Albasini, S.; Leonardi, M.C.; Jereczek-Fossa, B.A.; Veronesi, P.; et al. Safety of autologous fat grafting in breast cancer: A multicenter Italian study among 17 Senonetwork breast units. Breast Cancer Res. Treat. 2022, 191, 355–363. [Google Scholar] [CrossRef]
- Maione, L.; Vinci, V.; Caviggioli, F.; Klinger, F.; Banzatti, B.; Catania, B.; Lisa, A.; Klinger, M. Autologous fat graft in postmastectomy pain syndrome following breast-conservative surgery and radiotherapy. Aesthetic Plast. Surg. 2014, 38, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Demiri, E.C.; Tsimponis, A.; Pagkalos, A.; Georgiadou, E.; Goula, O.C.; Spyropoulou, G.A.; Dionyssiou, D. Fat-augmented latissimus dorsi versus deep inferior epigastric perforator flap: Comparative study in delayed autologous breast reconstruction. J. Reconstr. Microsurg. 2021, 37, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Blondeel, P.N.; Van Landuyt, K.; Monstrey, S.J.; Bozkurt, M.; Dap, L.; Neumann, G.; Mees, R.; Sverdlov, A.; Vandeweyer, E.; Van Laere, L.; et al. The DIEAP flap: A new standard in breast reconstruction? Br. J. Plast. Surg. 2003, 56, 619–630. [Google Scholar]
- Melbourne Breast Cancer Surgery. Smoking and Breast Surgery Complications. Available online: https://www.melbournebreastcancersurgery.com.au/smoking-and-breast-surgery-complications.html (accessed on 20 January 2025).
| AGE (y.o.) | AICAP or LICAP | BMI (kg/m2) | SMOKING |
|---|---|---|---|
| 65 | AICAP | 29.76 | no |
| 65 | AICAP | 25.64 | yes |
| 45 | AICAP | 18.22 | no |
| 39 | AICAP | 20.81 | no |
| 47 | AICAP | 19.61 | yes |
| 76 | AICAP | 26.84 | no |
| 57 | AICAP | 21.56 | no |
| 66 | LICAP | 24.03 | no |
| 40 | AICAP | 23.05 | no |
| 49 | AICAP | 23.11 | no |
| SMOKERS (Total No. 2) | NON-SMOKERS (Total No. 8) | Total (No. 10) | |
|---|---|---|---|
| PARTIAL FLAP LOSS (%) | 100 | 0 | 20 |
| TISSUE NECROSIS (%) | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klinger, F.; Cavallero, M.F.; Lisa, A.V.E.; Rosatti, F.; Catania, B.; Klinger, M.; Di Giuli, R.; Furlan, S.; Comunian, R.; Vaccari, S.; et al. Immediate Breast Reconstruction with Fat-Graft-Augmented ICAP Flaps. Life 2025, 15, 1017. https://doi.org/10.3390/life15071017
Klinger F, Cavallero MF, Lisa AVE, Rosatti F, Catania B, Klinger M, Di Giuli R, Furlan S, Comunian R, Vaccari S, et al. Immediate Breast Reconstruction with Fat-Graft-Augmented ICAP Flaps. Life. 2025; 15(7):1017. https://doi.org/10.3390/life15071017
Chicago/Turabian StyleKlinger, Francesco, Mattia Federico Cavallero, Andrea Vittorio Emanuele Lisa, Fernando Rosatti, Barbara Catania, Marco Klinger, Riccardo Di Giuli, Simone Furlan, Roberta Comunian, Stefano Vaccari, and et al. 2025. "Immediate Breast Reconstruction with Fat-Graft-Augmented ICAP Flaps" Life 15, no. 7: 1017. https://doi.org/10.3390/life15071017
APA StyleKlinger, F., Cavallero, M. F., Lisa, A. V. E., Rosatti, F., Catania, B., Klinger, M., Di Giuli, R., Furlan, S., Comunian, R., Vaccari, S., & Vinci, V. (2025). Immediate Breast Reconstruction with Fat-Graft-Augmented ICAP Flaps. Life, 15(7), 1017. https://doi.org/10.3390/life15071017







