Immunophenotype of Kawasaki Disease: Insights into Pathogenesis and Treatment Response
Abstract
1. Introduction
2. Literature Search
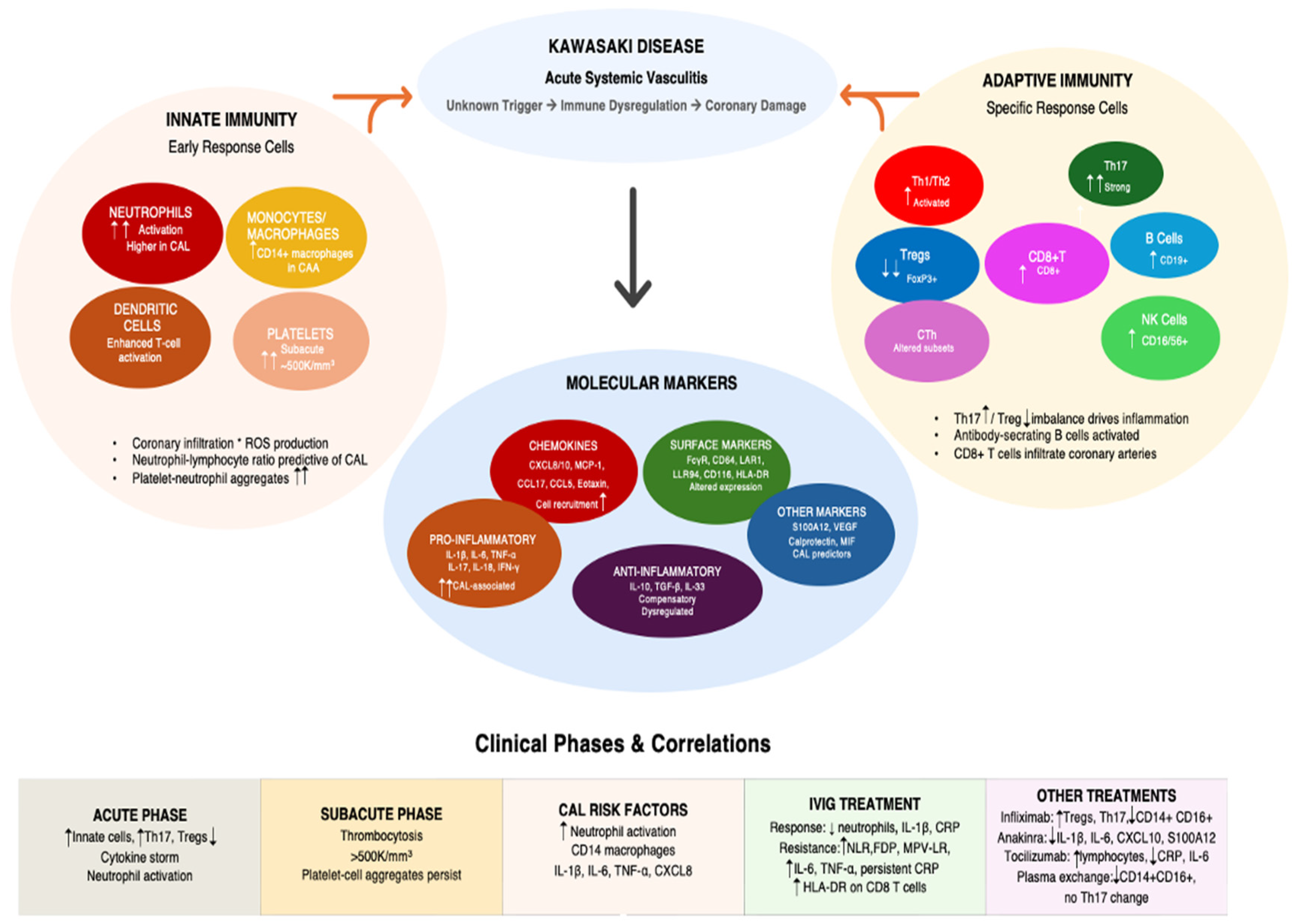
3. Immunophenotyping Kawasaki Disease: Insights for Disease Pathogenesis
3.1. Cellular Mechanisms
3.1.1. Innate Immunity
3.1.2. Adaptive Immunity
3.2. Secreted Proteins
3.2.1. Inflammatory Cytokines
3.2.2. Chemokines and Cell Adhesion Molecules
3.2.3. Complement Factors
3.2.4. Other
4. Immunophenotype and Response to Treatment
4.1. Response to IVIG Treatment
4.1.1. Cellular Changes
4.1.2. Cytokine and Biomarker Changes
4.2. Response to Other Treatments
5. Clinical Applications
5.1. Diagnosis
5.2. Prediction of IVIG Resistance
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADAM17 | A disintegrin and metalloproteinase 17 |
| ActRIIA | activin type IIA receptor |
| CAA | coronary artery abnormalities |
| CAL | coronary artery lesions |
| CCL | C-C motif ligand |
| CCR | C-C chemokine receptor |
| CRP | C-reactive protein |
| cTfh | circulating follicular helper T |
| CTLA-4 | cytotoxic T-lymphocyte associated protein 4 |
| CXCL | CXC chemokine ligand |
| CXCR | CXC chemokine receptor |
| DC | dendritic cell |
| FcγR | Fcγ receptor |
| FoxP3 | forkhead box P3 |
| GITR | glucocorticoid-induced TNF receptor family-related protein |
| HLA-DR | human leukocyte antigen-DR isotype |
| ICOS | inducible costimulatory |
| IFN-γ | interferon gamma |
| IL | interleukin |
| IVIG | intravenous immunoglobulin |
| KD | Kawasaki disease |
| KDSS | Kawasaki disease shock syndrome |
| LAIR-1 | leukocyte-associated Ig-like receptor-1 |
| MAS | macrophage activation syndrome |
| MCP-1 | monocyte chemoattractant protein-1 |
| mDC | myeloid dendritic cell |
| MPA | monocyte–platelet aggregates |
| NK | natural killer |
| PAF | platelet-activating factor |
| PD-1 | programmed cell death protein 1 |
| pDC | plasmacytoid dendritic cell |
| RORgt | retinoic acid receptor-related orphan receptor gt |
| ROS | reactive oxygen species |
| Th1/2 | T helper cell type ½ |
| TNF-α | tumor necrosis factor α |
| Treg | T regulatory cell |
References
- Elakabawi, K.; Lin, J.; Jiao, F.; Guo, N.; Yuan, Z. Kawasaki disease: Global burden and genetic background. Cardiol. Res. 2020, 11, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Rowley, A.H.; Shulman, S.T. The epidemiology and pathogenesis of Kawasaki disease. Front. Pediatr. 2018, 6, 374. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Jindal, A.K.; Pilania, R.K. Diagnosis of Kawasaki disease. Int. J. Rheum. Dis. 2018, 21, 36–44. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kosaki, F.; Okawa, S.; Shigematsu, I.; Yanagawa, H. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics 1974, 54, 271–276. [Google Scholar] [CrossRef]
- Burns, J.C. The etiologies of Kawasaki disease. J. Clin. Investig. 2024, 134, e176938. [Google Scholar] [CrossRef]
- Sapountzi, E.; Kotanidou, E.P.; Tsinopoulou, V.R.; Kalinderi, K.; Fidani, L.; Giannopoulos, A.; Galli-Tsinopoulou, A. Kawasaki disease: An update on genetics and pathophysiology. Genet. Test. Mol. Biomark. 2024, 28, 373–383. [Google Scholar] [CrossRef]
- Shuhan, H.; Zhimeng, H.; Yaxuan, L.; Jingxuan, F.; Ruiqi, C.; Wenxing, G.; Huifen, Z.; Xiaoqing, Y.; Wu, J.; Lilin, Z. Ozone exposure is positively correlated with the occurrence of Kawasaki disease in Chinese children. Pediatr. Res. 2025, 1–9. [Google Scholar] [CrossRef]
- Duarte, R.; Cisneros, S.; Fernandez, G.; Castellon, D.; Cattani, C.; A Melo, C.; Apocada, A. Kawasaki disease: A review with emphasis on cardiovascular complications. Insights Imaging 2010, 1, 223–231. [Google Scholar] [CrossRef]
- Herold, N.C.; Mitra, P. Immunophenotyping. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK558927/ (accessed on 1 May 2023).
- Hara, T.; Yamamura, K.; Sakai, Y. The up-to-date pathophysiology of Kawasaki disease. Clin. Transl. Immunol. 2021, 10, e1284. [Google Scholar] [CrossRef]
- Li, W.; Zhang, L.; Wang, Z.; He, X.; Lin, H.; Wang, Y.; Yuan, J.; Xie, X.; Zhang, X.; Qin, Y.; et al. Predictors for intravenous immunoglobulin resistance in patients with Kawasaki disease. Int. J. Clin. Pract. 2022, 2022, 2726686. [Google Scholar] [CrossRef]
- Sapountzi, E.; Fidani, L.; Giannopoulos, A.; Galli-Tsinopoulou, A. Association of genetic polymorphisms in Kawasaki disease with the response to immunoglobulin therapy. Pediatr. Cardiol. 2023, 44, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Kawashima, H.; Kashiwagi, Y.; Hoshika, A. Inflammatory cytokines as predictors of resistance to intravenous immunoglobulin therapy in Kawasaki disease patients. Int. J. Rheum. Dis. 2013, 16, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Huang, Y.; Li, X.; Lun, Y.; Li, X.; He, Y.; Wu, S.; Wang, S.; Sun, J.; Zhang, J. Atlas of circulating immune cells in Kawasaki disease. Int. Immunopharmacol. 2022, 102, 108396. [Google Scholar] [CrossRef]
- Hara, T.; Nakashima, Y.; Sakai, Y.; Nishio, H.; Motomura, Y.; Yamasaki, S. Kawasaki disease: A matter of innate immunity. Clin. Exp. Immunol. 2016, 186, 134–143. [Google Scholar] [CrossRef]
- Hu, J.; Qian, W.; Yu, Z.; Xu, T.; Ju, L.; Hua, Q.; Wang, Y.; Ling, J.J.; Lv, H. Increased neutrophil respiratory burst predicts the risk of coronary artery lesion in Kawasaki disease. Front. Pediatr. 2020, 8, 391. [Google Scholar] [CrossRef]
- Sarejloo, S.; Shahri, M.M.; Azami, P.; Clark, A.; Hass, E.; Salimi, M.; Lucke-Wold, B.; Sadeghvand, S.; Khanzadeh, S. Neutrophil to lymphocyte ratio as a biomarker for predicting the coronary artery abnormality in Kawasaki disease: A meta-analysis. Dis. Markers 2022, 2022, 6421543. [Google Scholar] [CrossRef]
- Furukawa, S.; Matsubara, T.; Yabuta, K. Mononuclear cell subsets and coronary artery lesions in Kawasaki disease. Arch. Dis. Child. 1992, 67, 706–708. [Google Scholar] [CrossRef]
- Suda, K.; Kishimoto, S.; Takahashi, T.; Nishino, H.; Okamura, H. Circulating myeloid dendritic cells is decreased in the acute phase of Kawasaki disease. Exp. Clin. Cardiol. 2013, 4, 272. [Google Scholar]
- Burns, J.; Song, Y.; Bujold, M.; Shimizu, C.; Kanegaye, J.; Tremoulet, A.; Franco, A. Immune-monitoring in Kawasaki disease patients treated with infliximab and intravenous immunoglobulin. Clin. Exp. Immunol. 2013, 174, 337–344. [Google Scholar] [CrossRef]
- Wang, N.; Chen, Z.; Zhang, F.; Zhang, Q.; Sun, L.; Lv, H.; Wang, B.; Shen, J.; Zhou, X.; Chen, F.; et al. Intravenous immunoglobulin therapy restores the quantity and phenotype of circulating dendritic cells and CD4+ T cells in children with acute Kawasaki disease. Front. Immunol. 2022, 13, 802690. [Google Scholar] [CrossRef]
- Yilmaz, A.; Rowley, A.; Schulte, D.J.; Doherty, T.M.; Schröder, N.W.; Fishbein, M.C.; Kalelkar, M.; Cicha, I.; Schubert, K.; Daniel, W.G.; et al. Activated myeloid dendritic cells accumulate and co-localize with CD3+ T cells in coronary artery lesions in patients with Kawasaki disease. Exp. Mol. Pathol. 2007, 83, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Choi, A.; Kim, S.; Han, J.W. The incidence of periungual desquamation and thrombocytosis in Kawasaki disease and the importance of systematic observation in the subacute phase. Front. Pediatr. 2024, 12, 1384015. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Choi, H.J. Clinical implications of thrombocytosis in acute phase Kawasaki disease. Eur. J. Pediatr. 2021, 180, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Ueno, K.; Nomura, Y.; Morita, Y.; Eguchi, T.; Masuda, K.; Kawano, Y. Circulating platelet-neutrophil aggregates play a significant role in Kawasaki disease. Circ. J. 2015, 79, 1349–1356. [Google Scholar] [CrossRef]
- Xing, H.; Tian, G. Increased Interleukin-35 suppresses peripheral CD14+ monocytes function in patients with Kawasaki disease. BMC Immunol. 2020, 21, 17. [Google Scholar] [CrossRef]
- Brogan, P.A.; Shah, V.; Clarke, L.A.; Dillon, M.J.; Klein, N. T cell activation profiles in Kawasaki syndrome. Clin. Exp. Immunol. 2008, 151, 267–274. [Google Scholar] [CrossRef]
- Brown, T.J.; Crawford, S.E.; Cornwall, M.L.; Garcia, F.; Shulman, S.T.; Rowley, A.H. CD8 T lymphocytes and macrophages infiltrate coronary artery aneurysms in acute Kawasaki disease. J. Infect. Dis. 2001, 184, 940–943. [Google Scholar] [CrossRef]
- Ehara, H.; Kiyohara, K.; Izumisawa, Y.; Ito, T. Early activation does not translate into effector differentiation of peripheral CD8 T cells during the acute phase of Kawasaki disease. Cell. Immunol. 2010, 265, 57–64. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Gong, F.; Fu, S.; Zhang, Q.; Hu, J.; Qi, Y.; Xie, C.; Zhang, Y. Evaluation of intravenous immunoglobulin resistance and coronary artery lesions in relation to Th1/Th2 cytokine profiles in patients with Kawasaki disease. Arthritis Rheum. 2013, 65, 805–814. [Google Scholar] [CrossRef]
- Jia, S.; Li, C.; Wang, G.; Yang, J.; Zu, Y. The T helper type 17/regulatory T cell imbalance in patients with acute Kawasaki disease. Clin. Exp. Immunol. 2010, 162, 131–137. [Google Scholar] [CrossRef]
- Rasouli, M.; Heidari, B.; Kalani, M. Downregulation of Th17 cells and the related cytokines with treatment in Kawasaki disease. Immunol. Lett. 2014, 162, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Ni, F.F.; Li, C.R.; Li, Q.; Xia, Y.; Wang, G.B.; Yang, J. Regulatory T cell microRNA expression changes in children with acute Kawasaki disease. Clin. Exp. Immunol. 2014, 178, 384–393. [Google Scholar] [CrossRef]
- Guo, M.M.H.; Tseng, W.N.; Ko, C.H.; Pan, H.M.; Hsieh, K.S.; Kuo, H.C. Th17- and Treg-related cytokine and mRNA expression are associated with acute and resolving Kawasaki disease. Allergy 2015, 70, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, G.; Xiong, L.J.; Yin, W.; Liu, J.; Liu, F.; Wang, R.G.; Xia, K.; Zhang, S.L.; Zhao, L. Profiles of responses of immunological factors to different subtypes of Kawasaki disease. BMC Musculoskelet. Disord. 2015, 16, 315. [Google Scholar] [CrossRef]
- Sugahara-Tobinai, A.; Inui, M.; Metoki, T.; Watanabe, Y.; Onuma, R.; Takai, T.; Kumaki, S. Augmented ILT3/LILRB4 expression of peripheral blood antibody-secreting cells in the acute phase of Kawasaki disease. Pediatr. Infect. Dis. J. 2019, 38, 431–438. [Google Scholar] [CrossRef]
- Xu, M.; Jiang, Y.; Wang, J.; Liu, J.; Liu, C.; Liu, D.; Yang, S. Distinct variations of antibody-secreting cells and memory B cells during the course of Kawasaki disease. BMC Immunol. 2019, 20, 16. [Google Scholar] [CrossRef]
- Xu, M.; Jiang, Y.; Zhang, J.; Zheng, Y.; Liu, D.; Guo, L.; Yang, S. Variation in IL-21-secreting circulating follicular helper T cells in Kawasaki disease. BMC Immunol. 2018, 19, 43. [Google Scholar] [CrossRef]
- Xu, M.; Jiang, Y.; Wang, J.; Liu, D.; Wang, S.; Yi, H.; Yang, S. Distribution of distinct subsets of circulating T follicular helper cells in Kawasaki disease. BMC Pediatr. 2019, 19, 43. [Google Scholar] [CrossRef]
- Rivas, M.N.; Arditi, M. Kawasaki disease: Pathophysiology and insights from mouse models. Nat. Rev. Rheumatol. 2020, 16, 391. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Shamie, I.; Lee, J.C.; Nowell, C.J.; Peng, W.; Angulo, S.; Le, L.N.; Liu, Y.; Miao, H.; Xiong, H.; et al. Immune response to intravenous immunoglobulin in patients with Kawasaki disease and MIS-C. J. Clin. Investig. 2021, 131, e147076. [Google Scholar] [CrossRef]
- Si, F.; Wu, Y.; Gao, F.; Feng, S.; Liu, R.; Yi, Q. Relationship between IL-27 and coronary arterial lesions in children with Kawasaki disease. Clin. Exp. Med. 2017, 17, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Teraura, H.; Kotani, K.; Minami, T.; Takeshima, T.; Shimooki, O.; Kajii, E. The serum concentration of soluble interleukin-2 receptor in patients with Kawasaki disease. Ann. Clin. Biochem. 2017, 54, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Shao, W.; Shang, S.; Zhang, T.; Hu, J.; Zhang, C.C. A comprehensive assessment of the value of laboratory indices in diagnosing Kawasaki disease. Arthritis Rheumatol. 2015, 67, 1943–1950. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, F.F.; Xu, Y.; Wang, J.J.; Samadli, S.; Wu, Y.F.; Liu, H.H.; Chen, W.X.; Luo, H.H.; Zhang, D.D.; et al. Interleukin-6 is prone to be a candidate biomarker for predicting incomplete and IVIG non-responsive Kawasaki disease rather than coronary artery aneurysm. Clin. Exp. Med. 2019, 19, 173–181. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Q.; Zou, L.; Wu, J.; Guo, L.; Teng, L.; Zheng, R.; Jung, L.K.L.; Lu, M. Kawasaki disease shock syndrome: Clinical characteristics and possible use of IL-6, IL-10 and IFN-γ as biomarkers for early recognition. Pediatr. Rheumatol. 2019, 17, 1. [Google Scholar] [CrossRef]
- Nandi, A.; Pal, P.; Basu, S. A comparison of serum IL6 and CRP levels with respect to coronary changes and treatment response in Kawasaki disease patients: A prospective study. Rheumatol. Int. 2019, 39, 1797–1801. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, S.Y.; Yuan, Y.; Wang, Q.; Gao, L.; Chen, X.; Yu, X.; Zhen, Z. Do cytokines correlate with refractory Kawasaki disease in children? Clin. Chim. Acta 2020, 506, 222–227. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, E.H.; Kil, H.R. Association between adipokines and coronary artery lesions in children with Kawasaki Disease. J. Korean Med. Sci. 2014, 29, 1385–1390. [Google Scholar] [CrossRef]
- Dai, L.; Zhang, L.; He, J.; Huang, R.; Tang, W.; Guo, H.; Shang, X. Diagnostic value of syndecan-1 for coronary artery lesions and correlation analysis of laboratory indicators in Kawasaki disease patients. Ital. J. Pediatr. 2024, 50, 209. [Google Scholar] [CrossRef]
- Fujimaru, T.; Ito, S.; Masuda, H.; Oana, S.; Kamei, K.; Ishiguro, A.; Kato, H.; Abe, J. Decreased levels of inflammatory cytokines in immunoglobulin-resistant Kawasaki disease after plasma exchange. Cytokine 2014, 70, 156–160. [Google Scholar] [CrossRef]
- Hachiya, A.; Kobayashi, N.; Matsuzaki, S.; Takeuchi, Y.; Akazawa, Y.; Shigemura, T.; Motoki, N.; Masumoto, J.; Agematsu, K. Analysis of biomarker serum levels in IVIG and infliximab-refractory Kawasaki disease patients. Clin. Rheumatol. 2018, 37, 1937–1943. [Google Scholar] [CrossRef] [PubMed]
- Netea, S.A.; Biesbroek, G.; van Stijn, D.; Ijspeert, H.; van der Made, C.I.; Jansen, M.H.; Geissler, J.; van den Berg, J.M.M.; van der Kuip, M.; Gruppen, M.P.; et al. Transient anti-cytokine autoantibodies superimpose the hyperinflammatory response in Kawasaki disease and multisystem inflammatory syndrome in children: A comparative cohort study on correlates of disease. EBioMedicine 2023, 95, 104736. [Google Scholar] [CrossRef] [PubMed]
- Lin, I.C.; Suen, J.L.; Huang, S.K.; Chou, M.H.; Kuo, H.C.; Lo, M.H.; Kuo, K.C.; Wang, L. Involvement of IL-17A/IL-17 receptor A with neutrophil recruitment and the severity of coronary arteritis in Kawasaki disease. J. Clin. Immunol. 2024, 44, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.N.; Lo, M.H.; Guo, M.M.H.; Hsieh, K.S.; Chang, W.C.; Kuo, H.C. IL-31 associated with coronary artery lesion formation in Kawasaki disease. PLoS ONE 2014, 9, e105195. [Google Scholar] [CrossRef][Green Version]
- Brodeur, K.E.; Liu, M.; Ibanez, D.; de Groot, M.J.; Chen, L.; Du, Y.; Seyal, E.; Laza-Briviesca, R.; Baker, A.; Chang, J.C.; et al. Elevation of IL-17 cytokines distinguishes Kawasaki disease from other pediatric inflammatory disorders. Arthritis Rheumatol. 2024, 76, 285–292. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, Y.; Shi, C.; Chai, H.; Shen, Y.; Ma, X.; Liu, Y. Expression of serum ferritin, human neutrophil lipocalin, procalcitonin, and inflammatory factors in children with Kawasaki disease and their relationship to coronary artery lesions. Am. J. Transl. Res. 2025, 17, 286–293. [Google Scholar] [CrossRef]
- Jinkawa, A.; Shimizu, M.; Nishida, K.; Kaneko, S.; Usami, M.; Sakumura, N.; Irabu, H.; Takakuwa, M.; Inoue, N.; Mizuta, M.; et al. Cytokine profile of macrophage activation syndrome associated with Kawasaki disease. Cytokine 2019, 119, 52–56. [Google Scholar] [CrossRef]
- Weng, K.P.; Hsieh, K.S.; Huang, S.H.; Ou, S.F.; Lai, T.J.; Tang, C.W.; Lin, C.C.; Ho, T.Y.; Liou, H.H.; Ger, L.P. Interleukin-18 and coronary artery lesions in patients with Kawasaki disease. J. Chin. Med. Assoc. 2013, 76, 438–445. [Google Scholar] [CrossRef]
- Qi, Y.; Xu, J.; Lin, Z.; Tao, Y.; Zheng, F.; Wang, Y.; Sun, Y.; Fu, S.; Wang, W.; Xie, C.; et al. The network of pro-inflammatory factors CD147, DcR3, and IL-33 in the development of Kawasaki disease. J. Inflamm. Res. 2021, 14, 6043–6053. [Google Scholar] [CrossRef]
- Ko, T.M.; Kuo, H.C.; Chang, J.S.; Chen, S.P.; Liu, Y.M.; Chen, H.W.; Tsai, F.J.; Lee, Y.C.; Chen, C.H.; Wu, J.Y.; et al. CXCL10/IP-10 is a biomarker and mediator for Kawasaki disease. Circ. Res. 2015, 116, 876–883. [Google Scholar] [CrossRef]
- Su, Y.; Feng, S.; Luo, L.; Liu, R.; Yi, Q. Association between IL-35 and coronary arterial lesions in children with Kawasaki disease. Clin. Exp. Med. 2019, 19, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Zhuge, Y.; Zhang, S.; Ni, C.; Wang, L.; Wu, R.; Niu, C.; Wen, Z.; Rong, X.; Qiu, H.; et al. IL-37b alleviates endothelial cell apoptosis and inflammation in Kawasaki disease through IL-1R8 pathway. Cell Death Dis. 2021, 12, 575. [Google Scholar] [CrossRef] [PubMed]
- Matsuguma, C.; Wakiguchi, H.; Suzuki, Y.; Okada, S.; Furuta, T.; Ohnishi, Y.; Azuma, Y.; Ohga, S.; Hasegawa, S. Dynamics of immunocyte activation during intravenous immunoglobulin treatment in Kawasaki disease. Scand. J. Rheumatol. 2019, 48, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Jiang, G.M.; Wu, Y.; Huang, B.Y.; Liu, S.Y.; Zhang, D.D.; Xu, Y.; Wu, Y.F.; Xia, X.; Wei, W.; et al. TNF-α is superior to conventional inflammatory mediators in forecasting IVIG non-response and coronary arteritis in Chinese children with Kawasaki disease. Clin. Chim. Acta 2017, 471, 76–80. [Google Scholar] [CrossRef]
- Kato, M.; Ayusawa, M.; Watanabe, H.; Komori, A.; Abe, Y.; Nakamura, T.; Kamiyama, H.; Takahashi, S. Cardiac function on 3-D speckle tracking imaging and cytokines in Kawasaki disease. Pediatr. Int. 2018, 60, 342–348. [Google Scholar] [CrossRef]
- Shimizu, M.; Mizuta, M.; Usami, M.; Inoue, N.; Sakakibara, Y.; Yamada, K.; Konishi, M.; Ohta, K.; Yachie, A. Clinical significance of serum soluble TNF receptor II level and soluble TNF receptor II/I ratio as indicators of coronary artery lesion development in Kawasaki disease. Cytokine 2018, 108, 168–172. [Google Scholar] [CrossRef]
- Heidari, B.; Amin, R.; Kashef, S.; Alyasin, S.; Moghtaderi, M.; Aminshahidi, M.; Kalani, M. Expression of CD11b as an adhesion molecule on neutrophils in children with Kawasaki disease. Iran. J. Allergy Asthma Immunol. 2014, 13, 265–270. [Google Scholar]
- Kobayashi, T.; Kimura, H.; Okada, Y.; Inoue, Y.; Kobayashi, T.; Shinohara, M.; Morikawa, A. Increased CD11b expression on polymorphonuclear leucocytes and cytokine profiles in patients with Kawasaki disease. Clin. Exp. Immunol. 2007, 148, 112–118. [Google Scholar] [CrossRef]
- Zou, Q.M.; Li, X.H.; Song, R.X.; Xu, N.P.; Zhang, T.; Zhang, M.M.; Lin, Y.; Shi, L.; Fu, J.; Cui, X.D. Early decreased plasma levels of factor B and C5a are important biomarkers in children with Kawasaki disease. Pediatr. Res. 2015, 78, 205–211. [Google Scholar] [CrossRef][Green Version]
- Song, R.X.; Zou, Q.M.; Li, X.H.; Xu, N.P.; Zhang, T.; Fu, J.; Cui, X.D. Plasma MASP-1 concentration and its relationship to recovery from coronary artery lesion in children with Kawasaki disease. Pediatr. Res. 2016, 79, 301–307. [Google Scholar] [CrossRef][Green Version]
- Feng, S.; Yadav, S.K.; Gao, F.; Yi, Q. Plasma levels of monokine induced by interferon-gamma/chemokine (C-X-X motif) ligand 9, thymus and activation-regulated chemokine/chemokine (C-C motif) ligand 17 in children with Kawasaki disease. BMC Pediatr. 2015, 15, 109. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, S.; Imagawa, K.; Yano, Y.; Lin, L.; Shiono, J.; Takahashi-Igari, M.; Hara, H.; Hayashi, D.; Imai, H.; Morita, A.; et al. The CXCL10-CXCR3 axis plays an important role in Kawasaki disease. Clin. Exp. Immunol. 2024, 216, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.P.; Huang, Y.H.; Hsu, Y.W.; Yang, K.D.; Chien, H.C.; Yu, H.R.; Yang, Y.L.; Wang, C.L.; Chang, W.C.; Kuo, H.C. TARC/CCL17 gene polymorphisms and expression associated with susceptibility and coronary artery aneurysm formation in Kawasaki disease. Pediatr. Res. 2013, 74, 545–551. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, J.; Zhao, C.; Zhang, S. Semaphorin 7A promotes endothelial permeability and inflammation via plexin C1 and integrin β1 in Kawasaki disease. BMC Pediatr. 2024, 24, 285. [Google Scholar] [CrossRef]
- Huang, J.; Wu, S.; Cao, S.; Zhu, X.; Zhang, S. Neutrophil-derived semaphorin 4D induces inflammatory cytokine production of endothelial cells via different plexin receptors in Kawasaki disease. BioMed Res. Int. 2020, 2020, 6663291. [Google Scholar] [CrossRef]
- Guo, M.; Fan, S.; Chen, Q.; Jia, C.; Qiu, M.; Bu, Y.; Tang, W.H.; Zhang, Y. Platelet-derived microRNA-223 attenuates TNF-α induced monocytes adhesion to arterial endothelium by targeting ICAM-1 in Kawasaki disease. Front. Immunol. 2022, 13, 922868. [Google Scholar] [CrossRef]
- Reindel, R.; Bischof, J.; Kim, K.Y.; Orenstein, J.M.; Soares, M.B.; Baker, S.C.; Shulman, S.T.; Perlman, E.J.; Lingen, M.W.; Pink, A.J.; et al. CD84 is markedly up-regulated in Kawasaki disease arteriopathy. Clin. Exp. Immunol. 2014, 177, 203–211. [Google Scholar] [CrossRef]
- Shuai, S.; Zhang, H.; Zhang, R.; Tang, M.; Luo, E.; Yang, Y.; Gao, Y.; Yue, S.; Liang, H.; Cai, J. Prediction of coronary artery lesions based on C-reactive protein levels in children with Kawasaki disease: A retrospective cohort study. J. Pediatr. (Rio J.) 2023, 99, 406–412. [Google Scholar] [CrossRef]
- Xia, Y.; Tian, X.; Li, Q.; Nakajima, T.; Saito, H.; Terai, M. Expression of FcγRs on monocytes among Kawasaki disease patients with coronary artery lesions. Int. Immunopharmacol. 2017, 45, 1–5. [Google Scholar] [CrossRef]
- Kim, K.Y.; Ahn, Y.; Kim, D.Y.; Kim, H.S.; Kim, D.S. Elevated serum YKL-40 levels in patients with Kawasaki disease. Biomarkers 2016, 22, 326–330. [Google Scholar] [CrossRef]
- Wakiguchi, H.; Hasegawa, S.; Suzuki, Y.; Kudo, K.; Ichiyama, T. Relationship between T-cell HLA-DR expression and intravenous immunoglobulin treatment response in Kawasaki disease. Pediatr. Res. 2015, 77, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zeng, A.; Yang, P.; Zhang, J.; Liu, D.; Li, M.; Jing, F.; Yi, Q. Role of leukocyte-associated Ig-like receptor-1 in the pathogenesis of Kawasaki disease and coronary artery aneurysms. Immunol. Lett. 2025, 271, 106948. [Google Scholar] [CrossRef] [PubMed]
- Armaroli, G.; Verweyen, E.; Pretzer, C.; Kessel, K.; Hirono, K.; Ichida, F.; Okabe, M.; Cabral, D.A.; Foell, D.; Brown, K.L.; et al. Monocyte-derived interleukin-1β as the driver of S100A12-induced sterile inflammatory activation of human coronary artery endothelial cells: Implications for the pathogenesis of Kawasaki disease. Arthritis Rheumatol. 2019, 71, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Zhang, J.; Zhong, J.; Zheng, Y. Elevated levels of platelet activating factor and its acetylhydrolase indicate high risk of Kawasaki disease. J. Interferon. Cytokine. Res. 2020, 40, 159–167. [Google Scholar] [CrossRef]
- Wu, Q.; Weng, R.; Xu, Y.; Wang, L.; Huang, Y.; Yang, J. Activin A suppresses peripheral CD8+ T lymphocyte activity in acute-phase Kawasaki disease. BMC Immunol. 2021, 22, 17. [Google Scholar] [CrossRef]
- Si, F.; Lu, Y.; Wen, Y.; Chen, T.; Zhang, Y.; Yang, Y. Cathelicidin (LL-37) causes expression of inflammatory factors in coronary artery endothelial cells of Kawasaki disease by activating TLR4-NF-κB-NLRP3 signaling. Immun. Inflamm. Dis. 2023, 11, e1032. [Google Scholar] [CrossRef]
- Bordea, M.A.; Costache, C.; Grama, A.; Florian, A.I.; Lupan, I.; Samasca, G.; Deleanu, D.; Makovicky, P.; Makovicky, P.; Rimarova, K. Cytokine cascade in Kawasaki disease versus Kawasaki-like syndrome. Physiol. Res. 2022, 71, 17–27. [Google Scholar] [CrossRef]
- Porritt, R.A.; Chase Huizar, C.; Dick, E.J.; Kumar, S.; Escalona, R.; Gomez, A.C.; Marek-Iannucci, S.; Noval Rivas, M.; Patterson, J.; Forsthuber, T.G.; et al. Inhibition of IL-6 in the LCWE mouse model of Kawasaki disease inhibits acute phase reactant serum amyloid A but fails to attenuate vasculitis. Front. Immunol. 2021, 12, 630196. [Google Scholar] [CrossRef]
- Yeung, R.S. Kawasaki disease: Update on pathogenesis. Curr. Opin. Rheumatol. 2010, 22, 551–560. [Google Scholar] [CrossRef]
- Li, J.; Li, D.; Hu, M.; Huang, H.; Xu, S.; Li, H. Red blood cell distribution width and tumor necrosis factor-α for the early prediction of coronary artery lesion in Kawasaki disease: A retrospective study. Eur. J. Pediatr. 2022, 181, 903–909. [Google Scholar] [CrossRef]
- Stringer, E.; Yeung, R.S.M. Pathogenesis of Kawasaki disease: The central role of TNF-α. Future Rheumatol. 2008, 3, 69–77. [Google Scholar] [CrossRef]
- Alphonse, M.P.; Duong, T.T.; Tam, S.; Yeung, R.S.M. Mercury increases IL-1β and IL-18 secretion and intensifies coronary arteritis in an animal model of Kawasaki disease. Front. Immunol. 2023, 14, 1126154. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Shimizu, M.; Shimbo, A.; Irabu, H.; Yokoyama, K.; Furuno, K.; Tanaka, T.; Ueno, K.; Fujita, S.; Iwata, N.; et al. Clinical significance of serum cytokine profiles for differentiating between Kawasaki disease and its mimickers. Cytokine 2023, 169, 156280. [Google Scholar] [CrossRef]
- Patel, B.; Silwal, A.; Eltokhy, M.A.; Gaikwad, S.; Curcic, M.; Patel, J.; Prasad, S. Deciphering CD59: Unveiling its role in immune microenvironment and prognostic significance. Cancers 2024, 16, 3699. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Zandstra, J.; van de Geer, A.; Tanck, M.W.T.; van Stijn-Bringas Dimitriades, D.; Aarts, C.E.M.; Dietz, S.M.; van Bruggen, R.; Schweintzger, N.A.; Zenz, W.; Emonts, M.; et al. Biomarkers for the discrimination of acute Kawasaki disease from infections in childhood. Front. Pediatr. 2020, 8, 355. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, X. The predictive values of MMP-9, PLTs, ESR, and CRP levels in Kawasaki disease with cardiovascular injury. Evid.-Based Complement. Altern. Med. 2022, 2022, 6913315. [Google Scholar] [CrossRef]
- Hokibara, S.; Kobayashi, N.; Kobayashi, K.; Shigemura, T.; Nagumo, H.; Takizawa, M.; Yamazaki, T.; Agematsu, K. Markedly elevated CD64 expression on neutrophils and monocytes as a biomarker for diagnosis and therapy assessment in Kawasaki disease. Inflamm. Res. 2016, 65, 579–585. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, Z.; Huang, Y.; Wang, L.; Weng, R.; Yang, J. Effect of Activin A on activation status of monocytes in acute-phase Kawasaki disease. Clin. Exp. Med. 2021, 21, 407–414. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Nookala, V. Biochemistry of platelet activating factor. In StatPearls; StatPearls: St. Petersburg, FL, USA, 2025. [Google Scholar]
- Rife, E.; Gedalia, A. Kawasaki disease: An update. Curr. Rheumatol. Rep. 2020, 22, 75. [Google Scholar] [CrossRef]
- Shulman, S.T.; Rowley, A.H. Kawasaki disease: Insights into pathogenesis and approaches to treatment. Nat. Rev. Rheumatol. 2015, 11, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Fang, X. The clinical value of dynamic monitoring of complete blood count in predicting immunoglobulin resistance in Chinese children with Kawasaki disease. Sci. Rep. 2025, 15, 18041. [Google Scholar] [CrossRef]
- Furukawa, S.; Matsubara, T.; Jujoh, K.; Sasai, K.; Nakachi, S.; Sugawara, T.; Yabuta, K.; Kato, H. Reduction of peripheral blood macrophages/monocytes in Kawasaki disease by intravenous gammaglobulin. Eur. J. Pediatr. 1990, 150, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Yang, H.J.; Kee, S.J.; Choi, I.; Ha, K.; Ki, K.K.; Jeong, I.S.; Cho, H.J. The “intermediate” CD14+CD16+ monocyte subpopulation plays a role in IVIG responsiveness of children with Kawasaki disease. Pediatr. Rheumatol. Online J. 2021, 19, 76. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, J.; Fan, R. Efficacy of glucocorticoid plus intravenous immunoglobulin in children with immunoglobulin-insensitive Kawasaki disease. J. Healthc. Eng. 2022, 2022, 9011259, Retracted in J. Healthc. Eng. 2023, 2023, 9824018. [Google Scholar] [CrossRef]
- McAlpine, S.M.; Roberts, S.E.; Heath, J.J.; Käsermann, F.; Issekutz, A.C.; Issekutz, T.B.; Derfalvi, B. High dose intravenous IgG therapy modulates multiple NK cell and T cell functions in patients with immune dysregulation. Front. Immunol. 2021, 12, 660506. [Google Scholar] [CrossRef]
- Kong, W.X.; Ma, F.Y.; Fu, S.L.; Wang, W.; Xie, C.H.; Zhang, Y.Y.; Gong, F.Q. Biomarkers of intravenous immunoglobulin resistance and coronary artery lesions in Kawasaki disease. World J. Pediatr. 2019, 15, 168–175. [Google Scholar] [CrossRef]
- Zhang, H.; Song, H.-B.; Wang, D.X.; Deng, H.Y.; Sun, W.L. Correlation between the level of inflammatory cytokines and prognosis in children with IVIG-sensitive Kawasaki disease and IVIG-resistant Kawasaki disease. Pak. J. Med. Sci. 2022, 38, 1165–1169. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, X.; Shen, J.; Lu, X.; Zhang, J.; Chen, S. Changes in peripheral blood neutrophils, lymphocytes and IL-10 in children with Kawasaki disease from different age groups undergoing intravenous immunoglobulin: A retrospective study. Mediat. Inflamm. 2020, 2020, 5213451. [Google Scholar] [CrossRef]
- Li, G.; Wang, T.; Gou, Y.; Zeng, R.; Liu, D.; Duan, Y.; Liu, B. Value of C-reactive protein/albumin ratio in predicting intravenous immunoglobulin-resistant Kawasaki disease—A data from multi-institutional study in China. Int. Immunopharmacol. 2020, 89 Pt A, 107037. [Google Scholar] [CrossRef]
- Portman, M.A.; Dahdah, N.S.; Slee, A.; Olson, A.K.; Choueiter, N.F.; Soriano, B.D.; Buddhe, S.; Altman, C.A.; EATAK Investigators. Etanercept with IVIg for acute Kawasaki disease: A randomized controlled trial. Pediatrics 2019, 143, e20183675. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Xie, F.; Zhou, Q.; Ouyang, Q.; Li, L.; Zhao, W.; Liu, X. Case series on the efficacy and safety of tocilizumab in IVIG-resistant Kawasaki disease: A retrospective analysis of five patients. J. Inflamm. Res. 2024, 17, 10991–10998. [Google Scholar] [CrossRef] [PubMed]
- Kessel, C.; Koné-Paut, I.; Tellier, S.; Belot, A.; Masjosthusmann, K.; Wittkowski, H.; Fuehner, S.; Rossi-Semerano, L.; Dusser, P.; Marie, I.; et al. An immunological axis involving interleukin 1β and leucine-rich-α2-glycoprotein reflects therapeutic response of children with Kawasaki disease: Implications from the KAWAKINRA trial. J. Clin. Immunol. 2022, 42, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- Nomura, O.; Fukuda, S.; Ota, E.; Ono, H.; Ishiguro, A.; Kobayashi, T. Monoclonal antibody and anti-cytokine biologics for Kawasaki disease: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2021, 51, 1045–1056. [Google Scholar] [CrossRef]
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; Council on Epidemiology and Prevention. Diagnosis, treatment, and long-term management of Kawasaki disease: A scientific statement for health professionals from the American Heart Association. Circulation 2017, 135, e927–e999. [Google Scholar]
- Koizumi, K.; Hoshiai, M.; Katsumata, N.; Toda, T.; Kise, H.; Hasebe, Y.; Kono, Y.; Sunaga, Y.; Yoshizawa, M.; Watanabe, A.; et al. Infliximab regulates monocytes and regulatory T cells in Kawasaki disease. Pediatr. Int. 2018, 60, 796–802. [Google Scholar] [CrossRef]
- Koizumi, K.; Hoshiai, M.; Moriguchi, T.; Katsumata, N.; Toda, T.; Kise, H.; Hasebe, Y.; Kono, Y.; Sunaga, Y.; Yoshizawa, M.; et al. Plasma exchange downregulates activated monocytes and restores regulatory T cells in Kawasaki disease. Ther. Apher. Dial. 2019, 23, 92–98. [Google Scholar] [CrossRef]
- Duan, M.; Geng, Z.; Gao, L.; Zhao, Y.; Li, Z.; Chen, L.; Kuosmanen, P.; Qi, G.; Gong, F.; Yu, G. An interpretable machine learning-assisted diagnostic model for Kawasaki disease in children. Sci. Rep. 2025, 15, 7927. [Google Scholar] [CrossRef]
- Kobayashi, T.; Inoue, Y.; Takeuchi, K.; Okada, Y.; Tamura, K.; Tomomasa, T.; Kobayashi, T.; Morikawa, A. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation 2006, 113, 2606–2612. [Google Scholar] [CrossRef]
- Egami, K.; Muta, H.; Ishii, M.; Suda, K.; Sugahara, Y.; Iemura, M.; Matsuishi, T. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J. Pediatr. 2006, 149, 237–240. [Google Scholar] [CrossRef]
- Sano, T.; Kurotobi, S.; Matsuzaki, K.; Yamamoto, T.; Maki, I.; Miki, K.; Kogaki, S.; Hara, J. Prediction of non-responsiveness to standard high-dose gamma-globulin therapy in patients with acute Kawasaki disease before starting initial treatment. Eur. J. Pediatr. 2007, 166, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Kuniyoshi, Y.; Tsujimoto, Y.; Banno, M.; Taito, S.; Ariie, T.; Takahashi, N.; Tokutake, H.; Takada, T. Prediction models for intravenous immunoglobulin resistance in Kawasaki disease: A meta-analysis. Pediatrics 2023, 151, e2022059175. [Google Scholar] [CrossRef] [PubMed]
- Piram, M.; Darce Bello, M.; Tellier, S.; Di Filippo, S.; Boralevi, F.; Madhi, F.; Meinzer, U.; Cimaz, R.; Piedvache, C.; Koné-Paut, I. Defining the risk of first intravenous immunoglobulin unresponsiveness in non-Asian patients with Kawasaki disease. Sci. Rep. 2020, 10, 3125. [Google Scholar] [CrossRef]
- Sleeper, L.A.; Minich, L.L.; McCrindle, B.M.; Li, J.S.; Mason, W.; Colan, S.D.; Atz, A.M.; Printz, B.F.; Baker, A.; Vetter, V.L.; et al. Pediatric Heart Network Investigators. Evaluation of Kawasaki disease risk-scoring systems for intravenous immunoglobulin resistance. J. Pediatr. 2011, 158, 831–835.e3. [Google Scholar] [CrossRef]
- Takeshita, S.; Kanai, T.; Kawamura, Y.; Yoshida, Y.; Nonoyama, S. A comparison of the predictive validity of the combination of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio and other risk scoring systems for intravenous immunoglobulin (ivig)-resistance in Kawasaki disease. PLoS ONE 2017, 12, e0176957. [Google Scholar] [CrossRef]
- Mirata, D.; Tiezzi, A.C.; Buffoni, L.; Pagnini, I.; Maccora, I.; Marrani, E.; Mastrolia, M.V.; Simonini, G.; Giani, T. Learning-based models for predicting IVIG resistance and coronary artery lesions in Kawasaki disease: A review of technical aspects and study features. Pediatr. Drugs 2025, 27, 465–479. [Google Scholar] [CrossRef]
| Marker | Role in KD | Change at Specific KD Phase or After Treatment a | References | |
|---|---|---|---|---|
| Pathogenesis | Treatment Response | |||
| Cytokines | ||||
| IL-1β | Y | Y | Serum levels: KD + CALs >> KD no CALs >> febrile and healthy controls ↓↓ after IVIG | [41,42] |
| IL-2 receptor | Y | Y | Serum levels: KD >> controls or reference range ↓↓ after IVIG | [43] |
| IL-6 | Y | Y | Serum levels ↑ in KD, ↑ in acute KD KD + CALs >> KD no CALs | [33,34,42,44,45,46,47,48,49,50] |
| ↓↓ after IVIG, plasma exchange, infliximab, or anakinra | [30,33,45,47,50,51,52] | |||
| IL-8 | Y | - | ↑ in acute KD, KD >> MIS-C >> healthy controls | [53] |
| IL-10 | Y | Y | ↑ in KD, ↑ in IVIG-resistant KD patients. ↓↓ after IVIG or anakinra | [30,42,48,50] |
| IL-17 | Y | Y | KD >> febrile and healthy controls | [34,42,54,55,56] |
| ↓↓ after plasma exchange and gradually after IVIG | [34,54] | |||
| IL-18 | Y | Y | KD >> healthy controls Associated with CAL. | [57,58] |
| ↓↓ after IVIG | [59] | |||
| IL-23 | Y | - | KD + CALs >> KD no CALs >> infectious disease and healthy controls | [31] |
| IL-27 | Y | - | Serum levels: KD + CALs >> KD no CALs >> healthy controls | [42] |
| IL-31 | Y | Y | Serum levels KD >> febrile and healthy controls Significantly associated with CALs | [34,55] |
| ↑↑ after IVIG | [55] | |||
| IL-33 | Y | Y | Acute KD >> healthy controls; KD << febrile controls ↓↓ after IVIG | [60,61] |
| IL-35 | Y | - | KD << febrile and healthy controls KD + CAL << no CAL | [62] |
| KD >> healthy controls | [26] | |||
| IL-37 | Y | - | KD << febrile and healthy controls | [63] |
| IFN-γ | Y | Y | KD + KDSS >> KD >> MIS-C >> healthy controls ↓↓ after IVIG | [30,46,53] |
| TNF-α | Y | Y | Acute KD >> healthy controls ↑↑ KD + CALs | [30,58,64] |
| ↓↓ after plasma exchange and infliximab treatment. ↓↓ after IVIG in KD patients without CALs and in IVIG responders ↑ after IVIG in KD patients with CALs and in IVIG-resistant patients; predictor of IVIG-resistance | [30,48,51,52,65] | |||
| Soluble TNFR1 and TNFR2 | Y | Y | Acute >> subacute KD TNFR2 and TNFR1/2 ratio: KD + CALs >> no CALs ↓↓ after plasma exchange and infliximab treatment TNFR1 remains high in infliximab-resistant patients. | [51,52,66,67] |
| G-CSF | Y | Y | KD >> MIS-C >> healthy controls ↓↓ after plasma exchange, infliximab, and IVIG treatment Remains high in infliximab-resistant patients | [41,51,52,53] |
| sCD40L | Y | - | KD >> febrile controls | [61] |
| IP-10 | Y | Y | ↑ in KD ↓ after infliximab treatment | [52] |
| TGF-β | Y | - | ↑ in acute KD >> infectious disease and healthy controls | [31] |
| Complement receptors | ||||
| CD11b | Y | Y | Mean CD11b ↓ in KD before and after IVIG | [68,69] |
| CD59 | Y | - | subacute KD << acute KD KD + CAL << no CAL | [70,71] |
| Chemokines and cell adhesion molecules | ||||
| CXCL9 and CXCL10 | Y | Y | Both ↑ in acute KD; CXCL9: KD + CAL >> no CAL ↓↓ after IVIG CXCL10: ↓↓ after anakinra treatment | [50,61,72,73] |
| MCP-1 (CCL2) | Y | Y | ↑ in acute KD ↓↓ after IVIG | [73] |
| CCL5 | Y | - | ↑ in acute KD, KD >> healthy controls | [53] |
| Eotaxin (CCL11) | Y | Y | ↑ in acute KD ↓↓ after IVIG | [73] |
| CCL17 | Y | Y | KD >> healthy controls; KD + CAL >> no CAL | [73] |
| ↓↓ after IVIG | [74] | |||
| Semaphorin 7A | Y | - | Serum levels ↑ in KD | [75] |
| Semaphorin 4D | Y | - | Serum levels ↑ in acute KD and in patients with CALs ↓ in convalescent phase | [76] |
| P-Selectin (CD62P) | Y | - | 2~3-fold higher expression in KD platelets than in healthy platelets | [77] |
| Other | ||||
| VEGF (angiogenic factor) | Y | - | KD + CALs >> no CALs | [78] |
| CD84 (Signaling lymphocyte activation molecule) | Y | - | Robust expression in inflammatory cells in arterial walls in 6/7 acute and 4/5 chronic cases. | [78] |
| CRP | Y | Y | ↑ in KD + CAL Level >100 mg/L at diagnosis is an independent risk factor of IVIG resistance. | [11,79] |
| Fcγ receptors | Y | Y | FcγRIII and FcγRIIa levels: KD >> controls | [80] |
| FcγRIIb: KD << controls; KD + CALs << no CALs | [80] | |||
| FcγRI (CD64): ↑↑ expression on neutrophils and monocytes at the onset of KD flare-ups. ↓↓ after IVIG | [81] | |||
| LILRs/ILTs (receptors involved in immune regulation) | Y | Y | LILRB4 (ILT3/LIR-5/CD85k): ↑ in acute KD, expressed uniquely on antibody-secreting B cells; ↓ after IVIG LILRB1 (ILT2/CD85j): ↑ in acute KD and after IVIG in naïve and memory B cells, antibody-secreting cells, and monocytes. | [36] |
| HLA-DR (MHC molecule) | - | Y | ↑ in IVIG-resistant KD patients. | [82] |
| LAIR-1 (receptor involved in immune regulation) | Y | - | Significantly increased in KD >> healthy controls; KD + CAL >> no CAL | [83] |
| YKL-40 (endothelial marker) | Y | - | Acute KD >> disease and healthy controls | [81] |
| S100A12 (calcium-binding protein) | Y | Y | ↑↑ in acute KD KD + CAL >> no CAL ↓↓ after IVIG, no change in non-responsive patients ↓↓ after anakinra treatment | [50,84] |
| PAF (phospholipid mediator) | Y | - | Acute KD >> febrile and healthy controls KD + CAL >> no CAL | [85] |
| Activin receptor IIA | Y | - | Increased expression on CD8+ T cells and CD19+ B cells in KD. | [86] |
| Cathelicidin (LL-37) (anti-microbial peptide) | - | KD >> pneumonia and healthy controls | [87] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agrafiotou, A.; Sapountzi, E.; Margoni, A.; Fotis, L. Immunophenotype of Kawasaki Disease: Insights into Pathogenesis and Treatment Response. Life 2025, 15, 1012. https://doi.org/10.3390/life15071012
Agrafiotou A, Sapountzi E, Margoni A, Fotis L. Immunophenotype of Kawasaki Disease: Insights into Pathogenesis and Treatment Response. Life. 2025; 15(7):1012. https://doi.org/10.3390/life15071012
Chicago/Turabian StyleAgrafiotou, Aikaterini, Evdoxia Sapountzi, Angeliki Margoni, and Lampros Fotis. 2025. "Immunophenotype of Kawasaki Disease: Insights into Pathogenesis and Treatment Response" Life 15, no. 7: 1012. https://doi.org/10.3390/life15071012
APA StyleAgrafiotou, A., Sapountzi, E., Margoni, A., & Fotis, L. (2025). Immunophenotype of Kawasaki Disease: Insights into Pathogenesis and Treatment Response. Life, 15(7), 1012. https://doi.org/10.3390/life15071012






