Controversies and Perspectives in the Current Management of Patients with Locally Advanced Rectal Cancer—A Systematic Review
Abstract
1. Introduction
2. Materials and Methods (See Table 1 and Table S1)
| Items | Specification |
|---|---|
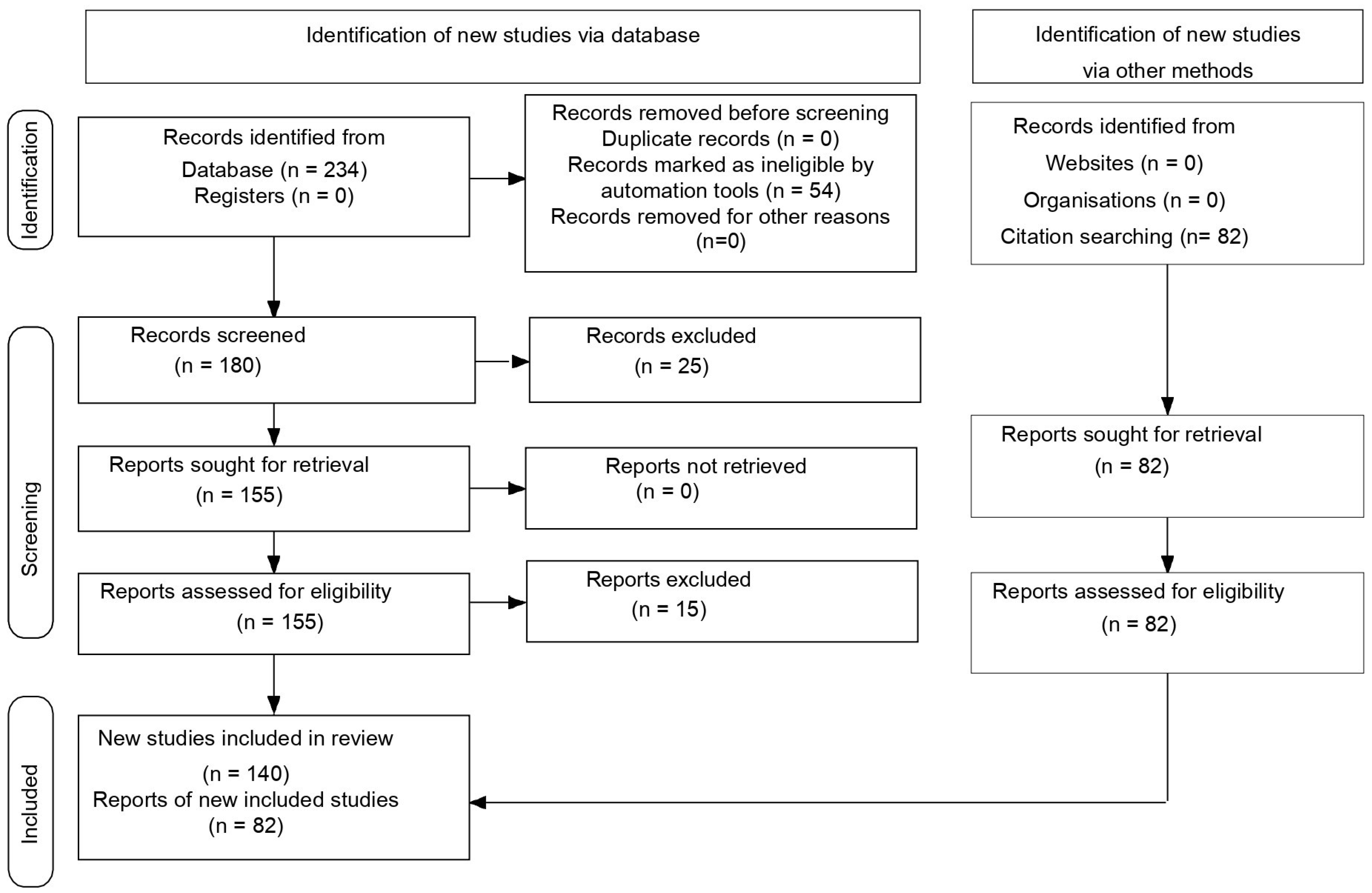
| Database and other sources searched Search terms used (including MeSH and free text search terms and filters) Timeframe Inclusion and exclusion criteria (study type, language restrictions, etc.). Selection process | PubMed Central (PMC) Search strategy (see Table S1) 2015–2025 Inclusion criteria: meta-analyses; trials studies; clinical trials & updates of clinical trials; reviews; original articles; only studies/papers/journals written in English. Exclusion criteria: unpublished data from abstracts contained in volumes from various congresses or conferences; papers that were not in English RDB performed the search in the databases according to the presented criteria. RDB conducted the database search based on the specified criteria. A total of 234 records were identified using the advanced search builder with the applied filters (Table S1). Of these, 54 articles were excluded using automation tools. The remaining 180 articles were screened by two independent reviewers, RDB and RES, resulting in the exclusion of 25 based on title and abstract. The remaining 155 reports were assessed for eligibility. If a study was deemed irrelevant by either RDB or RES, the full text was reviewed by a third reviewer (MG) for final evaluation. As a result, 15 reports were excluded. Additionally, the reference lists of the included articles were examined for further relevant studies, and 82 additional records were identified through citation screening (Figure 1). |
3. Therapeutic Options over Time
4. Current Indications for Total Neoadjuvant Therapy
4.1. TNM Stage—T3/T4N+
4.2. Contraindications for TNT
5. Radiotherapy in LARC
6. The Importance of Chemotherapy in the Management of LARC
6.1. 5FU and Oxaliplatin
6.2. The Role of Adjuvant Chemotherapy (CT)
6.3. TNT vs. Standard CRT
6.4. Neoadjuvant Induction vs. Consolidation Chemotherapy
6.5. Strategies with Intensified Neoadjuvant Chemotherapy
6.6. The Role of Targeted Therapy in Patients with LARC
7. Evaluation of Response to Neoadjuvant Treatment
8. Effectiveness of TNT
8.1. Markers of Neoadjuvant Treatment Effectiveness
8.2. Rate of pCR
8.3. Local Recurrence Rate
8.4. Distant Survival
8.5. NOM Rate
9. Surgical Management
9.1. Current Surgical Principles
9.2. Postoperative Complications
10. Non-Operative Management (NOM)
11. Perspectives
12. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RC | rectal cancer |
| MRF | mesorectal fascia |
| CRM | circumferential resection margins |
| CRT | chemoradiotherapy |
| CT | chemotherapy |
| LCRT | long-course radiotherapy |
| S-RT | short-course radiotherapy |
| pCR | pathologic complete response |
| cCR | clinic complete response |
| NOM | non-operative management |
| WW | wait-and-watch strategy |
References
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Sadanandam, A.; Wang, X.; de Sousa E Melo, F.; Gray, J.W.; Vermeulen, L.; Hanahan, D.; Medema, J.P. Reconciliation of classification systems defining molecular subtypes of colorectal cancer: Interrelationships and clinical implications. Cell Cycle 2014, 13, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundand, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- Hammarström, K.; Mezheyeuski, A.; Korsavidou Hult, N.; Sjöblom, T.; Glimelius, B. Stage distribution utilizing magnetic resonance imaging in an unselected population of primary rectal cancers. Eur. J. Surg. Oncol. 2018, 44, 1858–1864. [Google Scholar] [CrossRef]
- Hung, A.Y.; Canning, C.A.; Patel, K.M.; Holland, J.M.; Kachnic, L.A. Radiation therapy for gastrointestinal cancer. Hematol. Oncol. Clin. N. Am. 2006, 20, 287–320. [Google Scholar] [CrossRef]
- Liscu, H.D.; Miron, A.I.; Rusea, A.R.; Oprea, A.N.; Mitre, R.; Herdea, A.; Negreanu, R. Short-Course Radiotherapy versus Long-Course Radio-Chemotherapy as Neoadjuvant Treatment for Locally Advanced Rectal Cancer: Meta-Analysis from a Toxicity Perspective. Maedica 2021, 16, 382–388. [Google Scholar] [CrossRef]
- Liu, S.X.; Zhou, Z.R.; Chen, L.X.; Yang, Y.J.; Hu, Z.D.; Zhang, T.S. Short-course Versus Long-course Preoperative Radiotherapy plus Delayed Surgery in the Treatment of Rectal Cancer: A Meta-analysis. Asian Pac. J. Cancer Prev. 2015, 16, 5755–5762. [Google Scholar] [CrossRef]
- Zhou, Z.R.; Liu, S.X.; Zhang, T.S.; Chen, L.X.; Xia, J.; Hu, Z.D.; Li, B. Short-course preoperative radiotherapy with immediate surgery versus long-course chemoradiation with delayed surgery in the treatment of rectal cancer: A systematic review and meta-analysis. Surg. Oncol. 2014, 23, 211–221. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, J.; He, X.; Wang, C.; Lian, L.; Liu, H.; Wang, J.; Lan, P. Postoperative adjuvant chemotherapy for stage II colorectal cancer: A systematic review of 12 randomized controlled trials. J. Gastrointest. Surg. 2012, 16, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Wolmark, N.; Wieand, H.S.; Hyams, D.M.; Colangelo, L.; Dimitrov, N.V.; Romond, E.H.; Wexler, M.; Prager, D.; Cruz, A.B.; Gordon, P.H.; et al. Randomized trial of postoperative adjuvant chemotherapy with or without radiotherapy for carcinoma of the rectum: National Surgical Adjuvant Breast and Bowel Project Protocol R-02. J. Natl. Cancer Inst. 2000, 92, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Castan, F.; Etienne, P.L.; Rio, E.; Mesgouez-Nebout, N.; Evesque, L.; Vendrely, V.; Artignan, X.; Bouché, O.; Gargot, D.; et al. Total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiotherapy in patients with locally advanced rectal cancer: Long-term results of the UNICANCER-PRODIGE 23 trial. Ann. Oncol. 2024, 35, 873–881. [Google Scholar] [CrossRef]
- Dijkstra, E.A.; Nilsson, P.J.; Hospers, G.A.P.; Bahadoer, R.R.; Meershoek-Klein Kranenbarg, E.; Roodvoets, A.G.H.; Putter, H.; Berglund, Å.; Cervantes, A.; Crolla, R.M.P.H.; et al. Locoregional Failure During and After Short-course Radiotherapy Followed by Chemotherapy and Surgery Compared With Long-course Chemoradiotherapy and Surgery: A 5-Year Follow-up of the RAPIDO Trial. Ann. Surg. 2023, 278, e766–e772. [Google Scholar] [CrossRef]
- Heald, R.J.; Ryall, R.D. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986, 1, 1479–1482. [Google Scholar] [CrossRef]
- Emile, S.H.; de Lacy, F.B.; Keller, D.S.; Martin-Perez, B.; Alrawi, S.; Lacy, A.M.; Chand, M. Evolution of transanal total mesorectal excision for rectal cancer: From top to bottom. World J. Gastrointest. Surg. 2018, 10, 28–39. [Google Scholar] [CrossRef]
- Diaz-Gonzalez, J.A.; Arbea, L.; Aristu, J. Rectal cancer treatment: Improving the picture. World J. Gastroenterol. 2007, 13, 5805–5812. [Google Scholar] [CrossRef]
- Swedish Rectal Cancer Trial; Cedermark, B.; Dahlberg, M.; Glimelius, B.; Påhlman, L.; Rutqvist, L.E.; Wilking, N. Improved survival with preoperative radiotherapy in resectable rectal cancer. N. Engl. J. Med. 1997, 336, 980–987. [Google Scholar] [CrossRef]
- Kapiteijn, E.; Marijnen, C.A.; Nagtegaal, I.D.; Putter, H.; Steup, W.H.; Wiggers, T.; Rutten, H.J.; Pahlman, L.; Glimelius, B.; van Krieken, J.H.; et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N. Engl. J. Med. 2001, 345, 638–646. [Google Scholar] [CrossRef]
- Frykholm, G.J.; Glimelius, B.; Påhlman, L. Preoperative or postoperative irradiation in adenocarcinoma of the rectum: Final treatment results of a randomized trial and an evaluation of late secondary effects. Dis. Colon. Rectum 1993, 36, 564–572. [Google Scholar] [CrossRef]
- Molinari, C.; Passardi, A. Why is neoadjuvant chemoradiation therapy underused for locally advanced rectal cancer? Expert Rev. Gastroenterol. Hepatol. 2016, 10, 1317–1319. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Cho, S.; Kim, Y. Patterns of Rectal Cancer Radiotherapy Adopting Evidence-Based Medicine: An Analysis of the National Database from2005 to 2016. Cancer Res. Treat. 2018, 50, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Folkesson, J.; Birgisson, H.; Pahlman, L.; Cedermark, B.; Glimelius, B.; Gunnarsson, U. Swedish Rectal Cancer Trial: Long lasting benefits from radiotherapy on survival and local recurrence rate. J. Clin. Oncol. 2005, 23, 5644–5650. [Google Scholar] [CrossRef]
- Sauer, R.; Becker, H.; Hohenberger, W.; Rödel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef]
- Buckley, A.M.; Lynam-Lennon, N.; O’Neill, H.; O’Sullivan, J. Targeting hallmarks of cancer to enhance radiosensitivity in gastrointestinal cancers. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 298–313. [Google Scholar] [CrossRef]
- van der Sluis, F.J.; Couwenberg, A.M.; de Bock, G.H.; Intven, M.P.W.; Reerink, O.; van Leeuwen, B.L.; van Westreenen, H.L. Population-based study of morbidity risk associated with pathological complete response after chemoradiotherapy for rectal cancer. Br. J. Surg. 2020, 107, 131–139. [Google Scholar] [CrossRef]
- Dinaux, A.M.; Amri, R.; Bordeianou, L.G.; Hong, T.S.; Wo, J.Y.; Blaszkowsky, L.S.; Allen, J.N.; Murphy, J.E.; Kunitake, H.; Berger, D.L. The Impact of Pathologic Complete Response in Patients with Neoadjuvantly Treated Locally Advanced Rectal Cancer-a Large Single-Center Experience. J. Gastrointest. Surg. 2017, 21, 1153–1158. [Google Scholar] [CrossRef]
- Couch, D.G.; Hemingway, D.M. Complete radiotherapy response in rectal cancer: A review of the evidence. World J. Gastroenterol. 2016, 22, 467–470. [Google Scholar] [CrossRef]
- Jin, C.; Deng, X.; Li, Y.; He, W.; Yang, X.; Liu, J. Lymph node ratio is an independent prognostic factor for rectal cancer after neoadjuvant therapy: A meta-analysis. J. Evid. Based Med. 2018, 11, 169–175. [Google Scholar] [CrossRef]
- Du, R.; Chang, Y.; Zhang, J.; Cheng, Y.; Li, Y.; Zhang, C.; Zhang, J.; Xu, L.; Liu, Y. Whether the watch-and-wait strategy has application value for rectal cancer with clinical complete response after neoadjuvant chemoradiotherapy? A network meta-analysis. Asian J. Surg. 2024, 47, 853–863. [Google Scholar] [CrossRef]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv22–iv40. [Google Scholar] [CrossRef]
- Sclafani, F.; Corrò, C.; Koessler, T. Debating pros and cons of total neoadjuvant therapy in rectal cancer. Cancers 2021, 13, 6361. [Google Scholar] [CrossRef] [PubMed]
- Wo, J.Y.; Anker, C.J.; Ashman, J.B.; Bhadkamkar, N.A.; Bradfield, L.; Chang, D.T.; Dorth, J.; Garcia-Aguilar, J.; Goff, D.; Jacqmin, D.; et al. Radiation therapy for rectal cancer: Executive summary of an ASTRO clinical practice guideline. Pract. Radiat. Oncol. 2021, 11, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Chadi, S.A.; Malcomson, L.; Ensor, J.; Riley, R.D.; Vaccaro, C.A.; Rossi, G.L.; Daniels, I.R.; Smart, N.J.; Osborne, M.E.; Beets, G.L.; et al. Factors affecting local regrowth after watch and wait for patients with a clinical complete response following chemoradiotherapy in rectal cancer (InterCoRe consortium): An individual participant data meta-analysis. Lancet Gastroenterol. Hepatol. 2018, 3, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Azad, N.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. Rectal Cancer, Version 2.202, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 1139–1167. [Google Scholar] [CrossRef]
- Bahadoer, R.R.; Dijkstra, E.A.; van Etten, B.; Marijnen, C.A.M.; Putter, H.; Kranenbarg, E.M.-K.; Roodvoets, A.G.H.; Nagtegaal, I.D.; Beets-Tan, R.G.H.; Blomqvist, L.K.; et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 29–42. [Google Scholar] [CrossRef]
- Conroy, T.; Bosset, J.-F.; Etienne, P.-L.; Rio, E.; François, E.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouché, O.; Gargot, D.; et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 702–715. [Google Scholar] [CrossRef]
- Glimelius, B. Recent advances in rectal cancer treatment—Are we on the right track? Ups. J. Med. Sci. 2024, 21, 129. [Google Scholar] [CrossRef]
- Schmiegel, W.; Buchberger, B.; Follmann, M.; Graeven, U.; Heinemann, V.; Langer, T.; Nothacker, M.; Porschen, R.; Rödel, C.; Rösch, T.; et al. S3-Leitlinie: Kolorektaleskarzinom. Z. Gastroenterol. 2017, 55, 1344–1498. [Google Scholar] [CrossRef]
- Schrag, D.; Shi, Q.; Weiser, M.R.; Gollub, M.J.; Saltz, L.B.; Musher, B.L.; Goldberg, J.; Al Baghdadi, T.; Goodman, K.A.; McWilliams, R.R.; et al. Preoperative Treatment of Locally Advanced Rectal Cancer. N. Engl. J. Med. 2023, 389, 322–334. [Google Scholar] [CrossRef]
- Ruppert, R.; Junginger, T.; Kube, R.; Strassburg, J.; Lewin, A.; Baral, J.; Maurer, C.A.; Sauer, J.; Lauscher, J.; Winde, G.; et al. Risk-Adapted Neoadjuvant Chemoradiotherapy in Rectal Cancer: Final Report of the OCUM Study. J. Clin. Oncol. 2023, 41, 4025–4034. [Google Scholar] [CrossRef] [PubMed]
- Chatila, W.K.; Kim, J.K.; Walch, H.; Marco, M.R.; Chen, C.T.; Wu, F.; Omer, D.M.; Khalil, D.N.; Ganesh, K.; Qu, X.; et al. Genomic and transcriptomic determinants of response to neoadjuvant therapy in rectal cancer. Nat. Med. 2022, 28, 1646–1655. [Google Scholar] [CrossRef] [PubMed]
- De Mattia, E.; Polesel, J.; Mezzalira, S.; Palazzari, E.; Pollesel, S.; Toffoli, G.; Cecchin, E. Predictive and prognostic value of oncogene mutations and microsatellite instability in locally-advanced rectal cancer treated with neoadjuvant radiation-based therapy: A systematic review and meta-analysis. Cancers 2023, 15, 1469. [Google Scholar] [CrossRef]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef]
- Papke, D.J.; Yurgelun, M.B.; Noffsinger, A.E.; Turner, K.O.; Genta, R.M.; Redston, M. Prevalence of Mismatch-Repair Deficiency in Rectal Adenocarcinomas. N. Engl. J. Med. 2022, 387, 1714–1716. [Google Scholar] [CrossRef]
- Khalij, Y.; Belaid, I.; Chouchane, S.; Amor, D.; Omezzine, A.; Ben Rejeb, N.; Ben Ahmed, S.; Bouslama, A. DPYD and TYMS polymorphisms as predictors of 5 fluorouracil toxicity in colorectal cancer patients. J. Chemother. 2023, 35, 425–434. [Google Scholar] [CrossRef]
- Park, H.J.; Yoon, S.M.; Yu, C.S.; Kim, J.H.; Kim, T.W.; Kim, J.C. Randomized phase 3 trial comparing preoperative and postoperative chemoradiotherapy with capecitabine for locally advanced rectal cancer. Cancer 2011, 117, 3703–3712. [Google Scholar] [CrossRef]
- Fokas, E.; Liersch, T.; Fietkau, R.; Hohenberger, W.; Beissbarth, T.; Hess, C.; Becker, H.; Ghadimi, M.; Mrak, K.; Merkel, S.; et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: Updated results of the CAO/ARO/AIO-94 trial. J. Clin. Oncol. 2014, 32, 1554–1562. [Google Scholar] [CrossRef]
- Garcia-Aguilar, J.; Patil, S.; Kim, J.K.; Yuval, J.B.; Thompson, H.; Verheij, F.; Lee, M.; Saltz, L.B. Preliminary results of the organ preservation of rectal adenocarcinoma (OPRA) trial. J. Clin. Oncol. 2020, 38, 4008. [Google Scholar] [CrossRef]
- Sebag-Montefiore, D.; Stephens, R.J.; Steele, R.; Monson, J.; Grieve, R.; Khanna, S.; Quirke, P.; Couture, J.; de Metz, C.; Myint, A.S.; et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): A multicentre, randomised trial. Lancet 2009, 373, 811–820. [Google Scholar] [CrossRef]
- Pettersson, D.; Cedermark, B.; Holm, T.; Radu, C.; Pahlman, L.; Glimelius, B.; Martling, A. Interim analysis of the Stockholm III trial of preoperative radiotherapy regimens for rectal cancer. Br. J. Surg. 2010, 97, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Sloothaak, D.A.; Geijsen, D.E.; van Leersum, N.J.; Punt, C.J.; Buskens, C.J.; Bemelman, W.A.; Tanis, P.J.; Dutch Surgical Colorectal Audit. Optimal time interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br. J. Surg. 2013, 100, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Tulchinsky, H.; Shmueli, E.; Figer, A.; Klausner, J.M.; Rabau, M. An interval >7 weeks between neoadjuvant therapy and surgery improves pathologic complete response and disease-free survival in patients with locally advanced rectal cancer. Ann. Surg. Oncol. 2008, 15, 2661–2667. [Google Scholar] [CrossRef]
- Riou, O.; Gourgou, S.; Conroy, T. Comment on Locoregional Failure During and After Short-Course Radiotherapy Followed by Chemotherapy and Surgery Compared to Long-Course Chemoradiotherapy and Surgery: A Five-Year Follow-Up of the RAPIDO Trial: The RAPIDO Trial Does Not Achieve Its Primary Endpoint. Ann. Surg. Open 2023, 4, e288. [Google Scholar] [CrossRef]
- Vailati, B.B.; Cerdán-Santacruz, C.; São Julião, G.P.; Corbi, L.; Perez, R.O. Short-Course Radiation and Consolidation Chemotherapy for Rectal Cancer-the Rise and Fall of a Treatment Strategy-Rest in Peace. Dis. Colon Rectum 2023, 66, 1297–1299. [Google Scholar] [CrossRef]
- Liao, C.K.; Kuo, Y.T.; Lin, Y.C.; Chern, Y.J.; Hsu, Y.J.; Yu, Y.L.; Chiang, J.M.; Hsieh, P.S.; Yeh, C.Y.; You, J.F. Neoadjuvant Short-Course Radiotherapy followed by consolidation chemotherapy before surgery for treating locally advanced rectal Cancer: A systematic review and Meta-analysis. Curr. Oncol. 2022, 29, 3708–3727. [Google Scholar] [CrossRef]
- Ngan, S.Y.; Burmeister, B.; Fisher, R.J.; Solomon, M.; Goldstein, D.; Joseph, D.; Ackland, S.P.; Schache, D.; McClure, B.; McLachlan, S.A.; et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J. Clin. Oncol. 2012, 30, 3827–3833. [Google Scholar] [CrossRef]
- Jin, J.; Tang, Y.; Hu, C.; Jiang, L.M.; Jiang, J.; Li, N.; Liu, W.-Y.; Chen, S.-L.; Li, S.; Lu, N.-N.; et al. Multicenter, Randomized, Phase III Trial of Short-Term Radiotherapy Plus Chemotherapy versus Long-Term Chemoradiotherapy in Locally Advanced Rectal Cancer (STELLAR). J. Clin. Oncol. 2022, 40, 1681–1692. [Google Scholar] [CrossRef]
- Fokas, E.; Allgauer, M.; Polat, B.; Klautke, G.; Grabenbauer, G.G.; Fietkau, R.; Kuhnt, T.; Staib, L.; Brunner, T.; Grosu, A.L.; et al. Randomized Phase II Trial of Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: CAO/ARO/AIO-12. J. Clin. Oncol. 2019, 37, 3212–3222. [Google Scholar] [CrossRef]
- Cho, M.S.; Bae, H.W.; Chang, J.S.; Yang, S.Y.; Kim, T.H.; Koom, W.S.; Shin, S.J.; Choi, G.-S.; Kim, N.K. Short-term outcomes and cost-effectiveness between long-course chemoradiation and short-course Radiotherapy for locally advanced rectal Cancer. Yonsei Med. J. 2023, 64, 395–403. [Google Scholar] [CrossRef]
- Wang, S.; Wen, F.; Zhang, P.; Wang, X.; Li, Q. Cost-effectiveness analysis of long-course oxaliplatin and bolus of fluorouracil based preoperative chemoradiotherapy vs. 5 × 5Gy radiation plus FOLFOX4 for locally advanced resectable rectal cancer. Radiat. Oncol. 2019, 14, 113. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, J.G.; Oh, S.T.; Lee, M.; Chun, H.G.; Kim, T.H.; Kim, S.Y.; Baek, J.Y.; Park, J.W.; Oh, J.H.; et al. Two-week course of preoperative chemoradiotherapy followed by delayed surgery for rectal cancer: A phase II multi-institutional clinical trial (KROG 11-02). Radiother. Oncol. 2014, 110, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Dahlberg, M.; Glimelius, B.; Graf, W.; Pahlman, L. Preoperative irradiation affects functional results after surgery for rectal cancer: Results from a randomized study. Dis. Colon Rectum 1998, 41, 543–549, discussion 549–551. [Google Scholar] [CrossRef] [PubMed]
- Bruheim, K.; Guren, M.G.; Skovlund, E.; Hjermstad, M.J.; Dahl, O.; Frykholm, G.; Carlsen, E.; Tveit, K.M. Late side effects and quality of life afte65r radiotherapy for rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 1005–1011. [Google Scholar] [CrossRef]
- Pollack, J.; Holm, T.; Cedermark, B.; Altman, D.; Holmstrom, B.; Glimelius, B.; Mellgren, A. Late adverse effects of short-course preoperative radiotherapy in rectal cancer. Br. J. Surg. 2006, 93, 1519–1525. [Google Scholar] [CrossRef]
- Fabiani, C.; Ferrante, M.G.; Meneghini, C.; Licata, E.; Paciotti, G.; Gallo, M.; Schiavi, M.; Spina, V.; Guarino, A.; Caserta, D.; et al. Female fertility preservation: Impact of cancer on ovarian function and oocyte quality. Int. J. Gynaecol. Obstet. 2021, 156, 166–171. [Google Scholar] [CrossRef]
- De Felice, F.; Marchetti, C.; Marampon, F.; Cascialli, G.; Muzii, L.; Tombolini, V. Radiation effects on male fertility. Andrology 2019, 7, 2–7. [Google Scholar] [CrossRef]
- Schmoll, H.J.; Haustermans, K.; Price, T.J.; Price, T.; Hofheinz, R.D.; Nordlinger, B.; Daisne, J.F.; Janssens, J.; Brenner, B.; Reinel, H.; et al. Preoperative chemoradiotherapy and postoperative chemotherapy with capecitabine +/− oxaliplatin in locally advanced rectal cancer: Final results of PETACC-6. JCO 2018, 36 (Suppl. 15), 3500. [Google Scholar] [CrossRef]
- Banwell, V.C.; Phillips, H.A.; Duff, M.J.; Speake, D.; McLean, C.; Williams, L.J.; He, Y.; Paterson, H.M. Five-year oncological outcomes after selective neoadjuvant radiotherapy for resectable rectal cancer. Acta Oncol. 2019, 58, 1267–1272. [Google Scholar] [CrossRef]
- Gérard, J.P.; Conroy, T.; Bonnetain, F.; Bouché, O.; Chapet, O.; Closon-Dejardin, M.T.; Untereiner, M.; Leduc, B.; Francois, E.; Maurel, J.; et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3–4 rectal cancers: Results of FFCD 9203. J. Clin. Oncol. 2006, 24, 4620–4625. [Google Scholar] [CrossRef]
- Bosset, J.F.; Calais, G.; Mineur, L.; Maingon, P.; Stojanovic-Rundic, S.; Bensadoun, R.J.; Bardet, E.; Beny, A.; Ollier, J.C.; Bolla, M.; et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: Long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014, 15, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Willett, C.G. Management of Locoregional Rectal Cancer. J. Natl. Compr. Cancer Netw. 2018, 16, 617–619. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, M.J.; Colangelo, L.H.; Beart, R.W.; Petrelli, N.J.; Allegra, C.J.; Sharif, S.; Pitot, H.C.; Shields, A.F.; Landry, J.C.; Ryan, D.P.; et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: Surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R-04. J. Clin. Oncol. 2014, 32, 1927–1934. [Google Scholar] [CrossRef]
- Brændengen, M.; Glimelius, B. Preoperative radiotherapy or chemoradiotherapy in rectal cancer—Is survival improved? An update of the ‘Nordic’ LARC study in non-resectable cancers. Radiother. Oncol. 2018, 127, 392–395. [Google Scholar] [CrossRef]
- Sauer, R.; Liersch, T.; Merkel, S.; Fietkau, R.; Hohenberger, W.; Hess, C.; Becker, H.; Raab, H.R.; Villanueva, M.T.; Witzigmann, H.; et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J. Clin. Oncol. 2012, 30, 1926–1933. [Google Scholar] [CrossRef]
- Kjellstrom, J.; Kjellen, E.; Johnsson, A. In vitro radiosensitization by oxaliplatin and 5-fluorouracil in a human colon cancer cell line. Acta Oncol. 2005, 44, 687–693. [Google Scholar] [CrossRef]
- Cividalli, A.; Ceciarelli, F.; Livdi, E.; Altavista, P.; Cruciani, G.; Marchetti, P.; Danesi, D.T. Radiosensitization by oxaliplatin in a mouse adenocarcinoma: Influence of treatment schedule. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 1092–1098. [Google Scholar] [CrossRef]
- André, T.; Boni, C.; Mounedji-Boudiaf, L.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Zaninelli, M.; Clingan, P.; Bridgewater, J.; et al. Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) Investigators Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N. Engl. J. Med. 2004, 350, 2343–2351. [Google Scholar] [CrossRef]
- Kogler, P.; DeVries, A.F.; Eisterer, W.; Thaler, J.; Sölkner, L.; Öfner, D.; TAKO 05/ABCSG R-02 Trial Investigators. Intensified preoperative chemoradiation by adding oxaliplatin in locally advanced, primary operable (cT3NxM0) rectal cancer: Impact on long-term outcome. Results of the phase II TAKO 05/ABCSG R-02 trial. Strahlenther. Onkol. 2018, 194, 41–49. [Google Scholar] [CrossRef]
- Aschele, C.; Cionini, L.; Lonardi, S.; Pinto, C.; Cordio, S.; Rosati, G.; Artale, S.; Tagliagambe, A.; Ambrosini, G.; Rosetti, P.; et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: Pathologic results of the STAR-01 randomized phase III trial. J. Clin. Oncol. 2011, 29, 2773–2780. [Google Scholar] [CrossRef]
- Deng, Y.; Chi, P.; Lan, P.; Wang, L.; Chen, W.; Cui, L.; Chen, D.; Cao, J.; Wei, H.; Peng, X.; et al. Neoadjuvant Modified FOLFOX6 with or without Radiation Versus Fluorouracil Plus Radiation for Locally Advanced Rectal Cancer: Final Results of the Chinese FOWARC Trial. J. Clin. Oncol. 2019, 37, 3223–3233. [Google Scholar] [CrossRef] [PubMed]
- Gerard, J.P.; Azria, D.; Gourgou-Bourgade, S.; Martel-Lafay, I.; Hennequin, C.; Etienne, P.L.; Vendrely, V.; Francois, E.; de La Roche, G.; Bouche, O.; et al. Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J. Clin. Oncol. 2012, 30, 4558–4565. [Google Scholar] [CrossRef] [PubMed]
- Allegra, C.J.; Yothers, G.; O’Connell, M.J.; Beart, R.W.; Wozniak, T.F.; Pitot, H.C.; Shields, A.F.; Landry, J.C.; Ryan, D.P.; Arora, A.; et al. Neoadjuvant 5-FU or Capecitabine Plus Radiation with or without Oxaliplatin in Rectal Cancer Patients: A Phase III Randomized Clinical Trial. J. Natl. Cancer Inst. 2015, 107, djv248. [Google Scholar] [CrossRef]
- Jiao, D.; Zhang, R.; Gong, Z.; Liu, F.; Chen, Y.; Yu, Q.; Sun, L.; Duan, H.; Zhu, S.; Liu, F.; et al. Fluorouracil-based preoperative chemoradiotherapy with or without oxaliplatin for stage II/III rectal cancer: A 3-year follow-up study. Chin. J. Cancer Res. 2015, 27, 588–596. [Google Scholar] [CrossRef]
- Schmoll, H.J.; Stein, A.; Van Cutsem, E.; Price, T.; Hofheinz, R.D.; Nordlinger, B.; Daisne, J.F.; Janssens, J.; Brenner, B.; Reinel, H.; et al. Pre- and Postoperative Capecitabine without or with Oxaliplatin in Locally Advanced Rectal Cancer: PETACC 6 Trial by EORTC GITCG and ROG, AIO, AGITG, BGDO, and FFCD. J. Clin. Oncol. 2021, 39, 17–29. [Google Scholar] [CrossRef]
- Rodel, C.; Graeven, U.; Fietkau, R.; Hohenberger, W.; Hothorn, T.; Arnold, D.; Hofheinz, R.D.; Ghadimi, M.; Wolff, H.A.; Lang-Welzenbach, M.; et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): Final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015, 16, 979–989. [Google Scholar] [CrossRef]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Cederquist, L.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Engstrom, P.F.; et al. Rectal Cancer, Version 2. 2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 874–901. [Google Scholar] [CrossRef]
- Petersen, S.H.; Harling, H.; Kirkeby, L.T.; Wille-Jorgensen, P.; Mocellin, S. Postoperative adjuvant chemotherapy in rectal cancer operated for cure. Cochrane Database Syst. Rev. 2012, 3, CD004078. [Google Scholar] [CrossRef]
- Breugom, A.J.; van Gijn, W.; Muller, E.W.; Berglund, A.; van den Broek, C.B.; Fokstuen, T.; Gelderblom, H.; Kapiteijn, E.; Leer, J.W.; Marijnen, C.A.; et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: A Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann. Oncol. 2015, 26, 696–701. [Google Scholar] [CrossRef]
- Hong, Y.S.; Kim, S.Y.; Lee, J.S.; Nam, B.H.; Kim, K.P.; Kim, J.E.; Park, Y.S.; Park, J.O.; Baek, J.Y.; Kim, T.Y.; et al. Oxaliplatin-Based Adjuvant Chemotherapy for Rectal Cancer After Preoperative Chemoradiotherapy (ADORE): Long-Term Results of a Randomized Controlled Trial. J. Clin. Oncol. 2019, 37, 3111–3123. [Google Scholar] [CrossRef] [PubMed]
- Breugom, A.J.; Swets, M.; Bosset, J.F.; Collette, L.; Sainato, A.; Cionini, L.; Glynne-Jones, R.; Counsell, N.; Bastiaannet, E.; van den Broek, C.B.; et al. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: A systematic review and meta-analysis of individual patient data. Lancet Oncol. 2015, 16, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Zeng, T.; Xie, X.; Li, J.; Li, D.; Kejin, Y.; Chen, F.; Zhu, H. Adjuvant chemotherapy does not improve cancer-specific survival for pathologic stage II/III rectal adenocarcinoma after neoadjuvant chemoradiotherapy and surgery: Evidence based on long-term survival analysis from SEER data. Int. J. Color. Dis. 2023, 38, 134. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.L.; Denost, Q.; Simillis, C.; Pellino, G.; Rasheed, S.; Kontovounisios, C.; Tekkis, P.P.; Rullier, E. The effect of adjuvant chemotherapy on survival and recurrence after curative rectal cancer surgery in patients who are histologically node negative after neoadjuvant chemoradiotherapy. Color. Dis. 2017, 19, 980–986. [Google Scholar] [CrossRef]
- Gérard, J.P.; Azria, D.; Gourgou-Bourgade, S.; Martel-Laffay, I.; Hennequin, C.; Etienne, P.L.; Vendrely, V.; François, E.; de La Roche, G.; Bouché, O.; et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: Results of the phase III trial ACCORD 12/0405-Prodige 2. J. Clin. Oncol. 2010, 28, 1638–1644. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, J.; Hou, Y.; Liu, H.; Fan, X.; Luo, S.; Liu, Z.; Hu, H.; Lai, S.; Kang, L.; et al. Clinical significance of adjuvant chemotherapy for pathological complete response rectal cancer patients with acellular mucin pools after neoadjuvant chemoradiotherapy. Therap. Adv. Gastroenterol. 2023, 16, 17562848221117875. [Google Scholar] [CrossRef]
- Lai, S.H.; Vogel, J.D.; Vemuru, S.; Messersmith, W.; Lieu, C.; McCarter, M.D.; Birnbaum, E.; Chapman, B.C. Improved survival after adjuvant therapy in locally advanced rectal cancer patients with pathologic complete response. Dis. Colon Rectum 2023, 66, 983–993. [Google Scholar] [CrossRef]
- Dijkstra, E.A.; Zwart, W.H.; Nilsson, P.J.; Putter, H.; Roodvoets, A.G.; Meershoek-Klein Kranenbarg, E.; Frödin, J.E.; Nygren, P.; Østergaard, L.; Kersten, C.; et al. The value of post-operative chemotherapy after chemoradiotherapy in patients with high-risk locally advanced rectal cancer-results from the RAPIDO trial. ESMO Open 2023, 8, 101158. [Google Scholar] [CrossRef]
- Boublikova, L.; Novakova, A.; Simsa, J.; Lohynska, R. Total neoadjuvant therapy in rectal cancer: The evidence and expectations. Crit. Rev. Oncol. Hematol. 2023, 192, 104196. [Google Scholar] [CrossRef]
- Brouwer, N.P.; Bos, A.C.; Lemmens, V.E.; Tanis, P.J.; Hugen, N.; Nagtegaal, I.D.; de Wilt, J.H.; Verhoeven, R.H. An overview of 25 years of incidence, treatment and outcome of colorectal cancer patients. Int. J. Cancer 2018, 143, 2758–2766. [Google Scholar] [CrossRef]
- Fernandez-Martos, C.; Garcia-Albeniz, X.; Pericay, C.; Maurel, J.; Aparicio, J.; Montagut, C.; Safont, M.J.; Salud, A.; Vera, R.; Massuti, B.; et al. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: Long-term results of the Spanish GCR-3 phase II randomized trial. Ann. Oncol. 2015, 26, 1722–1728. [Google Scholar] [CrossRef] [PubMed]
- Cercek, A.; Roxburgh, C.S.; Strombom, P.; Smith, J.J.; Temple, L.K.; Nash, G.M.; Guillem, J.G.; Paty, P.B.; Yaeger, R.; Stadler, Z.K.; et al. Adoption of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer. JAMA Oncol. 2018, 4, e180071. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhou, H.; Jiang, J.; Zhu, Y.; Zou, S.; Jiang, L.; Tang, Y.; Liang, J.; Sun, Y.; Jiang, Z.; et al. Neoadjuvant chemotherapy with modified FOLFOXIRI for locally advanced rectal cancer to transform effectively EMVI and MRF from positive to negative: Results of a long-term single center phase 2 clinical trial. BMC Cancer 2023, 23, 592. [Google Scholar] [CrossRef] [PubMed]
- Fokas, E.; Schlenska-Lange, A.; Polat, B.; Klautke, G.; Grabenbauer, G.G.; Fietkau, R.; Kuhnt, T.; Staib, L.; Brunner, T.; Grosu, A.L.; et al. Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Patients with Locally Advanced Rectal Cancer: Long-term Results of the CAO/ARO/AIO-12 Randomized Clinical Trial. JAMA Oncol. 2022, 8, e215445. [Google Scholar] [CrossRef]
- Garcia-Aguilar, J.; Patil, S.; Gollub, M.J.; Kim, J.K.; Yuval, J.B.; Thompson, H.M.; Verheij, F.S.; Omer, D.M.; Lee, M.; Dunne, R.F.; et al. Organ Preservation in Patients with Rectal Adenocarcinoma Treated with Total Neoadjuvant Therapy. J. Clin. Oncol. 2022, 40, 2546–2556. [Google Scholar] [CrossRef]
- Verheij, F.S.; Omer, D.M.; Williams, H.; Lin, S.T.; Qin, L.X.; Buckley, J.T.; Thompson, H.M.; Yuval, J.B.; Kim, J.K.; Dunne, R.F.; et al. Long-Term Results of Organ Preservation in Patients with Rectal Adenocarcinoma Treated with Total Neoadjuvant Therapy: The Randomized Phase II OPRA Trial. J. Clin. Oncol. 2024, 42, 500–506. [Google Scholar] [CrossRef]
- Habr-Gama, A.; São Julião, G.P.; Ortega, C.D.; Vailati, B.B.; Araujo, S.; Jorge, T.; Sabbaga, J.; Rossi, G.L.; D’Alpino, R.; Kater, F.R.; et al. A multi-centre randomized controlled trial investigating Consolidation Chemotherapy with and without oxaliplatin in distal rectal cancer and Watch & Wait. BMC Cancer 2023, 23, 546. [Google Scholar] [CrossRef]
- Gollins, S.; West, N.; Sebag-Montefiore, D.; Susnerwala, S.; Falk, S.; Brown, N.; Saunders, M.; Quirke, P.; Ray, R.; Parsons, P.; et al. A prospective phase II study of pre-operative chemotherapy then short-course radiotherapy for high risk rectal cancer: COPERNICUS. Br. J. Cancer 2018, 119, 697–706. [Google Scholar] [CrossRef]
- Deng, Y.; Chi, P.; Lan, P.; Wang, L.; Chen, W.; Cui, L.; Chen, D.; Cao, J.; Wei, H.; Peng, X.; et al. Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: Initial results of the Chinese FOWARC multicenter, open-label, randomized three-arm phase III trial. J. Clin. Oncol. 2016, 34, 3300–3307. [Google Scholar] [CrossRef]
- Matsuda, C.; Kudo, T.; Morimoto, Y.; Kagawa, Y.; Tei, M.; Ide, Y.; Miyoshi, N.; Takahashi, H.; Uemura, M.; Takemasa, I.; et al. A phase II study of neoadjuvant capecitabine, oxaliplatin, and irinotecan (XELOXIRI) in patients with locally advanced rectal cancer. Ann. Gastroenterol. Surg. 2023, 7, 81–90. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, X.; Liu, A.; Zhu, Y.; Zhang, T.; Liu, L.; Jia, J.; Tan, S.; Wu, J.; Wang, X.; et al. Long-term outcome of a phase III trial on neoadjuvant chemoradiation with capecitabine and irinotecan in patients with locally advanced rectal cancer: Updated results of the CinClare trial. J. Clin. Oncol. 2021, 39 (Suppl. 15), 3603. [Google Scholar] [CrossRef]
- Sebag-Montefiore, D.; Adams, R.; Gollins, S.; Samuel, L.M.; Glynne-Jones, R.; Harte, R.; West, N.; Quirke, P.; Myint, A.S.; Bach, S.P.; et al. ARISTOTLE: A phase III trial comparing concurrent capecitabine with capecitabine and irinotecan (Ir) chemoradiation as preoperative treatment for MRI-defined locally advanced rectal cancer (LARC). J. Clin. Oncol. 2020, 38, 4101. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Lenz, H.J.; Köhne, C.H.; Heinemann, V.; Tejpar, S.; Melezínek, I.; Beier, F.; Stroh, C.; Rougier, P.; van Krieken, J.H.; et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J. Clin. Oncol. 2015, 33, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Cremolini, C.; Loupakis, F.; Antoniotti, C.; Lupi, C.; Sensi, E.; Lonardi, S.; Mezi, S.; Tomasello, G.; Ronzoni, M.; Zaniboni, A.; et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: Updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015, 16, 1306–1315. [Google Scholar] [CrossRef]
- Masi, G.; Vivaldi, C.; Fornaro, L.; Lonardi, S.; Buccianti, P.; Sainato, A.; Marcucci, L.; Martignetti, A.; Luca Urso, E.D.; Castagna, M.; et al. Total neoadjuvant approach with FOLFOXIRI plus bevacizumab followed by chemoradiotherapy plus bevacizumab in locally advanced rectal cancer: The TRUST trial. Eur. J. Cancer 2019, 110, 32–41. [Google Scholar] [CrossRef]
- Borg, C.; Rullier, E.; Marchal, F.; Etienne, P.-L.; Rio, E.; Francois, E.; Mesgouez-Nebout, N.; Vendre, V.; Artignan, X.; Bouche, O.; et al. LBA21 Neoadjuvant mFOLFIRINOX and preoperative chemoradiation (CRT) versus preoperative CRT in patients with T3-4 rectal cancer: Surgical and quality of life results of PRODIGE 23 phase III trial. Ann. Oncol. 2020, 31, S1152. [Google Scholar] [CrossRef]
- Li, S.; Kim, J.S.; Kim, J.M.; Cho, M.J.; Yoon, W.H.; Song, K.S.; Yeo, S.G.; Kim, J.S. Epidermal growth factor receptor as a prognostic factor in locally advanced rectal-cancer patients treated with preoperative chemoradiation. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 705–712. [Google Scholar] [CrossRef]
- Leichman, C.G.; McDonough, S.L.; Smalley, S.R.; Billingsley, K.G.; Lenz, H.J.; Beldner, M.A.; Hezel, A.F.; Velasco, M.R.; Guthrie, K.A.; Blanke, C.D.; et al. Cetuximab Combined With Induction Oxaliplatin and Capecitabine, Followed by Neoadjuvant Chemoradiation for Locally Advanced Rectal Cancer: SWOG 0713. Clin. Color. Cancer 2018, 17, e121–e125. [Google Scholar] [CrossRef]
- Sclafani, F.; Gonzalez, D.; Cunningham, D.; Hulkki Wilson, S.; Peckitt, C.; Giralt, J.; Glimelius, B.; Roselló Keränen, S.; Wotherspoon, A.; Brown, G.; et al. RAS mutations and cetuximab in locally advanced rectal cancer: Results of the EXPERT-C trial. Eur. J. Cancer 2014, 50, 1430–1436. [Google Scholar] [CrossRef]
- Dewdney, A.; Cunningham, D.; Tabernero, J.; Capdevila, J.; Glimelius, B.; Cervantes, A.; Tait, D.; Brown, G.; Wotherspoon, A.; Gonzalez de Castro, D.; et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin; capecitabine; and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C). J. Clin. Oncol. 2012, 30, 1620–1627. [Google Scholar] [CrossRef]
- Bando, H.; Tsukada, Y.; Inamori, K.; Togashi, Y.; Koyama, S.; Kotani, D.; Fukuoka, S.; Yuki, S.; Komatsu, Y.; Homma, S.; et al. Preoperative chemoradiotherapy plus nivolumab before surgery in patients with microsatellite stable and microsatellite instability-high locally advanced rectal cancer. Clin. Cancer Res. 2022, 28, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, L.; Bensi, M.; Corallo, S.; Bergamo, F.; Pellegrini, I.; Rasola, C.; Borelli, B.; Tamburini, E.; Randon, G.; Galuppo, S.; et al. Phase II study of preoperative (PREOP) chemoradiotherapy (CTRT) plus avelumab (AVE) in patients (PTS) with locally advanced rectal cancer (LARC): The AVANA study. J. Clin. Oncol. 2021, 39 (Suppl. 15), 3511. [Google Scholar] [CrossRef]
- Shamseddine, A.; Zeidan, Y.H.; El Husseini, Z.; Kreidieh, M.; Al Darazi, M.; Turfa, R.; Kattan, J.; Khalifeh, I.; Mukherji, D.; Temraz, S.; et al. Efficacy and safety-in analysis of short-course radiation followed by mFOLFOX-6 plus avelumab for locally advanced rectal adenocarcinoma. Radiat. Oncol. 2020, 15, 233. [Google Scholar] [CrossRef]
- Lin, Z.; Cai, M.; Zhang, P.; Li, G.; Liu, T.; Li, X.; Cai, K.; Nie, X.; Wang, J.; Liu, J.; et al. Phase II; single-arm trial of preoperative short-course radiotherapy followed by chemotherapy and camrelizumab in locally advanced rectal cancer. J. Immunother. Cancer 2021, 9, e003554. [Google Scholar] [CrossRef]
- Akama-Garren, E.H.; Morris, Z.S.; Sikora, A.G.; Weichselbaum, R.; Schoenfeld, J.D. Prospective clinical investigation of the efficacy of combination radiation therapy with immune checkpoint inhibition. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 1165–1175. [Google Scholar] [CrossRef]
- Li, M.; Xiao, Q.; Venkatachalam, N.; Hofheinz, R.D.; Veldwijk, M.R.; Herskind, C.; Ebert, M.P.; Zhan, T. Predicting response to neoadjuvant chemoradiotherapy in rectal cancer: From biomarkers to tumor models. Ther. Adv. Med. Oncol. 2022, 14, 17588359221077972. [Google Scholar] [CrossRef]
- Hammarström, K.; Imam, I.; Mezheyeuski, A.; Ekström, J.; Sjöblom, T.; Glimelius, B. A Comprehensive Evaluation of Associations Between Routinely Collected Staging Information and The Response to (Chemo)Radiotherapy in Rectal Cancer. Cancers 2020, 13, 16. [Google Scholar] [CrossRef]
- Steel, M.C.; Woods, R.; Mackay, J.M.; Chen, F. Extent of mesorectal invasion is a prognostic indicator in T3 rectal carcinoma. ANZ J. Surg. 2002, 72, 483–487. [Google Scholar] [CrossRef]
- Brown, G.; Radcliffe, A.G.; Newcombe, R.G.; Dallimore, N.S.; Bourne, M.W.; Williams, G.T. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br. J. Surg. 2003, 90, 355–364. [Google Scholar] [CrossRef]
- MERCURY Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: Prospective observational study. BMJ 2006, 333, 779. [Google Scholar] [CrossRef]
- McClelland, D.; Murray, G.I. A comprehensive study of extramural venous invasion in colorectal cancer. PLoS ONE 2015, 10, e0144987. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, K.S.; Hosseini-Nik, H.; Thipphavong, S.; Assarzadegan, N.; Menezes, R.J.; Kennedy, E.D.; Kirsch, R. MRI detection of extramural venous invasion in rectal cancer: Correlation with histopathology using elastin stain. AJR Am. J. Roentgenol. 2016, 206, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.J.; Barbachano, Y.; Norman, A.R.; Swift, R.I.; Abulafi, A.M.; Brown, G. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br. J. Surg. 2008, 95, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Chand, M.; Evans, J.; Swift, R.I.; Tekkis, P.P.; West, N.P.; Stamp, G.; Heald, R.J.; Brown, G. The prognostic significance of postchemoradiotherapy high-resolution MRI and histopathology detected extramural venous invasion in rectal cancer. Ann. Surg. 2015, 261, 473–479. [Google Scholar] [CrossRef]
- Battersby, N.J.; How, P.; Moran, B.; Stelzner, S.; West, N.P.; Branagan, G.; Strassburg, J.; Quirke, P.; Tekkis, P.; Pedersen, B.G.; et al. Prospective Validation of a Low Rectal Cancer Magnetic Resonance Imaging Staging System and Development of a Local Recurrence Risk Stratification Model: The MERCURY II Study. Ann. Surg. 2016, 263, 751–760. [Google Scholar] [CrossRef]
- Maas, M.; Nelemans, P.J.; Valentini, V.; Das, P.; Rödel, C.; Kuo, L.-J.; Calvo, F.A.; García-Aguilar, J.; Glynne-Jones, R.; Haustermans, K.; et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol. 2010, 11, 835–844. [Google Scholar] [CrossRef]
- Kim, S.H.; Chang, H.J.; Kim, D.Y.; Park, J.W.; Baek, J.Y.; Kim, S.Y.; Park, S.C.; Oh, J.H.; Yu, A.; Nam, B.H. What Is the Ideal Tumor Regression Grading System in Rectal Cancer Patients after Preoperative Chemoradiotherapy? Cancer Res. Treat. 2016, 48, 998–1009. [Google Scholar] [CrossRef]
- Hermanek, P.; Merkel, S.; Hohenberger, W. Prognosis of rectal carcinoma after multimodal treatment: ypTNM classification and tumor regression grading are essential. Anticancer Res. 2013, 33, 559–566. [Google Scholar] [PubMed]
- Lee, S.D.; Kim, T.H.; Kim, D.Y.; Baek, J.Y.; Kim, S.Y.; Chang, H.J.; Park, S.C.; Park, J.W.; Oh, J.H.; Jung, K.H. Lymph node ratio is an independent prognostic factor in patients with rectal cancer treated with preoperative chemoradiotherapy and curative resection. Eur. J. Surg. Oncol. 2012, 38, 478–483. [Google Scholar] [CrossRef]
- Maas, M.; Lambregts, D.M.J.; Nelemans, P.J.; Heijnen, L.A.; Martens, M.H.; Leijtens, J.W.A.; Sosef, M.; Hulsewé, K.W.E.; Hoff, C.; Breukink, S.O.; et al. Assessment of Clinical Complete Response After Chemoradiation for Rectal Cancer with Digital Rectal Examination; Endoscopy; and MRI: Selection for Organ-Saving Treatment. Ann. Surg. Oncol. 2015, 22, 3873–3880. [Google Scholar] [CrossRef]
- Lynn, P.; Strombom, P.; Garcia-Aguilar, J. Organ-Preserving Strategies for the Management of Near-Complete Responses in Rectal Cancer after Neoadjuvant Chemoradiation. Clin. Colon Rectal Surg. 2017, 30, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Habr-Gama, A.; Perez, R.O.; Wynn, G.; Marks, J.; Kessler, H.; Gama-Rodrigues, J. Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: Characterization of clinical and endoscopic findings for standardization. Dis. Colon Rectum 2010, 53, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- Patel, U.B.; Brown, G.; Rutten, H.; West, N.; Sebag-Montefiore, D.; Glynne-Jones, R.; Rullier, E.; Peeters, M.; Van Cutsem, E.; Ricci, S.; et al. Comparison of magnetic resonance imaging and histopathological response to chemoradiotherapy in locally advanced rectal cancer. Ann. Surg. Oncol. 2012, 19, 2842–2852. [Google Scholar] [CrossRef] [PubMed]
- Rullier, E.; Rouanet, P.; Tuech, J.J.; Valverde, A.; Lelong, B.; Rivoire, M.; Faucheron, J.L.; Jafari, M.; Portier, G.; Meunier, B.; et al. Organ preservation for rectal cancer (GRECCAR 2): A prospective; randomised; open-label; multicentre; phase 3 trial. Lancet 2017, 390, 469–479. [Google Scholar] [CrossRef]
- Hupkens, B.J.P.; Maas, M.; Martens, M.H.; van der Sande, M.E.; Lambregts, D.M.J.; Breukink, S.O.; Melenhorst, J.; Houwers, J.B.; Hoff, C.; Sosef, M.N.; et al. Organ Preservation in Rectal Cancer After Chemoradiation: Should We Extend the Observation Period in Patients with a Clinical Near-Complete Response? Ann. Surg. Oncol. 2018, 25, 197–203. [Google Scholar] [CrossRef]
- Santiago, I.; Barata, M.; Figueiredo, N.; Parés, O.; Henriques, V.; Galzerano, A.; Carvalho, C.; Matos, C.; Heald, R.J. The split scar sign as an indicator of sustained complete response after neoadjuvant therapy in rectal cancer. Eur. Radiol. 2020, 30, 224–238. [Google Scholar] [CrossRef]
- Popita, A.-R.; Lisencu, C.; Rusu, A.; Popita, C.; Cainap, C.; Irimie, A.; Resiga, L.; Munteanu, A.; Fekete, Z.; Badea, R. MRI evaluation of complete and near-complete response after neoadjuvant therapy in patients with locally advanced rectal cancer. Diagnostics 2022, 12, 921. [Google Scholar] [CrossRef]
- Yuan, Y.; Zheng, K.; Zhou, L.; Chen, F.; Zhang, S.; Lu, H.; Lu, J.; Shao, C.; Meng, R.; Zhang, W.; et al. Predictive value of modified MRI-based split scar sign (mrSSS) score for pathological complete response after neoadjuvant chemoradiotherapy for patients with rectal cancer. Int. J. Color. Dis. 2023, 38, 40. [Google Scholar] [CrossRef]
- Ale Ali, H.; Kirsch, R.; Razaz, S.; Jhaveri, A.; Thipphavong, S.; Kennedy, E.D.; Jhaveri, K.S. Extramural venous invasion in rectal cancer: Overview of imaging; histopathology; and clinical implications. Abdom. Radiol. 2019, 44, 1–10. [Google Scholar] [CrossRef]
- Cen, P.; Chan, K.H.; Van Eps, J.; Haewon Rowe, J.; Wray, C.J. Total neoadjuvant oxaliplatin-based therapy (TNT) before (induction) and after (consolidation) chemoradiation for advanced rectal cancer: MRI correlation on complete response and outcome. JCO 2023, 41, 165. [Google Scholar] [CrossRef]
- Deantonio, L.; Caroli, A.; Puta, E.; Ferrante, D.; Apicella, F.; Turri, L.; Sacchetti, G.; Brambilla, M.; Krengli, M. Does baseline [18F] FDG-PET/CT correlate with tumor staging; response after neoadjuvant chemoradiotherapy; and prognosis in patients with rectal cancer? Radiat. Oncol. 2018, 13, 211. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.O.; Habr-Gama, A.; Gama-Rodrigues, J.; Proscurshim, I.; Julião, G.P.; Lynn, P.; Ono, C.R.; Campos, F.G.; Silva e Sousa, A.H., Jr.; Imperiale, A.R.; et al. Accuracy of positron emission tomography/computed tomography and clinical assessment in the detection of complete rectal tumor regression after neoadjuvant chemoradiation. Cancer 2011, 118, 3501–3511. [Google Scholar] [CrossRef] [PubMed]
- Cascini, G.L.; Avallone, A.; Delrio, P.; Guida, C.; Tatangelo, F.; Marone, P.; Aloj, L.; De Martinis, F.; Comella, P.; Parisi, V.; et al. 18F-FDG PET is an early predictor of pathologic tumor response to preoperative radiochemotherapy in locally advanced rectal cancer. J. Nucl. Med. 2006, 47, 1241–1248. [Google Scholar] [PubMed]
- Joye, I.; Deroose, C.M.; Vandecaveye, V.; Haustermans, K. The role of diffusion-weighted MRI and 18F-FDG PET/CT in the prediction of pathologic complete response after radiochemotherapy for rectal cancer: A systematic review. Radiother. Oncol. 2014, 113, 158–165. [Google Scholar] [CrossRef]
- Dasari, A.; Morris, V.K.; Allegra, C.J.; Atreya, C.; Benson, A.B., III; Boland, P. ctDNA applications and integration in colorectal cancer: An NCI Colon and Rectal-Anal Task Forces whitepaper. Nat. Rev. Clin. Oncol. 2020, 17, 757–770. [Google Scholar] [CrossRef]
- Vidal, J.; Casadevall, D.; Bellosillo, B.; Pericay, C.; Garcia-Carbonero, R.; Losa, F.; Layos, L.; Alonso, V.; Capdevila, J.; Gallego, J.; et al. Clinical impact of presurgery circulating tumor DNA after total neoadjuvant treatment in locally advanced rectal cancer: A biomarker study from the GEMCAD 1402 trial. Clin. Cancer Res. 2021, 27, 2890–2898. [Google Scholar] [CrossRef]
- Kotani, D.; Oki, E.; Nakamura, Y.; Yukami, H.; Mishima, S.; Bando, H.; Shirasu, H.; Yamazaki, K.; Watanabe, J.; Kotaka, M.; et al. Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer. Nat. Med. 2023, 29, 127–134. [Google Scholar] [CrossRef]
- Habr-Gama, A.; Perez, R.O.; Nadalin, W.; Sabbaga, J.; Ribeiro, U., Jr.; Silva e Sousa, A.H., Jr.; Campos, F.G.; Kiss, D.R.; Gama-Rodrigues, J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy. Ann. Surg. 2004, 240, 711–718. [Google Scholar] [CrossRef]
- Goffredo, P.; Quezada-Diaz, F.F.; Garcia-Aguilar, J.; Smith, J.J. Non-operative management of patients with rectal cancer: Lessons learnt from the OPRA trial. Cancers 2022, 14, 3204. [Google Scholar] [CrossRef]
- Fokas, E.; Appelt, A.; Glynne-Jones, R.; Beets, G.; Perez, R.; Garcia-Aguilar, J.; Rullier, E.; Smith, J.J.; Marijnen, C.; Peters, F.P.; et al. International consensus recommendations on key outcome measures for organ preservation after (chemo)radiotherapy in patients with rectal cancer. Nat. Rev. Clin. Oncol. 2021, 18, 805–816. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Akiyoshi, T.; Fukunaga, Y.; Sakamoto, T.; Mukai, T.; Hiyoshi, Y.; Nagasaki, T.; Taguchi, S.; Chino, A.; Shinozaki, E.; et al. Adding Induction Chemotherapy Before Chemoradiotherapy with Total Mesorectal Excision and Selective Lateral Lymph Node Dissection for Patients with Poor-Risk; Locally Advanced; Mid-to-Low Rectal Cancer May Improve Oncologic Outcomes: A Propensity Score-Matched Analysis. Ann. Surg. Oncol. 2023, 30, 4716–4724. [Google Scholar] [CrossRef] [PubMed]
- Holch, J.W.; Demmer, M.; Lamersdorf, C.; Michl, M.; Schulz, C.; von Einem, J.C.; Modest, D.P.; Heinemann, V. Pattern and Dynamics of Distant Metastases in Metastatic Colorectal Cancer. Visc. Med. 2017, 33, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.G.R.J.; Park, J.; Helewa, R.M.; Goldenberg, B.A.; Nashed, M.; Hyun, E. Total neoadjuvant therapy for rectal cancer: A guide for surgeons. Can. J. Surg. 2023, 66, E196–E201. [Google Scholar] [CrossRef]
- Wasmuth, H.H.; Rekstad, L.C.; Tranø, G. The outcome and the frequency of pathological complete response after neoadjuvant radiotherapy in curative resections for advanced rectal cancer: A population-based study. Color. Dis. 2016, 18, 67–72. [Google Scholar] [CrossRef]
- Zorcolo, L.; Rosman, A.S.; Restivo, A.; Pisano, M.; Nigri, G.R.; Fancellu, A.; Melis, M. Complete pathologic response after combined modality treatment for rectal cancer and long-term survival: A meta-analysis. Ann. Surg. Oncol. 2012, 19, 2822–2832. [Google Scholar] [CrossRef]
- Li, J.Y.; Huang, X.Z.; Gao, P.; Song, Y.X.; Chen, X.W.; Lv, X.E.; Fu, Y.; Xiao, Q.; Ye, S.Y.; Wang, Z.N. Survival landscape of different tumor regression grades and pathologic complete response in rectal cancer after neoadjuvant therapy based on reconstructed individual patient data. BMC Cancer 2021, 21, 1214. [Google Scholar] [CrossRef]
- Fokas, E.; Glynne-Jones, R.; Appelt, A.; Beets-Tan, R.; Beets, G.; Haustermans, K.; Marijnen, C.; Minsky, B.D.; Ludmir, E.; Quirke, P.; et al. Outcome measures in multimodal rectal cancer trials. Lancet Oncol. 2020, 21, e252–e264. [Google Scholar] [CrossRef]
- Valentini, V.; van Stiphout, R.G.; Lammering, G.; Gambacorta, M.A.; Barba, M.C.; Bebenek, M.; Bonnetain, F.; Bosset, J.F.; Bujko, K.; Cionini, L.; et al. Selection of appropriate end-points (pCR vs 2yDFS) for tailoring treatments with prediction models in locally advanced rectal cancer. Radiother. Oncol. 2015, 114, 302–309. [Google Scholar] [CrossRef]
- Thompson, H.M.; Omer, D.M.; Lin, S.; Kim, J.K.; Yuval, J.B.; Verheij, F.S.; Qin, L.X.; Gollub, M.J.; Wu, A.J.; Lee, M.; et al. Organ Preservation and Survival by Clinical Response Grade in Patients With Rectal Cancer Treated With Total Neoadjuvant Therapy: A Secondary Analysis of the OPRA Randomized Clinical Trial. JAMA Netw. Open 2024, 7, e2350903. [Google Scholar] [CrossRef]
- van der Valk, M.J.M.; Hilling, D.E.; Bastiaannet, E.; Meershoek-Klein Kranenbarg, E.; Beets, G.L.; Figueiredo, N.L.; Habr-Gama, A.; Perez, R.O.; Renehan, A.G.; van de Velde, C.J.H.; et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): An international multicentre registry study. Lancet 2018, 391, 2537–2545. [Google Scholar] [CrossRef]
- Rullier, E.; Perez, R.O. Surgery or a watch-and-wait approach for rectal cancer? Lancet Oncol. 2019, 20, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.D.; Schultheiss, T.E.; Smith, D.D.; Fakih, M.G.; Wong, J.Y.C.; Chen, Y.J. Effect of increasing radiation dose on pathologic complete response in rectal cancer patients treated with neoadjuvant chemoradiation therapy. Acta Oncol. 2016, 55, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Turri, G.; Ostuzzi, G.; Vita, G.; Barresi, V.; Scarpa, A.; Milella, M.; Mazzarotto, R.; Ruzzenente, A.; Barbui, C.; Pedrazzani, C. Treatment of Locally Advanced Rectal Cancer in the Era of Total Neoadjuvant Therapy: A Systematic Review and Network Meta-Analysis. JAMA Netw. Open 2024, 7, e2414702. [Google Scholar] [CrossRef]
- Bedrikovetski, S.; Traeger, L.; Seow, W.; Dudi-Venkata, N.N.; Selva-Nayagam, S.; Penniment, M.; Sammour, T. Oncological Outcomes and Response Rate After Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: A Network Meta-Analysis Comparing Induction vs. Consolidation Chemotherapy vs. Standard Chemoradiation. Clin. Color. Cancer 2024, 23, 326–336.e9. [Google Scholar] [CrossRef] [PubMed]
- Beppu, N.; Ikeda, M.; Kataoka, K.; Kimura, K.; Ikeuchi, H.; Uchino, M.; Nakamoto, Y.; Okamoto, R.; Yanagi, H. Total Neoadjuvant Chemotherapy in Rectal Cancer: Current Facts and Future Strategies. J. Anus Rectum Colon 2023, 7, 1–7. [Google Scholar] [CrossRef]
- Fokas, E.; Williams, H.; Diefenhardt, M.; Lin, S.; Qin, L.X.; Piso, P.; Dapper, H.; Germer, C.T.; Grützmann, R.; Tim Friede, J.; et al. Chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for locally advanced rectal cancer: Pooled analysis of the CAO/ARO/AIO-12 and the OPRA randomized phase 2 trials. Eur. J. Cancer 2024, 210, 114291. [Google Scholar] [CrossRef]
- Cisel, B.; Pietrzak, L.; Michalski, W.; Wyrwicz, L.; Rutkowski, A.; Kosakowska, E.; Cencelewicz, A.; Spalek, M.; Polkowski, W.; Jankiewicz, M.; et al. Long-course preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for clinical T4 and fixed clinical T3 rectal cancer: Long-term results of the randomized Polish II study. Ann. Oncol. 2019, 30, 1298–1303. [Google Scholar] [CrossRef]
- Beyond TME Collaborative. Consensus statement on the multidisciplinary management of patients with recurrent and primary rectal cancer beyond total mesorectal excision planes. Br. J. Surg. 2013, 100, 1009–1014. [Google Scholar] [CrossRef]
- Gunderson, L.L.; Sargent, D.J.; Tepper, J.B.; Wolmark, N.; O’Connell, M.J.; Begovic, M.; Allmer, C.; Colangelo, L.; Smalley, S.R.; Haller, D.G.; et al. Impact of T and N stage and treatment on survival and relapse in adjuvant rectal cancer: A pooled analysis. J. Clin. Oncol. 2004, 22, 1785–1796. [Google Scholar] [CrossRef]
- Greene, F.L.; Stewart, A.K.; Norton, H.J. New tumor-node-metastasis staging strategy for node-positive (stage III) rectal cancer: An analysis. J. Clin. Oncol. 2004, 22, 1778–1784. [Google Scholar] [CrossRef]
- 183 Fleshman, J.; Branda, M.; Sargent, D.J.; Boller, A.M.; George, V.; Abbas, M.; Peters, W.R., Jr.; Maun, D.; Chang, G.; Herline, A.; et al. Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes. The ACOSOGZ6051 randomized clinical trial. JAMA 2015, 314, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, A.R.L.; Solomon, M.J.; Lumley, J.W.; Hewett, P.; Clouston, A.D.; Gebski, V.J.; Davies, L.; Wilson, K.; Hague, W.; Simes, J.; et al. Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer. The ALacaRT randomized clinical trial. JAMA 2015, 314, 1356–1363. [Google Scholar] [CrossRef]
- Fleshman, J.; Branda, M.E.; Sargent, D.J.; Boller, A.M.; George, V.V.; Abbas, M.A.; Peters, W.R., Jr.; Maun, D.C.; Chang, G.J.; Herline, A.; et al. Disease-free survival and local recurrence for laparoscopic resection compared with open resection of stage II to III rectal cancer: Follow-up results of the ACOSOG Z6051 randomized controlled trial. Ann. Surg. 2019, 269, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Yuan, W.; Li, T.; Tang, B.; Jia, B.; Zhou, Y.; Zhang, W.; Zhao, R.; Zhang, C.; Cheng, L.; et al. Robotic versus laparoscopic surgery for middle and low rectal cancer (REAL): Short-term outcomes of a multicentrerandomised controlled trial. Lancet Gastroenterol. Hepatol. 2022, 7, 991–1004. [Google Scholar] [CrossRef]
- Ito, M.; Kobayashi, A.; Fujita, S.; Mizusawa, J.; Kanemitsu, Y.; Kinugasa, Y.; Komori, K.; Ohue, M.; Ota, M.; Akazai, Y.; et al. Urinary dysfunction after rectal cancer surgery: Results from a randomized trial comparing mesorectal excision with and without lateral lymph node dissection for clinical stage II or III lower rectal cancer. Eur. J. Surg. Oncol. 2018, 44, 463–468. [Google Scholar] [CrossRef]
- Paun, B.C.; Cassie, S.; MacLean, A.R.; Dixon, E.; Buie, W.D. Postoperative complications following surgery for rectal cancer. Ann. Surg. 2010, 251, 807–818. [Google Scholar] [CrossRef]
- Perry, W.R.G.; Abd El Aziz, M.A.; Duchalais, E.; Grass, F.; Behm, K.T.; Mathis, K.L.; Kelley, S.R. Sexual dysfunction followingsurgery for rectal cancer: A single-institution experience. Updates Surg. 2021, 73, 2155–2159. [Google Scholar] [CrossRef]
- Lange, M.M.; Maas, C.P.; Marijnen, C.A.; Wiggers, T.; Rutten, H.J.; Kranenbarg, E.K.; van de Velde, C.J.H. Urinary dysfunction after rectal cancer treatment is mainly caused by surgery. Br. J. Surg. 2008, 95, 1020–1028. [Google Scholar] [CrossRef]
- Sturiale, A.; Martellucci, J.; Zurli, L.; Vaccaro, C.; Brusciano, L.; Limongelli, P.; Docimo, L.; Valeri, A. Long-term functionalfollow-up after anterior rectal resection for cancer. Int. J. Color. Dis. 2017, 32, 83–88. [Google Scholar] [CrossRef]
- Vinchurkar, K.; Togale, M.; Maste, P.; Chaudhary, S.; Ahmed, I.; Krishnamurthy, S.; Bhise, R.; Mane, J.; Kumbar, P. Truly Inevitable—Our Perspective on the Complications After Surgery for Rectal Cancer. Indian J. Surg. Oncol. 2024. [Google Scholar] [CrossRef]
- Peeters, K.C.M.J. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: Increased bowel dysfunction in irradiated patients–a Dutch colorectal cancer group study. J. Clin. Oncol. 2005, 23, 6199–6206. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Kang, J.; Hur, H.; Min, B.S.; Baik, S.H.; Lee, K.Y.; Kim, N.K. Different Clinical Features According to the Anastomotic Leakage Subtypes after Rectal Cancer Surgeries: Contained vs. Free Leakages. PLoS ONE 2018, 13, e0208572. [Google Scholar] [CrossRef]
- Borstlap, W.A.A.; Westerduin, E.; Aukema, T.S.; Bemelman, W.A.; Tanis, P.J.; Dutch Snapshot Research Group. Anastomotic Leakage and Chronic Presacral Sinus Formation after Low Anterior Resection: Results from a Large Cross-Sectional Study. Ann. Surg. 2017, 266, 870–877. [Google Scholar] [CrossRef]
- Yasui, M.; Takemasa, I.; Miyake, Y.; Hata, T.; Ikeda, M.; Miyake, Y.; Hasegawa, J.; Ota, H.; Matsuda, C.; Mizushima, T.; et al. Tumor size as an independent risk factor for postoperative complications in laparoscopic low anterior resection for advanced rectal cancer: A multicenter Japanese study. Surg. Laparosc. Endosc. Percutaneous Tech. 2017, 27, 98–103. [Google Scholar] [CrossRef]
- Jahnson, S.; Christofferson, R.H.; Gerdin, B. Reduced mucosal perianastomotic capillary density in rat small in testine with chronic radiation damage. Radiat. Res. 1998, 150, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Ma, T.; Deng, Y.; Zheng, J.; Zhou, Z.; Wang, H.; Wang, L.; Wang, J. Impact of Preoperative Radiotherapy on Anastomotic Leakage and Stenosis after Rectal Cancer Resection: Post Hoc Analysis of a Randomized Controlled Trial. Dis. Colon Rectum 2016, 59, 934–942. [Google Scholar] [CrossRef]
- Yang, J.; Luo, Y.; Tian, T.; Dong, P.; Fu, Z. Effects of Neoadjuvant Radiotherapy on Postoperative Complications in Rectal Cancer: A Meta-Analysis. J. Oncol. 2022, 2022, 8197701. [Google Scholar] [CrossRef]
- Ma, B.; Gao, P.; Wang, H.; Xu, Q.; Song, Y.; Huang, X.; Sun, J.; Zhao, J.; Luo, J.; Sun, Y.; et al. What Has Preoperative Radio(Chemo)Therapy Brought to Localized Rectal Cancer Patients in Terms of Perioperative and Long-Term Outcomes over the Past Decades? A Systematic Review and Meta-Analysis Based on 41;121 Patients: PRT/PCRT-Perioperative and Long-Term Outcomes over the Past Decades. Int. J. Cancer 2017, 141, 1052–1065. [Google Scholar] [CrossRef]
- Foppa, C.; Carvello, M.; Maroli, A.; Sacchi, M.; Gramellini, M.; Montorsi, M.; Spinelli, A. Single-stapled anastomosis is associated with a lower anastomotic leak rate than double-stapled technique after minimally invasive total mesorectal excision for MRI-defined low rectal cancer. Surgery 2023, 173, 1367–1373. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Y.; Tian, G.; Liu, Y.; Jiang, Y.; Li, X.; Song, M. Meta-Analysis on the Efficacy of Indocyanine Green Fluorescence Angiography for Reduction of Anastomotic Leakage After Rectal Cancer Surgery. Am. Surg. 2021, 87, 1910–1919. [Google Scholar] [CrossRef]
- Armstrong, G.; Croft, J.; Corrigan, N.; Brown, J.M.; Goh, V.; Quirke, P.; Hulme, C.; Tolan, D.; Kirby, A.; Cahill, R.; et al. IntAct: Intra-operative fluorescence angiography to prevent anastomotic leak in rectal cancer surgery: A randomized controlled trial. Color. Dis. 2018, 20, O226–O234. [Google Scholar] [CrossRef] [PubMed]
- 204. vanGijn, W.; Marijnen, C.A.; Nagtegaal, I.D.; Kranenbarg, E.M.; Putter, H.; Wiggers, T.; Rutten, H.J.; Påhlman, L.; Glimelius, B.; van de Velde, C.J.; Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre; randomised controlled TME trial. Lancet Oncol. 2011, 12, 575–582. [Google Scholar] [CrossRef]
- Sun, W.; Dou, R.; Chen, J.; Lai, S.; Zhang, C.; Ruan, L.; Kang, L.; Deng, Y.; Lan, P.; Wang, L.; et al. Impact of long-course neo adjuvant radiation on postoperative low anterior resection syn drome and quality of life in rectal cancer: Post hoc analysis of a randomized controlled trial. Ann. Surg. Oncol. 2019, 26, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Pucciarelli, S.; Del Bianco, P.; Efficace, F.; Toppan, P.; Serpentini, S.; Friso, M.L.; Lonardi, S.; De Salvo, G.L.; Nitti, D. Health-related quality of life; faecal continence and bowel function in rectal cancer patients after chemoradiotherapy followed by radical surgery. Support. Care Cancer 2010, 18, 601–608. [Google Scholar] [CrossRef]
- Ekkarat, P.; Boonpipattanapong, T.; Tantiphlachiva, K.; Sangkhathat, S. Factors determining low anterior resection syndrome after rectal cancer resection: A study in Thai patients. Asian J. Surg. 2016, 39, 225–231. [Google Scholar] [CrossRef]
- Brown, C.J.; Fenech, D.S.; McLeod, R.S. Reconstructive techniques after rectal resection for rectal cancer. Cochrane Database Syst. Rev. 2008, 16, CD006040. [Google Scholar] [CrossRef]
- Croese, A.D.; Lonie, J.M.; Trollope, A.F.; Vangaveti, V.N.; Ho, Y. A meta-analysis of the prevalence of low anterior resection syndrome and systematic review of risk factors. Int. J. Surg. 2018, 56, 234–241. [Google Scholar] [CrossRef]
- Bryant, C.L.; Lunniss, P.J.; Knowles, C.H.; Thaha, M.A.; Chan, C.L. Anterior resection syndrome. Lancet Oncol. 2012, 13, e403–e408. [Google Scholar] [CrossRef]
- Qiu, Y.; Pu, Y.; Guan, H.; Fan, W.; Wang, S.; Du, G.; Yang, H.; Xiao, W. Low Anterior Resection Syndrome in Adults with Rectal Cancer in China: A Case Series Analysis. Indian J. Surg. 2021, 83, 1496–1501. [Google Scholar] [CrossRef]
- Nicotera, A.; Falletto, E.; Arezzo, A.; Mistrangelo, M.; Passera, R.; Morino, M. Risk factors for Low Anterior Resection Syndrome (LARS) in patients undergoing laparoscopic surgery for rectal cancer. Surg. Endosc. 2022, 36, 6059–6066. [Google Scholar] [CrossRef]
- Cedermark, B.; Johansson, H.; Rutqvist, L.E.; Wilking, N. The Stockholm I trial of preoperative short term radiotherapy in operable rectal carcinoma.A prospective randomized trial. Stockholm Colorectal Cancer Study Group. Cancer 1995, 75, 2269–2275. [Google Scholar] [CrossRef] [PubMed]
- El-Gazzaz, G.; Kiran, R.P.; Lavery, I. Wound compli cations in rectal cancer patients undergoing primary closure of the perineal wound after abdominoperineal resection. Dis. Colon Rectum 2009, 52, 1962–1966. [Google Scholar] [CrossRef] [PubMed]
- Musters, G.D.; Buskens, C.J.; Bemelman, W.A.; Tanis, P.J. Perineal wound healing after abdominoperineal resection for rectal cancer. Dis. Colon Rectum 2014, 57, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, L.; Wan, J.; Zhang, H.; Wu, R.; Wang, J.; Wang, Y.; Xu, Y.; Cai, S.; Zhang, Z.; et al. Short-course radiotherapy combined with CAPOX and Toripalimab for the total neoadjuvant therapy of locally advanced rectal cancer: A randomized; prospective; multicentre; double-arm; phase II trial (TORCH). BMC Cancer 2022, 22, 274. [Google Scholar] [CrossRef]
- Akiyoshi, T.; Shinozaki, E.; Taguchi, S.; Chino, A.; Hiratsuka, M.; Tominaga, T.; Nonaka, T.; Toda, S.; Matoba, S.; Matsui, S.; et al. Non-operative management after chemoradiotherapy plus consolidation or sandwich (induction with bevacizumab and consolidation) chemotherapy in patients with locally advanced rectal cancer: A multicentre; randomised phase II trial (NOMINATE trial). BMJ Open 2022, 12, e055140. [Google Scholar] [CrossRef]
- Lord, A.C.; Corr, A.; Chandramohan, A.; Hodges, N.; Pring, E.; Airo-Farulla, C.; Moran, B.; Jenkins, J.T.; Di Fabio, F.; Brown, G. Assessment of the2020 NICE criteria for preoperative radiotherapy in patients with rectal cancer treated by surgery alone in comparison with proven MRI prognostic factors: A retrospective cohort study. Lancet Oncol. 2022, 23, 793–801. [Google Scholar] [CrossRef]
- Xiao, W.W.; Li, M.; Guo, Z.W.; Zhang, R.; Xi, S.Y.; Zhang, X.G.; Li, Y.; Wu, D.Q.; Ren, Y.F.; Pang, X.L.; et al. A genotype signature for predicting pathologic complete response in locally advanced rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 482–491. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, X.; Yang, L.; Zhu, J.; Wan, J.; Shen, L.; Xia, F.; Fu, G.; Deng, Y.; Pan, M.; et al. Patient-Derived Organoids Predict Chemoradiation Responses of Locally Advanced Rectal Cancer. Cell Stem Cell 2020, 26, 17–26.e6. [Google Scholar] [CrossRef]
- Kim, S.; Kim, Y.; Lee, S.; Kim, Y.; Jeon, B.; Kim, H.; Park, H. Live biotherapeutic lactococcus lactis GEN3013 enhances antitumor efficacy of cancer treatment via modulation of cancer progression and immune system. Cancers 2022, 14, 4083. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefan, R.E.; Birla, R.D.; Gheorghe, M.; Dinu, D.E.; Hoara, P.A.; Ciuc, D.; Dinca, V.-G.; Constantinoiu, S. Controversies and Perspectives in the Current Management of Patients with Locally Advanced Rectal Cancer—A Systematic Review. Life 2025, 15, 1011. https://doi.org/10.3390/life15071011
Stefan RE, Birla RD, Gheorghe M, Dinu DE, Hoara PA, Ciuc D, Dinca V-G, Constantinoiu S. Controversies and Perspectives in the Current Management of Patients with Locally Advanced Rectal Cancer—A Systematic Review. Life. 2025; 15(7):1011. https://doi.org/10.3390/life15071011
Chicago/Turabian StyleStefan, Roxana Elena, Rodica Daniela Birla, Mircea Gheorghe, Daniela Elena Dinu, Petre Angel Hoara, Diana Ciuc, Valeriu-Gabi Dinca, and Silviu Constantinoiu. 2025. "Controversies and Perspectives in the Current Management of Patients with Locally Advanced Rectal Cancer—A Systematic Review" Life 15, no. 7: 1011. https://doi.org/10.3390/life15071011
APA StyleStefan, R. E., Birla, R. D., Gheorghe, M., Dinu, D. E., Hoara, P. A., Ciuc, D., Dinca, V.-G., & Constantinoiu, S. (2025). Controversies and Perspectives in the Current Management of Patients with Locally Advanced Rectal Cancer—A Systematic Review. Life, 15(7), 1011. https://doi.org/10.3390/life15071011








