The Role of Nutrition in HIV-Associated Neurocognitive Disorders: Mechanisms, Risks, and Interventions
Abstract
1. Introduction
2. Metabolic Syndrome and Body Weight in People with HIV
2.1. ART-Driven Dysbiosis and MetS
2.2. Metabolic Syndrome and Neurocognitive Impairments in People with HIV
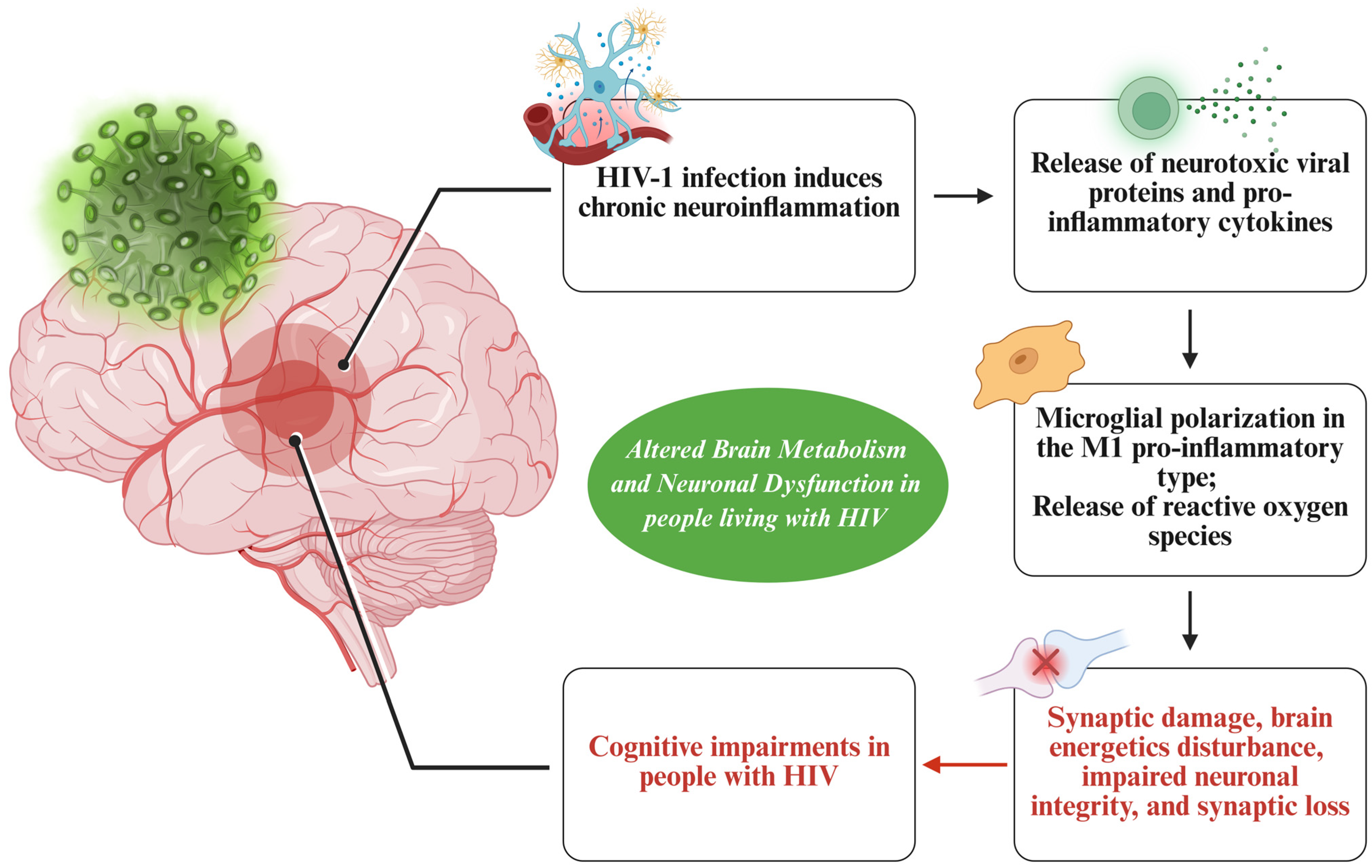
3. Altered Brain Metabolism and Neuronal Dysfunction Underlying Neurocognitive Impairments in People with HIV
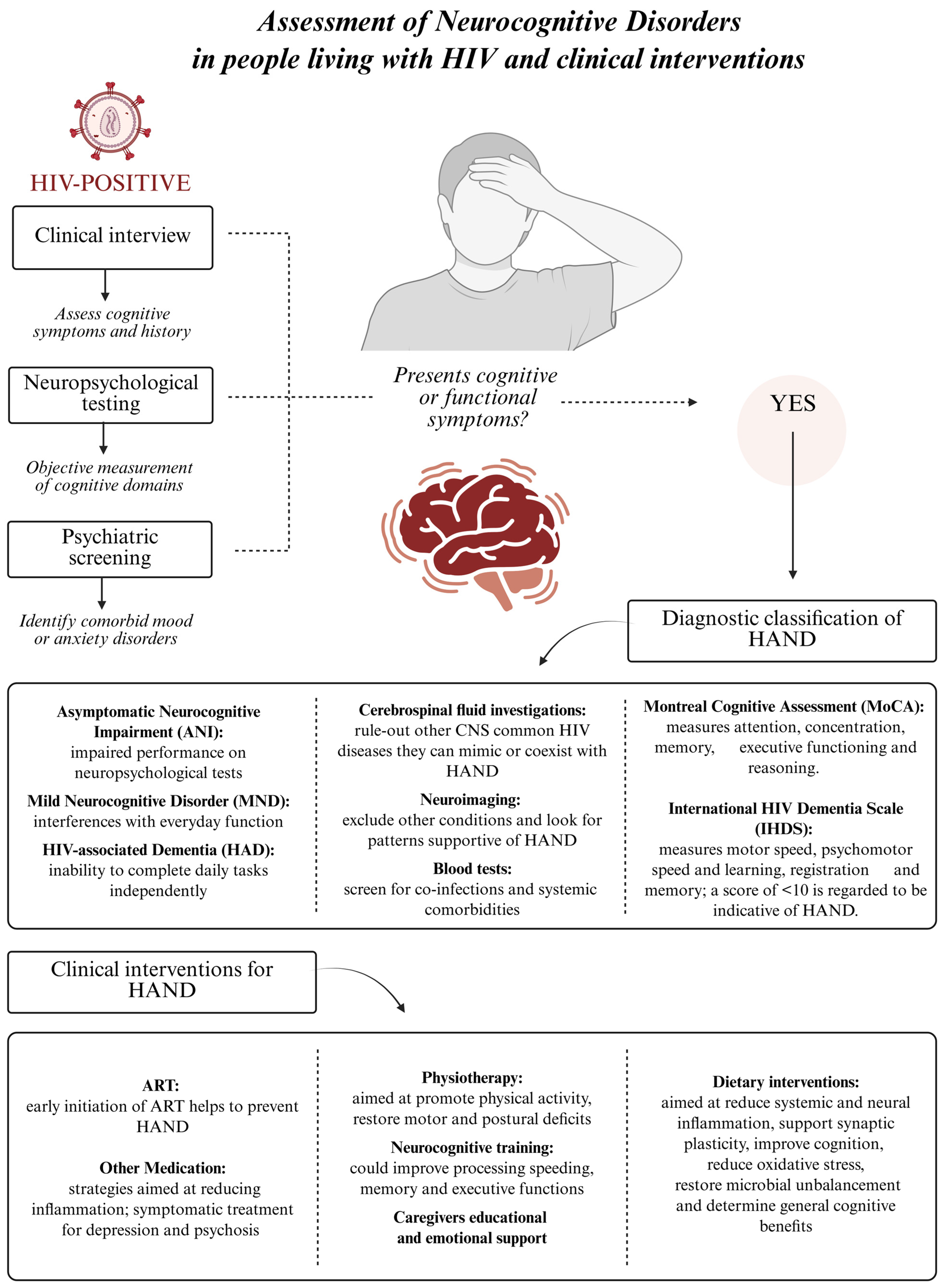
4. From HIV to HAND: Exploring the Role of Nutrition
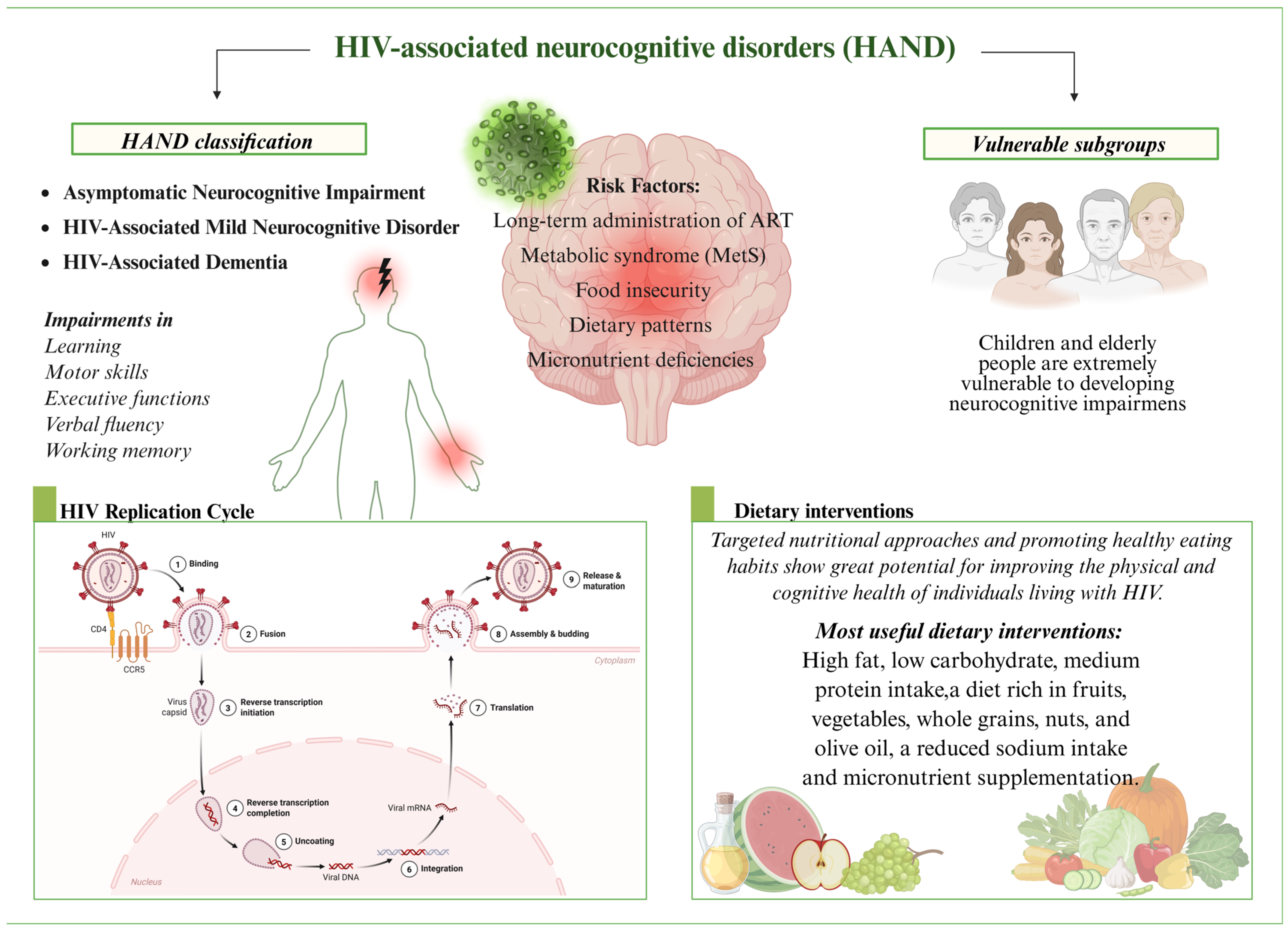
4.1. HAND: Classification, Mechanisms and Risk Factors
4.2. Micronutrient Deficiencies and Their Impact on HAND
5. Dietary Interventions and Their Influence on HAND
6. Diet, Nutrition, and HAND: Insights from Vulnerable HIV Subgroups
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gottlieb, M.S.; Schroff, R.; Schanker, H.M.; Weisman, J.D.; Fan, P.T.; Wolf, R.A.; Saxon, A. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: Evidence of a new acquired cellular immunodeficiency. N. Engl. J. Med. 1981, 305, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Masur, H.; Michelis, M.A.; Greene, J.B.; Onorato, I.; Stouwe, R.A.; Holzman, R.S.; Brettman, L.; Lange, M.; Murray, H.W.; Cunningham-Rundles, S. An outbreak of community-acquired Pneumocystis carinii pneumonia: Initial manifestation of cellular immune dysfunction. N. Eng. J. Med. 1981, 305, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Epidemiologic aspects of the current outbreak of Kaposi’s sarcoma and opportunistic infections. N. Eng. J. Med. 1982, 306, 248–252. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Pneumocystis pneumonia—Los Angeles. MMWR Morb. Mortal. Wkly. Rep. 1981, 30, 250–252. [Google Scholar]
- National Institute of Health (Reviewed 2025 March 31). The Stages of HIV Infection. Available online: https://hivinfo.nih.gov/understanding-hiv/fact-sheets/stages-hiv-infection (accessed on 18 June 2025).
- UNAIDS. 2024. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 11 June 2025).
- Günthard, H.F.; Saag, M.S.; Benson, C.A.; del Rio, C.; Eron, J.J.; Gallant, J.E.; Hoy, J.F.; Mugavero, M.J.; Sax, P.E.; Thompson, M.A.; et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2016 Recommendations of the International Antiviral Society-USA Panel. JAMA 2016, 316, 191–210. [Google Scholar] [CrossRef]
- Kemnic, T.R.; Gulick, P.G. HIV Antiretroviral Therapy. [Updated 20 September 2022]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513308/ (accessed on 11 June 2025).
- Rodger, A.J.; Cambiano, V.; Bruun, T.; Vernazza, P.; Collins, S.; Van Lunzen, J.; Corbelli, G.M.; Estrada, V.; Geretti, A.M.; Beloukas, A.; et al. Sexual Activity Without Condoms and Risk of HIV Transmission in Serodifferent Couples When the HIV-Positive Partner Is Using Suppressive Antiretroviral Therapy. JAMA 2016, 316, 171–181. [Google Scholar] [CrossRef]
- Jericó, C.; Knobel, H.; Montero, M.; Ordoñez-Llanos, J.; Guelar, A.; Gimeno, J.L.; Saballs, P.; López-Colomés, J.L.; Pedro-Botet, J. Metabolic syndrome among HIV-infected patients: Prevalence, characteristics, and related factors. Diabetes Care 2005, 28, 132–1377. [Google Scholar] [CrossRef]
- Elendu, C.; Aguocha, C.M.; Okeke, C.V.; Okoro, C.B.; Peterson, J.C. HIV-related neurocognitive disorders: Diagnosis, Treatment, and Mental Health Implications: A Review. Medicine 2023, 102, e35652. [Google Scholar] [CrossRef] [PubMed]
- Heaton, R.K.; Clifford, D.B.; Franklin, D.R., Jr.; Woods, S.P.; Ake, C.; Vaida, F.; Ellis, R.J.; Letendre, S.L.; Marcotte, T.D.; Atkinson, J.H.; et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010, 75, 2087–2096. [Google Scholar] [CrossRef]
- Nightingale, S.; Ances, B.; Cinque, P.; Dravid, A.; Dreyer, A.J.; Gisslén, M.; Joska, J.A.; Kwasa, J.; Meyer, A.-C.; Mpongo, N.; et al. Cognitive impairment in people living with HIV: Consensus recommendations for a new approach. Nat. Rev. Neurol. 2023, 19, 424–433. [Google Scholar] [CrossRef]
- Vastag, Z.; Fira-Mladinescu, O.; Rosca, E.C. HIV-Associated Neurocognitive Disorder (HAND): Obstacles to Early Neuropsychological Diagnosis. Int. J. Gen. Med. 2022, 15, 4079–4090. [Google Scholar] [CrossRef]
- Ancuta, P.; Kamat, A.; Chew, G.M. HIV-associated chronic immune activation and neuroinflammation. Curr. Opin. HIV AIDS 2016, 11, 191–198. [Google Scholar]
- McArthur, J.C.; Johnson, T.P. Chronic inflammation mediates brain injury in HIV infection: Relevance for cure strategies. Curr. Opin. Neurol. 2020, 33, 397–404. [Google Scholar] [CrossRef]
- Antinori, A.; Arendt, G.; Becker, J.T.; Brew, B.J.; Byrd, D.A.; Cherner, M.; Clifford, D.B.; Cinque, P.; Epstein, L.G.; Goodkin, K.; et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007, 69, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Rumbaugh, J.A.; Tyor, W. HIV-associated neurocognitive disorders: Five new things. Neurol. Clin. Pr. 2015, 5, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Hinkin, C.H.; Hardy, D.J.; Mason, K.I.; Castellon, S.A.; Durvasula, R.S.; Lam, M.N.; Stefaniak, M. Medication adherence in HIV-infected adults: Effect of patient age, cognitive status, and substance abuse. Aids 2004, 18 (Suppl. S1), S19–S25. [Google Scholar] [CrossRef]
- Samji, H.; Cescon, A.; Hogg, R.S.; Modur, S.P.; Althoff, K.N.; Buchacz, K.; Burchell, A.N.; Cohen, M.; Gebo, K.A.; Gill, M.J.; et al. Closing the gap: Increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS ONE 2013, 8, e81355. [Google Scholar] [CrossRef] [PubMed]
- Paton, N.; Sangeetha, S.; Earnest, A.; Bellamy, R. The impact of malnutrition on survival and the CD4 count response in HIV-infected patients starting antiretroviral therapy. HIV Med. 2006, 7, 323–330. [Google Scholar] [CrossRef]
- Sax, P.E.; Erlandson, K.M.; Lake, J.E.; Mccomsey, G.A.; Orkin, C.; Esser, S.; Brown, T.T.; Rockstroh, J.K.; Wei, X.; Carter, C.C.; et al. Weight Gain Following Initiation of Antiretroviral Therapy: Risk Factors in Randomized Comparative Clinical Trials. Clin. Infect. Dis. 2020, 71, 1379–1389. [Google Scholar] [CrossRef]
- Capeau, J.; Lagathu, C.; Béréziat, V. Recent data on the role of antiretroviral therapy in weight gain and obesity in persons living with HIV. Curr. Opin. HIV AIDS 2024, 19, 14–20. [Google Scholar] [CrossRef]
- Ruderman, S.A.; Crane, H.M.; Nance, R.M.; Whitney, B.M.; Harding, B.N.; Mayer, K.H.; Moore, R.D.; Eron, J.J.; Geng, E.; Mathews, W.C.; et al. Brief Report: Weight Gain Following ART Initiation in ART-Naïve People Living With HIV in the Current Treatment Era. Am. J. Ther. 2021, 86, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Fields-Gardner, C.; Campa, A.M. Position of the American Dietetic Association: Nutrition intervention and human immunodeficiency virus infection. J. Am. Diet. Assoc. 2010, 110, 1105–1119. [Google Scholar] [CrossRef] [PubMed]
- Mankal, P.K.; Kotler, D.P. From wasting to obesity, changes in nutritional concerns in HIV/AIDS. Endocrinol. Metab. Clin. 2014, 43, 647–663. [Google Scholar] [CrossRef]
- Butterfield, T.R.; Landay, A.L.; Anzinger, J.J. Dysfunctional Immunometabolism in HIV Infection: Contributing Factors and Implications for Age-Related Comorbid Diseases. Curr. HIV/AIDS Rep. 2020, 17, 125–137. [Google Scholar] [CrossRef]
- Palmer, C.S.; Cherry, C.L.; Sada-Ovalle, I.; Singh, A.; Crowe, S.M. Glucose Metabolism in T Cells and Monocytes: New Perspectives in HIV Pathogenesis. eBioMedicine 2016, 6, 31–41. [Google Scholar] [CrossRef]
- Pan American Health Organization. Available online: https://www.paho.org/fr/node/4919 (accessed on 18 June 2025).
- Cleveland Clinic. Available online: https://my.clevelandclinic.org/health/treatments/antiretroviral-therapy (accessed on 18 June 2025).
- World Health Organization. Updated Recommendations on First-Line and Second-Line Antiretroviral Regimens and Post-Exposure Prophylaxis and Recommendations on Early Infant Diagnosis of HIV: Interim Guidelines: Supplement to the 2016 Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Dressman, J.; Kincer, J.; Matveev, S.V.; Guo, L.; Greenberg, R.N.; Guerin, T.; Meade, D.; Li, X.A.; Zhu, W.; Uittenbogaard, A.; et al. HIV protease inhibitors promote atherosclerotic lesion formation independent of dyslipidemia by increasing CD36-dependent cholesteryl ester accumulation in macrophages. J. Clin. Investig. 2003, 111, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Fontas, E.; van Leth, F.; Sabin, C.A.; Friis-Møller, N.; Rickenbach, M.; d’Arminio Monforte, A.; Kirk, O.; Dupon, M.; Morfeldt, L.; Mateu, S.; et al. Lipid profiles in HIV-infected patients receiving combination antiretroviral therapy: Are different antiretroviral drugs associated with different lipid profiles? J. Infect. Dis. 2004, 189, 1056–1074. [Google Scholar] [CrossRef]
- Trachunthong, D.; Tipayamongkholgul, M.; Chumseng, S.; Darasawang, W.; Bundhamcharoen, K. Burden of metabolic syndrome in the global adult HIV-infected population: A systematic review and meta-analysis. BMC Public Health 2024, 24, 2657. [Google Scholar] [CrossRef]
- Canadian Agency for Drugs and Technologies in Health. Table 1, Summary of Results of the GEMINI-1 and GEMINI-2 Trials. In Clinical Review Report: Dolutegravir/Lamivudine (Dovato): (ViiV Healthcare ULC): Indication: As a Complete Regimen for the Treatment of Human Immunodeficiency Virus Type 1 (HIV-1) Infection in Adults and Adolescents 12 Years of Age and Older and Weighing at Least 40 kg [Internet]; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, USA, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551951/table/cl1.tab2/ (accessed on 11 June 2025).
- Bourgi, K.; Jenkins, C.A.; Rebeiro, P.F.; Palella, F.; Moore, R.D.; Altoff, K.N.; Gill, J.; Rabkin, C.S.; Gange, S.J.; Horberg, M.A.; et al. Weight gain among treatment-naïve persons with HIV starting integrase inhibitors compared to non-nucleoside reverse transcriptase inhibitors or protease inhibitors in a large observational cohort in the United States and Canada. J. Int. AIDS Soc. 2020, 23, e25484. [Google Scholar] [CrossRef]
- Eckard, A.R.; McComsey, G.A. Weight gain and integrase inhibitors. Curr. Opin. Infect. Dis. 2020, 33, 10–19. [Google Scholar] [CrossRef]
- Jevtović, D.J.; Dragović, G.; Salemović, D.; Ranin, J.; Djurković-Djaković, O. The metabolic syndrome, an epidemic among HIV-infected patients on HAART. Biomed. Pharmacother. 2009, 63, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.A.; Peer, N.; Mills, E.J.; Kengne, A.P. A meta-analysis of the metabolic syndrome prevalence in the global HIV-infected population. PLoS ONE 2016, 11, e0150970. [Google Scholar] [CrossRef] [PubMed]
- Bares, S.H.; Smeaton, L.M.; Xu, A.; Godfrey, C.; McComsey, G.A. HIV-infected women gain more weight than HIV-infected men following the initiation of antiretroviral therapy. J. Women’s Health 2018, 27, 1162–1169. [Google Scholar] [CrossRef]
- Chillo, P.; Muhihi, A.; Danaei, G.; Bakari, M.; Kwesigabo, G.; Njelekela, M.; Ulenga, N.; Fawzi, W.W.; Mugusi, F.; Sudfeld, C.R. Sociodemographic and Clinical Predictors of Weight Gain During the First Year of Antiretroviral Therapy among Adults Living With HIV in Urban Tanzania. J. Int. Assoc. Provid. AIDS Care 2024, 23, 23259582241281010. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.A.; Yang, C.A.; Ojuri, V.; Buckley, K.; Bedi, B.; Musonge-Effoe, J.; Soibi-Harry, A.; Lahiri, C.D. Sex Differences in Metabolic Disorders of Aging and Obesity in People with HIV. Curr. HIV/AIDS 2024, 22, 3. [Google Scholar] [CrossRef]
- Brenchley, J.M.; Schacker, T.W.; Ruff, L.E.; Price, D.A.; Taylor, J.H.; Beilman, G.J.; Nguyen, P.L.; Khoruts, A.; Larson, M.; Haase, A.T.; et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 2004, 200, 749–759. [Google Scholar] [CrossRef]
- Nowak, P.; Troseid, M.; Avershina, E.; Barqasho, B.; Neogi, U.; Holm, K.; Hov, J.R.; Noyan, K.; Vesterbacka, J.; Svärd, J.; et al. Gut microbiota diversity predicts immune status in HIV-1 infection. AIDS 2015, 29, 2409–2418. [Google Scholar] [CrossRef]
- Bandera, A.; De Benedetto, I.; Bozzi, G.; Gori, A. Altered gut microbiome composition in HIV infection: Causes, effects and potential intervention. Curr. Opin. HIV AIDS 2018, 13, 73–80. [Google Scholar] [CrossRef]
- Ray, S.; Narayanan, A.; Giske, C.G.; Neogi, U.; Sönnerborg, A.; Nowak, P. Altered Gut Microbiome under Antiretroviral Therapy: Impact of Efavirenz and Zidovudine. ACS Infect. Dis. 2020, 7, 1104–1115. [Google Scholar] [CrossRef]
- Villanueva-Millán, M.J.; Pérez-Matute, P.; Recio-Fernández, E.; Lezana Rosales, J.M.; Oteo, J.A. Characterization of gut microbiota composition in HIV-infected patients with metabolic syndrome. J. Physiol. Biochem. 2019, 75, 299–309. [Google Scholar] [CrossRef]
- Baltazar-Díaz, T.A.; Amador-Lara, F.; Andrade-Villanueva, J.F.; González-Hernández, L.A.; Cabrera-Silva, R.I.; Sánchez-Reyes, K.; Álvarez-Zavala, M.; Valenzuela-Ramírez, A.; Del Toro-Arreola, S.; Bueno-Topete, M.R. Gut Bacterial Communities in HIV-Infected Individuals with Metabolic Syndrome: Effects of the Therapy with Integrase Strand Transfer Inhibitor-Based and Protease Inhibitor-Based Regimens. Microorganisms 2023, 11, 951. [Google Scholar] [CrossRef] [PubMed]
- Ezkurdia, A.; Ramírez, M.J.; Solas, M. Metabolic Syndrome as a Risk Factor for Alzheimer’s Disease: A Focus on Insulin Resistance. Int. J. Mol. Sci. 2023, 24, 4354. [Google Scholar] [CrossRef]
- Atti, A.R.; Valente, S.; Iodice, A.; Caramella, I.; Ferrari, B.; Albert, U.; Mandelli, L.; De Ronchi, D. Metabolic Syndrome, Mild Cognitive Impairment, and Dementia: A Meta-Analysis of Longitudinal Studies. Am. J. Geriatr. Psychiatry 2019, 27, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Leszek, J.; Mikhaylenko, E.V.; Belousov, D.M.; Koutsouraki, E.; Szczechowiak, K.; Kobusiak-Prokopowicz, M.; Mysiak, A.; Diniz, B.S.; Somasundaram, S.G.; Kirkland, C.E.; et al. The Links between Cardiovascular Diseases and Alzheimer’s Disease. Curr. Neuropharmacol. 2021, 19, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Athanasaki, A.; Melanis, K.; Tsantzali, I.; Stefanou, M.I.; Ntymenou, S.; Paraskevas, S.G.; Kalamatianos, T.; Boutati, E.; Lambadiari, V.; Voumvourakis, K.I.; et al. Type 2 Diabetes Mellitus as a Risk Factor for Alzheimer’s Disease: Review and Meta-Analysis. Biomedicines 2022, 10, 778. [Google Scholar] [CrossRef]
- Reitz, C. Dyslipidemia and the risk of Alzheimer’s disease. Curr. Atheroscler. Rep. 2013, 15, 307. [Google Scholar] [CrossRef]
- Masenga, S.K.; Liweleya, S.; Kirabo, A. High salt intake and HIV infection on endothelial glycocalyx shedding in salt-sensitive hypertension. Front. Cell Dev. Biol. 2024, 12, 1395885. [Google Scholar] [CrossRef]
- Kallianpur, A.R.; Gittleman, H.; Letendre, S.; Ellis, R.; Barnholtz-Sloan, J.S.; Bush, W.S.; Heaton, R.; Samuels, D.C.; Franklin, D.R., Jr.; Rosario-Cookson, D.; et al. Cerebrospinal Fluid Ceruloplasmin, Haptoglobin, and Vascular Endothelial Growth Factor Are Associated with Neurocognitive Impairment in Adults with HIV Infection. Mol. Neurobiol. 2018, 56, 3808–3818. [Google Scholar] [CrossRef]
- McCutchan, J.A.; Marquie-Beck, J.A.; Fitzsimons, C.A.; Letendre, S.L.; Ellis, R.J.; Heaton, R.K.; Wolfson, T.; Rosario, D.; Alexander, T.J.; Marra, C.; et al. Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology 2012, 78, 485–492. [Google Scholar] [CrossRef]
- Yang, J.; Jacobson, L.P.; Becker, J.T.; Levine, A.; Martin, E.M.; Munro, C.A.; Palella, F.J.; Lake, J.E.; Sacktor, N.C.; Brown, T.T. Impact of glycemic status on longitudinal cognitive performance in men with and without HIV infection. AIDS 2018, 32, 1849–1860. [Google Scholar] [CrossRef]
- Khuder, S.S.; Chen, S.; Letendre, S.; Marcotte, T.; Grant, I.; Franklin, D.; Rubin, L.H.; Margolick, J.B.; Jacobson, L.P.; Sacktor, N.; et al. Impaired insulin sensitivity is associated with worsening cognition in HIV-infected patients. Neurology 2019, 92, e1344–e1353. [Google Scholar] [CrossRef] [PubMed]
- Osborne, O.; Peyravian, N.; Nair, M.; Daunert, S.; Toborek, M. The Paradox of HIV Blood-Brain Barrier Penetrance and Antiretroviral Drug Delivery Deficiencies. Trends Neurosci. 2020, 43, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Mukerji, S.S.; Locascio, J.J.; Misra, V.; Lorenz, D.R.; Holman, A.; Dutta, A.; Penugonda, S.; Wolinsky, S.M.; Gabuzda, D. Lipid profiles and apoe4 allele impact midlife cognitive decline in HIV-Infected men on antiretroviral therapy. Clin. Infect. Dis. 2016, 63, 1130–1139. [Google Scholar] [CrossRef]
- Yadav, A.; Betts, M.R.; Collman, R.G. Statin modulation of monocyte phenotype and function: Implications for HIV-1-associated neurocognitive disorders. J. NeuroVirol. 2016, 22, 584–596. [Google Scholar] [CrossRef]
- Okafor, C.N.; Kelso, N.E.; Bryant, V.; Burrell, L.E.; Míguez, M.J., 2nd; Gongvatana, A.; Tashima, K.T.; de la Monte, S.; Cook, R.L.; Cohen, R.A. Body mass index, inflammatory biomarkers and neurocognitive impairment in HIV-infected persons. Psychol. Health Med. 2017, 22, 289–302. [Google Scholar] [CrossRef]
- Yu, B.; Pasipanodya, E.; Montoya, J.L.; Moore, R.C.; Gianella, S.; McCutchan, A.; Ellis, R.; Heaton, R.K.; Jeste, D.V.; Moore, D.J.; et al. Metabolic Syndrome and Neurocognitive Deficits in HIV Infection. J. Acquir. Immune Defic. Syndr. 2019, 81, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Marquine, M.J.; Kamalyan, L.; Zlatar, Z.Z.; Yassai-Gonzalez, D.; Perez-Tejada, A.; Umlauf, A.; Al-Rousan, T.; González, V.; Breton, J.; Guareña, L.A.; et al. Disparities in Metabolic Syndrome and Neurocognitive Function Among Older Hispanics/Latinos with Human Immunodeficiency Virus. AIDS Patient Care STDS 2024, 38, 195–205. [Google Scholar] [CrossRef]
- Ellis, R.J.; Marquine, M.J.; Kaul, M.; Fields, J.A.; Schlachetzki, J.C.M. Mechanisms underlying HIV-associated cognitive impairment and emerging therapies for its management. Nat. Rev. Neurol. 2023, 19, 668–687. [Google Scholar] [CrossRef]
- Reuter, M.A.; Pombo, C.; Betts, M.R. Cytokine production and dysregulation in HIV pathogenesis: Lessons for development of therapeutics and vaccines. Cytokine Growth Factor. Rev. 2012, 23, 181–191. [Google Scholar] [CrossRef]
- Chen, L.; Gao, B.; Zhang, Y.; Lu, H.; Li, X.; Pan, L.; Yin, L.; Zhi, X. PAR2 promotes M1 macrophage polarization and inflammation via FOXO1 pathway. J. Cell. Biochem. 2019, 120, 9799–9809. [Google Scholar] [CrossRef]
- Hong, S.; Banks, W.A. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav. Immun. 2015, 45, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.N.S.; Arjona, S.P.; Santerre, M.; De Lucia, C.; Koch, W.J.; Sawaya, B.E. Metabolic Reprogramming in HIV-Associated Neurocognitive Disorders. Front. Cell. Neurosci. 2022, 16, 812887. [Google Scholar] [CrossRef] [PubMed]
- Caron, M.; Auclair, M.; Vissian, A.; Vigouroux, C.; Capeau, J. Contribution of mitochondrial dysfunction and oxidative stress to cellular premature senescence induced by antiretroviral thymidine analogues. Antivir. Ther. 2008, 13, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Roca-Bayerri, C.; Robertson, F.; Pyle, A.; Hudson, G.; Payne, B.A.I. Mitochondrial DNA Damage and Brain Aging in Human Immunodeficiency Virus. Clin. Infect. Dis. 2021, 73, e466–e473. [Google Scholar] [CrossRef]
- Fiala, M.; Murphy, T.; MacDougall, J.; Yang, W.; Luque, A.; Iruela-Arispe, L.; Cashman, J.; Buga, G.; Byrns, R.E.; Barbaro, G.; et al. HAART drugs induce mitochondrial damage and intercellular gaps and gp120 causes apoptosis. Cardiovasc. Toxicol. 2004, 4, 327–337. [Google Scholar] [CrossRef]
- Pinti, M.; Salomoni, P.; Cossarizza, A. Anti-HIV drugs and the mitochondria. Biochim. Biophys. Acta Bioenerg. 2006, 1757, 700–707. [Google Scholar] [CrossRef]
- Lewis, W.; Copeland, W.C.; Day, B.J. Mitochondrial DNA depletion, oxidative stress, and mutation: Mechanisms of dysfunction from nucleoside reverse transcriptase inhibitors. Lab. Investig. 2001, 81, 777–790. [Google Scholar] [CrossRef]
- Funes, H.A.; Blas-Garcia A: Esplugues, J.V.; Apostolova, N. Efavirenz alters mitochondrial respiratory function in cultured neuron and glial cell lines. J. Antimicrob. Chemothe 2015, 70, 2249–2254. [Google Scholar] [CrossRef]
- Ma, Q.; Vaida, F.; Wong, J.; Sanders, C.A.; Kao, Y.T.; Croteau, D.; Clifford, D.B.; Collier, A.C.; Gelman, B.B.; Marra, C.M.; et al. Long-term efavirenz use is associated with worse neurocognitive functioning in HIV-infected patients. J. Neurovirol. 2016, 22, 170–178. [Google Scholar] [CrossRef]
- Avdoshina, V.; Fields, J.A.; Castellano, P.; Dedoni, S.; Palchik, G.; Trejo, M.; Adame, A.; Rockenstein, E.; Eugenin, E.; Masliah, E.; et al. The HIV Protein gp120 Alters Mitochondrial Dynamics in Neurons. Neurotox. Res. 2016, 29, 583–593. [Google Scholar] [CrossRef]
- Rozzi, S.J.; Avdoshina, V.; Fields, J.A.; Mocchetti, I. Human immunodeficiency virus Tat impairs mitochondrial fission in neurons. Cell Death Discov. 2018, 4, 8. [Google Scholar] [CrossRef]
- Schweinsburg, B.C.; Taylor, M.J.; Alhassoon, O.M.; Gonzalez, R.; Brown, G.G.; Ellis, R.J.; Letendre, S.; Videen, J.S.; McCutchan, J.A.; Patterson, T.L.; et al. HNRC Group. Brain mitochondrial injury in human immunodeficiency virus-seropositive (HIV+) individuals taking nucleoside reverse transcriptase inhibitors. J. Neurovirol. 2005, 11, 356–364. [Google Scholar] [CrossRef]
- Jensen, B.K.; Monnerie, H.; Mannell, M.V.; Gannon, P.J.; Espinoza, C.A.; Erickson, M.A.; Bruce-Keller, A.J.; Gelman, B.B.; Briand, L.A.; Pierce, R.C.; et al. Altered Oligodendrocyte Maturation and Myelin Maintenance: The Role of Antiretrovirals in HIV-Associated Neurocognitive Disorders. J. Neuropathol. Exp. Neurol. 2015, 74, 1093–1118. [Google Scholar] [CrossRef] [PubMed]
- Hinman, J.D.; Chen, C.D.; Oh, S.Y.; Hollander, W.; Abraham, C.R. Age-dependent accumulation of ubiquitinated 2′,3′-cyclic nucleotide 3′-phosphodiesterase in myelin lipid rafts. Glia 2008, 56, 118–133. [Google Scholar] [CrossRef]
- Riviere-Cazaux, C.; Cornell, J.; Shen, Y.; Zhou, M. The role of CCR5 in HIV-associated neurocognitive disorders. Heliyon 2022, 8, e09950. [Google Scholar] [CrossRef] [PubMed]
- Burdo, T.H.; Lackner, A.; Williams, K.C. Monocyte/macrophages and their role in HIV neuropathogenesis. Immunol. Rev. 2013, 254, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Benki-Nugent, S.F.; Martopullo, I.; Laboso, T.; Tamasha, N.; Wamalwa, D.C.; Tapia, K.; Langat, A.; Maleche-Obimbo, E.; Marra, C.M.; Bangirana, P.; et al. High Plasma Soluble CD163 During Infancy Is a Marker for Neurocognitive Outcomes in Early-Treated HIV-Infected Children. Am. J. Ther. 2019, 81, 102–109. [Google Scholar] [CrossRef]
- Sasaoka, T.; Wada, T.; Tsuneki, H. Insulin resistance and cognitive function. Nihon Rinsho 2014, 72, 633–640. [Google Scholar]
- Kim, B.H.; Kelschenbach, J.; Borjabad, A.; Hadas, E.; He, H.; Potash, M.J.; Nedelcovych, M.T.; Rais, R.; Haughey, N.J.; McArthur, J.C.; et al. Intranasal insulin therapy reverses hippocampal dendritic injury and cognitive impairment in a model of HIV-associated neurocognitive disorders in EcoHIV-infected mice. AIDS 2019, 33, 973–984. [Google Scholar] [CrossRef]
- Deeks, S.G.; Lewin, S.R.; Havlir, D.V. The end of AIDS: HIV infection as a chronic disease. Lancet 2013, 382, 1525–1533. [Google Scholar] [CrossRef]
- Mitra, P.; Sharman, T. HIV Neurocognitive Disorders. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK555954/ (accessed on 9 June 2025).
- Eugenin, E.A.; Osiecki, K.; Lopez, L.; Goldstein, H.; Calderon, T.M.; Berman, J.W. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: A potential mechanism of HIV-CNS invasion and NeuroAIDS. J. Neurosci. 2006, 26, 1098–1106. [Google Scholar] [CrossRef]
- Valcour, V.; Chalermchai, T.; Sailasuta, N.; Marovich, M.; Lerdlum, S.; Suttichom, D.; Suwanwela, N.C.; Jagodzinski, L.; Michael, N.; Spudich, S.; et al. Central Nervous System Viral Invasion and Inflammation during Acute HIV Infection. J. Infect. Dis. 2012, 206, 275–282. [Google Scholar] [CrossRef]
- Olivier, I.S.; Cacabelos, R.; Naidoo, V. Risk Factors and Pathogenesis of HIV-Associated Neurocognitive Disorder: The Role of Host Genetics. Int. J. Mol. Sci. 2018, 19, 3594. [Google Scholar] [CrossRef]
- Dinçer, M.; Köse, N.; Ünal, E. The role of socioeconomic and behavioral factors in HIV-related deaths. Humanit. Soc. Sci. Commun. 2024, 11, 1588. [Google Scholar] [CrossRef]
- Evian, C. AIDS and the cycle of poverty. Nurs. RSA 1993, 8, 45. [Google Scholar] [PubMed]
- Kalichman, S.C.; Hernandez, D.; Kegler, C.; Cherry, C.; Kalichman, M.O.; Grebler, T. Dimensions of Poverty and Health Outcomes Among People Living with HIV Infection: Limited Resources and Competing Needs. J. Community Health 2015, 40, 702–708. [Google Scholar] [CrossRef]
- Anema, A.; Vogenthaler, N.; Frongillo, E.A.; Kadiyala, S.; Weiser, S.D. Food insecurity and HIV/AIDS: Current knowledge, gaps, and research priorities. Curr. HIV/AIDS Rep. 2009, 6, 224–231. [Google Scholar] [CrossRef]
- Spinelli, M.A.; Frongillo, E.A.; Sheira, L.A.; Palar, K.; Tien, P.C.; Wilson, T.; Merenstein, D.; Cohen, M.; Adedimeji, A.; Wentz, E.; et al. Food Insecurity is Associated with Poor HIV Outcomes Among Women in the United States. AIDS Behav. 2017, 21, 3473–3477. [Google Scholar] [CrossRef] [PubMed]
- Koethe, J.R.; Heimburger, D.C. Nutritional aspects of HIV-associated wasting in sub-Saharan Africa. Am. J. Clin. Nutr. 2010, 91, 1138S–1142S. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Budhathoki, C.; Farley, J.E. Effectiveness of macronutrient supplementation on nutritional status and HIV/AIDS progression: A systematic review and meta-analysis. Clin. Nutr. ESPEN 2018, 27, 66–74. [Google Scholar] [CrossRef]
- Visser, M.E.; Durao, S.; Sinclair, D.; Irlam, J.H.; Siegfried, N. Micronutrient supplementation in adults with HIV infection. Cochrane Database Syst. Rev. 2017, 5, CD003650. [Google Scholar] [CrossRef] [PubMed]
- Basta, D.; Latinovic, O.S.; Tagaya, Y.; Silvestri, G. Potential Advantages of a Well-balanced Nutrition Regimen for People Living with Human Immunodeficiency Virus Type—1. J. AIDS HIV Treat. 2024, 6, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Nxasana, N.; Oladimeji, K.E.; Pulido-Estrada, G.A.; Apalata, T.R. Prevalence of Micronutrient Deficiency among People Living with HIV in Selected Rural Districts of the Eastern Cape Province of South Africa. Nutrients 2023, 15, 3017. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Graham, N.M.; Caiaffa, W.T.; Margolick, J.B.; Clement, L.; Vlahov, D. Increased mortality associated with vitamin A deficiency during human immunodeficiency virus type 1 infection. Arch. Intern. Med. 1993, 153, 2149–2154. [Google Scholar] [CrossRef]
- June, H.L.; Yang, A.R.T.; Bryant, J.L.; Jones, O.; Royal, W., 3rd. Vitamin A deficiency and behavioral and motor deficits in the human immunodeficiency virus type 1 transgenic rat. J. Neurovirol. 2009, 15, 380–389. [Google Scholar] [CrossRef]
- Baldewicz, T.; Goodkin, K.; Feaster, D.J.; Blaney, N.T.; Kumar, M.; Kumar, A.; Shor-Posner, G.; Baum, M. Plasma pyridoxine deficiency is related to increased psychological distress in recently bereaved homosexual men. Psychosom. Med. 1998, 60, 297–308. [Google Scholar] [CrossRef]
- Adhikari, M.P.; Acharya, S.D.; Ramapuram, J.T.; Rao, S.B.; Vadapalli, K.; Chowta, M.N.; Ullal, S.D. Serum pyridoxine levels in HIV-positive patients and its association with tuberculosis and neuropsychiatric manifestations. Int. J. Nutr. Pharmacol. Neurol. Dis. 2016, 6, 157–161. [Google Scholar]
- Vergori, A.; Pinnetti, C.; Lorenzini, P.; Brita, A.; Libertone, R.; Mastrorosa, I.; Cicalini, S.; Antinori, A.; Ammassari, A. Vitamin D deficiency is associated with neurocognitive impairment in HIV-infected subjects. Infection 2019, 47, 929–935. [Google Scholar] [CrossRef]
- Dong, R.; Lin, H.; Chen, X.; Shi, R.; Yuan, S.; Li, J.; Zhu, B.; Xu, X.; Shen, W.; Wang, K.; et al. Gut Microbiota and Fecal Metabolites Associated With Neurocognitive Impairment in HIV-Infected Population. Front. Cell. Infect. Microbiol. 2021, 11, 723840. [Google Scholar] [CrossRef]
- Pastor-Ibáñez, R.; Blanco-Heredia, J.; Etcheverry, F.; Sánchez-Palomino, S.; Díez-Fuertes, F.; Casas, R.; Navarrete-Muñoz, M.Á.; Castro-Barquero, S.; Lucero, C.; Fernández, I.; et al. Adherence to a Supplemented Mediterranean Diet Drives Changes in the Gut Microbiota of HIV-1-Infected Individuals. Nutrients 2021, 13, 1141. [Google Scholar] [CrossRef]
- Tsiodras, S.; Poulia, K.A.; Yannakoulia, M.; Chimienti, S.N.; Wadhwa, S.; Karchmer, A.W.; Mantzoros, C.S. Adherence to Mediterranean diet is favorably associated with metabolic parameters in HIV-positive patients with the highly active antiretroviral therapy-induced metabolic syndrome and lipodystrophy. Metabolism 2009, 58, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Aparecida Silveira, E.; Falco, M.O.; Santos, A.S.E.A.C.; Noll, M.; de Oliveira, C. Nutritional Intervention Reduces Dyslipidemia, Fasting Glucose and Blood Pressure in People Living with HIV/AIDS in Antiretroviral Therapy: A Randomized Clinical Trial Comparing Two Nutritional Interventions. Nutrients 2020, 12, 2970. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, T.; Frongillo, E.A.; Turan, J.M.; Sheira, L.A.; Adedimeji, A.P.; Wilson, T.; Merenstein, D.; Cohen, M.; Adimora, A.A.; Ofotokun, I.; et al. Association of Higher Intake of Plant-Based Foods and Protein With Slower Kidney Function Decline in Women With HIV. Am. J. Ther. 2023, 94, 203–210. [Google Scholar] [CrossRef]
- Chinna-Meyyappan, A.; Gomes, F.A.; Koning, E.; Fabe, J.; Breda, V.; Brietzke, E. Effects of the ketogenic diet on cognition: A systematic review. Nutr. Neurosci. 2023, 26, 1258–1278. [Google Scholar] [CrossRef]
- Rong, L.; Peng, Y.; Shen, Q.; Chen, K.; Fang, B.; Li, W. Effects of ketogenic diet on cognitive function of patients with Alzheimer’s disease: A systematic review and meta-analysis. J. Nutr. Health Aging 2024, 28, 100306. [Google Scholar] [CrossRef]
- Morrison, S.A.; Fazeli, P.L.; Gower, B.; Younger, J.; Willig, A.; Sneed, N.M.; Vance, D.E. The ketogenic diet as a non-pharmacological treatment for HIV-associated neurocognitive disorder: A descriptive analysis. J. Psychiatry Behav. Sci. 2018, 3, 1014. [Google Scholar] [CrossRef]
- Fazeli, P.L.; Woods, S.P.; Heaton, R.K.; Umlauf, A.; Gouaux, B.; Rosario, D.; Moore, R.C.; Grant, I.; Moore, D.J.; HNRP Group. An active lifestyle is associated with better neurocognitive functioning in adults living with HIV infection. J. Neurovirology 2014, 20, 233–242. [Google Scholar] [CrossRef]
- Rubin, L.H.; Gustafson, D.R.; Warrior, L.; Sheira, L.; Fitzgerald, K.C.; Dastgheyb, R.; Weber, K.M.; Tien, P.C.; French, A.; Spence, A.B.; et al. Dietary intake is associated with neuropsychological impairment in women with HIV. Am. J. Clin. Nutr. 2021, 114, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Royer, M.F.; Guerithault, N.; Braden, B.B.; Laska, M.N.; Bruening, M. Food Insecurity Is Associated with Cognitive Function: A Systematic Review of Findings across the Life Course. Int. J. Transl. 2021, 1, 205–222. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, J.; Yin, D. Impact of food insecurity on cognitive health in older adults: Insights from the NHANES 2011-2014 data. Front. Nutr. 2024, 11, 1421970. [Google Scholar] [CrossRef]
- Tan, J.Y.; Sheira, A.; Frongillo, E.A.; Adimora, A.A.; Tien, P.C.; Konkle-Parker, D.; Golub, E.T.; Merenstein, D.; Levin, S.; Cohen, M.; et al. Food insecurity and neurocognitive function among women living with or at risk for HIV in the United States. Am. J. Clin. Nutr. 2020, 112, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, J.A.; Meade, C.S.; Campa, A.; Martinez, S.S.; Li, T.; Sherman, K.E.; Baum, M.K. Food Insecurity and Cognitive Impairment in the Miami Adult Studies on HIV (MASH) Cohort. J. Nutr. 2021, 151, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Weiser, S.D.; Young, S.L.; Cohen, C.R.; Kushel, M.B.; Tsai, A.C.; Tien, P.C.; Hatcher, A.M.; Frongillo, E.A.; Bangsberg, D.R. Conceptual framework for understanding the bidirectional links between food insecurity and HIV/AIDS. Am. J. Clin. Nutr. 2011, 94, 1729S–1739S. [Google Scholar] [CrossRef] [PubMed]
- Hobkirk, A.L.; Towe, S.L.; Patel, P.; Meade, C.S. Food Insecurity Is Associated with Cognitive Deficits Among HIV-Positive, But Not HIV-Negative, Individuals in a United States Sample. AIDS Behav. 2017, 21, 783–791. [Google Scholar] [CrossRef]
- Henry, B.L.; Quintana, E.; Moore, D.J.; Garcia, J.; Montoya, J.L. Focus groups inform a mobile health intervention to promote adherence to a Mediterranean diet and engagement in physical activity among people living with HIV. BMC Public Health 2019, 19, 101. [Google Scholar] [CrossRef]
- Rezazadeh, L.; Ostadrahimi, A.; Tutunchi, H.; Naemi Kermanshahi, M.; Pourmoradian, S. Nutrition interventions to address nutritional problems in HIV-positive patients: Translating knowledge into practice. J. Health Popul. Nutr. 2023, 42, 94. [Google Scholar] [CrossRef]
- van den Brink, A.C.; Brouwer-Brolsma, E.M.; Berendsen, A.A.M.; van de Rest, O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diets Are Associated with Less Cognitive Decline and a Lower Risk of Alzheimer’s Disease-A Review. Adv. Nutr. 2019, 10, 1040–1065. [Google Scholar] [CrossRef]
- Nguyen, N.; Holodniy, M. HIV infection in the elderly. Clin. Interv. Aging 2008, 3, 453–472. [Google Scholar]
- Barroso, S.M.; Sousa, K.C.R. Neurocognitive Disorder and Emotional Symptoms in HIV+ Brazilian Elderly: Influence of Gender, Income, Diet, and Sleep. Front. Hum. Neurosci. 2021, 15, 721029. [Google Scholar] [CrossRef]
- Morrison, S.A.; Fazeli, P.L.; Gower, B.; Willig, A.L.; Younger, J.; Sneed, N.M.; Vance, D.E. Cognitive Effects of a Ketogenic Diet on Neurocognitive Impairment in Adults Aging With HIV: A Pilot Study. J. Assoc. Nurses AIDS Care 2020, 31, 312–324. [Google Scholar] [CrossRef]
- McHenry, M.S.; McAteer, C.I.; Oyungu, E.; McDonald, B.C.; Bosma, C.B.; Mpofu, P.B.; Deathe, A.R.; Vreeman, R.C. Neurodevelopment in Young Children Born to HIV-Infected Mothers: A Meta-analysis. Pediatrics 2018, 141, e20172888. [Google Scholar] [CrossRef] [PubMed]
- McHenry, M.S.; Oyungu, E.; Yang, Z.; Ombitsa, A.R.; Cherop, C.; Vreeman, R.C. Neurodevelopmental Outcomes of Young Children Born to HIV-Infected Mothers: A Pilot Study. Front. Pediatr. 2021, 9, 697091. [Google Scholar] [CrossRef] [PubMed]
- Ruiseñor-Escudero, H.; Familiar-Lopez, I.; Sikorskii, A.; Jambulingam, N.; Nakasujja, N.; Opoka, R.; Bass, J.; Boivin, M. Nutritional and Immunological Correlates of Memory and Neurocognitive Development Among HIV-Infected Children Living in Kayunga, Uganda. J. Acquir. Immune Defic. Syndr. (1999) 2016, 71, 522–529. [Google Scholar] [CrossRef] [PubMed]
- White, M.; Connor, K.L. In Utero HIV Exposure and the Early Nutritional Environment Influence Infant Neurodevelopment: Findings from an Evidenced Review and Meta-Analysis. Nutrients 2020, 12, 3375. [Google Scholar] [CrossRef] [PubMed]
- Brown Belfort, M. The Science of Breastfeeding and Brain Development. Breastfeed. Med. 2017, 12, 459–461. [Google Scholar] [CrossRef]
- Wedderburn, C.J.; Yeung, S.; Rehman, A.M.; Stadler, J.A.M.; Nhapi, R.T.; Barnett, W.; Myer, L.; Gibb, D.M.; Zar, H.J.; Stein, D.J.; et al. Neurodevelopment of HIV-exposed uninfected children in South Africa: Outcomes from an observational birth cohort study. Lancet Child. Adolesc. Health 2019, 3, 803–813. [Google Scholar] [CrossRef]
- Strehlau, R.; van Aswegen, T.; Burke, M.; Kuhn, L.; Potterton, J. A description of early neurodevelopment in a cohort of HIV-exposed uninfected children. Aids Care 2020, 32, 1421–1428. [Google Scholar] [CrossRef]
- Chaudhury, S.; Williams, P.L.; Mayondi, G.K.; Leidner, J.; Holding, P.; Tepper, V.; Nichols, S.; Magetse, J.; Sakoi, M.; Moabi, K.; et al. Neurodevelopment of HIV-Exposed and HIV-Unexposed Uninfected Children at 24 Months. Pediatrics 2017, 140, e20170988. [Google Scholar] [CrossRef]
- McHenry, M.S.; Dixit, A.; Vreeman, R.C. A Systematic Review of Nutritional Supplementation in HIV-Infected Children in Resource-Limited Settings. J. Int. Assoc. Provid. AIDS Care 2015, 14, 313–323. [Google Scholar] [CrossRef]
- McGrath, N.; Bellinger, D.; Robins, J.; Msamanga, G.I.; Tronick, E.; Fawzi, W.W. Effect of maternal multivitamin supplementation on the mental and psychomotor development of children who are born to HIV-1-infected mothers in Tanzania. Pediatrics 2006, 117, e216–e225. [Google Scholar] [CrossRef]
- Manji, K.P.; McDonald, C.M.; Kupka, R.; Bosch, R.J.; Kisenge, R.; Aboud, S.; Bellinger, D.C.; Fawzi, W.W.; Duggan, C.P. Effect of multivitamin supplementation on the neurodevelopment of HIV-exposed Tanzanian infants: A randomized, double-blind, placebo-controlled clinical trial. J. Trop. Pediatr. 2014, 60, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Carrieri, M.P.; Protopopescu, C.; Marcellin, F.; Rosellini, S.; Wittkop, L.; Esterle, L.; Zucman, D.; Raffi, F.; Rosenthal, E.; Poizot-Martin, I.; et al. ANRS CO13 HEPAVIH Study Group. Protective effect of coffee consumption on all-cause mortality of French HIV-HCV co-infected patients. J. Hepatol. 2017, 67, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Zarębska-Michaluk, D.; Rzymski, P.; Kanecki, K.; Tyszko, P.; Lewtak, K.; Goryński, P.; Genowska, A.; Parczewski, M.; Flisiak, R. Hospitalizations and deaths among people coinfected with HIV and HCV. Sci. Rep. 2024, 14, 28586. [Google Scholar] [CrossRef] [PubMed]
- Fialho, R.; Pereira, M.; Bucur, M.; Fisher, M.; Whale, R.; Rusted, J. Cognitive impairment in HIV and HCV co-infected patients: A systematic review and meta-analysis. AIDS Care 2016, 28, 1481–1494. [Google Scholar] [CrossRef]
- Sheppard, D.P.; Iudicello, J.E.; Morgan, E.E.; Kamat, R.; Clark, L.R.; Avci, G.; Bondi, M.W.; Woods, S.P. Accelerated and Accentuated Neurocognitive Aging in HIV Infection. J. Neurovirol. 2017, 23, 492–500. [Google Scholar] [CrossRef]
- Antwerpes, S.; Protopopescu, C.; Morlat, P.; Marcellin, F.; Wittkop, L.; Di Beo, V.; Salmon-Céron, D.; Sogni, P.; Michel, L.; Carrieri, M.P.; et al. Coffee Intake and Neurocognitive Performance in HIV/HCV Coinfected Patients (ANRS CO13 HEPAVIH). Nutrients 2020, 12, 2532. [Google Scholar] [CrossRef]
| Cohort of HIV Patients | Pharmacological Regimen | Outcome on the Gut Microbiome Composition | Reference |
|---|---|---|---|
| 16 viremic patients before and after one year of ART treatment | NNRTI (Zidovudine and Efavirenz) | Reduction in α-diversity | [46] |
| 11 HIV-MetS patients 40 HIV non MetS patients | NRTIs + PIs, 4/11 NRTIs + NNRTIs, 4/11 NRTIs + INSTIs, 2/11 NRTIs + NNRTIs, 18/40 NRTIs + INSTIs, 6/40 NRTIs + PIs, 11/40 | Decrease in the abundance of seven genera and seven bacterial species, including some anti-inflammatory bacteria, was observed in the HIV-MetS group | [47] |
| HIV-MetS patients | INSTI + MetS and PI + MetS | Higher relative abundances of Bacteroidetes and Proteobacteria in the INSTI + MetS group compared to the PI + MetS; A more pronounced dysbiosis in the INSTI + MetS group characterized by a reduction in α-diversity and an increase in several bacterial genera | [48] |
| Dietary Intervention | Key Features | Impact on Cognition | Proposed Mechanism | Reference | Study Population |
|---|---|---|---|---|---|
| Ketogenic Diet (KD) | Emphasizes high fat, low carbohydrate, and medium protein intake | Improves psychomotor speed, executive function and inhibition, processing speed, and verbal memory | Ketone bodies provide an alternative energy source, reduce neuroinflammation, and enhance mitochondrial function | [114] | People with HAND |
| Improved Diet Quality and Higher Caloric Intake | -- | Associated with improved cognition | Supports overall brain function by optimizing nutrient intake and metabolic health | [115] | People with HIV (mean age 56) |
| Frequent Processed Meat and Sweet Beverage Intake | -- | Associated with lower neurocognitive performance | May contribute to metabolic dysfunction, neuroinflammation, and oxidative stress | [116] | Women with HIV |
| Vegetable-Rich Diet | -- | Linked to reduced likelihood of neuropsychological impairment | Provides antioxidants, reduces inflammation, and supports gut microbiome | [116] | Women with HIV |
| Whole Milk Consumption | -- | Linked to poorer performance in attention, working memory, motor function, and executive function | Potential impact on lipid metabolism and insulin resistance affecting brain function | [116] | Women with HIV |
| Mediterranean Diet (via iSTEP Intervention) | Emphasizes physical activity along with a diet rich in fruits, vegetables, whole grains, nuts, and olive oil | Improved neurocognitive function | Reduces inflammation and supports cardiovascular and metabolic health | [123] | People with HIV |
| Other Nutritional Interventions | Education, Counseling, Micronutrient Supplementation, Food Assistance | Improved quality of life and nutritional symptoms | Enhances overall nutrition, mitigates malnutrition-related cognitive decline | [124] | Adults with HIV |
| DASH, Mediterranean, and MIND Diets | Emphasizes a diet rich in fruits, vegetables, whole grains, nuts, and olive oil, as well as a reduced sodium intake | Effective in reducing neurocognitive impairments and dementia risk | Lower inflammation, enhance vascular health, optimize brain metabolism | [125] | People with or without dementia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddi, C.; Balla, J.; Agbey, C.; Fadda, P.; Dedoni, S. The Role of Nutrition in HIV-Associated Neurocognitive Disorders: Mechanisms, Risks, and Interventions. Life 2025, 15, 982. https://doi.org/10.3390/life15060982
Siddi C, Balla J, Agbey C, Fadda P, Dedoni S. The Role of Nutrition in HIV-Associated Neurocognitive Disorders: Mechanisms, Risks, and Interventions. Life. 2025; 15(6):982. https://doi.org/10.3390/life15060982
Chicago/Turabian StyleSiddi, Carlotta, Jihane Balla, Christy Agbey, Paola Fadda, and Simona Dedoni. 2025. "The Role of Nutrition in HIV-Associated Neurocognitive Disorders: Mechanisms, Risks, and Interventions" Life 15, no. 6: 982. https://doi.org/10.3390/life15060982
APA StyleSiddi, C., Balla, J., Agbey, C., Fadda, P., & Dedoni, S. (2025). The Role of Nutrition in HIV-Associated Neurocognitive Disorders: Mechanisms, Risks, and Interventions. Life, 15(6), 982. https://doi.org/10.3390/life15060982






