Clinical Outcomes and Optical Bench Analysis of a Novel Enhanced Monofocal Intraocular Lens
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Patient Evaluation
2.3. Intraocular Lenses
2.4. Surgery
2.5. Statistical Analysis
2.6. Optical Bench Analysis
3. Results
3.1. Refractive and Visual Outcomes
3.2. ELP
3.3. Aberrometric Parameters and Optical Quality
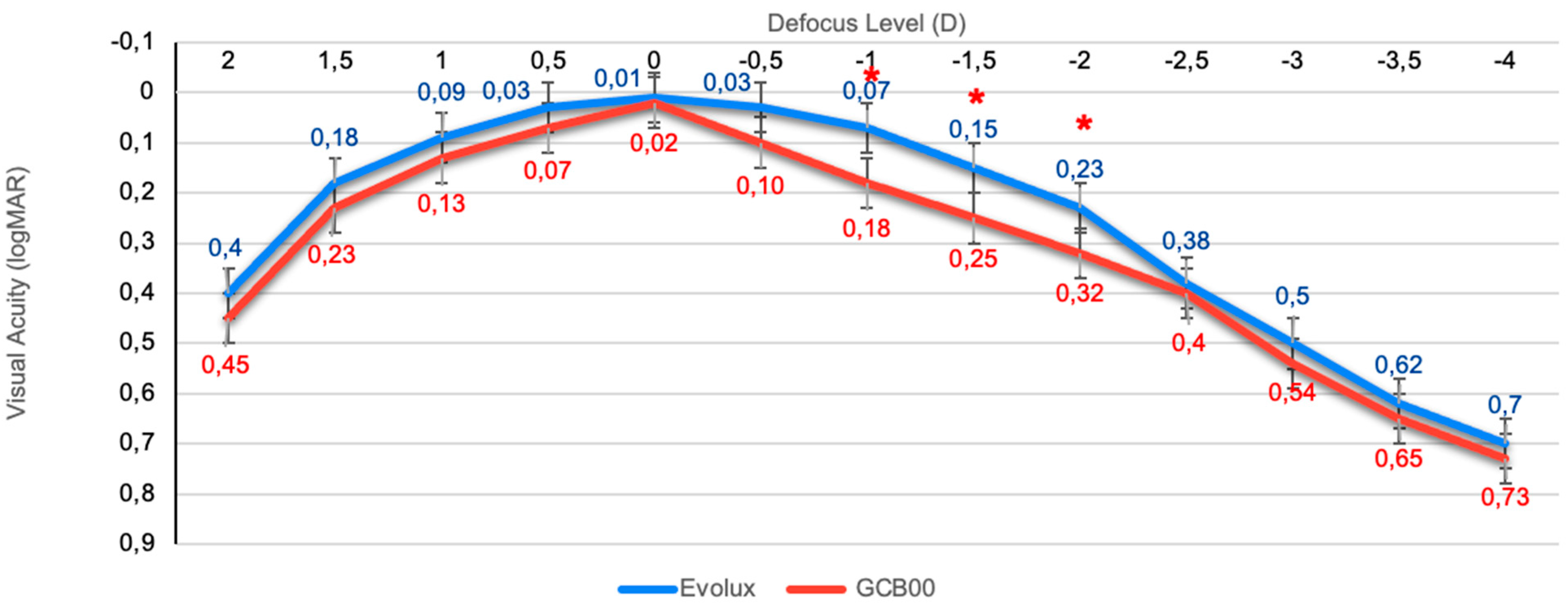
3.4. Binocular Defocus Curve
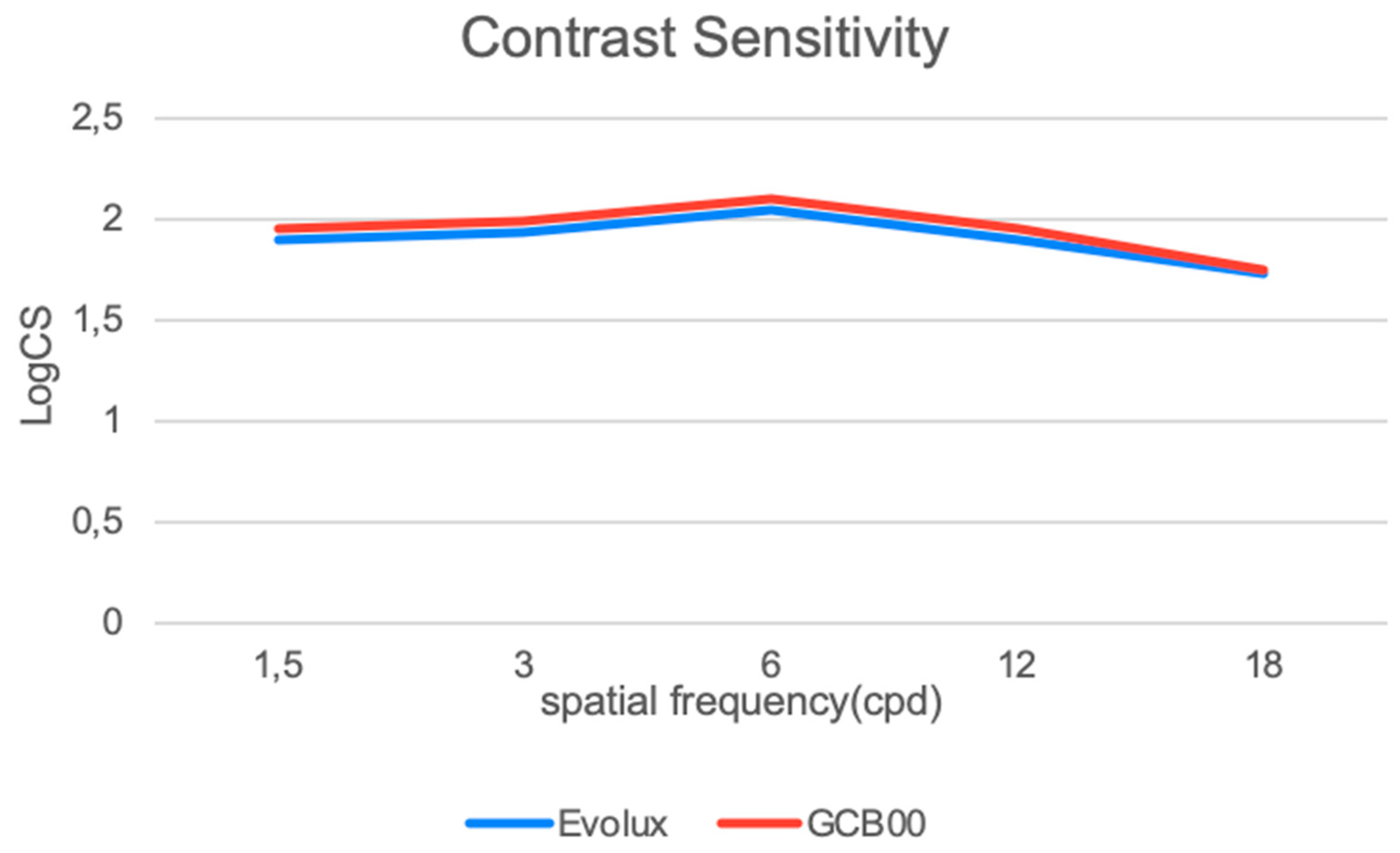
3.5. Binocular Contrast Sensitivity
3.6. Halometry
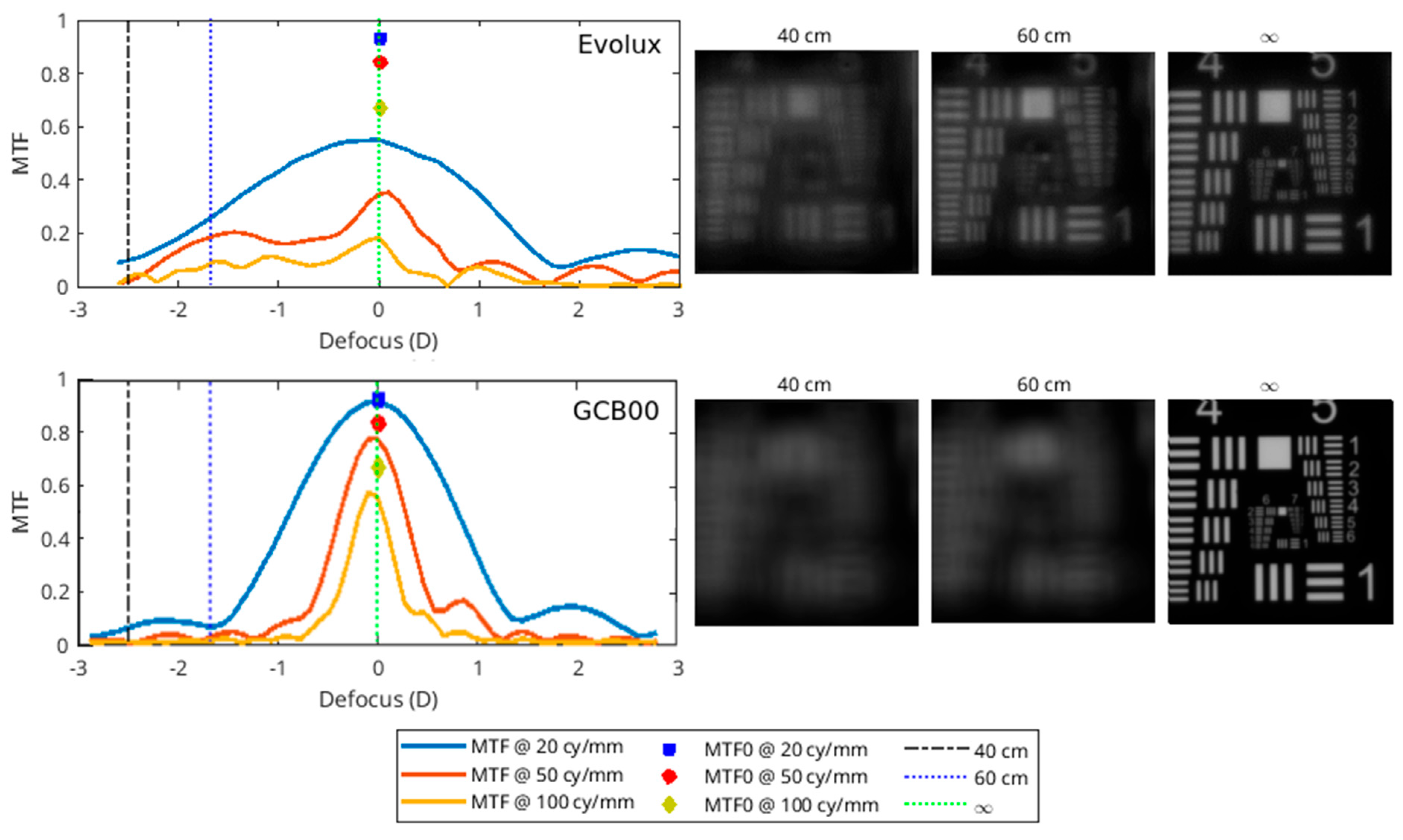
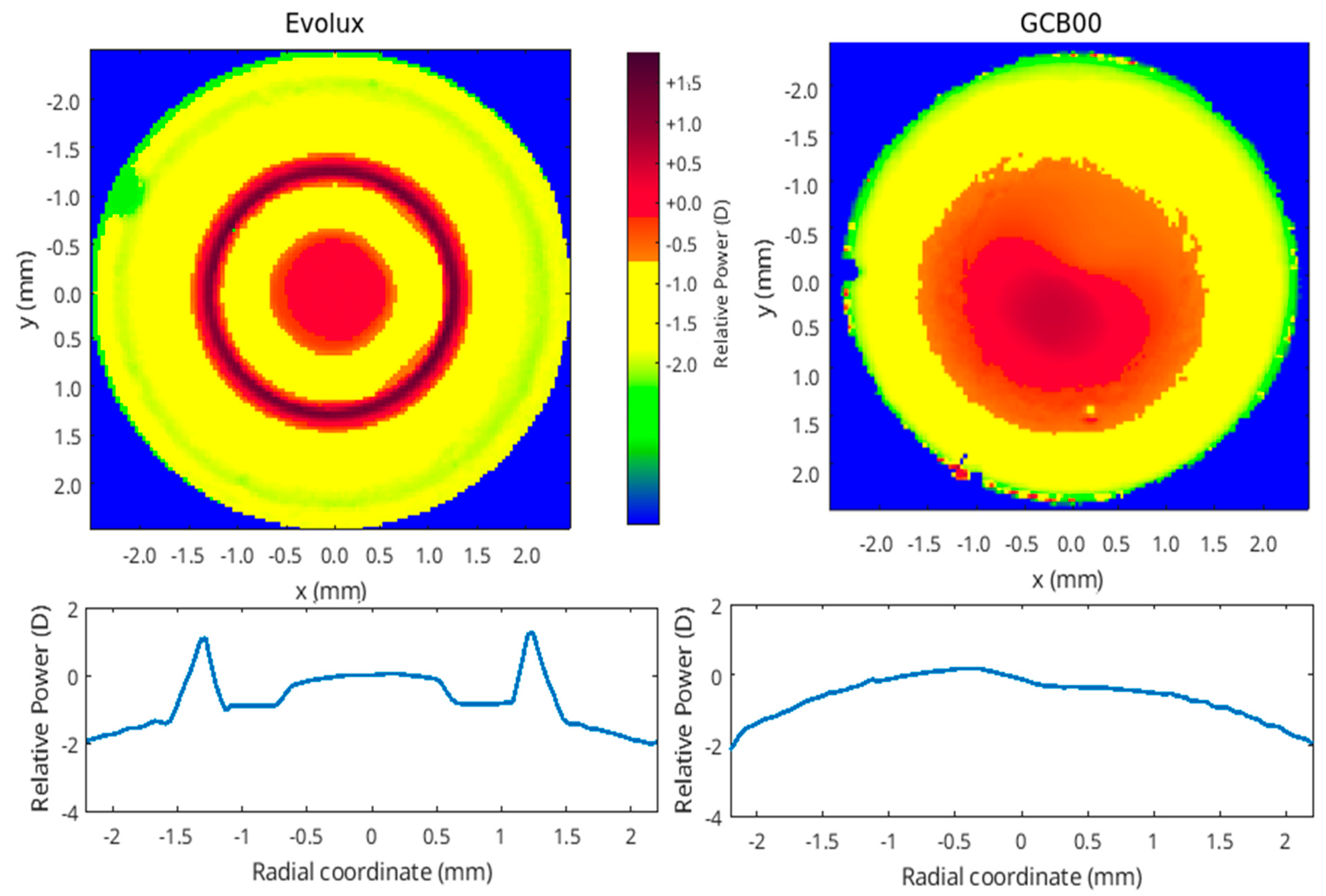
3.7. Optical Bench Analysis Results
3.8. Questionnaire
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosen, E.; Alió, J.L.; Dick, H.B.; Dell, S.; Slade, S. Efficacy and safety of multifocal intraocular lenses following cataract and refractive lens exchange: Metaanalysis of peer-reviewed publications. J. Cataract Refract. Surg. 2016, 42, 310–328. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, B.; Zhang, Y.; Hao, Y.; Wang, Z.; Liu, C.; Jiang, S. Comparative efficacy and safety of all kinds of intraocular lenses in presbyopia-correcting cataract surgery: A systematic review and meta-analysis. BMC Ophthalmol. 2024, 24, 172. [Google Scholar] [CrossRef] [PubMed]
- Dell, S.J.; Hannan, S.J.; Venter, J.A.; Teenan, D.; Hannan, N.C.; Raju, D.; Berry, C.W.; Kiss, H.J.; Schallhorn, J.M. Comparative analysis of clinical and patient-reported outcomes of a new enhanced monofocal IOL and a conventional monofocal IOL. Clin. Ophthalmol. 2024, 18, 1157–1169. [Google Scholar] [CrossRef]
- Breyer, D.R.H.; Kaymak, H.; Ax, T.; Kretz, F.T.A.; Auffarth, G.U.; Hagen, P.R. Multifocal intraocular lenses and extended depth of focus intraocular lenses. Asia-Pac. J. Ophthalmol. 2017, 6, 339–349. [Google Scholar]
- Alio, J.L.; Plaza-Puche, A.B.; Piero, D.P.; Amparo, F.; Rodríguez-Prats, J.L.; Ayala, M.J. Quality of life evaluation after implantation of 2 multifocal intraocular lens models and a mon-ofocal model. J. Cataract Refract. Surg. 2011, 37, 638–648. [Google Scholar] [CrossRef]
- Wan, K.H.; Au, A.C.K.; Kua, W.N.; Ng, A.L.K.; Cheng, G.P.M.; Lam, N.M.; Chow, V.W.S. Enhanced Monofocal Versus Con-ventional Monofocal Intraocular Lens in Cataract Surgery: A Meta-analysis. J. Refract. Surg. 2022, 38, 538–546. [Google Scholar] [CrossRef]
- Sachdev, G.S.; Sachdev, M. Optimizing outcomes with multifocal intraocular lenses. Indian J. Ophthalmol. 2017, 65, 1294–1300. [Google Scholar] [CrossRef]
- De Silva, S.R.; Evans, J.R.; Kirthi, V.; Ziaei, M.; Leyland, M. Multifocal versus monofocal intraocular lenses after cataract extraction. Cochrane Database Syst. Rev. 2016, 12, CD003169. [Google Scholar] [CrossRef]
- Coassin, M.; Di Zazzo, A.; Antonini, M.; Gaudenzi, D.; Gallo Afflitto, G.; Kohnen, T. Extended depth-of-focus intraocular lenses: Power calculation and outcomes. J. Cataract Refract. Surg. 2020, 46, 1554–1560. [Google Scholar] [CrossRef]
- Mencucci, R.; Cennamo, M.; Venturi, D.; Vignapiano, R.; Favuzza, E. Visual outcome, optical quality, and patient satisfaction with a new monofocal IOL, enhanced for intermediate vision: Preliminary results. J. Cataract Refract. Surg. 2020, 46, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Mencucci, R.; Morelli, A.; Cennamo, M.; Roszkowska, A.M.; Favuzza, E. Enhanced monofocal intraocular lenses: A retrospective, comparative study between three different models. J. Clin. Med. 2023, 12, 3588. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Rocha-de-Lossada, C.; Zamorano-Martín, F.; Rodríguez-Calvo-de-Mora, M.; Rodríguez-Vallejo, M. Positioning of enhanced monofocal intraocular lenses between conventional monofocal and extended depth of focus lenses: A scoping review. BMC Ophthalmol. 2023, 23, 101. [Google Scholar] [CrossRef] [PubMed]
- Beltraminelli, T.; Rizzato, A.; Toniolo, K.; Galli, A.; Menghini, M. Comparison of visual performances of enhanced mon-ofocal versus standard monofocal IOLs in a mini-monovision approach. BMC Ophthalmol. 2023, 23, 170. [Google Scholar] [CrossRef] [PubMed]
- Mencucci, R.; Romualdi, G.; De Vitto, C.; Cennamo, M. Enhanced Monofocal Intraocular Lenses in Fuchs’ Endothelial Dystrophy Patients: Results from Triple Descemet Mem-brane Endothelial Keratoplasty Procedure. Life 2024, 14, 243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ribeiro, F.; Dick, H.B.; Kohnen, T.; Findl, O.; Nuijts, R.; Cochener, B.; Fernández, J. Evidence-based functional classification of simultaneous vision intraocular lenses: Seeking a global consensus by the ESCRS Functional Vision Working Group. J. Cataract Refract. Surg. 2024, 50, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Ribeiro, F.; Rocha-de-Lossada, C.; Rodríguez-Vallejo, M. Functional Classification of Intraocular Lenses Based on Defocus Curves: A Scoping Review and Cluster Analysis. J. Refract. Surg. 2024, 40, e108–e116. [Google Scholar] [CrossRef] [PubMed]
- Güell, J.L.; Pujol, J.; Arjona, M.; Diaz-Douton, F.; Artal, P. Optical Quality Analysis System; Instrument for objective clinical evaluation of ocular optical quality. J. Cataract Refract. Surg. 2004, 30, 1598–1599. [Google Scholar] [CrossRef]
- Castro, J.J.; Jiménez, J.R.; Ortiz, C.; Alarcón, A.; Anera, R.G. New testing software for quantifying discrimination capacity in subjects with ocular pathologies. J. Biomed. Opt. 2011, 16, 015001. [Google Scholar] [CrossRef]
- Buckhurst, P.J.; Naroo, S.A.; Davies, L.N.; Shah, S.; Buckhurst, H.; Kingsnorth, A.; Drew, T.; Wolffsohn, J.S. Tablet App halometer for the assessment of dysphotopsia. J. Cataract Refract. Surg. 2015, 41, 2424–2429. [Google Scholar] [CrossRef] [PubMed]
- Eom, Y.; Kang, S.Y.; Song, J.S.; Kim, H.M. Comparison of the actual amount of axial movement of 3 aspheric intraocular lenses using anterior segment optical coherence tomography. J. Cataract Refract. Surg. 2013, 39, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- Morlock, R.; Wirth, R.J.; Tally, S.R.; Garufis, C.; Heichel, C.W.D. Patient-Reported Spectacle Independence Questionnaire (PRSIQ): Development and Validation. Am. J. Ophthalmol. 2017, 178, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Mojzis, P.; Majerova, K.; Hrckova, L.; Piñero, D.P. Implantation of a diffractive trifocal intraocular lens: One-year follow-up. J. Cataract Refract. Surg. 2015, 41, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.A.; Varón, C.; Cardona, G.; Vega, F.; Buil, J.A. Comparison of far and near contrast sensitivity in patients symmetrically implanted with multifocal and monofocal IOLs. Eur. J. Ophthalmol. 2014, 24, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Stem, M.S.; Oren, G.; Shtein, R.; Lichter, P.R. Patient-Centered and visual quality outcomes of premium cataract surgery: A Systematic Review. Eur. J. Ophthalmol. 2017, 27, 387–401. [Google Scholar] [CrossRef]
- Mencucci, R.; Favuzza, E.; Caporossi, O.; Savastano, A.; Rizzo, S. Comparative analysis of visual outcomes, reading skills, contrast sensitivity, and patient satisfaction with two models of trifocal diffractive intraocular lenses and an extended range of vision intraocular lens. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 1913–1922. [Google Scholar] [CrossRef]
- Cano-Ortiz, A.; Sánchez-Ventosa, Á.; González-Cruces, T.; Villalba González, M.; López Pérez, M.D.; Díaz-Ramos, J.C.; Prados Carmona, J.J.; Tejerina Fernández, V.; Elies Amat, D.; Villarrubia, A. Evaluation of Evolux™ Intraocular Lenses in Cataract Surgery: Clinical Outcomes and Patient Satisfaction. J. Clin. Med. 2024, 13, 7404. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spagnuolo, V.; Vicini, G.; Marincolo, G.; Franchini, A.; Giansanti, F.; Mazzini, C. Comparison of 3-month clinical outcomes between two enhanced monofocal intraocular lenses: A single-center prospective study. Eur. J. Ophthalmol. 2024, 15, 11206721241298031. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Ribeiro, F.; Burguera, N.; Rodríguez-Calvo-de-Mora, M.; Rodríguez-Vallejo, M. Visual and patient-reported outcomes of an enhanced versus monofocal intraocular lenses in cataract surgery: A systematic review and meta-analysis. Eye 2025, 39, 883–898. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Characteristics | Evolux IOL | GCB00 IOL | p-Value * |
|---|---|---|---|
| Patients (n) | 25 | 25 | |
| Eyes (n) | 50 | 50 | |
| Age (years) | 66.3 ± 2.5 | 65.6 ± 2.1 | 0.145 |
| AL (mm) | 23.32 ± 0.56 | 24.17 ± 0.63 | 0.089 |
| CYL (D) | 0.48 ± 0.21 | 0.40 ± 0.33 | 0.064 |
| SE (D) | 1.54 ± 0.73 | 1.34 ± 0.68 | 0.076 |
| UDVA (logMAR) | 0.48 ± 0.15 | 0.45 ± 0.09 | 0.110 |
| BCDVA (logMAR) | 0.41 ± 0.12 | 0.42 ± 0.10 | 0.097 |
| Anterior chamber depth (mm) | 3.02 ± 0.25 | 3.11 ± 0.18 | 0.104 |
| IOL power (D) | 22.2 ± 2.0 | 22.0 ± 1.8 | 0.143 |
| Outcome | Evolux IOL | GCB00 IOL | p-Value * | CI |
|---|---|---|---|---|
| SE (D) | −0.14 ± 0.31 | −0.22 ± 0.20 | 0.098 | [0.01; −0.17] |
| UDVA (LogMAR) | 0.03 ± 0.03 | 0.03 ± 0.02 | 0.953 | [−0.01; 0.01] |
| BCDVA (LogMAR) | 0.01 ± 0.02 | 0.01 ± 0.02 | 0.811 | [−0.01; 0.01] |
| UIVA (LogMAR) | 0.10 ± 0.06 | 0.26 ± 0.09 | <0.001 * | [−0.21; −0.11] |
| CIVA (LogMAR) | 0.02 ± 0.02 | 0.03 ± 0.03 | 0.106 | [−0.03; 0.01] |
| DCIVA (LogMAR) | 0.14 ± 0.06 | 0.20 ± 0.06 | 0.004 * | [−0.09; −0.02] |
| UNVA (LogMAR) | 0.36 ± 0.12 | 0.44 ± 0.17 | 0.071 | [−0.16; 0.01] |
| CNVA (LogMAR) | 0.02 ± 0.03 | 0.05 ± 0.06 | 0.067 | [−0.05; 0.01] |
| ADD NEAR (D) | 1.35 ± 0.43 | 2.57 ± 0.23 | 0.143 | [0.41; 2.85] |
| Postoperative Time | Evolux ELP (mm) | GCB00 ELP (mm) | p-Value * |
|---|---|---|---|
| 1 month | 4.10 ± 0.23 | 4.03 ± 0.18 | 0.298 |
| 3 months | 4.12 ± 0.24 | 4.08 ± 0.17 | 0.123 |
| 6 months | 4.15 ± 0.21 | 4.10 ± 0.22 | 0.145 |
| Characteristics | Evolux IOL | GCB00 IOL | p-Value * |
|---|---|---|---|
| Internal HOA (RMS) | 0.16 ± 0.03 | 0.15 ± 0.02 | 0.098 |
| Internal SA (RMS) | 0.08 ± 0.03 | 0.05 ± 0.04 | 0.124 |
| OSI | 1.39 ± 0.63 | 1.41 ± 0.51 | 0.179 |
| MTF cutoff (c/deg) | 30.4 ± 7.5 | 31.0 ± 4.1 | 0.129 |
| Strehl ratio | 0.16 ± 0.03 | 0.16 ± 0.05 | 0.096 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romualdi, G.; Buzzi, M.; Ruggeri, P.G.; Tommasi, F.; Giorgetti, A.; Cavalieri, S.; Mencucci, R. Clinical Outcomes and Optical Bench Analysis of a Novel Enhanced Monofocal Intraocular Lens. Life 2025, 15, 984. https://doi.org/10.3390/life15060984
Romualdi G, Buzzi M, Ruggeri PG, Tommasi F, Giorgetti A, Cavalieri S, Mencucci R. Clinical Outcomes and Optical Bench Analysis of a Novel Enhanced Monofocal Intraocular Lens. Life. 2025; 15(6):984. https://doi.org/10.3390/life15060984
Chicago/Turabian StyleRomualdi, Giovanni, Matilde Buzzi, Pier Giuseppe Ruggeri, Federico Tommasi, Alessio Giorgetti, Stefano Cavalieri, and Rita Mencucci. 2025. "Clinical Outcomes and Optical Bench Analysis of a Novel Enhanced Monofocal Intraocular Lens" Life 15, no. 6: 984. https://doi.org/10.3390/life15060984
APA StyleRomualdi, G., Buzzi, M., Ruggeri, P. G., Tommasi, F., Giorgetti, A., Cavalieri, S., & Mencucci, R. (2025). Clinical Outcomes and Optical Bench Analysis of a Novel Enhanced Monofocal Intraocular Lens. Life, 15(6), 984. https://doi.org/10.3390/life15060984