Protective Impacts of Chlorella vulgaris on Cisplatin-Induced Toxicity in Liver, Kidney, and Spleen of Rats: Role of Oxidative Stress, Inflammation, and Nrf2 Modulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Chemicals
2.2.1. Cisplatin
2.2.2. Chlorella
2.3. Experimental Groups and Design of Work
2.3.1. Hematological Sampling
2.3.2. Body Weight and Absolute Organ Weight
2.3.3. Clinical Biochemical Studies
Liver Function Tests
Kidney Function Tests
Lipid Profile Tests
Ions of Blood
Inflammatory Cytokine, Oxidative Biomarker and Total Antioxidant Parameters
2.3.4. Histological and Immunohistochemical Preparation
Histological Preparation
Immunohistochemical Preparation
Electron Microscopy Analysis
2.4. Statistical Analysis of Data
3. Results
3.1. Total Phenolic and Flavonoid Compounds in C. vulgaris Supplement
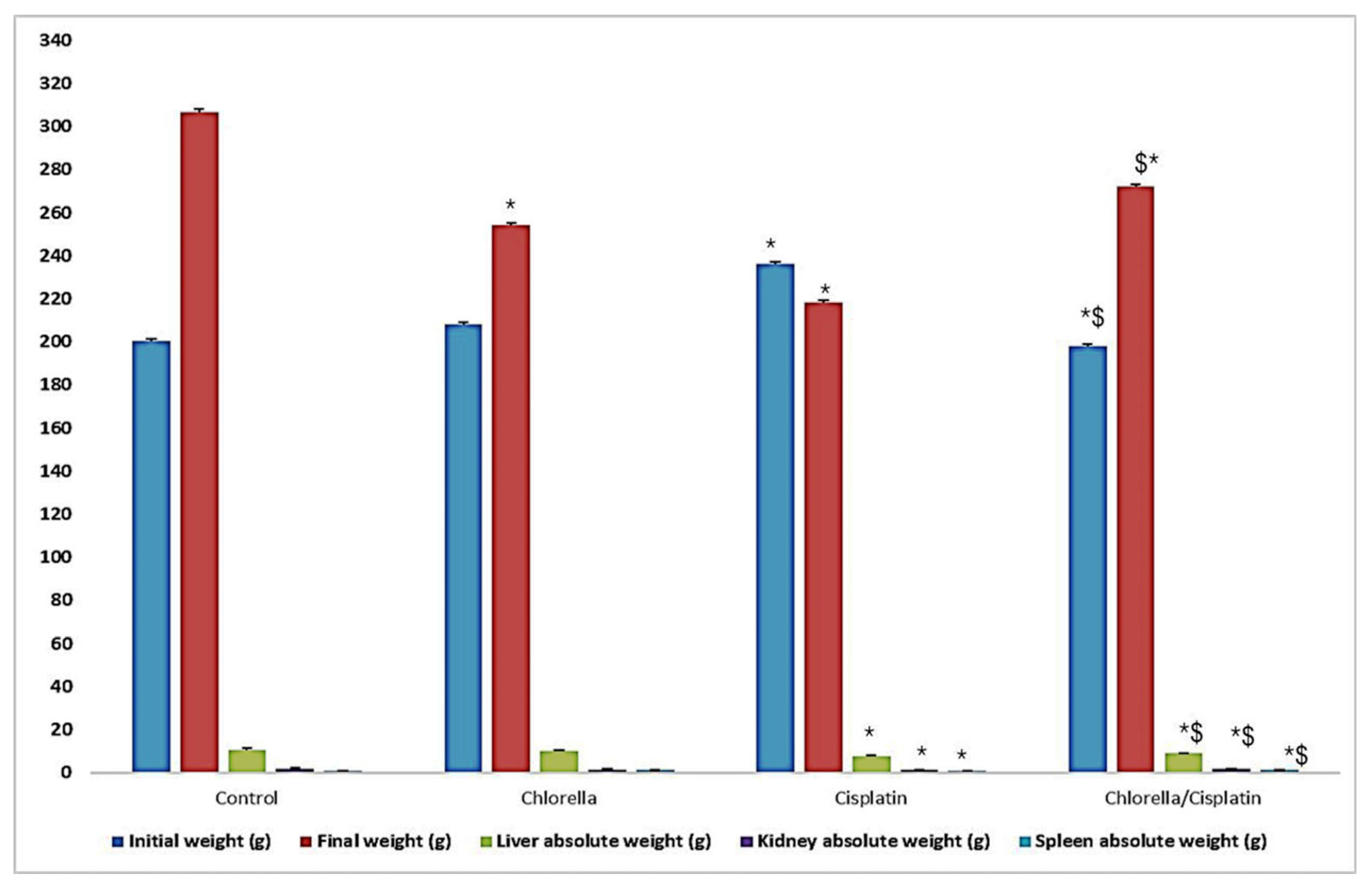
3.2. Effect of Cisplatin and Chlorella Supplement on Clinical Observations, Body Weight, and Organ Weights (Liver, Kidney, and Spleen)
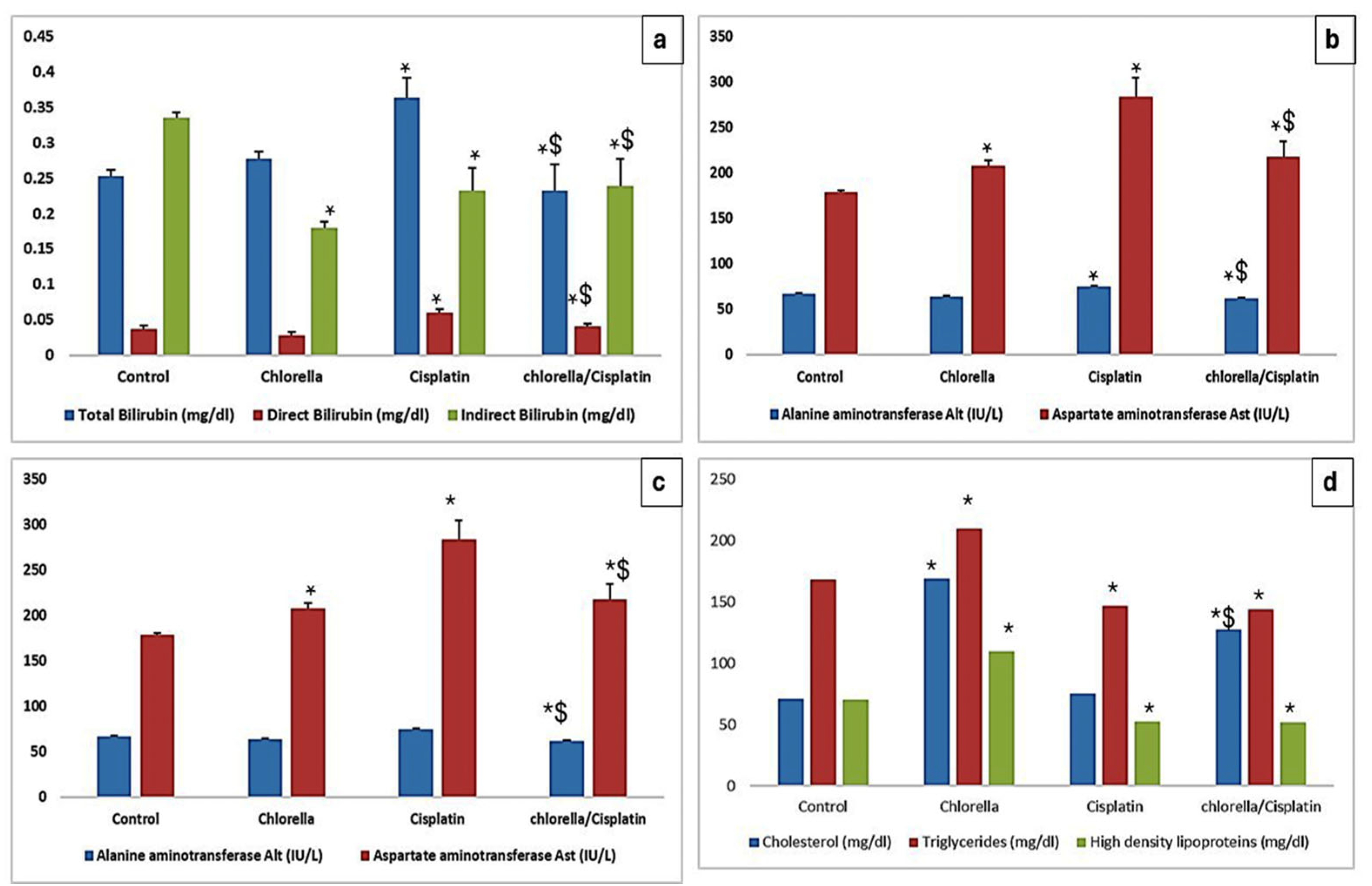
3.3. Effect of Cisplatin and Chlorella Supplement on Biochemical Parameters
3.3.1. Hepatocellular Injury Markers
3.3.2. Lipid Profile
3.3.3. Kidney Function
3.3.4. Blood Ions
3.4. Effect of Cisplatin and Chlorella Supplement on an Oxidative Biomarker, Total Antioxidant Parameters, and Inflammatory Cytokine in the Serum of Rats
3.5. Histological Examination
3.5.1. Histological Examination of Liver
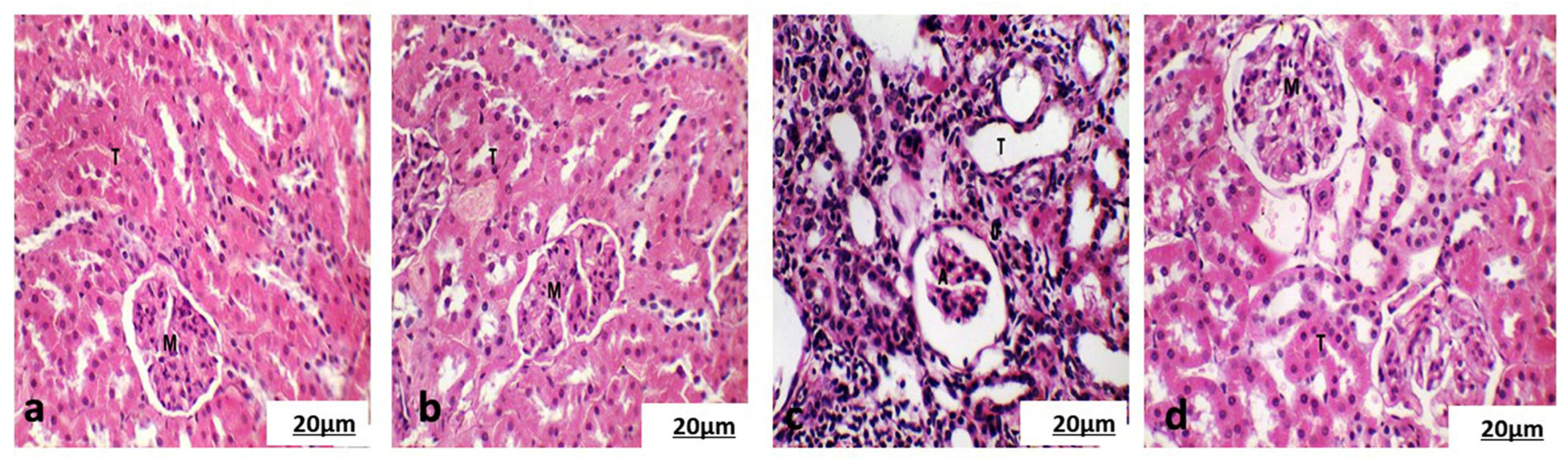
3.5.2. Histological Examination of the Cortical Region of Kidney
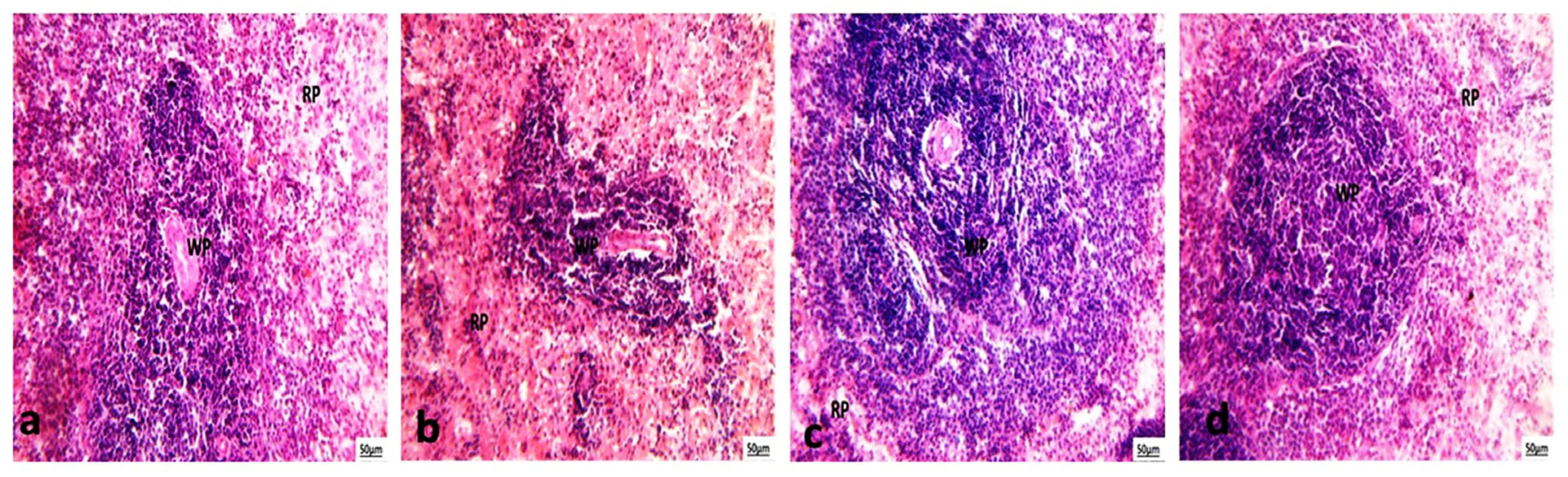
3.5.3. Histological Examination of Spleen
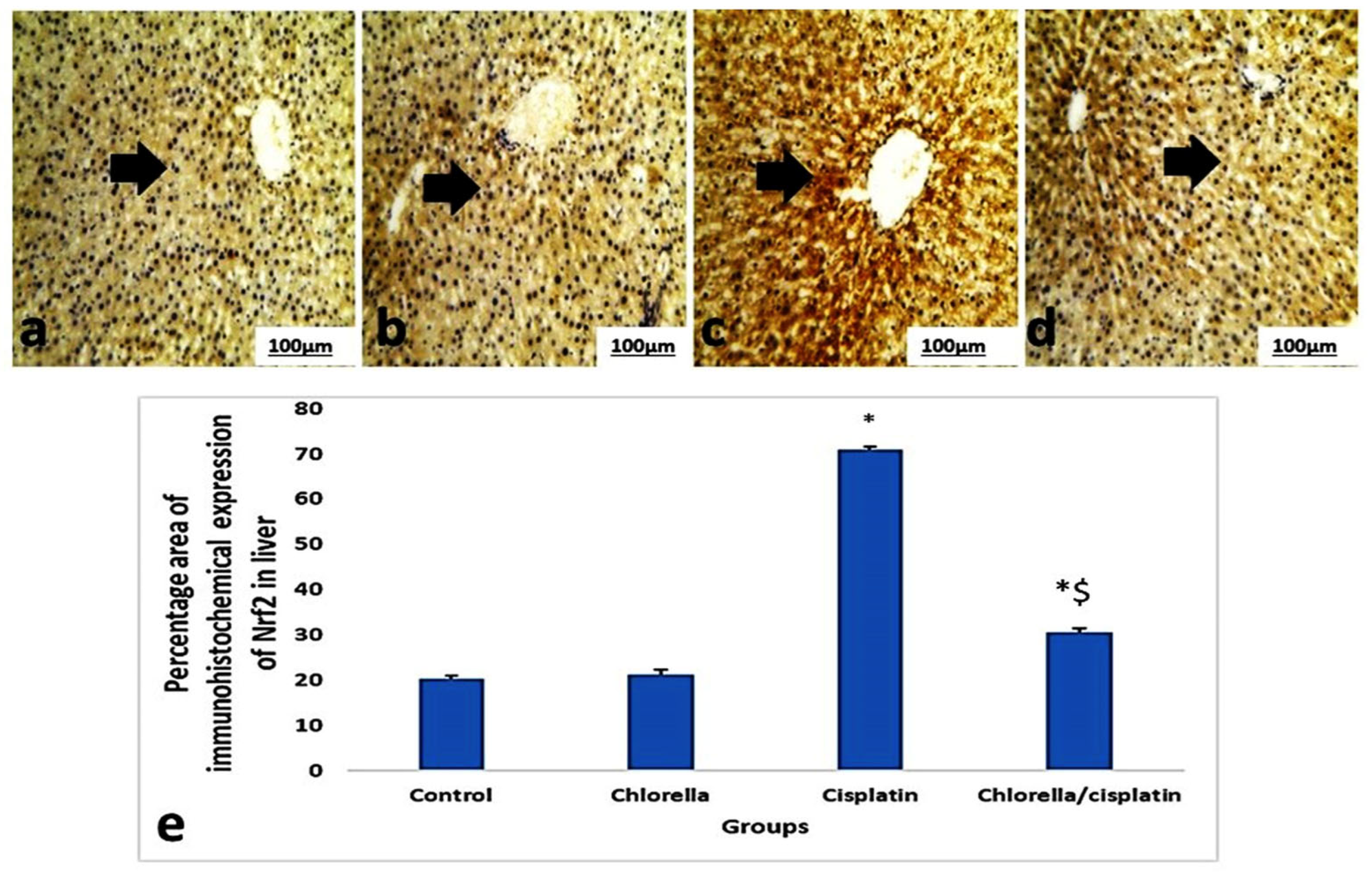
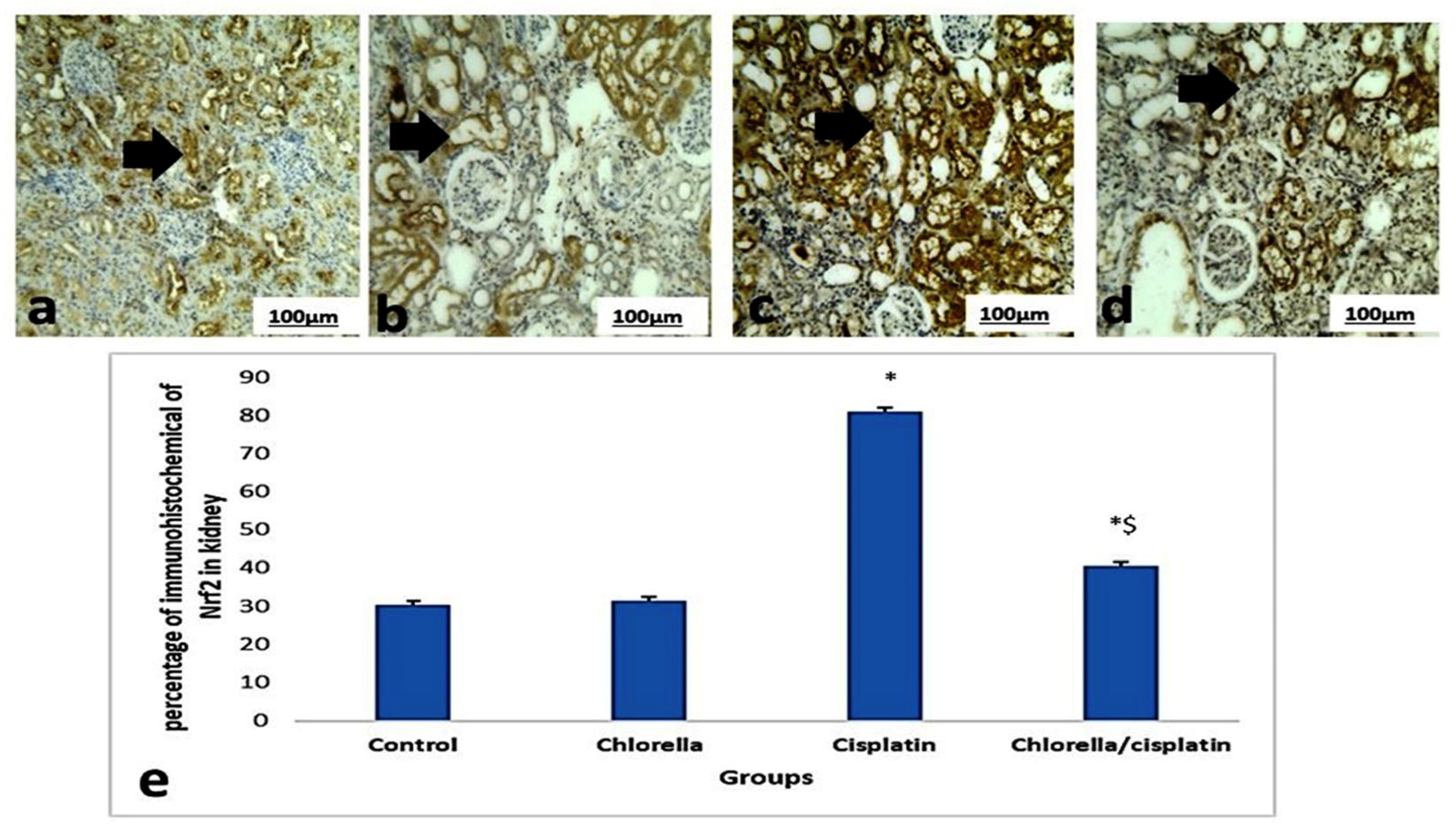
3.6. Immunohistochemical Expression Nrf2 of Liver and Kidney
3.7. Ultrastructure Examination
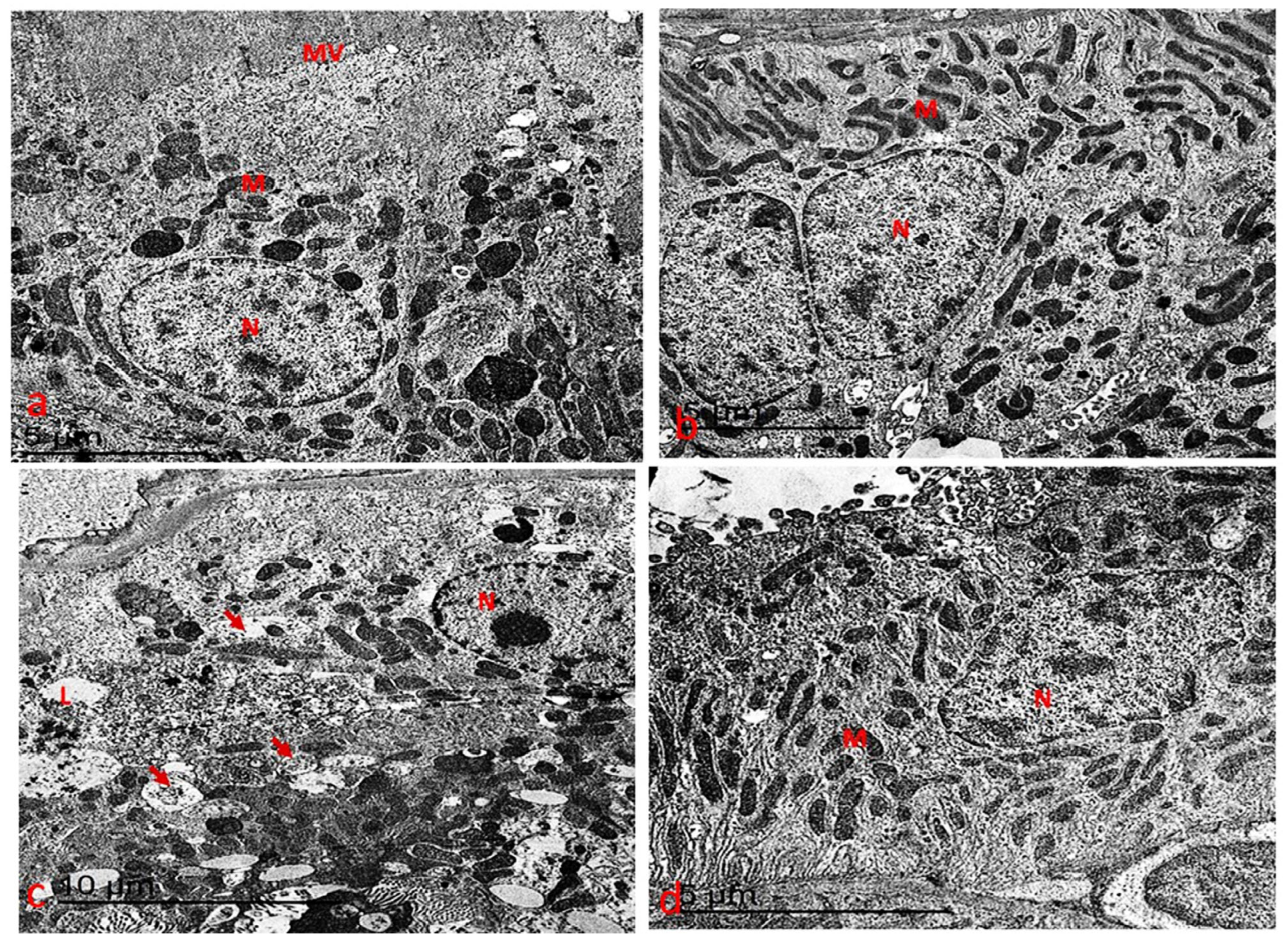
3.7.1. Ultrastructure Changes in Liver
3.7.2. Ultrastructure Changes in the Kidney
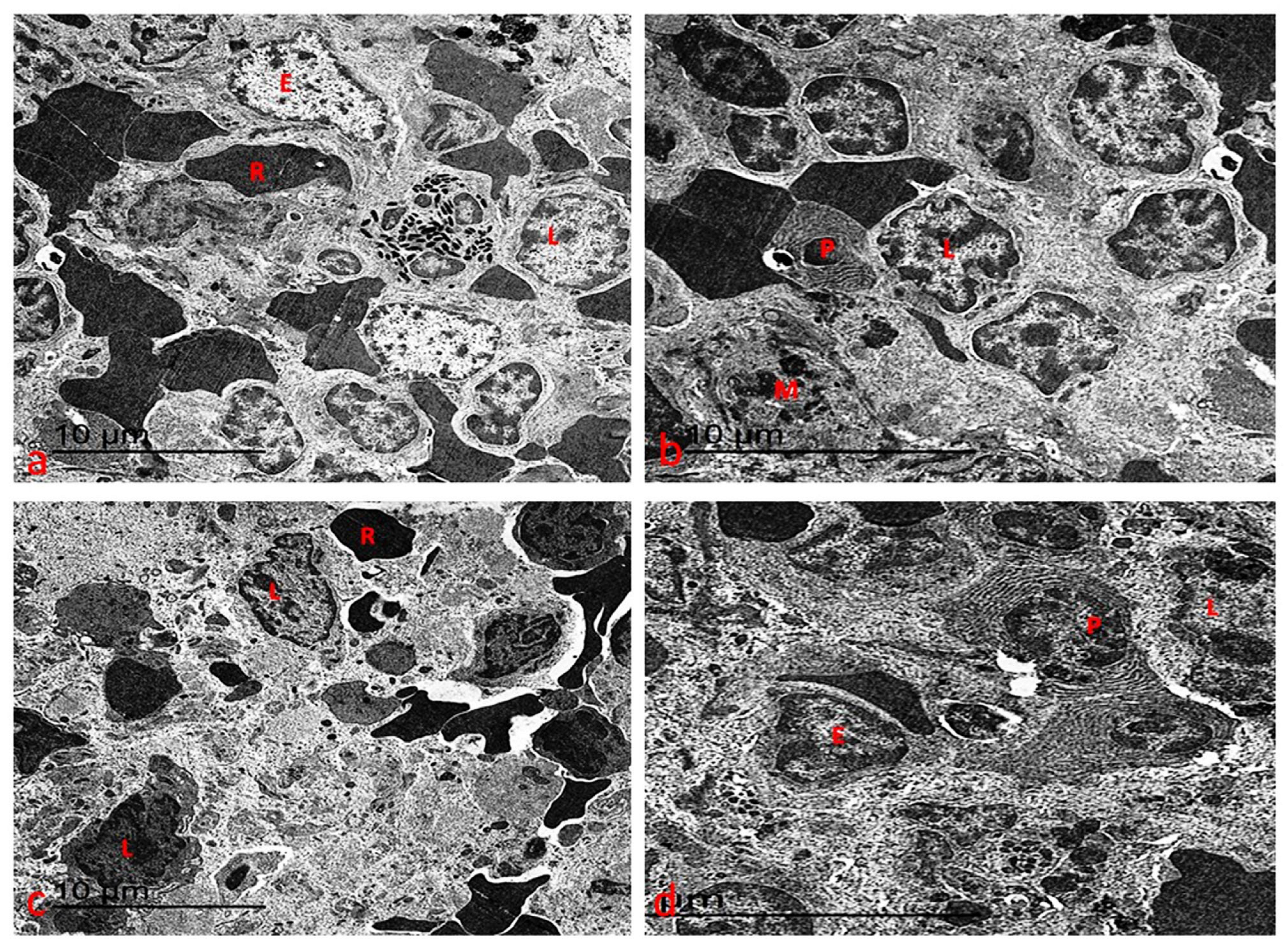
3.7.3. Ultrastructure Changes of Spleen
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rampling, R.; James, A.; Papanastassiou, V. The present and future management of malignant brain tumours: Surgery, radiotherapy, chemotherapy. J. Neurol. Neurosurg. Psychiatry 2004, 75, ii24–ii30. [Google Scholar] [CrossRef]
- Elkelish, A.; Abu-Elsaoud, A.M.M. Crosstalk Between Abiotic and Biotic Stress Responses in Plants: Mechanisms, Outcomes, and Implications for Crop Improvement. Spectr. Sci. J. 2024, 1, 27–34. [Google Scholar] [CrossRef]
- Astolfi, L.; Ghiselli, S.; Guaran, V.; Chicca, M.; Simoni, E.; Olivetto, E.; Lelli, G.; Martini, A. Correlation of adverse effects of cisplatin administration in patients affected by solid tumours: A retrospective evaluation. Oncol. Rep. 2013, 29, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Boulikas, T.; Vougiouka, M. Recent clinical trials using cisplatin, carboplatin and their combination chemotherapy drugs. Oncol. Rep. 2004, 11, 559–595. [Google Scholar] [CrossRef]
- Wang, D.; Lippard, S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005, 4, 307–320. [Google Scholar] [CrossRef]
- Palipoch, S.; Punsawad, C. Biochemical and histological study of rat liver and kidney injury induced by cisplatin. J. Toxicol. Pathol. 2013, 26, 293–299. [Google Scholar] [CrossRef]
- Raynaud, F.I.; Boxall, F.E.; Goddard, P.M.; Valenti, M.; Jones, M.; Murrer, B.A.; Abrams, M.; Kelland, L.R. cis-Amminedichloro (2-methylpyridine) platinum (II) (AMD473), a novel sterically hindered platinum complex: In vivo activity, toxicology, and pharmacokinetics in mice. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1997, 3, 2063–2074. [Google Scholar]
- Barabas, K.; Milner, R.; Lurie, D.; Adin, C. Cisplatin: A review of toxicities and therapeutic applications. Vet. Comp. Oncol. 2008, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chabner, B.A.; Amrein, P.C.; Druker, B.J.; Michaelson, M.D.; Mitsiades, C.S.; Goss, P.E.; Ryan, D.P.; Ramachandra, S.; Richardson, P.G.; Supko, J.G. Antineoplastic agents. In The Pharmacological Basis of Therapeutics, 11th ed.; The McGraw-Hill Co., Inc.: New York, NY, USA, 2005; pp. 1315–1403. [Google Scholar]
- Khodeer, D.M. Beyond Glycemic Control: Cardiovascular, Renal, and Hepatic Benefits of GLP-1 Receptor Agonists. Spectr. Sci. J. 2024, 1, 15–26. [Google Scholar] [CrossRef]
- Abdallah, H.M.; Abdel-Rahman, R.F.; El Awdan, S.A.; Allam, R.M.; El-Mosallamy, A.E.; Selim, M.S.; Mohamed, S.S.; Arbid, M.S.; Farrag, A.R.H. Protective effect of some natural products against chemotherapy-induced toxicity in rats. Heliyon 2019, 5, e01590. [Google Scholar] [CrossRef]
- Elkelish, A. New Plant Extracts toward Multidrug Resistance: The Convergence of Nanotechnology and Nanoscience. Spectr. Sci. J. 2024, 1, 1–14. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.-T.; Show, P.-L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Vartak, A.; Sonawane, S.; Alim, H.; Patel, N.; Hamrouni, L.; Khan, J.; Ali, A. Medicinal and Aromatic Plants in the Cosmetics Industry. In Medicinal and Aromatic Plants of India; Springer: Berlin/Heidelberg, Germany, 2022; Volume 1, pp. 341–364. [Google Scholar]
- Zainul Azlan, N.; Mohd Yusof, Y.A.; Makpol, S. Chlorella vulgaris ameliorates oxidative stress and improves the muscle regenerative capacity of young and old Sprague-Dawley rats. Nutrients 2020, 12, 3752. [Google Scholar] [CrossRef]
- Yadavalli, R.; Peasari, J.R.; Mamindla, P.; Praveenkumar; Mounika, S.; Ganugapati, J. Phytochemical screening and in silico studies of flavonoids from Chlorella pyrenoidosa. Inform. Med. Unlocked 2018, 10, 89–99. [Google Scholar] [CrossRef]
- Saleem, A.; Harris, C.S.; Asim, M.; Cuerrier, A.; Martineau, L.; Haddad, P.S.; Arnason, J.T. A RP-HPLC-DAD-APCI/MSD method for the characterisation of medicinal Ericaceae used by the Eeyou Istchee Cree First Nations. Phytochem. Anal. 2010, 21, 328–339. [Google Scholar] [CrossRef] [PubMed]
- El-Gendy, H.F.; El-Bahrawy, A.; Mansour, D.A.; Sheraiba, N.I.; Abdel-Megeid, N.S.; Selim, S.; Alhotan, R.A.; Ayyoub, A.; El Hanbally, S. Unraveling the potential of Saccharum officinarum and Chlorella vulgaris towards 5-fluorouracil-induced nephrotoxicity in rats. Pharmaceuticals 2024, 17, 885. [Google Scholar] [CrossRef] [PubMed]
- Lipnick, R.; Cotruvo, J.; Hill, R.; Bruce, R.; Stitzel, K.; Walker, A.P.; Chu, I.; Goddard, M.; Segal, L.; Springer, J. Comparison of the up-and-down, conventional LD50, and fixed-dose acute toxicity procedures. Food Chem. Toxicol. 1995, 33, 223–231. [Google Scholar] [CrossRef]
- Abdi-Azar, H.; Maleki, S. Comparison of the anesthesia with thiopental sodium alone and their combination with Citrus aurantium L. (Rutaseae) essential oil in male rat. Bull. Environ. Pharmacol. Life Sci. 2014, 3, 37–44. [Google Scholar]
- Parasuraman, S.; Zhen, K.M.; Raveendran, R. Retro-orbital sample collection in Rats—A video article. Pharmacol. Toxicol. Biomed. Rep. 2015, 1, 37–40. [Google Scholar] [CrossRef]
- Šuk, J.; Jašprová, J.; Biedermann, D.; Petrásková, L.; Valentová, K.; Křen, V.; Muchová, L.; Vítek, L. Isolated silymarin flavonoids increase systemic and hepatic bilirubin concentrations and lower lipoperoxidation in mice. Oxidative Med. Cell. Longev. 2019, 2019, 6026902. [Google Scholar] [CrossRef]
- Tan, H.; Liao, S.; Pan, T.; Zhang, J.; Chen, J. Rapid and simultaneous analysis of direct and indirect bilirubin indicators in serum through reagent-free visible-near-infrared spectroscopy combined with chemometrics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 233, 118215. [Google Scholar] [CrossRef]
- Fernández-Real, J.; Ortega, F.; Gómez-Ambrosi, J.; Salvador, J.; Frühbeck, G.; Ricart, W. Circulating osteocalcin concentrations are associated with parameters of liver fat infiltration and increase in parallel to decreased liver enzymes after weight loss. Osteoporos. Int. 2010, 21, 2101–2107. [Google Scholar] [CrossRef]
- Stavreva Veselinovska, S. The activities of the aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase enzymes in the blood serum of rats in conditions of chronic lead poisoning. In Proceedings of the Seminar of Ecology with International Participation—2015, Section “Biology”, Sofia, Bulgaria, 23–24 April 2015; pp. 149–157. [Google Scholar]
- Guobing, X.; Lili, J.; Lihua, Z.; Tiean, X. Application of an improved biuret method to the determination of total protein in urine and cerebrospinal fluid without concentration step by use of Hitachi 7170 auto-analyzer. J. Clin. Lab. Anal. 2001, 15, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.-J.; Tseng, C.-C.; Ju, W.-J.; Wang, H.-L.; Fu, L.-M. A rapid paper-based detection system for determination of human serum albumin concentration. Chem. Eng. J. 2018, 352, 241–246. [Google Scholar] [CrossRef]
- Busher, J.T. Serum albumin and globulin. Clin. Methods Hist. Phys. Lab. Exam. 1990, 3, 497–499. [Google Scholar]
- Higgins, C. Urea and the clinical value of measuring blood urea concentration. Acutecaretesting. Org. 2016, 22, 1–6. Available online: https://acutecaretesting.org/en/articles/urea-and-the-clinical-value-of-measuring-blood-urea-concentration (accessed on 20 March 2025).
- Dutt, V.E.; Mottola, H. Determination of uric acid at the microgram level by a kinetic procedure based on a pseudo-induction period. Anal. Chem. 1974, 46, 1777–1781. [Google Scholar] [CrossRef]
- Jacobs, R.M.; Lumsden, J.H.; Taylor, J.A.; Grift, E. Effects of interferents on the kinetic Jaffé reaction and an enzymatic colorimetric test for serum creatinine concentration determination in cats, cows, dogs and horses. Can. J. Vet. Res. 1991, 55, 150. [Google Scholar]
- Amundson, D.M.; Zhou, M. Fluorometric method for the enzymatic determination of cholesterol. J. Biochem. Biophys. Methods 1999, 38, 43–52. [Google Scholar] [CrossRef]
- Sardesai, V.M.; Manning, J.A. The determination of triglycerides in plasma and tissues. Clin. Chem. 1968, 14, 156–161. [Google Scholar] [CrossRef]
- Van Duijnhoven, F.J.; Bueno-De-Mesquita, H.B.; Calligaro, M.; Jenab, M.; Pischon, T.; Jansen, E.H.; Frohlich, J.; Ayyobi, A.; Overvad, K.; Toft-Petersen, A.P. Blood lipid and lipoprotein concentrations and colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition. Gut 2011, 60, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.W. Ionized calcium in normal serum, ultrafiltrates, and whole blood determined by ion-exchange electrodes. J. Clin. Investig. 1970, 49, 318–334. [Google Scholar] [CrossRef] [PubMed]
- Sava, L.; Pillai, S.; More, U.; Sontakke, A. Serum calcium measurement: Total versus free (ionized) calcium. Indian J. Clin. Biochem. 2005, 20, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Perez-Gracia, J.L.; Prior, C.; Guillén-Grima, F.; Segura, V.; Gonzalez, A.; Panizo, A.; Melero, I.; Grande-Pulido, E.; Gurpide, A.; Gil-Bazo, I. Identification of TNF-α and MMP-9 as potential baseline predictive serum markers of sunitinib activity in patients with renal cell carcinoma using a human cytokine array. Br. J. Cancer 2009, 101, 1876–1883. [Google Scholar] [CrossRef]
- Jakovljevic, B.; Novakov-Mikic, A.; Brkic, S.; Bogavac, M.A.; Tomic, S.; Miler, V. Lipid peroxidation in the first trimester of pregnancy. J. Matern.-Fetal Neonatal Med. 2012, 25, 1316–1318. [Google Scholar] [CrossRef]
- Rubio, C.P.; Hernández-Ruiz, J.; Martinez-Subiela, S.; Tvarijonaviciute, A.; Ceron, J.J. Spectrophotometric assays for total antioxidant capacity (TAC) in dog serum: An update. BMC Vet. Res. 2016, 12, 231. [Google Scholar] [CrossRef]
- Sakai, M.; Tsuda, H.; Tanebe, K.; Sasaki, Y.; Saito, S. Interleukin-12 secretion by peripheral blood mononuclear cells is decreased in normal pregnant subjects and increased in preeclamptic patients. Am. J. Reprod. Immunol. 2002, 47, 91–97. [Google Scholar] [CrossRef]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harb. Protoc. 2008, 2008, pdb.prot4986. [Google Scholar] [CrossRef]
- Sato, S.; Adachi, A.; Sasaki, Y.; Ghazizadeh, M. Oolong tea extract as a substitute for uranyl acetate in staining of ultrathin sections. J. Microsc. 2008, 229, 17–20. [Google Scholar] [CrossRef]
- Yadavalli, R.; Ratnapuram, H.; Motamarry, S.; Reddy, C.N.; Ashokkumar, V.; Kuppam, C. Simultaneous production of flavonoids and lipids from Chlorella vulgaris and Chlorella pyrenoidosa. Biomass Convers. Biorefin. 2022, 12, 683–691. [Google Scholar] [CrossRef]
- Prabakaran, G.; Moovendhan, M.; Arumugam, A.; Matharasi, A.; Dineshkumar, R.; Sampathkumar, P. Evaluation of chemical composition and in vitro antiinflammatory effect of marine microalgae Chlorella vulgaris. Waste Biomass Valorization 2019, 10, 3263–3270. [Google Scholar] [CrossRef]
- Abdel-Karim, O.H.; Gheda, S.F.; Ismail, G.A.; Abo-Shady, A.M. Phytochemical Screening and antioxidant activity of Chlorella vulgaris. Delta J. Sci. 2019, 41, 79–91. [Google Scholar] [CrossRef]
- Hickman, D.L.; Swan, M. Use of a body condition score technique to assess health status in a rat model of polycystic kidney disease. J. Am. Assoc. Lab. Anim. Sci. 2010, 49, 155–159. [Google Scholar] [PubMed]
- Heindel, J.J.; Blumberg, B.; Cave, M.; Machtinger, R.; Mantovani, A.; Mendez, M.A.; Nadal, A.; Palanza, P.; Panzica, G.; Sargis, R. Metabolism disrupting chemicals and metabolic disorders. Reprod. Toxicol. 2017, 68, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-T.; Ko, J.-L.; Liu, T.-C.; Chao, P.-T.; Ou, C.-C. Protective effect of D-methionine on body weight loss, anorexia, and nephrotoxicity in cisplatin-induced chronic toxicity in rats. Integr. Cancer Ther. 2018, 17, 813–824. [Google Scholar] [CrossRef]
- Blundell, J.; Gibbons, C.; Caudwell, P.; Finlayson, G.; Hopkins, M. Appetite control and energy balance: Impact of exercise. Obes. Rev. 2015, 16, 67–76. [Google Scholar] [CrossRef]
- Vijayavel, K.; Anbuselvam, C.; Balasubramanian, M. Antioxidant effect of the marine algae Chlorella vulgaris against naphthalene-induced oxidative stress in the albino rats. Mol. Cell. Biochem. 2007, 303, 39–44. [Google Scholar] [CrossRef]
- Hidaka, S.; Okamoto, Y.; Arita, M. A hot water extract of Chlorella pyrenoidosa reduces body weight and serum lipids in ovariectomized rats. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2004, 18, 164–168. [Google Scholar] [CrossRef]
- Michael, B.; Yano, B.; Sellers, R.S.; Perry, R.; Morton, D.; Roome, N.; Johnson, J.K.; Schafer, K. Evaluation of organ weights for rodent and non-rodent toxicity studies: A review of regulatory guidelines and a survey of current practices. Toxicol. Pathol. 2007, 35, 742–750. [Google Scholar] [CrossRef]
- Lala, V.; Zubair, M.; Minter, D. Liver Function Tests; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482489/ (accessed on 10 April 2025).
- Omar, H.A.; Mohamed, W.R.; Arafa, E.-S.A.; Shehata, B.A.; El Sherbiny, G.A.; Arab, H.H.; Elgendy, A.N.A. Hesperidin alleviates cisplatin-induced hepatotoxicity in rats without inhibiting its antitumor activity. Pharmacol. Rep. 2016, 68, 349–356. [Google Scholar] [CrossRef]
- Das, K.; Samanta, T.T.; Samanta, P.; Nandi, D.K. Effect of extract of Withania Somnifera on dehydration-induced oxidative stress-related uremia in male rats. Saudi J. Kidney Dis. Transplant. 2010, 21, 75–80. [Google Scholar]
- Hashash, J.G.; Koutroumpakis, F.; Anderson, A.M.; Rivers, C.R.; Hosni, M.; Koutroubakis, I.E.; Ahsan, M.; Gkiaouraki, E.; Dunn, M.A.; Schwartz, M. Elevated serum globulin fraction as a biomarker of multiyear disease severity in inflammatory bowel disease. Ann. Gastroenterol. 2022, 35, 609. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi-Mameghani, M.; Sadeghi, Z.; Farhangi, M.A.; Vaghef-Mehrabany, E.; Aliashrafi, S. Glucose homeostasis, insulin resistance and inflammatory biomarkers in patients with non-alcoholic fatty liver disease: Beneficial effects of supplementation with microalgae Chlorella vulgaris: A double-blind placebo-controlled randomized clinical trial. Clin. Nutr. 2017, 36, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, G.; Venanzi, S.; Verdura, C.; Saronio, P.; Esposito, A.; Timio, M. Association of uric acid with change in kidney function in healthy normotensive individuals. Am. J. Kidney Dis. 2010, 56, 264–272. [Google Scholar] [CrossRef]
- Tang, C.; Livingston, M.J.; Safirstein, R.; Dong, Z. Cisplatin nephrotoxicity: New insights and therapeutic implications. Nat. Rev. Nephrol. 2023, 19, 53–72. [Google Scholar] [CrossRef]
- Al-Halaseh, L.K.; Sweiss, M.A.; Issa, R.A.; AlKassasbeh, R.; Abbas, M.A.; Al-Jawabri, N.A.; Hajleh, M.N.A.; Al-Samydai, A.M. Nephroprotective activity of green microalgae, chlorella sorokiniana isolated from Jordanian water. J. Pure Appl. Microbiol. 2022, 16, 2775–2782. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; Mahdy, E.A.; El-Hady, E.; Abou-Zeid, S.M.; Mawed, S.A.; Azzam, M.M.; Crescenzo, G.; Abo-Elmaaty, A.M.J.B. Benefits of Chlorella vulgaris against cadmium chloride-induced hepatic and renal toxicities via restoring the cellular redox homeostasis and modulating Nrf2 and NF-KB pathways in male rats. Biomedicines 2023, 11, 2414. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.-S.; Kim, H.-J.; Shen, A.; Lee, S.B.; Khadka, D.; Pandit, A.; So, H.-S. Cisplatin-induced kidney dysfunction and perspectives on improving treatment strategies. Electrolytes Blood Press. EBP 2014, 12, 55. [Google Scholar] [CrossRef]
- Mirzaei, S.; Hushmandi, K.; Zabolian, A.; Saleki, H.; Torabi, S.M.R.; Ranjbar, A.; SeyedSaleh, S.; Sharifzadeh, S.O.; Khan, H.; Ashrafizadeh, M. Elucidating role of reactive oxygen species (ROS) in cisplatin chemotherapy: A focus on molecular pathways and possible therapeutic strategies. Molecules 2021, 26, 2382. [Google Scholar] [CrossRef]
- Abdelghaffar, E.G.; Hafney, H.A.; Ebaid, H.M.; Gad EL-Hak, H.N. Histological and Ultrastructural Studies on the Protective Effects of Chlorella vulgaris on Healthy Testis against Toxicity of The Therapeutic Regimen of Cisplatin. Egypt. Acad. J. Biol. Sci. D Histol. Histochem. 2023, 15, 51–58. [Google Scholar] [CrossRef]
- El-Chaghaby, G.; Rashad, S.; Abdel-Kader, S.F.; Rawash, E.-S.A.; Abdul Moneem, M. Assessment of phytochemical components, proximate composition and antioxidant properties of Scenedesmus obliquus, Chlorella vulgaris and Spirulina platensis algae extracts. Egypt. J. Aquat. Biol. Fish. 2019, 23, 521–526. [Google Scholar] [CrossRef]
- Sibi, G.; Rabina, S. Inhibition of pro-inflammatory mediators and cytokines by Chlorella vulgaris extracts. Pharmacogn. Res. 2016, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]




| Retention Time (RT) | Compound | Concentration mg/g |
|---|---|---|
| 13.1 | gallic acid | 10.6 |
| 27.8 | catechin | 5.6 |
| 31.49 | chlorogenic acid | 6.48 |
| 33.65 | caffeic acid | 32.78 |
| 48.8 | hesperidin | 6.00 |
| 50.37 | rutin | 12.83 |
| 52.46 | ellagic acid | 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almutairi, L.A.; Abdelghaffar, E.G.; Hafney, H.A.; Ebaid, H.M.; Alkhodair, S.A.; Shaalan, A.A.M.; EL-Hak, H.N.G. Protective Impacts of Chlorella vulgaris on Cisplatin-Induced Toxicity in Liver, Kidney, and Spleen of Rats: Role of Oxidative Stress, Inflammation, and Nrf2 Modulation. Life 2025, 15, 934. https://doi.org/10.3390/life15060934
Almutairi LA, Abdelghaffar EG, Hafney HA, Ebaid HM, Alkhodair SA, Shaalan AAM, EL-Hak HNG. Protective Impacts of Chlorella vulgaris on Cisplatin-Induced Toxicity in Liver, Kidney, and Spleen of Rats: Role of Oxidative Stress, Inflammation, and Nrf2 Modulation. Life. 2025; 15(6):934. https://doi.org/10.3390/life15060934
Chicago/Turabian StyleAlmutairi, Layla A., Ebtehal G. Abdelghaffar, Hany A. Hafney, Hala M. Ebaid, Sahar A. Alkhodair, Aly A. M. Shaalan, and Heba N. Gad EL-Hak. 2025. "Protective Impacts of Chlorella vulgaris on Cisplatin-Induced Toxicity in Liver, Kidney, and Spleen of Rats: Role of Oxidative Stress, Inflammation, and Nrf2 Modulation" Life 15, no. 6: 934. https://doi.org/10.3390/life15060934
APA StyleAlmutairi, L. A., Abdelghaffar, E. G., Hafney, H. A., Ebaid, H. M., Alkhodair, S. A., Shaalan, A. A. M., & EL-Hak, H. N. G. (2025). Protective Impacts of Chlorella vulgaris on Cisplatin-Induced Toxicity in Liver, Kidney, and Spleen of Rats: Role of Oxidative Stress, Inflammation, and Nrf2 Modulation. Life, 15(6), 934. https://doi.org/10.3390/life15060934







