Abstract
Air pollutants are significant environmental factors that contribute to the exacerbation of respiratory, cardiopulmonary, and skin diseases in East Asia, and their impact is based on particle size. Natural products represent a promising and sustainable strategy for reducing the adverse effects of air pollutants on health. Cordyceps spp. have been integral to traditional Chinese medicine. Recently, their fruiting bodies and related supplements have gained popularity. The physiological effects of Cordyceps species are well documented and attributed to their chemical constituents, such as cordycepin, polysaccharides, cordymin, glycoprotein, ergosterol, and other bioactive extracts. Cordyceps supplementation may support lung health and enhance respiratory function. Although further clinical data are necessary, many preclinical studies have found a connection between Cordyceps and improved lung health. In addition, preclinical and clinical studies have indicated that Cordyceps and its derivatives (e.g., Ningxinbao, Corbrin, and Jinshuibao capsules) protect against vascular diseases by modulating key molecular pathways. This review provides insights into the potential of Cordyceps for clinical application in the management of air pollutant-related respiratory and vascular diseases.
1. Introduction
Exposure to air pollutants is associated with numerous health issues, including unexpected death among individuals with vascular disorders, asthma, irregular heartbeat, heart attack, lung disease, and reduced lung function [1]. Naturopathic medicines derived from natural sources, including microorganisms, plants, animals, and marine organisms, are recognized for their beneficial effects in the prevention and treatment of various diseases. Naturopathic remedies typically have fewer side effects than allopathic medicines. Traditionally, these remedies have been utilized worldwide in minimally invasive yet effective ways and are rich in antioxidants and anti-inflammatory compounds, which may counteract the adverse effects of particulate matter (PM) on the human body. Medicinal mushrooms have been used in naturopathic medicine to prevent and treat various diseases. Among them, the genus Cordyceps, which comprises approximately 750 varieties and a group of ascomycete parasitic fungi, is primarily used as a functional ingredient in traditional Oriental medicine in Asia [2,3]. Cordyceps militaris (C. militaris) has been reported to have immunoprotective, antimicrobial, antiviral, anticancer, anti-inflammatory, and antioxidant properties [4]. Consequently, it has gained significant attention as a potential novel agent for combating respiratory and vascular diseases related to exposure to air pollutants. Recent advances in fungal taxonomy based on molecular phylogenetic analyses have led to the reclassification of the genus Cordyceps, with several species reassigned to new genera, such as Ophiocordyceps and Metacordyceps. Despite these revisions, the term Cordyceps remains widely used in the pharmacological, clinical, and ethnobotanical literature to collectively describe the traditional group of entomopathogenic fungi with medicinal value. In this study, we adopted the classical concept of Cordyceps (sensu lato, abbreviated as s.l.) to include all conventionally recognized medicinal species, both formerly and currently classified under the genus Cordyceps [5,6]. This approach ensures continuity with the existing literature and supports its widespread relevance in therapeutic research and development.
2. Genus Cordyceps: Promising Health Benefits for Managing Various Disease Conditions
Historically, the term “Cordyceps” has primarily referred to C. sinensis. The two most extensively studied species of the Cordyceps genus are C. sinensis and C. militaris. Cordyceps species exhibit various biological functions, showing potential for treating respiratory and liver dysfunctions, heart diseases, cardiovascular diseases, uncontrolled perspiration, fatigue, impotence, spermatorrhea, and cancers [7] (Figure 1 and Figure 2). The State Food and Drug Administration of China has approved 50 medicines and 2 dietary supplements containing Cordyceps militaris and Cordyceps sinensis [8]. The market efficacy of these two Cordyceps species has significantly increased since the 1980s. In 2023, the global market for C. militaris was estimated to be worth USD 1.02 billion. Its annual growth rate (CAGR) of 11.79% from 2023 to 2033 is expected to result in significant expansion. By 2033, the global market for C. militaris is projected to reach USD 3.11 billion [9]. This demand has resulted in the overharvesting of C. sinensis, resulting in its near-total depletion. Due to their rarity and high cost, most wild Cordyceps species are now artificially cultivated. Numerous studies on artificially cultured species have shown promising results, indicating that these cultivated species possess the same potent bioactivities as their wild counterparts [3]. Owing to the limited presence of C. militaris in natural habitats, we opted to cultivate it on germinated Rhynchosia nulubilis (GRC) [3]. Notably, when cultivated on germinated soybeans (GSC), C. militaris exhibits immunostimulatory [10], anticancer [11], and antiallergic properties [12].

Figure 1.
Images of Cordyceps species (a) Cordyceps sinensis (Ophiocordyceps sinensis); (b) Cordyceps militaris; (c) Cordyceps ophioglossoides (Tolypocladium ophioglossoides); (d) Cordyceps taii (Metacordyceps taii); (e) Cordyceps jiangxiensis (Ophiocordyceps jiangxiensis); (f) Cordyceps cicadae (Isaria cicadae). The images are sourced from MeFSAT [13] and Wikimedia Commons in the public domain.
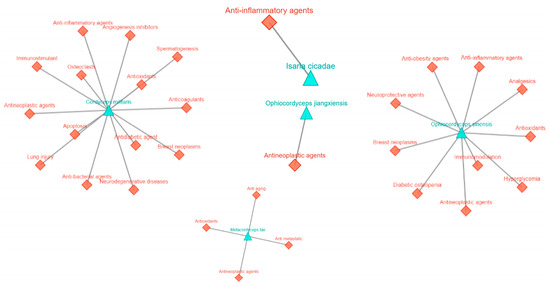
Figure 2.
Health benefits of the genus Cordyceps in managing various disease conditions. Images modified from MeFSAT (https://cb.imsc.res.in/mefsat/ accessed on 22 March 2025).
2.1. Cordyceps sinensis
C. sinensis, now renamed as “Ophiocordyceps sinensis”, is one of the most widely studied species. It has been used for a long time in traditional Chinese medicine (TCM). The consumption of C. sinensis is recommended for preventing infections and flu because of its efficacy in reducing phlegm, cough, and bronchial disease symptoms [11]. The C. sinensis capsule significantly reduced the frequency and severity of acute exacerbations in patients with chronic bronchitis compared to the placebo. It also improved symptoms such as expectoration and wheezing, with no significant difference in adverse event rates between the groups [14]. Adjuvant Cordyceps sinensis treatment in patients with lung cancer improves tumor response, quality of life, and immunity while reducing adverse reactions and radiation pneumonitis [15]. Cordymin, a peptide purified from C. sinensis, has anti-inflammatory and antinociceptive effects [16]. Consequently, Cordyceps spp. have been used to treat lung fibrosis, particularly in patients with severe acute respiratory syndrome (SARS). These therapeutic effects align with TCM principles, attributing them to the capacity of C. sinensis to balance lung yin and yang [17]. In addition, C. sinensis enhances cardiac energy metabolism, prevents calcium overload during myocardial ischemia, and alleviates blood–brain barrier disruption induced by cerebral ischemia [18]. It also helps prevent or treat hypertension, thrombosis, atherosclerosis, and arrhythmia linked to heart ischemia. Fermented C. sinensis powder reduces inflammation and pulmonary arteriole remodeling in hypoxia-induced pulmonary hypertension in rats by blocking the p38 MAPK and NF-κB signaling pathways [14].
2.2. Cordyceps militaris
C. militaris, which is often cultivated and used in TCM, shares several medicinal qualities with C. sinensis. C. militaris is used to treat a spectrum of conditions, including pulmonary and kidney impairment, elevated blood sugar levels, abnormal lipid profiles, breathing difficulties, exhaustion, night sweats, reproductive issues, irregular heart rhythms, and other heart ailments. Its pharmacological properties include anti-inflammatory, antioxidant, anticancer, antimetastatic, immunomodulatory, hypoglycemic, and steroidogenic activities [19]. CM1, a purified polysaccharide from C. militaris, significantly reduced atherosclerotic plaque formation and improved lipid profiles in LDLR (−/−) mice. It regulates lipid metabolism through multiple pathways, including enhancing VLDLR expression, suppressing hepatic lipid synthesis, and promoting intestinal lipid excretion [20]. Hyperlipidemia, a key cardiovascular risk factor, is often treated with synthetic drugs that have adverse effects. Cordycepin from C. militaris reduces triglycerides, cholesterol, LDL, and VLDL in hyperlipidemic rats by activating AMPK and inhibiting hepatic lipase and lipoproteins, offering natural therapeutic potential [19]. C. militaris cocktail inhibits inflammation and fibrosis in lung tissue caused by PM [21]. Cordyceps militaris ARA301 extract (CME) significantly attenuated lipopolysaccharide (LPS)-induced lung injury in mice by reducing immune cell infiltration, mucus production, and pro-inflammatory cytokine levels. It also suppressed NF-κB pathway activation in vitro, suggesting its potential as a functional supplement for lung protection [22]. A functional beverage containing fermented Cordyceps militaris (FCM) enhanced NK cell activity and reduced pro-inflammatory cytokines (IL-1β, IL-6) in healthy men and women without adverse effects. These studies indicate that FCM may be a safe and effective immune-enhancing supplement [23].
2.3. Other Cordyceps Species
Mushrooms grown on insect substrates, such as C. fumosorosea, are considered promising agents for managing various insect populations through biological control. Fumosoroseanosides A and B from C. fumosorosea exhibit anti-aging, antibacterial, and antifungal activities [24]. Serum metabolomic analysis in mice revealed the alleviating effect of C. fumosorosea against acute lung injury (ALI) [25].
Metacordyceps taii develops on the larvae or pupae of Hepialidae that reside in the soil or in tree trunks [26]. The chloroform extract of Metacordyceps taii (CFCT) demonstrated in vivo antitumor and antimetastatic activities, indicating its potential for development as a chemopreventive agent derived from Metacordyceps taii [27]. Metacordyceps taii polysaccharides demonstrated strong immune function-boosting, antioxidant effects, and free radical scavenging activity [28].
C. jiangxiensis thrives in moist soil, particularly on the larvae of Elateridae (Coleoptera) [26]. A novel compound, jiangxienone, isolated from C. jiangxiensis, exhibits strong cytotoxic effects on human lung carcinoma (A549) and human gastric adenocarcinoma (SGC-7901) cells [29].
Isaria cicadae may ameliorate kidney fibrosis by targeting the TGF-β1 and CTGF pathways in vivo [30]. Aqueous extracts of Isaria cicadae (Miq.) Massee have been reported to be a promising hydrating and antiwrinkle component that enhances hyaluronan concentration in human skin fibroblasts [31]. This species could reduce inflammation, oxidative stress, and renal fibrosis in the murine model with lupus nephritis by modulating the PI3K/mTOR-mediated autophagy pathway [31]. Isaria cicadae mycelium (CCM) ameliorates high intraocular pressure in animals and humans [32]. It also prevents early-stage cataracts in a UVB-induced murine cataract model [32].
C. ophioglossoides exhibits antitumor activity that is attributed to an alkali-soluble polysaccharide present in the fungus [33]. Ophiocordin isolated from C. ophioglossoides showed antifungal antibiotic effects [34].
2.4. Bioactive Constituents of Cordyceps Species
In addition to the major bioactive compounds described above, recent studies have identified over 100 distinct chemical constituents in various Cordyceps species. These include nucleosides (e.g., adenosine, cordycepin), polysaccharides, sterols (e.g., ergosterol, β-sitosterol), peptides, alkaloids, flavonoids, and cyclic dipeptides [2,4].
More than 30 compounds have been isolated from Cordyceps militaris, and over 70 compounds, including unique fatty acids and complex protein-bound polysaccharides, have been identified in C. sinensis [4].
This wide range of chemical constituents highlights the metabolic richness of individual Cordyceps species and supports their diverse pharmacological activities.
3. Fermented Cordyceps and Its Role in Immune Regulation: Evidence from Recent Studies
C. militaris is a fungal species that grows by parasitizing insect larvae. However, the production yield of C. militaris in nature is very low because of the requirement for specific hosts and stringent growth conditions. The commercial use of natural C. militaris is limited by its high cost and low extraction yield [3,35,36]. Cari Co., Ltd. grew C. militaris on germinated R. nulubilis (GRC) instead of using dead insects [3]. GRC fermented with Pediococcus pentosaceus SC11, a strain isolated from salted small octopus, possesses immune-enhancing and antiviral properties. The lactic acid bacteria fermentation process with SC11 significantly increased the contents of total flavonoids, cordycepin, and β-glucan in GRC. Notably, GRC-SC11 suppresses 3CL protease activity associated with severe acute respiratory syndrome coronavirus (SARS-CoV). Furthermore, studies involving immunocompromised mice have shown that GRC-SC11 significantly enhances the indices of the thymus and spleen [3]. Treatment with C. militaris, fermented by P. pentosaceus ON89A, a strain isolated from onion (GRC-ON89A), has been shown to restore immune function in mice treated with high doses of cyclophosphamide, a chemotherapeutic drug. This recovery is evidenced by increased phagocytic activity and nitric oxide (NO) production in mouse peritoneal macrophages. Additionally, GRC-ON89A mitigates the toxicity of anticancer agents by aiding immune system restoration [37]. Fermented C. militaris with a P. pentosaceus, a strain isolated from a small salted octopus (SC11), significantly reduced IgE-induced allergic reactions in BALB/c mice with passive cutaneous anaphylaxis (PCA) by decreasing inflammatory cell infiltration, vascular permeability, and ear swelling. This fermentation process enhanced the antiallergic effects of GRC, boosting both cordycepin content and antioxidant activity compared to non-fermented GRC [36]. Enzymatic breakdown and fungal fermentation significantly enhanced the flavor and nutritional value of C. militaris beverages. Enzymatic breakdown generates a more reduced form of sugar, providing an optimal fermentation medium for bacterial growth. A previous study revealed that using a mixture of cellulase and pectinase at a 2:3 ratio resulted in elevated cordycepin levels. After fermentation, the cordycepin concentration remained high at 98.02% [38].
4. Cordyceps Species and Their Bioactive Substances Effective for Air Pollutant-Induced Respiratory Diseases
The lungs are strongly influenced by environmental factors [39]. Air pollutant exposure significantly increases the incidence of respiratory diseases, with indoor and outdoor air pollution responsible for seven million fatalities worldwide [40]. Air pollution is caused by various chemical and biological elements present in both indoor and outdoor environments. Harmful outdoor air pollutants for the respiratory system include gas chemicals such as ozone (O3), nitrogen oxide including NO2, sulfur dioxide (SO2), inhaled diesel gas and PM, and allergens in the air, such as endotoxin (e.g., lipopolysaccharide), fungal spores, allergenic, and pollen [41]. The inhalation of particulate matter (PM) in ambient air increases the risk of acute lower respiratory infections by bacteria and viruses, pneumonia, and asthma in children [41,42]. Outdoor pollutants can also affect indoor air quality. Indoor air contains various harmful substances from numerous sources. In indoor environments, one can find environmental tobacco smoke (ETS), NO2, formaldehyde (HCHO), other volatile organic compounds (SO2, O3), and allergens derived from various plants, animals, and insects [43]. Exposure to indoor cigarette smoke increases the incidence of pediatric respiratory diseases, including asthma, rhinitis, and respiratory tract infections [43]. Cordyceps, a genus of parasitic fungi, has been used in traditional Oriental medicine for its potential health benefits in treating various lung disorders, including chronic obstructive pulmonary disease (COPD). This study provides an overview of its relevance and potential benefits in the management of lung conditions (Table 1).

Table 1.
Chemical compounds related to air pollutants of Cordyceps spp., and their mode of action for respiratory diseases.
4.1. COPD Treatment
Epidemiological and mechanistic studies have shown a close association between air pollution and the development of COPD. The primary cause of COPD is long-term exposure to harmful substances that damage the lungs, most commonly cigarette smoke. Other causes include air pollution, occupational dust, chemicals, and secondhand smoke [63]. Studies have shown that Cordyceps may improve lung function in patients diagnosed with GOLD stages 2 and 3 COPD. A systematic review and meta-analysis found that the combination of Cordyceps and Western medicine significantly improved lung function metrics, such as the volume exhaled at the end of the first second of forced expiration (FEV1)% predicted and the FEV1/forced vital capacity (FVC) ratio, which are critical measures of pulmonary function in patients with COPD [64]. C. sinensis treatment led to a significant reduction in airway wall thickening, including collagen deposition, smooth muscle hypertrophy, fibrosis, and epithelial hyperplasia in a COPD rat model [65]. Nucleosides isolated from C. sinensis alleviate cigarette smoke extract-triggered inflammation through the SIRT1-NF-κB/p65 pathway in RAW264.7 macrophages and mice with COPD. Cordyceps-derived nucleosides and ergosterol have demonstrated therapeutic effects in a COPD model. Nucleosides activate SIRT1 and suppress the NF-κB/p65 pathway while also reducing TGF-β1/Smad signaling and increasing Smad7 expression, thereby attenuating airway inflammation and fibrosis in RAW264.7 macrophages and mice with COPD [66]. Ergosterol suppresses cigarette smoke extract-induced COPD by mitigating inflammation, oxidative stress, and apoptosis both in vitro and in vivo [56]. Recent studies have demonstrated that Cordyceps militaris cultivated on germinated Rhynchosia nulubilis and encapsulated in chitosan nanoparticles (GCNs) significantly enhances the expression of superoxide dismutase 1 (SOD1) in the lung tissues of mice exposed to PM2.5-induced oxidative stress [67]. SOD1, a Cu-Zn-dependent cytosolic antioxidant enzyme, plays a crucial role in neutralizing reactive oxygen species (ROS) and protecting lung epithelial integrity. The recombinant Cu, Zn-SOD from Cordyceps militaris retained 80 ± 2% activity under physiological conditions and showed structural similarity to the native enzyme, suggesting stable antioxidant capacity. Given the role of oxidative stress in the pathogenesis of COPD, its functional resemblance and stability support the therapeutic potential of C. militaris-derived SOD in mitigating pulmonary oxidative damage [68]. Cordycepin from Cordyceps militaris exerts immunomodulatory effects by suppressing T cell activation through the inhibition of the TCR signaling cascade, as demonstrated in CFA-induced inflammation models. This mechanism may contribute to its therapeutic potential in COPD, where dysregulated T cell responses and chronic inflammation play a critical role [69]. The hot-water extract from C. guangdongensis reduced tobacco smoking-induced chronic bronchitis symptoms [44].
4.2. Anti-Asthma
Air pollutants, such as PM2.5 and formaldehyde (FA), exacerbate allergic asthma through oxidative stress. Asthma is a clinical condition characterized by intermittent respiratory symptoms, typically marked by generalized airway hyperreactivity and inflammation. C. sinensis and C. militaris were studied for their effects on pulmonary function using a Calu-3 cell line, a human airway epithelial model. Both extracts, along with their active compounds, cordycepin and adenosine, enhanced ion transport in a dose-dependent manner. The study found that these extracts influence anion movement in airway epithelia, involving the basolateral Na+–K+–2Cl− symporter and the apical cAMP-regulated CFTR Cl− channel. The effects of C. sinensis and C. militaris on anion movement contribute to the hydration of mucus in the airways, leading to a low-viscosity mucus layer. This reduced viscosity is expected to improve mucociliary clearance, which may be beneficial for treating certain pulmonary diseases such as asthma [70]. Cordycepin from C. militaris inhibits NF-κB pathway, suppresses Th2 cytokines (IL-4, IL-5, IL-13), reduces eosinophilic inflammation, and improves airway hyperresponsiveness [71]. Cordyceps polysaccharides regulate Th1/Th2 balance, reduce IgE levels, and inhibit mast cell degranulation [72]. Cordyceps militaris polysaccharide (CMP) alleviated allergic asthma in ovalbumin-induced mice by reducing lung and gut inflammation, improving oxidative stress, and modulating Nrf2/HO-1 and NF-κB signaling pathways. CMP also restored gut microbiota composition and function, suggesting gut–lung axis involvement in its therapeutic effects [73].
4.3. Antiviral Activity
Exposure to environmental PM may be associated with increased vulnerability to viral infections [74]. It is reported that Cordyceps exerts antiviral activities owing to the presence of active compounds. Cordycepin, currently being tested in clinical trial NCT00709215, shares structural resemblance with adenosine but lacks a 3′ hydroxyl group in its ribose structure. This difference allows cordycepin to act as a poly(A) polymerase inhibitor, leading to the premature termination of protein synthesis. Given that functional RNAs of the SARS-CoV-2 genome are highly 3′-polyadenylated, cordycepin interferes with the synthesis of all viral proteins [45]. Steroids extracted from C. militaris have been recognized as crucial for controlling the cytokine storm associated with COVID-19 [46]. Cordyceps appears to be a safe immune booster for the treatment of patients with mild-to-moderate COVID-19 [75]. Ohta et al. investigated the anti-influenza effects of C. militaris and observed a significant reduction in virus titers in both the lung tissue and bronchoalveolar lavage fluid of mice administered intranasally with an acidic polysaccharide (APS) extracted from C. militaris [47]. C. guangdongensis exerted anti-influenza viral activity [44]. The C. militaris group exhibited notably higher NK cell activity (p = 0.047) and IgA levels (p = 0.035) than the placebo group [76]. C. militaris powder could potentially reduce lung inflammation and fibrosis triggered by the SARS-CoV-2 spike protein and LPS through the TGF-β R1/Smad2 signaling pathway [77].
4.4. Lung Cancer Treatment
Air pollution is the second most common cause of lung cancer. When combined with smoking, it has a synergistic effect that worsens lung cancer survival [78]. In studies on non-small cell lung cancer (NSCLC), C. sinensis has demonstrated potential anticancer properties. It has been reported to inhibit tumor growth and metastasis by affecting pathways, such as the MAPK pathway, which is involved in cell proliferation and survival (BioMed Central). Several studies have demonstrated that cordycepin (3′-deoxyadenosine), a key bioactive compound extracted from C. militaris, inhibits cancer cell proliferation [58,79]. Cordycepin blocks the growth of Lewis lung carcinoma cells in vitro by activating adenosine A3 receptors [58]. Cordycepin triggers apoptosis in human lung cancer cells by blocking the nitric oxide-stimulated ERK/Slug signaling pathway [50]. Cordycepin treatment increases apoptosis in human lung adenocarcinoma by upregulating Foxo3a via caveolin-1-mediated JNK signaling [51]. Cordycepin reduced the viability of A549 and PC9 human lung adenocarcinoma cells by suppressing LPS-induced expression of iNOS, NO, phospho-ERK (p-ERK), and Slug. It induced apoptosis by inhibiting the ERK/Slug pathway via GSK3β activation, leading to increased Bax and caspase-3 expression in lung cancer cells [50]. C. sinensis ameliorates non-small cell lung cancer by blocking the MAPK pathway [80]. C. sinensis aqueous extract enhances the antitumor efficacy of cisplatin while mitigating therapy-related toxic effects against non-small cell lung cancer through AKT/MMP2/MMP9 and NFkB pathways [81]. C. sinensis extract suppresses breast cancer cell metastasis by inhibiting the expression of metastasis-related cytokines [82]. A polysaccharide-rich extract from Cordyceps sinensis (CS) significantly enhanced the cytotoxic and pro-apoptotic effects of cisplatin in H157 non-small cell lung cancer (NSCLC) cells. The combination treatment also reduced VEGF and bFGF expression, suggesting that CS may serve as a potential adjuvant in NSCLC chemotherapy [83]. Cordyceps acid from C. sinensis exhibits a significant tumor-suppressing effect on lung cancer by modulating the Nrf-2/HO-1/NLRP3/NF-κB signaling pathway [60]. C. sinensis (hot-water extract) inhibits B16 melanoma cell lung metastasis [84].
4.5. Acute Lung Injury Treatment
Chronic (rather than short-term) exposure to low-to-moderate levels of air pollutants, such as O3, NO2, SO2, carbon monoxide (CO), and PM ≤ 2.5 μm in aerodynamic diameter (PM2.5), is linked to the onset of acute lung injury (ALI) [85]. C. sinensis and its active compound cordycepin could have an anti-inflammatory and antioxidant effect on LPS-induced ALI by suppressing NF-κB p65 phosphorylation, as well as the expression of COX-2 and iNOS in the lungs [61]. Cordycepin, a natural compound derived from C. militaris, was found to reduce lung edema, the production of inflammatory cytokines (TNF-α, IL-1β, and IL-6) and nitric oxide, MPO activity, and malondialdehyde (MDA) content in LPS-induced ALI mice [48,86]. Studies have examined the ability of C. fumosorosea to alleviate ALI. Metabolomic analysis in mice indicated that this species could reduce lung inflammation and improve overall lung health by altering various metabolic pathways and reducing oxidative stress [25]. According to the Medicinal Fungi Secondary Metabolite And Therapeutics (MeFSAT) database, miR-1321 and miR-3188, both present in C. militaris, target the 3′-UTR of CXCR2 mRNA, thereby inhibiting its translation in the lungs of bleomycin-treated mice [52]. Cordycepin treatment significantly inhibited the increase in the lung wet/dry weight ratio, MPO activity, MDA content, and inflammatory cytokine production in LPS-induced ALI mice. Moreover, cordycepin inhibited LPS-induced NF-κB activation and increased Nrf2 and HO-1 protein expression in a dose-dependent manner [52]. Treatment with cigarette smoke extract (CSE) increased cellular senescence and activated the ROS/PI3K/AKT/mTOR pathway in human bronchial epithelial (16HBE) cells, both of which were reduced by C. sinensis. Blocking this signaling pathway can reduce CSE-induced cellular senescence [57]. Cordyceps militaris-derived polysaccharides significantly alleviated LPS-induced ALI in mice by reducing inflammatory cytokines (e.g., TNF-α, IL-6) and oxidative stress markers. These effects are mediated by the suppression of NF-κB activation and enhancement of endogenous antioxidant defenses [87]. Cordyceps militaris (GCN) encapsulated in chitosan nanoparticles significantly alleviated PM2.5-induced lung injury in mice by reducing oxidative stress, suppressing inflammation, and restoring epithelial barrier integrity. GCN also enhanced antioxidant enzyme expression and inhibited ECM degradation, showing potential as a therapeutic agent for pollution-related lung diseases [88].
4.6. Idiopathic Pulmonary Fibrosis (IPF) Treatment
Numerous studies support a causal link between air pollution and idiopathic pulmonary fibrosis (IPF) [89]. Idiopathic pulmonary fibrosis is a chronic, irreversible, and debilitating lung disease characterized by the expansion of fibroblasts and myofibroblasts and abnormal buildup of the extracellular matrix in the interstitium, resulting in breathing difficulties [54]. A combined pharmacokinetic and pharmacological analysis revealed that Cordyceps extract and its active components, such as cordycepin and adenosine, exhibit antifibrogenic properties by inhibiting epithelial–mesenchymal transition (EMT) [54]. The anamorph of C. sinensis attenuates bleomycin-induced pulmonary inflammation and fibrosis in vivo [90]. The phenotypic transition of cobblestone-shaped epithelial cells into more mobile myofibroblasts, known as EMT, along with the formation of fibroblastic foci, are prominent characteristics of both experimental and human lung fibrosis, such as IPF [54].
C. sinensis enhances hypoxia tolerance in human lung epithelial cells by increasing the expression of Heme Oxygenase-1 and metallothionein via Nrf2 activation in human lung epithelial cells [91]. Cordyceps sinensis ameliorates BLM-induced pulmonary fibrosis by regulating mitochondrial oxidative phosphorylation and inhibiting mitochondrial ROS overproduction. This protective mechanism involves the modulation of mitochondrial complex expression and activity, thereby preserving mitochondrial integrity and function [92].
Ko’s group reported that C. sinensis and C. militaris enhance respiratory health by modulating the activity of the Na+–K+–2Cl− cotransporter and the CFTR Cl− channel. These mushrooms improve ion transport and fluid balance in the airway epithelium, offering potential therapeutic benefits for respiratory diseases [70].
Extracts of Cordyceps militaris containing cordycepic acid (D-mannitol) have been suggested to alleviate mucus hypersecretion by modulating several pathological mechanisms associated with airway inflammation and epithelial dysfunction. Although the direct effect of cordycepic acid on mucus production remains unclear, studies have shown that C. militaris extracts can reduce airway goblet cell hyperplasia and mucus-related gene expression in respiratory inflammation models, indicating their potential indirect role in regulating mucus overproduction [93,94].
4.7. Silicosis Treatment
Silicosis is a fibrotic lung disorder caused by occupational and air pollutant exposure and is prevalent worldwide, particularly in developing countries. Fermented C. sinensis may alleviate silica-triggered pulmonary inflammation and fibrosis by suppressing Th1 and Th17 immune responses and preventing the augmentation of Th2 responses [49]. Fermented C. sinensis powder (FCP) mitigates silica-induced pulmonary fibrosis by modulating immune responses. Specifically, FCP suppresses overactive Th1 and Th17 responses and inhibits the enhancement of Th2 responses, thereby reducing inflammation and fibrotic progression in the lungs [49].
5. Cordyceps Undergoing Clinical Trials
Enhanced NK cell activity was observed in healthy volunteers who were administered C. militaris capsules containing 7.3% cordycepic acid, 0.13% adenosine, 0.001% cordycepin, and 32% cordyceps polysaccharide, indicating that C. militaris could potentially serve as an immune-stimulating agent [23] (Table 2).

Table 2.
Ongoing clinical trials on the use of Cordyceps spp.
Cordyceps sinensis (Cs-4) improves lung function and enhances exercise performance in healthy older adults. After 12 weeks of Cs-4 supplementation, the metabolic threshold, where lactate begins to accumulate, increased by 10.5%, from 0.83 ± 0.06 to 0.93 ± 0.08 L/min (p < 0.02). Similarly, the ventilatory threshold, which reflects the point where unbuffered H+ stimulates ventilation, increased by 8.5%, from 1.25 ± 0.11 to 1.36 ± 0.15 L/min. These findings suggest that Cs-4 can enhance lung function by improving oxygen uptake, aerobic capacity, ventilatory efficiency, and fatigue resistance in older adults [98]. The administration of Bailing capsules (Cs-C-Q80) in patients with chronic bronchitis significantly reduced the frequency of acute exacerbations and improved key respiratory symptoms, such as expectoration and wheezing. The treatment was well tolerated, with no significant difference in adverse events compared to those in the placebo group. These findings suggest that Bailing capsules may be an effective and safe adjunct therapy for the management of chronic bronchitis [14]. The fruiting bodies of C. militaris have shown the potential to alleviate urinary symptoms and decrease the size of the prostate gland [95]. C. militaris extract is expected to be safely used as a functional food to protect against the progression of fatty liver or cirrhosis by suppressing the lipid accumulation of hepatocytes in patients with mild liver dysfunction [96]. Adjuvant treatment with CS for lung cancer not only improves the tumor response rate, quality of life, and immune function but also decreases the occurrence of adverse drug reactions (ADRs) and radiation pneumonitis [15]. Paecilomyces hepiali (CBG-CS-2) contains Cordyceps polysaccharides and adenosine, which play a crucial role in initiating immune responses and inducing immunomodulatory effects. This is achieved by enhancing NK cell activity and phagocyte reactions through activation [101].
Despite increasing clinical interest, the standardization of Cordyceps spp. formulations remains a challenge due to variability in strains, cultivation methods, and extraction techniques. To ensure reproducibility and clinical translation, future studies should report the exact content of bioactive compounds (e.g., cordycepin, adenosine, and polysaccharides) and adopt chemical fingerprinting and HPLC-based quantification for quality control purposes.
6. Cordyceps Species and Their Bioactive Substances Effective for Air Pollutant-Induced Vascular Diseases
Air pollutants have been linked to an increase in the incidence of various diseases, including cardiovascular and cerebrovascular diseases [102,103]. Among air pollutants, PM with a diameter of 10 μm or smaller (PM10) and PM2.5 have recently attracted significant attention due to their substantial impact on public health [104], respiratory disorders [105], and higher rates of neonatal mortality [106]. Cardiovascular and cerebrovascular diseases (CCVDs) are major global health concerns because of their high morbidity and mortality rates, primarily driven by atherosclerosis and blood clot formation. The key risk factors include PM, genetic predisposition, obesity, smoking, elevated cholesterol levels, and high blood pressure. Acute ischemic and reperfusion injuries in the brain (cerebral ischemia/reperfusion injury [CI/RI]) and heart (myocardial ischemia/reperfusion injury [MI/RI]) arise from reduced blood supply due to vascular stenosis or atherosclerosis. These injuries involve complex pathological processes, including autophagy, necrosis, apoptosis, inflammation, calcium overload, and oxidative stress. Moreover, clinical and experimental evidence has highlighted the link between brain injury and cardiac dysfunction, emphasizing the need for treatments targeting multiple injury mechanisms. Since the late 20th century, Chinese medicine has provided increasing evidence of the potential of Cordyceps in treating CCVDs. Preclinical and clinical studies suggest that Cordyceps and its artificial derivatives, such as Ningxinbao, Corbrin, and Jinshuibao capsules, offer protective benefits against ischemic conditions by addressing the molecular mechanisms involved in these diseases. C. militaris demonstrates strong potential as a natural antihypertensive agent by inhibiting angiotensin-converting enzyme (ACE) activity. C. militaris extract and its active compound, cordycepin, may act as natural ACE inhibitors with promising therapeutic potential for managing hypertension [107].
6.1. Antithrombosis
Particulate matter exposure leads to vascular injury, the release of adhesion molecules, platelet activation, and thrombin generation, collectively contributing to a prothrombotic state [108]. A novel serine protease, CSP, was purified from the culture supernatant of Cordyceps mycelia. CSP exhibits fibrinolytic activity and efficiently degrades fibrinogen, fibrin, and casein. It has the potential to be a therapeutic agent for thrombosis treatment [108]. The antithrombotic activity of the ethanol extract of cultured C. militaris CMEE is related to its antiplatelet rather than the anticoagulation effect in an FeCl3-induced arterial thrombosis model [109]. C. sinensis (Cs)-4 polysaccharides significantly reduced CD62P expression and αIIbβ3 activation on platelets while inhibiting collagen-induced platelet activation and aggregation [109]. WIB-801CE, a cordycepin-enriched extract from C. militaris, exhibited significant antiplatelet effects by inhibiting platelet aggregation induced by ADP, collagen, and thrombin. This effect was associated with reduced thromboxane A2 (TXA2) and serotonin release, achieved by suppressing cyclooxygenase-1, TXA2 synthase, and cytosolic phospholipase A2 activity. These findings indicate that WIB-801CE may help prevent thrombotic diseases by reducing platelet aggregation [110].
6.2. Anti-Atherosclerosis
Atherosclerosis remains a major global health challenge, prompting ongoing research on innovative treatments. Studies on hypercholesterolemic rabbits and ApoE-null mice have revealed that ambient PM exposure accelerates atherosclerosis, with smaller particles having stronger proatherogenic effects [111]. Cordyceps, as used in TCM, has shown potential as a therapeutic agent for atherosclerosis owing to its anti-inflammatory, antioxidant, cholesterol-lowering, and platelet aggregation-inhibiting properties, as well as its effects on apoptosis and autophagy in vascular endothelial cells [112]. Studies have emphasized the pharmacological role of adenosine and cordycepin in the treatment of atherosclerosis through their anti-inflammatory, antioxidant, hypolipidemic, immunomodulatory, antiplatelet aggregation, and vascular smooth muscle relaxation effects. Cordyceps contains key amino acids, including glutamic acid, arginine, and aspartic acid, and its mycelia are rich in proteins, peptides, and amino acids, such as glutamic acid, phenylalanine, and aspartic acid, which contribute to energy production and exert antithrombotic and vasodilatory effects associated with their potential to prevent and manage atherosclerosis.
6.3. Anticerebral Ischemic/Reperfusion Injury
Increasing evidence indicates that exposure to airborne fine PM2.5 is associated with an increased risk of ischemic stroke [113]. Cordycepin, a bioactive compound derived from C. militaris, has demonstrated strong neuroprotective effects in cerebral ischemia/reperfusion models. In both mice and brain slices, cordycepin reduced neuronal degeneration, reduced the levels of excitatory amino acids such as aspartate and glutamate, boosted SOD activity, and reduced MDA, thus decreasing oxidative stress. It also inhibited matrix metalloproteinase-3 (MMP-3), thereby reducing inflammation. These results indicate the potential of cordycepin as a neuroprotective agent against ischemic brain injury [114]. In a rat model of cerebral ischemia–reperfusion (IR), C. sinensis mycelium (CSM) demonstrated neuroprotective effects by significantly reducing inflammation. It inhibited NF-kappaB activation, decreased inflammatory markers (IL-1β, TNF-α, iNOS, ICAM-1, and COX-2), and prevented PMN cell infiltration, indicating its potential to protect against cerebral IR injury [115].
6.4. Arrhythmia
A large cohort study revealed that both short-term and chronic exposure to outdoor PM air pollutants is associated with a heightened risk of arrhythmia [116]. Cordyceps has gained attention for its cardiovascular effects, working through various mechanisms, such as the direct dilation of blood vessels or the activation of M-cholinergic receptors, leading to improved coronary and cerebral blood circulation. In addition, Cordyceps has the potential to treat cardiac arrhythmias by correcting abnormalities in rhythmic contractions, indicating its therapeutic value for cardiovascular health [117]. Thirteen trials involving 1164 participants studied the effects of Ningxinbao capsules plus routine drugs on tachyarrhythmia, comparing 586 participants in the experimental group with 578 participants in the control group who received only routine treatment [118,119,120]. All participants received standard care for their primary condition and symptom relief. Eleven of these trials reported total effectiveness rates, showing that the experimental group had a significantly higher effectiveness rate than the control group, although notable heterogeneity was observed across the studies. Five trials used the effectiveness rate as the primary outcome, and an analysis of these five trials (n = 461) demonstrated a significantly higher effectiveness rate in the Ningxinbao group than in the routine treatment group (RR = 1.24; 95% CI, 1.15–1.35; z = 5.42; p < 0.00001; I2 = 37%) [121]. Cordyceps has demonstrated a positive effect on arrhythmia treatment, mainly by regulating adrenergic signaling in cardiomyocytes and modulating the PI3K-Akt signaling pathway [119,122].
7. Conclusions
This review highlights the therapeutic potential of the Cordyceps genus in treating air pollutant-induced respiratory and vascular diseases. Compounds such as adenosine, cordycepin, nucleotides, polysaccharides, fatty acids, sterols, and cyclic peptides in Cordyceps exhibit anti-inflammatory, immunomodulatory, lung-protective, and antiviral effects and alleviate ischemic cardiovascular conditions, as demonstrated in preclinical and clinical studies. Cordyceps species are currently being investigated in clinical trials for their potential to improve lung function following air pollutant exposure, aid in COVID-19 treatment, and serve as supportive therapy for lung cancer, a condition that is often aggravated by air pollution. Additionally, Cordyceps demonstrates potential in addressing thrombosis, providing neuroprotection in cerebral ischemia–reperfusion injury, and managing arrhythmias, which may be exacerbated by air pollutants. Key findings include its fibrinolytic and antiplatelet effects in preventing thrombosis, the neuroprotective properties of cordycepin and C. sinensis mycelium (CSM) against ischemic injury, and the ability to modulate adrenergic signaling and the PI3K-Akt pathway in arrhythmia management. Further research is essential to isolate and validate these bioactive compounds through well-designed, high-quality clinical trials targeting diseases associated with air pollution.
Funding
This study was supported by Konkuk University in 2025.
Conflicts of Interest
The author declares no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of Open Access Journals |
| TLA | Three-letter acronym |
| LD | Linear dichroism |
References
- Kim, K.-H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef]
- Krishna, K.V.; Ulhas, R.S.; Malaviya, A. Bioactive compounds from Cordyceps and their therapeutic potential. Crit. Rev. Biotechnol. 2024, 44, 753–773. [Google Scholar] [CrossRef]
- Dhong, K.R.; Kwon, H.K.; Park, H.J. Immunostimulatory Activity of Cordyceps militaris Fermented with Pediococcus pentosaceus SC11 Isolated from a Salted Small Octopus in Cyclophosphamide-Induced Immunocompromised Mice and Its Inhibitory Activity against SARS-CoV 3CL Protease. Microorganisms 2022, 10, 2321. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, O.J.; Tang, J.; Tola, A.; Auberon, F.; Oluwaniyi, O.; Ouyang, Z. The genus Cordyceps: An extensive review of its traditional uses, phytochemistry and pharmacology. Fitoterapia 2018, 129, 293–316. [Google Scholar] [CrossRef]
- Sung, G.H.; Hywel-Jones, N.L.; Sung, J.M.; Luangsa-Ard, J.J.; Shrestha, B.; Spatafora, J.W. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud. Mycol. 2007, 57, 5–59. [Google Scholar] [CrossRef]
- Shrestha, B.; Sung, G.H.; Sung, J.M. Current nomenclatural changes in Cordyceps sensu lato and its multidisciplinary impacts. Mycology 2017, 8, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Li, S. Cordyceps as an Herbal Drug; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Tuli, H.S.; Sandhu, S.S.; Sharma, A.K. Pharmacological and therapeutic potential of Cordyceps with special reference to Cordycepin. 3 Biotech 2014, 4, 1–12. [Google Scholar] [CrossRef]
- Kanhere, M. Cordyceps Militaris Market Report 2025 (Global Edition), 8th ed.; Pharma & Healthcare, 2025; Available online: https://www.globenewswire.com/news-release/2025/03/26/3049439/28124/en/Cordyceps-Militaris-Ferment-Filtrate-Market-Report-2025-Clean-Beauty-and-Wellness-Trends-Driving-Industry-Growth-at-8-75-CAGR.html?pdf=1 (accessed on 2 June 2025).
- Park, D.K.; Hayashi, T.; Park, H.-J. Arabinogalactan-type polysaccharides (APS) from Cordyceps militaris grown on germinated soybeans (GSC) induces innate immune activity of THP-1 monocytes through promoting their macrophage differentiation and macrophage activity. Food Sci. Biotechnol. 2012, 21, 1501–1506. [Google Scholar] [CrossRef]
- Mollah, M.L.; Park, D.K.; Park, H.-J. Cordyceps militaris Grown on Germinated Soybean Induces G2/M Cell Cycle Arrest through Downregulation of Cyclin B1 and Cdc25c in Human Colon Cancer HT-29 Cells. Evid.-Based Complement. Altern. Med. 2012, 2012, 249217. [Google Scholar] [CrossRef]
- Park, D.K.; Choi, W.S.; Park, H.-J. Antiallergic Activity of Novel Isoflavone Methyl-glycosides from Cordyceps militaris Grown on Germinated Soybeans in Antigen-Stimulated Mast Cells. J. Agric. Food Chem. 2012, 60, 2309–2315. [Google Scholar] [CrossRef]
- Vivek-Ananth, R.P.; Sahoo, A.K.; Kumaravel, K.; Mohanraj, K.; Samal, A. MeFSAT: A curated natural product database specific to secondary metabolites of medicinal fungi. RSC Adv. 2021, 11, 2596–2607. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Xu, D.; Qu, Y.; Shang, X.; Qiao, K.; Feng, C.; Cui, H.; Zhao, X.; Li, Y.; Peng, Y.; et al. Efficacy and safety of Cordyceps sinensis (Hirsutella sinensis, Cs-C-Q80) in chronic bronchitis. Front. Pharmacol. 2024, 15, 1428216. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Qi, Y. Adjuvant treatment with Cordyceps sinensis for lung cancer: A systematic review and meta-analysis of randomized controlled trials. J. Ethnopharmacol. 2024, 327, 118044. [Google Scholar] [CrossRef]
- Qian, G.M.; Pan, G.F.; Guo, J.Y. Anti-inflammatory and antinociceptive effects of cordymin, a peptide purified from the medicinal mushroom Cordyceps sinensis. Nat. Prod. Res. 2012, 26, 2358–2362. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Shin, H.-S.; Leyva-Gómez, G.; Prado-Audelo, M.L.D.; Cortes, H.; Singh, Y.D.; Panda, M.K.; Mishra, A.P.; Nigam, M.; Saklani, S.; et al. Cordyceps spp.: A Review on Its Immune-Stimulatory and Other Biological Potentials. Front. Pharmacol. 2021, 11, 602364. [Google Scholar] [CrossRef]
- Chen, S.; Li, Z.; Krochmal, R.; Abrazado, M.; Kim, W.; Cooper, C.B. Effect of Cs-4® (Cordyceps sinensis) on Exercise Performance in Healthy Older Subjects: A Double-Blind, Placebo-Controlled Trial. J. Altern. Complement. Med. 2010, 16, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Shweta; Abdullah, S.; Komal; Kumar, A. A brief review on the medicinal uses of Cordyceps militaris. Pharmacol. Res.—Mod. Chin. Med. 2023, 7, 100228. [Google Scholar] [CrossRef]
- Yin, F.; Lin, P.; Yu, W.Q.; Shen, N.; Li, Y.; Guo, S.D. The Cordyceps militaris-Derived Polysaccharide CM1 Alleviates Atherosclerosis in LDLR((-/-)) Mice by Improving Hyperlipidemia. Front. Mol. Biosci. 2021, 8, 783807. [Google Scholar] [CrossRef]
- Piao, Z.; Yoo, J.-K.; Park, B.-W.; Seo, S.B.; Park, S.-J.; Jeon, H.-Y.; Rahman, M.M.; Kim, N.; Kim, S. Therapeutic effects of shibashin misena® against fine-dust-induced pulmonary disorders in mice. Biomed. Pharmacother. 2020, 125, 110018. [Google Scholar] [CrossRef]
- Seong, H.K.; Kim, M.J.; Fauziah, A.N.; Jeong, H.S.; Kim, H.J.; Yang, C.Y.; Park, S.J.; Bae, S.Y.; Jung, S.K. The Inhibitory Effects of Cordyceps militaris ARA301 Extract on Lipopolysaccharide-Induced Lung Injury in vivo. J. Microbiol. Biotechnol. 2025, 35, e2412043. [Google Scholar] [CrossRef]
- Ontawong, A.; Pengnet, S.; Thim-Uam, A.; Munkong, N.; Narkprasom, N.; Narkprasom, K.; Kuntakhut, K.; Kamkeaw, N.; Amornlerdpison, D. A randomized controlled clinical trial examining the effects of Cordyceps militaris beverage on the immune response in healthy adults. Sci. Rep. 2024, 14, 7994. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhou, X.; Dong, M.; Yang, L.; Zhao, C.; Lu, R.; Bao, G.; Hu, F. Metabolites and novel compounds with anti-microbial or antiaging activities from Cordyceps fumosorosea. AMB Express 2022, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Zhou, J.; Guo, Y.; Hou, D.; Pi, N.; Yang, Y.; Yu, H. Mechanism of Alleviating Acute Lung Injury in Mice from Serum Metabolomics Analysis of Cordyceps fumosorosea. Separations 2024, 11, 74. [Google Scholar] [CrossRef]
- Zha, L.-S.; Huang, S.-K.; Xiao, Y.-P.; Boonmee, S.; Eungwanichayapant, P.D.; McKenzie, E.; Kryukov, V.; Wu, X.-L.; Hyde, K.; Wen, T.-C. An Evaluation of Common Cordyceps (Ascomycetes) Species Found in Chinese Markets. Int. J. Med. Mushrooms 2018, 20, 1149–1162. [Google Scholar] [CrossRef]
- Liu, R.-M.; Zhang, X.-J.; Liang, G.-Y.; Yang, Y.-F.; Zhong, J.-J.; Xiao, J.-H. Antitumor and antimetastatic activities of chloroform extract of medicinal mushroom Cordyceps taii in mouse models. BMC Complement. Altern. Med. 2015, 15, 216. [Google Scholar] [CrossRef]
- Xiao, J.H.; Xiao, D.M.; Chen, D.X.; Xiao, Y.; Liang, Z.Q.; Zhong, J.J. Polysaccharides from the Medicinal Mushroom Cordyceps taii Show Antioxidant and Immunoenhancing Activities in a D-Galactose-Induced Aging Mouse Model. Evid. Based Complement. Altern. Med. 2012, 2012, 273435. [Google Scholar] [CrossRef]
- Xiao, J.H.; Sun, Z.H.; Pan, W.D.; Lü, Y.H.; Chen, D.X.; Zhong, J.J. Jiangxienone, a new compound with potent cytotoxicity against tumor cells from traditional Chinese medicinal mushroom Cordyceps jiangxiensis. Chem. Biodivers. 2012, 9, 1349–1355. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, Y.P.; Deng, Y.Y.; Zheng, R.; Zhong, Y.F.; Wang, L.; Du, L.P. Cordyceps cicadae extracts ameliorate renal malfunction in a remnant kidney model. J. Zhejiang Univ. Sci. B 2011, 12, 1024–1033. [Google Scholar] [CrossRef]
- Shao, L.; Jiang, S.; Li, Y.; Yu, L.; Liu, H.; Ma, L.; Yang, S. Aqueous extract of Cordyceps cicadae (Miq.) promotes hyaluronan synthesis in human skin fibroblasts: A potential moisturizing and anti-aging ingredient. PLoS ONE 2023, 18, e0274479. [Google Scholar] [CrossRef]
- Hsu, J.-H.; Chang, W.-J.; Fu, H.-I.; Chang, H.-H.; Chen, C.-C. Clinical evaluation of the short-term effects of Cordyceps cicadae mycelium in lowering intraocular pressure. J. Funct. Foods 2022, 95, 105177. [Google Scholar] [CrossRef]
- Yamada, H.; Kawaguchi, N.; Ohmori, T.; Takeshita, Y.; Taneya, S.-i.; Miyazaki, T. Structure and antitumor activity of an alkali-soluble polysaccharide from Cordyceps ophioglossoides. Carbohydr. Res. 1984, 125, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Kneifel, H.; Konig, W.A.; Loeffler, W.; Müller, R. Ophiocordin, an antifungal antibiotic of Cordyceps ophioglossoides. Arch. Microbiol. 1977, 113, 121–130. [Google Scholar] [CrossRef]
- Kwon, H.K.; Jo, W.R.; Park, H.J. Immune-enhancing activity of C. militaris fermented with Pediococcus pentosaceus (GRC-ON89A) in CY-induced immunosuppressed model. BMC Complement. Altern. Med. 2018, 18, 75. [Google Scholar] [CrossRef]
- Phull, A.R.; Dhong, K.R.; Park, H.J. Lactic Acid Bacteria Fermented Cordyceps militaris (GRC-SC11) Suppresses IgE Mediated Mast Cell Activation and Type I Hypersensitive Allergic Murine Model. Nutrients 2021, 13, 3849. [Google Scholar] [CrossRef]
- Park, H.J. Current Uses of Mushrooms in Cancer Treatment and Their Anticancer Mechanisms. Int. J. Mol. Sci. 2022, 23, 10502. [Google Scholar] [CrossRef]
- Lao, Y.; Zhang, M.; Li, Z.; Bhandari, B. A novel combination of enzymatic hydrolysis and fermentation: Effects on the flavor and nutritional quality of fermented Cordyceps militaris beverage. LWT 2020, 120, 108934. [Google Scholar] [CrossRef]
- Air pollution: A major threat to lung health. Lancet 2019, 393, 1774. [CrossRef] [PubMed]
- Kuehn, B.M. WHO: More than 7 million air pollution deaths each year. JAMA 2014, 311, 1486. [Google Scholar] [CrossRef]
- Pope, C.A., 3rd. Air pollution and health—Good news and bad. N. Engl. J. Med. 2004, 351, 1132–1134. [Google Scholar] [CrossRef]
- Davila Cordova, J.E.; Tapia Aguirre, V.; Vasquez Apestegui, V.; Ordoñez Ibarguen, L.; Vu, B.N.; Steenland, K.; Gonzales, G.F. Association of PM(2.5) concentration with health center outpatient visits for respiratory diseases of children under 5 years old in Lima, Peru. Environ. Health 2020, 19, 7. [Google Scholar] [CrossRef]
- Olivieri, D.; Scoditti, E. Impact of environmental factors on lung defences. Eur. Respir. Rev. 2005, 14, 51. [Google Scholar] [CrossRef]
- Yan, W.; Li, T.; Zhong, Z. Anti-inflammatory effect of a novel food Cordyceps guangdongensis on experimental rats with chronic bronchitis induced by tobacco smoking. Food Funct. 2014, 5, 2552–2557. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K. Cordycepin: A bioactive metabolite of Cordyceps militaris and polyadenylation inhibitor with therapeutic potential against COVID-19. J. Biomol. Struct. Dyn. 2022, 40, 3745–3752. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Verma, H.; Gera, N.; Baddipadige, R.; Choudhary, S.; Bhandu, P.; Silakari, O. Evaluation of Cordyceps militaris steroids as anti-inflammatory agents to combat the Covid-19 cytokine storm: A bioinformatics and structure-based drug designing approach. J. Biomol. Struct. Dyn. 2024, 42, 5159–5177. [Google Scholar] [CrossRef]
- Ohta, Y.; Lee, J.B.; Hayashi, K.; Fujita, A.; Park, D.K.; Hayashi, T. In vivo anti-influenza virus activity of an immunomodulatory acidic polysaccharide isolated from Cordyceps militaris grown on germinated soybeans. J. Agric. Food Chem. 2007, 55, 10194–10199. [Google Scholar] [CrossRef]
- Lei, J.; Wei, Y.; Song, P.; Li, Y.; Zhang, T.; Feng, Q.; Xu, G. Cordycepin inhibits LPS-induced acute lung injury by inhibiting inflammation and oxidative stress. Eur. J. Pharmacol. 2018, 818, 110–114. [Google Scholar] [CrossRef]
- Pu, S.; Yang, Z.; Zhang, X.; Li, M.; Han, N.; Yang, X.; He, J.; Yu, G.; Meng, X.; Jia, Q.; et al. Fermented cordyceps powder alleviates silica-induced pulmonary inflammation and fibrosis in rats by regulating the Th immune response. Chin. Med. 2023, 18, 131. [Google Scholar] [CrossRef]
- Hwang, J.H.; Park, S.J.; Ko, W.G.; Kang, S.M.; Lee, D.B.; Bang, J.; Park, B.J.; Wee, C.B.; Kim, D.J.; Jang, I.S.; et al. Cordycepin induces human lung cancer cell apoptosis by inhibiting nitric oxide mediated ERK/Slug signaling pathway. Am. J. Cancer Res. 2017, 7, 417–432. [Google Scholar]
- Joo, J.C.; Hwang, J.H.; Jo, E.; Kim, Y.R.; Kim, D.J.; Lee, K.B.; Park, S.J.; Jang, I.S. Cordycepin induces apoptosis by caveolin-1-mediated JNK regulation of Foxo3a in human lung adenocarcinoma. Oncotarget 2017, 8, 12211–12224. [Google Scholar] [CrossRef]
- Liu, S.; Tang, J.; Huang, L.; Xu, Q.; Ling, X.; Liu, J. Cordyceps militaris Alleviates Severity of Murine Acute Lung Injury Through miRNAs-Mediated CXCR2 Inhibition. Cell Physiol. Biochem. 2015, 36, 2003–2011. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Z.; Feng, Q.; Xu, Q.; Lü, L.; Liu, J.-K.; Zhang, L.; Wu, B.; Li, Y.-Q. Unusual Spirodecane Sesquiterpenes and a Fumagillol Analogue from Cordyceps ophioglossoides. Helv. Chim. Acta 2013, 96, 76–84. [Google Scholar] [CrossRef]
- Chen, M.; Cheung, F.W.; Chan, M.H.; Hui, P.K.; Ip, S.P.; Ling, Y.H.; Che, C.T.; Liu, W.K. Protective roles of Cordyceps on lung fibrosis in cellular and rat models. J. Ethnopharmacol. 2012, 143, 448–454. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Qiu, S.; Chen, J.; Zheng, Y. Effects of the exopolysaccharide fraction (EPSF) from a cultivated Cordyceps sinensis on immunocytes of H22 tumor bearing mice. Fitoterapia 2008, 79, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Feng, X.; Zheng, D.; Li, A.; Li, C.; Li, S.; Zhao, Z. Ergosterol attenuates cigarette smoke extract-induced COPD by modulating inflammation, oxidative stress and apoptosis in vitro and in vivo. Clin. Sci. 2019, 133, 1523–1536. [Google Scholar] [CrossRef]
- Liu, A.; Wu, J.; Li, A.; Bi, W.; Liu, T.; Cao, L.; Liu, Y.; Dong, L. The inhibitory mechanism of Cordyceps sinensis on cigarette smoke extract-induced senescence in human bronchial epithelial cells. Int. J. Chron. Obs. Pulmon Dis. 2016, 11, 1721–1731. [Google Scholar] [CrossRef]
- Nakamura, K.; Yoshikawa, N.; Yamaguchi, Y.; Kagota, S.; Shinozuka, K.; Kunitomo, M. Antitumor effect of cordycepin (3′-deoxyadenosine) on mouse melanoma and lung carcinoma cells involves adenosine A3 receptor stimulation. Anticance. Res. 2006, 26, 43–47. [Google Scholar]
- Bok, J.W.; Lermer, L.; Chilton, J.; Klingeman, H.G.; Towers, G.H.N. Antitumor sterols from the mycelia of Cordyceps sinensis. Phytochemistry 1999, 51, 891–898. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.; Li, W.; Shan, L. Cordyceps acid alleviates lung cancer in nude mice. J. Biochem. Mol. Toxicol. 2021, 35, e22670. [Google Scholar] [CrossRef]
- Fu, S.; Lu, W.; Yu, W.; Hu, J. Protective effect of Cordyceps sinensis extract on lipopolysaccharide-induced acute lung injury in mice. Biosci. Rep. 2019, 39, BSR20190789. [Google Scholar] [CrossRef]
- Heo, J.C.; Nam, S.H.; Nam, D.Y.; Kim, J.G.; Lee, K.G.; Yeo, J.H.; Yoon, C.S.; Park, C.H.; Lee, S.H. Anti-asthmatic activities in mycelial extract and culture filtrate of Cordyceps sphecocephala J201. Int. J. Mol. Med. 2010, 26, 351–356. [Google Scholar]
- Duan, R.R.; Hao, K.; Yang, T. Air pollution and chronic obstructive pulmonary disease. Chronic Dis. Transl. Med. 2020, 6, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Mao, Y.; Shergis, J.; Coyle, M.; Wu, L.; Chen, Y.; Zhang, T.; Lin, L.; Xue, C.; Xu, Y. Effectiveness and Safety of Oral Cordyceps sinensis on Stable COPD of GOLD Stages 2–3: Systematic Review and Meta-Analysis. Evid.-Based Complement. Altern. Med. 2019, 2019, 4903671. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jiao, X.; Wu, J.; Zhao, J.; Liu, T.; Xu, J.; Ma, X.; Cao, L.; Liu, L.; Liu, Y.; et al. Cordyceps sinensis inhibits airway remodeling in rats with chronic obstructive pulmonary disease. Exp. Ther. Med. 2018, 15, 2731–2738. [Google Scholar] [CrossRef]
- Sun, X.; Dong, Z.; Li, N.; Feng, X.; Liu, Y.; Li, A.; Zhu, X.; Li, C.; Zhao, Z. Nucleosides isolated from Ophiocordyceps sinensis inhibit cigarette smoke extract-induced inflammation via the SIRT1-nuclear factor-kappaB/p65 pathway in RAW264.7 macrophages and in COPD mice. Int. J. Chron. Obstr. Pulmon. Dis. 2018, 13, 2821–2832. [Google Scholar] [CrossRef]
- Park, B.J.; Dhong, K.R.; Park, H.J. Cordyceps militaris Grown on Germinated Rhynchosia nulubilis (GRC) Encapsulated in Chitosan Nanoparticle (GCN) Suppresses Particulate Matter (PM)-Induced Lung Inflammation in Mice. Int. J. Mol. Sci. 2024, 25, 10642. [Google Scholar] [CrossRef]
- Mong, L.S.; Ho, J.Y.; Dae, S.H.; Rae, J.B. Genomic Structure of the Cu,Zn Superoxide Dismutase (SOD1) Gene of Paecillomyces tenuipes and Paecilomyces sp. Int. J. Ind. Entomol. Biomater. 2005, 10, 35–43. [Google Scholar]
- Wang, X.; Xi, D.; Mo, J.; Wang, K.; Luo, Y.; Xia, E.; Huang, R.; Luo, S.; Wei, J.; Ren, Z.; et al. Cordycepin exhibits a suppressive effect on T cells through inhibiting TCR signaling cascade in CFA-induced inflammation mice model. Immunopharmacol. Immunotoxicol. 2020, 42, 119–127. [Google Scholar] [CrossRef]
- Yue, G.G.-L.; Lau, C.B.-S.; Fung, K.-P.; Leung, P.-C.; Ko, W.-H. Effects of Cordyceps sinensis, Cordyceps militaris and their isolated compounds on ion transport in Calu-3 human airway epithelial cells. J. Ethnopharmacol. 2008, 117, 92–101. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; He, Y.; Li, T.; Wang, W.; Zhang, J.; Wei, J.; Deng, Y.; Lin, R. Cordycepin alleviates airway hyperreactivity in a murine model of asthma by attenuating the inflammatory process. Int. Immunopharmacol. 2015, 26, 401–408. [Google Scholar] [CrossRef]
- Kuo, M.C.; Chang, C.Y.; Cheng, T.L.; Wu, M.J. Immunomodulatory effect of exo-polysaccharides from submerged cultured Cordyceps sinensis: Enhancement of cytokine synthesis, CD11b expression, and phagocytosis. Appl. Microbiol. Biotechnol. 2007, 75, 769–775. [Google Scholar] [CrossRef]
- Song, L.; Yang, J.; Kong, W.; Liu, Y.; Liu, S.; Su, L. Cordyceps militaris polysaccharide alleviates ovalbumin-induced allergic asthma through the Nrf2/HO-1 and NF-κB signaling pathways and regulates the gut microbiota. Int. J. Biol. Macromol. 2023, 238, 124333. [Google Scholar] [CrossRef]
- Rebuli, M.E.; Brocke, S.A.; Jaspers, I. Impact of inhaled pollutants on response to viral infection in controlled exposures. J. Allergy Clin. Immunol. 2021, 148, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Dubhashi, S.; Sinha, S.; Dwivedi, S.; Ghanekar, J.; Kadam, S.; Samant, P.; Datta, V.; Singh, S.; Chaudry, I.H.; Gurmet, P.; et al. Early Trends to Show the Efficacy of Cordyceps militaris in Mild to Moderate COVID Inflammation. Cureus 2023, 15, e43731. [Google Scholar] [CrossRef]
- Jung, S.J.; Hwang, J.H.; Oh, M.R.; Chae, S.W. Evaluation of the Efficacy of Ethanolic Extract of Cordyceps in Enhancing Immunity and Preventing Upper Respiratory Tract Infections in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled Study. J. Nutr. Health 2019, 52, 258–267. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Liu, X.-H.; Li, Y.; Yin, L.-J.; Cao, P.; Hou, Y.-Y. Cordyceps militaris capsules inhibit pulmonary inflammation and relieve pulmonary fibrosis. Mycosystema 2021, 40, 1820–1832. [Google Scholar] [CrossRef]
- Berg, C.D.; Schiller, J.H.; Boffetta, P.; Cai, J.; Connolly, C.; Kerpel-Fronius, A.; Kitts, A.B.; Lam, D.C.L.; Mohan, A.; Myers, R.; et al. Air Pollution and Lung Cancer: A Review by International Association for the Study of Lung Cancer Early Detection and Screening Committee. J. Thorac. Oncol. 2023, 18, 1277–1289. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Huang, Z.; Tan, X.; Chen, G. Proteomic detection of changes in protein expression induced by cordycepin in human hepatocellular carcinoma BEL-7402 cells. Methods Find Exp. Clin. Pharmacol. 2008, 30, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Zhou, L.; Chu, Z.; Song, Y.; Wang, Q.; Zhao, M.; Dai, C.; Chen, L.; Cheng, G.; Wang, J.; et al. Cordyceps sinensis relieves non-small cell lung cancer by inhibiting the MAPK pathway. Chin. Med. 2024, 19, 54. [Google Scholar] [CrossRef]
- Huo, X.; Liu, C.; Bai, X.; Li, W.; Li, J.; Hu, X.; Cao, L. Correction: Aqueous extract of Cordyceps sinensis potentiates the antitumor effect of DDP and attenuates therapy-associated toxicity in non-small cell lung cancer via IκBα/NFκB and AKT/MMP2/MMP9 pathways. RSC Adv. 2017, 7, 41796–41798. [Google Scholar] [CrossRef]
- Cai, H.; Li, J.; Gu, B.; Xiao, Y.; Chen, R.; Liu, X.; Xie, X.; Cao, L. Extracts of Cordyceps sinensis inhibit breast cancer cell metastasis via down-regulation of metastasis-related cytokines expression. J. Ethnopharmacol. 2018, 214, 106–112. [Google Scholar] [CrossRef]
- Ji, N.F.; Yao, L.S.; Li, Y.; He, W.; Yi, K.S.; Huang, M. Polysaccharide of Cordyceps sinensis enhances cisplatin cytotoxicity in non-small cell lung cancer H157 cell line. Integr. Cancer Ther. 2011, 10, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Wan, R.; Kobayashi, M.; Tamesada, M. Effect of the hot-water extract of Cordyceps sinensis on lung metastasis of B16 melanoma cells. J. Tradit. Med. 2006, 23, 83–87. [Google Scholar] [CrossRef]
- Reilly, J.P.; Zhao, Z.; Shashaty, M.G.S.; Koyama, T.; Christie, J.D.; Lanken, P.N.; Wang, C.; Balmes, J.R.; Matthay, M.A.; Calfee, C.S.; et al. Low to Moderate Air Pollutant Exposure and Acute Respiratory Distress Syndrome after Severe Trauma. Am. J. Respir. Crit. Care Med. 2019, 199, 62–70. [Google Scholar] [CrossRef] [PubMed]
- He, Y.-Q.; Zhou, C.-C.; Yu, L.-Y.; Wang, L.; Deng, J.-l.; Tao, Y.-L.; Zhang, F.; Chen, W.-S. Natural product derived phytochemicals in managing acute lung injury by multiple mechanisms. Pharmacol. Res. 2021, 163, 105224. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, K.; Zhang, J.; Xu, G.; Guo, Z.; Lu, X.; Liang, C.; Gu, X.; Huang, L.; Liu, S.; et al. Cordyceps militaris solid medium extract alleviates lipopolysaccharide-induced acute lung injury via regulating gut microbiota and metabolism. Front. Immunol. 2024, 15, 1528222. [Google Scholar] [CrossRef]
- Kim, H.-M.; Kim, J.-H.; Park, B.-J.; Park, H.-J. Chitosan Nanoparticle-Encapsulated Cordyceps militaris Grown on Germinated Rhynchosia nulubilis Reduces Type II Alveolar Epithelial Cell Apoptosis in PM2.5-Induced Lung Injury. Int. J. Mol. Sci. 2025, 26, 1105. [Google Scholar] [CrossRef]
- Majewski, S.; Piotrowski, W.J. Air Pollution—An Overlooked Risk Factor for Idiopathic Pulmonary Fibrosis. J. Clin. Med. 2020, 10, 77. [Google Scholar] [CrossRef]
- Huang, T.-T.; Lai, H.-C.; Ko, Y.-F.; Ojcius, D.M.; Lan, Y.-W.; Martel, J.; Young, J.D.; Chong, K.-Y. Hirsutella sinensis mycelium attenuates bleomycin-induced pulmonary inflammation and fibrosis in vivo. Sci. Rep. 2015, 5, 15282. [Google Scholar] [CrossRef]
- Singh, M.; Tulsawani, R.; Koganti, P.; Chauhan, A.; Manickam, M.; Misra, K. Cordyceps sinensis increases hypoxia tolerance by inducing heme oxygenase-1 and metallothionein via Nrf2 activation in human lung epithelial cells. Biomed. Res. Int. 2013, 2013, 569206. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, L.; Cheng, G.; Zhou, Y.; Guo, Q.; Wu, J.; Wong, Y.; Zhang, J.; Tang, H.; Wang, J. Cordyceps sinensis ameliorates idiopathic pulmonary fibrosis in mice via inhibiting mitochondrion-mediated oxidative stress. MedComm—Future Med. 2024, 3, e91. [Google Scholar] [CrossRef]
- Daviskas, E.; Anderson, S.D.; Jaques, A.; Charlton, B. Inhaled mannitol improves the hydration and surface properties of sputum in patients with cystic fibrosis. Chest 2010, 137, 861–868. [Google Scholar] [CrossRef]
- Chen, J.; Chan, W.M.; Leung, H.Y.; Leong, P.K.; Yan, C.T.M.; Ko, K.M. Anti-Inflammatory Effects of a Cordyceps sinensis Mycelium Culture Extract (Cs-4) on Rodent Models of Allergic Rhinitis and Asthma. Molecules 2020, 25, 4051. [Google Scholar] [CrossRef]
- Hsieh, S.-A.; Lin, T.-H.; Wang, J.-S.; Chen, J.-J.; Hsu, W.-K.; Ying, L.-C.; Liang, Z.-C. The effects of Cordyceps militaris fruiting bodies in micturition and prostate size in benign prostatic hyperplasia patients: A pilot study. Pharmacol. Res.—Mod. Chin. Med. 2022, 4, 100143. [Google Scholar] [CrossRef]
- Heo, J.Y.; Baik, H.W.; Kim, H.J.; Lee, J.M.; Kim, H.W.; Choi, Y.S. The Efficacy and Safety of Cordyceps militaris in Korean Adults Who Have Mild Liver Dysfunction. J. Clin. Nutr. 2015, 7, 81–86. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, G.; Zhang, C.; Tang, G.; Chan, Y.; Wang, N.; Feng, Y. Randomized, waitlist-controlled trial of Cordyceps sinensis mycelium culture extract (Cs4) for long COVID patients in Hong Kong. Acta Mater. Medica 2025, 4, 250–261. [Google Scholar] [CrossRef]
- Yi, X.; Xi-zhen, H.; Jia-shi, Z. Randomized double-blind placebo-controlled clinical trial and assessment of fermentation product of Cordyceps sinensis (Cs-4) in enhancing aerobic capacity and respiratory function of the healthy elderly volunteers. Chin. J. Integr. Med. 2004, 10, 187–192. [Google Scholar] [CrossRef]
- Wang, N.-q.; Jiang, L.-d.; Zhang, X.; Li, Z.-x. Effect of Dongchong Xiacao capsule on airway inflammation of asthmatic patients. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Medica 2007, 32, 1566–1568. [Google Scholar]
- Ma, Y.; Chen, Y.; Zhang, N.; Xu, G.; Wang, Y.; Sun, Y.; Bai, C.; Zuo, Z. Efficacy and safety of pulmonary rehabilitation training on lung function, quality of life, and T cell immune function in patients with stable chronic obstructive pulmonary disease: A randomized controlled trial. Ann. Palliat. Med. 2022, 11, 1774–1785. [Google Scholar] [CrossRef]
- Jung, S.-J.; Jung, E.-S.; Choi, E.-K.; Sin, H.-S.; Ha, K.-C.; Chae, S.-W. Immunomodulatory effects of a mycelium extract of Cordyceps (Paecilomyces hepiali; CBG-CS-2): A randomized and double-blind clinical trial. BMC Complement. Altern. Med. 2019, 19, 77. [Google Scholar] [CrossRef]
- Hong, Y.-C.; Lee, J.-T.; Kim, H.; Ha, E.-H.; Schwartz, J.; Christiani, D.C. Effects of air pollutants on acute stroke mortality. Environ. Health Perspect. 2002, 110, 187–191. [Google Scholar] [CrossRef]
- Ostro, B.; Malig, B.; Broadwin, R.; Basu, R.; Gold, E.B.; Bromberger, J.T.; Derby, C.; Feinstein, S.; Greendale, G.A.; Jackson, E.A. Chronic PM2.5 exposure and inflammation: Determining sensitive subgroups in mid-life women. Environ. Res. 2014, 132, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, K.; Li, D.; Zhang, Y.; Liu, X.; Wu, K. Effects of fine particulate matter on the ocular surface: An in vitro and in vivo study. Biomed. Pharmacother. 2019, 117, 109177. [Google Scholar] [CrossRef] [PubMed]
- Tecer, L.H.; Alagha, O.; Karaca, F.; Tuncel, G.; Eldes, N. Particulate matter (PM2.5, PM10-2.5, and PM10) and children’s hospital admissions for asthma and respiratory diseases: A bidirectional case-crossover study. J. Toxicol. Environ. Health Part A 2008, 71, 512–520. [Google Scholar] [CrossRef]
- Son, J.-Y.; Lee, H.J.; Koutrakis, P.; Bell, M.L. Pregnancy and lifetime exposure to fine particulate matter and infant mortality in Massachusetts, 2001–2007. Am. J. Epidemiol. 2017, 186, 1268–1276. [Google Scholar] [CrossRef]
- Valdez-Solana, M.A.; Corral-Guerrero, I.A.; Téllez-Valencia, A.; Avitia-Domínguez, C.; Meza-Velázquez, J.A.; de Casa, A.G.; Sierra-Campos, E. Cordyceps militaris Inhibited Angiotensin-Converting Enzyme through Molecular Interaction between Cordycepin and ACE C-Domain. Life 2022, 12, 1450. [Google Scholar] [CrossRef]
- Li, H.P.; Hu, Z.; Yuan, J.L.; Fan, H.D.; Chen, W.; Wang, S.J.; Zheng, S.S.; Zheng, Z.L.; Zou, G.L. A novel extracellular protease with fibrinolytic activity from the culture supernatant of Cordyceps sinensis: Purification and characterization. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2007, 21, 1234–1241. [Google Scholar] [CrossRef]
- Choi, E.; Oh, J.; Sung, G.H. Antithrombotic and Antiplatelet Effects of Cordyceps militaris. Mycobiology 2020, 48, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Ok, W.; Nam, G.; Kim, M.; Kwon, H.-W.; Kim, H.-H.; Shin, J.-H.; Lim, D.; Kwon, H.-K.; Lee, C.-H.; Chung, S.-H.; et al. Antiplatelet Effects of Cordycepin-Enriched WIB-801CE from Cordyceps militaris: Involvement of Thromboxane A 2, Serotonin, Cyclooxygenase-1, Thromboxane A 2 Synthase, Cytosolic Phospholipase A 2. Biomed. Sci. Lett. 2016, 22, 127–139. [Google Scholar] [CrossRef]
- Araujo, J.A.; Nel, A.E. Particulate matter and atherosclerosis: Role of particle size, composition and oxidative stress. Part. Fibre Toxicol. 2009, 6, 24. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.J. Cordyceps as potential therapeutic agents for atherosclerosis. J. Integr. Med. 2024, 22, 102–114. [Google Scholar] [CrossRef]
- Gao, L.; Qin, J.X.; Shi, J.Q.; Jiang, T.; Wang, F.; Xie, C.; Gao, Q.; Zhi, N.; Dong, Q.; Guan, Y.T. Fine particulate matter exposure aggravates ischemic injury via NLRP3 inflammasome activation and pyroptosis. CNS Neurosci. Ther. 2022, 28, 1045–1058. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; He, W.; Zhou, X.; Lv, Q.; Xu, X.; Yang, S.; Zhao, C.; Guo, L. Cordycepin protects against cerebral ischemia/reperfusion injury in vivo and in vitro. Eur. J. Pharmacol. 2011, 664, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, P.; Zhao, D.; Tang, H.; Guo, J. Anti-inflammation effects of Cordyceps sinensis mycelium in focal cerebral ischemic injury rats. Inflammation 2011, 34, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Kang, J.; Hong, Y.S.; Chang, Y.; Ryu, S.; Park, J.; Cho, J.; Guallar, E.; Shin, H.C.; Zhao, D. Long-Term Particulate Matter Exposure and Incidence of Arrhythmias: A Cohort Study. J. Am. Heart Assoc. 2020, 9, e016885. [Google Scholar] [CrossRef]
- Zhu, J.-S.; Halpern, G.M.; Jones, K. The scientific rediscovery of a precious ancient Chinese herbal regimen: Cordyceps sinensis Part II. J. Altern. Complement. Med. 1998, 4, 429–457. [Google Scholar] [CrossRef]
- Tao, Y.; Zhao, X.; Liu, J.; Ren, S.; Zhou, W. Clinical application of Ningxinbao capsule combined with amiodarone in unstable angina pectoris with ventricular arrhythmias. Hainan Med. 2014, 25, 2266–2268. [Google Scholar]
- Wang, L.; Sun, H.; Yang, M.; Xu, Y.; Hou, L.; Yu, H.; Wang, X.; Zhang, Z.; Han, J. Bidirectional regulatory effects of Cordyceps on arrhythmia: Clinical evaluations and network pharmacology. Front. Pharmacol. 2022, 13, 948173. [Google Scholar] [CrossRef]
- Li, Y.; He, L.; Song, H.; Bao, X.; Niu, S.; Bai, J.; Ma, J.; Yuan, R.; Liu, S.; Guo, J. Cordyceps: Alleviating ischemic cardiovascular and cerebrovascular injury—A comprehensive review. J. Ethnopharmacol. 2024, 332, 118321. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, J. Treatment of Bradyarrhythmia with Ningxinbao Capsules. Mod. Distance Educ. Tradit. Chin. Med. China 2009, 7, 108. [Google Scholar] [CrossRef]
- Jia, J.-d.; Li, Y.-F.; Xiao, M.; Jiang, X.; Xiu, S.-Y.; Wang, J. Systematic review and Meta-analysis of efficacy and safety of Ningxinbao Capsules in treatment of arrhythmia. Zhongguo Zhong Yao Za Zhi 2021, 46, 1260–1267. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).