Extracellular Vesicles Mediate Epimastigogenesis in Trypanosoma cruzi: Strain-Specific Dynamics and Temperature-Dependent Differentiation
Abstract
1. Introduction
2. Methods
2.1. Parasite Cultivation
2.2. In Vitro Epimastigogenesis
2.3. Infection Assays
2.4. Isolation and Characterisation of Extracellular Vesicles
2.5. Epimastigogenesis in the Presence of EVs
2.6. Morphological Analysis
2.7. Statistical Analysis
3. Results
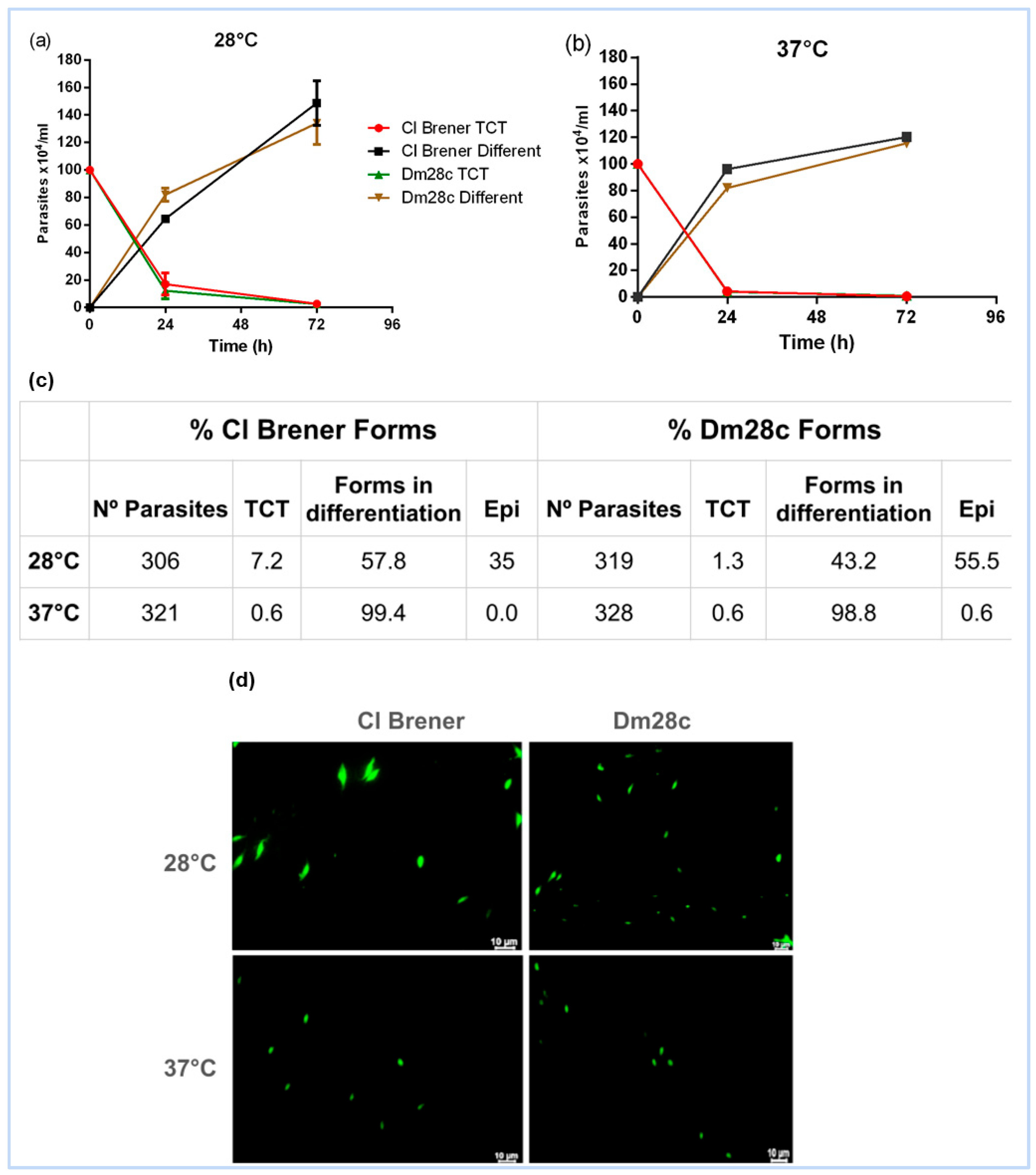
3.1. Epimastigogenesis: A Temperature-Driven Transformation
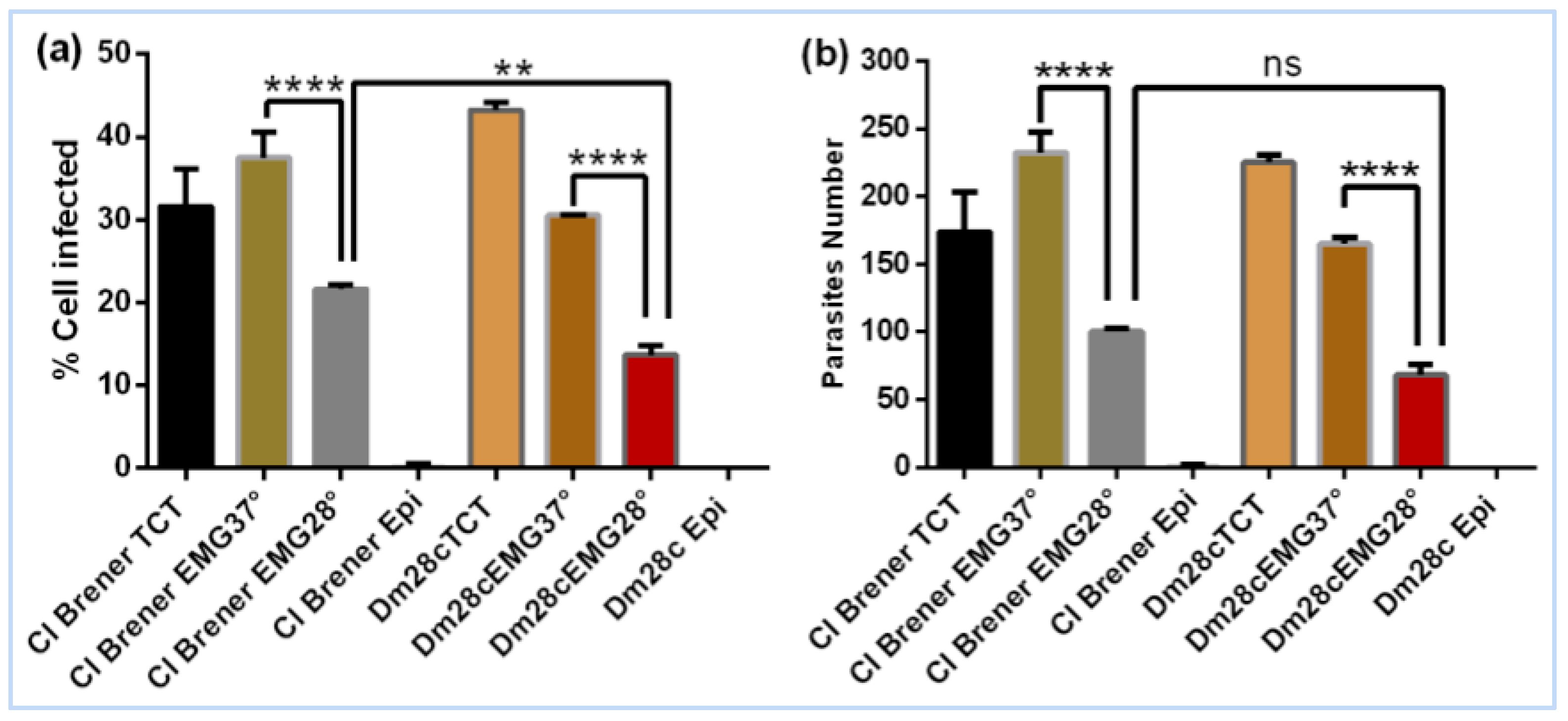
3.2. Evaluation of the Invasive Capacity of T. cruzi CL Brener and Dm28c After Epimastigogenesis
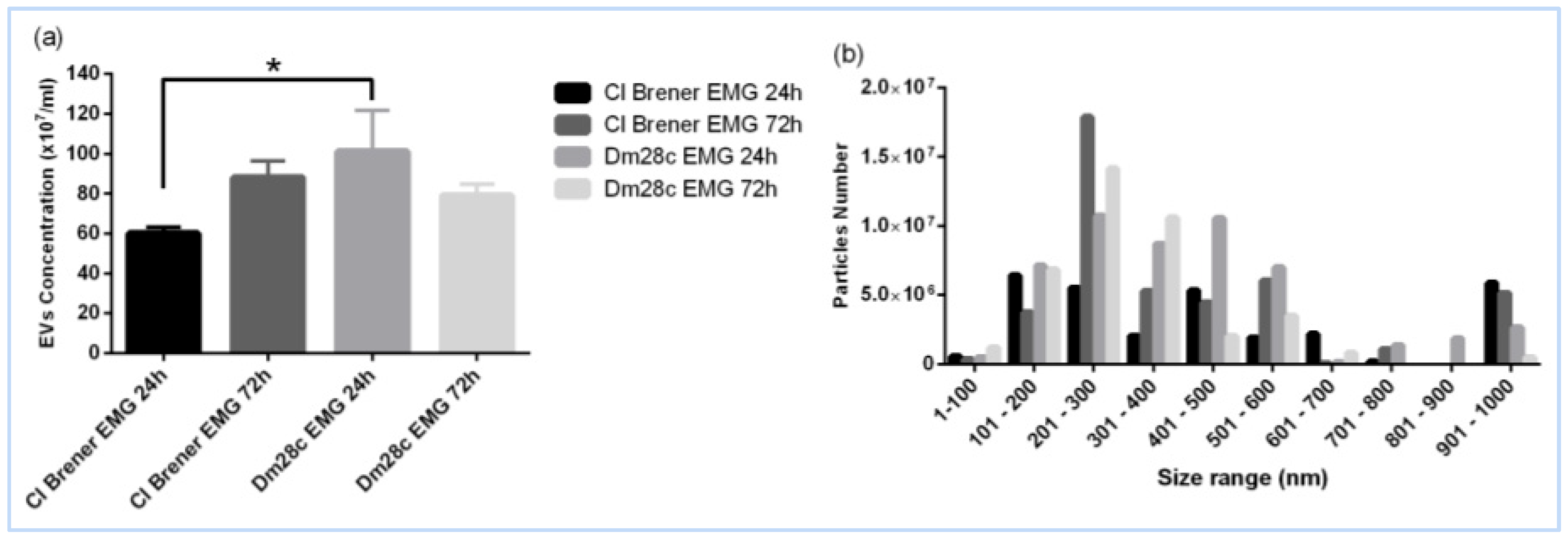
3.3. Extracellular Vesicles: Key Players in Epimastigogenesis
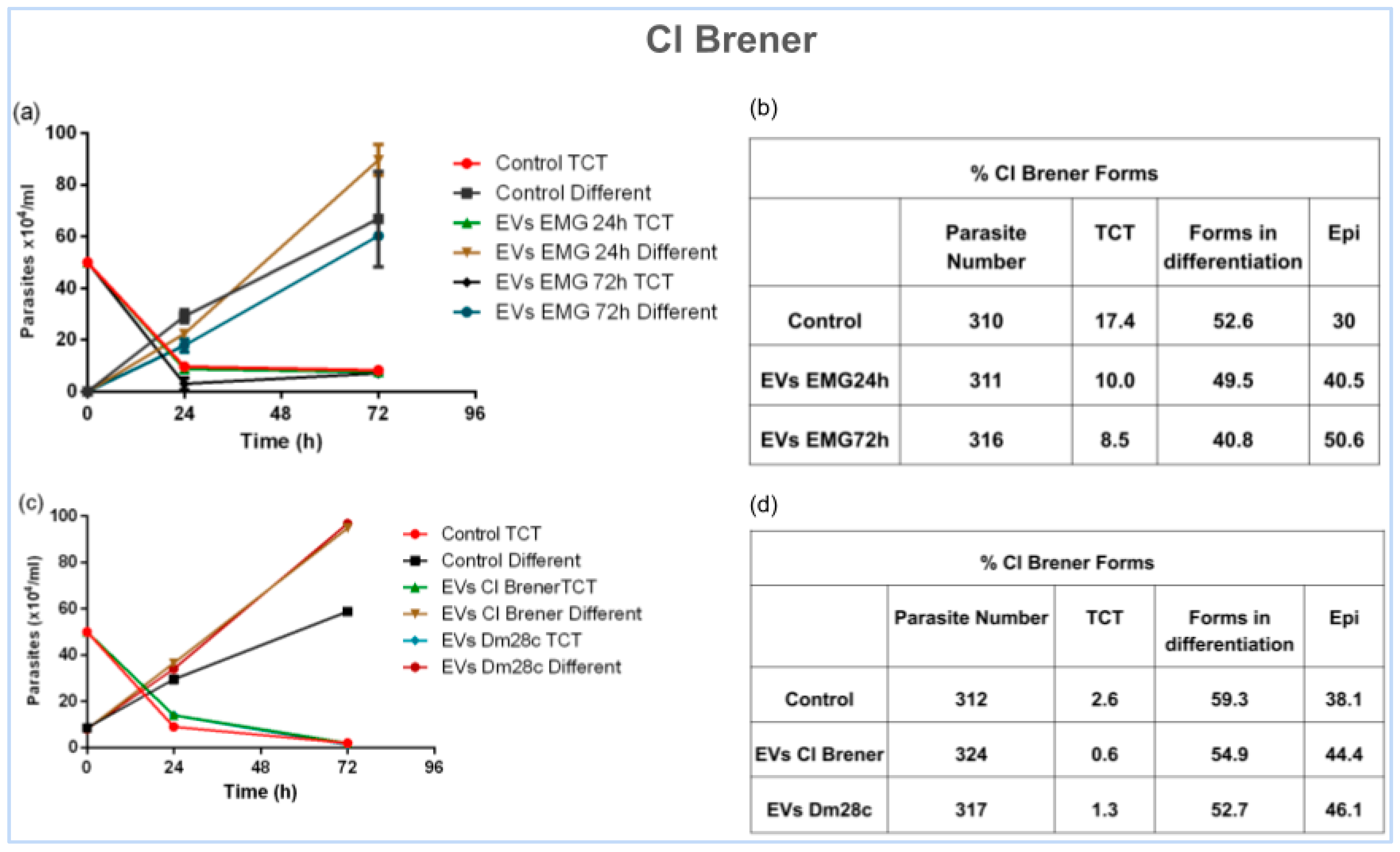
3.4. Unveiling the Role of EVs in Parasite Differentiation
4. Discussion
4.1. Temperature as a Key Driver of Epimastigogenesis
4.2. Loss of Invasive Capacity During Epimastigogenesis
4.3. Extracellular Vesicles: Mediators of Differentiation
4.4. Heterogeneity of EVs in Epimastigogenesis
4.5. Pioneering Evidence of EVs in Epimastigogenesis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torrecilhas, A.C.; Soares, R.P.; Schenkman, S.; Fernández-Prada, C.; Olivier, M. Extracellular vesicles in trypanosomatids: Host cell communication. Front. Cell. Infect. Microbiol. 2020, 10, 602502. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.F.P.; Guarneri, A.A.; Silber, A.M. The influence of environmental cues on the development of Trypanosoma cruzi in Triatominae vector. Front. Cell. Infect. Microbiol. 2020, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, D.P.; de Souza, W. The kinetoplast of trypanosomatids: From early studies of electron microscopy to recent advances in atomic force microscopy. Scanning 2018, 2018, 9603051. [Google Scholar] [CrossRef]
- Silva, B.; Gregorio, J.; Contreras, U.L.; Graterol, R.; Aguilera, N.; Navarro, M.C.; Contreras, A.; de Lima, R. Trypanosoma cruzi: Epimastigogenesis en condiciones axénicas. Cambios morfológicos, peptídicos, glicopeptídicos y antigénicos. Parasitol. Acta Científica Venez. 2008, 59, 1–11. [Google Scholar]
- Ferreira, A.Z.L.; de Araújo, C.N.; Cardoso, I.C.C.; de Souza Mangabeira, K.S.; Rocha, A.P.; Charneau, S.; Santana, J.M.; Motta, F.N.; Bastos, I.M.D. Metacyclogenesis as the starting point of Chagas disease. Int. J. Mol. Sci. 2023, 25, 117. [Google Scholar] [CrossRef] [PubMed]
- Rey, L. Parasitologia: Parasitas e Doenças Parasitárias do Homem nos Trópicos Ocidentais, 4th ed.; Guanabara Koogan: Rio de Janeiro, Brazil, 2008. [Google Scholar]
- Cestari, I.; Evans-Osses, I.; Schlapbach, L.J.; de Messias-Reason, I.; Ramirez, M.I. Mechanisms of complement lectin pathway activation and resistance by trypanosomatid parasites. Mol. Immunol. 2013, 53, 328–334. [Google Scholar] [CrossRef]
- De Lima, A.R.; Aparicio, A.; Berrocal, A.; Navarro, M.C.; Graterol, D.; Contreras, V.T. Epimastigogénesis de Trypanosoma cruzi en medio axénico: Cambios peptídicos, glicopeptídicos y enzimáticos. Salus 2007, 11, 39–47. [Google Scholar]
- Graterol, D.; Arteaga, R.Y.; Navarro, M.C.; Domínguez, M.; de Lima, A.R.; Contreras, V.T. El estadio amastigota precede la evolución del epimastigota durante la epimastigogénesis in vitro de Trypanosoma cruzi. Rev. Soc. Venez. Microbiol. 2013, 33, 72–79. [Google Scholar]
- Kessler, R.L.; Contreras, V.T.; Marliére, N.P.; Guarneri, A.A.; Silva, L.H.V.; Mazzarotto, G.A.C.A.; Batista, M.; Soccol, V.T.; Krieger, M.A.; Probst, C.M. Recently differentiated epimastigotes from Trypanosoma cruzi are infective to the mammalian host. Mol. Microbiol. 2017, 104, 712–736. [Google Scholar] [CrossRef]
- Hill, A.F. Extracellular vesicles and neurodegenerative diseases. J. Neurosci. 2019, 39, 9269–9273. [Google Scholar] [CrossRef]
- Urabe, F.; Kosaka, N.; Ito, K.; Kimura, T.; Egawa, S.; Ochiya, T. Extracellular vesicles as biomarkers and therapeutic targets for cancer. Am. J. Physiol. 2020, 318, C29–C39. [Google Scholar] [CrossRef] [PubMed]
- Salas, N.; Blasco Pedreros, M.; Dos Santos Melo, T.; Maguire, V.G.; Sha, J.; Wohlschlegel, J.A.; Pereira-Neves, A.; de Miguel, N. Role of cytoneme structures and extracellular vesicles in Trichomonas vaginalis parasite-parasite communication. eLife 2023, 12, e86067. [Google Scholar] [CrossRef] [PubMed]
- Kochanowsky, J.A.; Mira, P.M.; Elikaee, S.; Muratore, K.; Rai, A.K.; Riestra, A.M.; Johnson, P.J. Trichomonas vaginalis extracellular vesicles up-regulate and directly transfer adherence factors promoting host cell colonisation. Proc. Natl. Acad. Sci. USA 2024, 121, e2401159121. [Google Scholar] [CrossRef]
- Cestari, I.; Ansa-Addo, E.; Deolindo, P.; Inal, J.M.; Ramirez, M.I. Trypanosoma cruzi immune evasion mediated by host cell-derived microvesicles. J. Immunol. 2012, 188, 1942–1952. [Google Scholar] [CrossRef]
- Garcia-Silva, M.R.; das Neves, R.F.; Cabrera-Cabrera, F.; Sanguinetti, J.; Medeiros, L.C.; Robello, C.; Naya, H.; Fernandez-Calero, T.; Souto-Padron, T.; de Souza, W.; et al. Extracellular vesicles shed by Trypanosoma cruzi are linked to small RNA pathways, life cycle regulation, and susceptibility to infection of mammalian cells. Parasitol. Res. 2014, 113, 285–304. [Google Scholar] [CrossRef]
- Cortes-Serra, N.; Gualdron-Lopez, M.; Pinazo, M.J.; Torrecilhas, A.C.; Fernandez-Becerra, C. Extracellular Vesicles in Trypanosoma cruzi Infection: Immunomodulatory Effects and Future Perspectives as Potential Control Tools against Chagas Disease. J. Immunol. Res. 2022, 2022, 5230603. [Google Scholar] [CrossRef] [PubMed]
- Reis-Cunha, J.L.; Baptista, R.P.; Rodrigues-Luiz, G.F.; Coqueiro-Dos-Santos, A.; Valdivia, H.O.; de Almeida, L.V.; Cardoso, M.S.; D’Ávila, D.A.; Dias, F.H.C.; Fujiwara, R.T.; et al. Whole genome sequencing of Trypanosoma cruzi field isolates reveals extensive genomic variability and complex aneuploidy patterns within TcII DTU. BMC Genom. 2018, 19, 816. [Google Scholar] [CrossRef] [PubMed]
- Cronemberger-Andrade, A.; Xander, P.; Soares, R.P.; Pessoa, N.L.; Campos, M.A.; Ellis, C.C.; Grajeda, B.; Ofir-Birin, Y.; Almeida, I.C.; Regev-Rudzki, N.; et al. Trypanosoma cruzi-Infected Human Macrophages Shed Proinflammatory Extracellular Vesicles That Enhance Host-Cell Invasion via Toll-Like Receptor 2. Front. Cell Infect. Microbiol. 2020, 10, 99. [Google Scholar] [CrossRef]
- Retana Moreira, L.; Prescilla-Ledezma, A.; Cornet-Gomez, A.; Linares, F.; Jódar-Reyes, A.B.; Fernandez, J.; Ibarrola Vannucci, A.K.; De Pablos, L.M.; Osuna, A. Biophysical and Biochemical Comparison of Extracellular Vesicles Produced by Infective and Non-Infective Stages of Trypanosoma cruzi. Int. J. Mol. Sci. 2021, 22, 5183. [Google Scholar] [CrossRef]
- Rossi, I.V.; de Almeida, R.F.; Sabatke, B.; de Godoy, L.M.F.; Ramirez, M.I. Trypanosoma cruzi interaction with host tissues modulate the composition of large extracellular vesicles. Sci. Rep. 2024, 14, 5000. [Google Scholar] [CrossRef]
- Sana, A.; Rossi, I.V.; Sabatke, B.; Bonato, L.B.; Medeiros, L.C.S.; Ramirez, M.I. An Improved Method to Enrich Large Extracellular Vesicles Derived from Giardia intestinalis through Differential Centrifugation. Life 2023, 13, 1799. [Google Scholar] [CrossRef] [PubMed]
- Lander, N.; Chiurillo, M.A.; Docampo, R. Signalling pathways involved in environmental sensing in Trypanosoma cruzi. Mol. Microbiol. 2021, 115, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Lander, N.; Chiurillo, M.A.; Bertolini, M.S.; Storey, M.; Vercesi, A.E.; Docampo, R. Calcium-sensitive pyruvate dehydrogenase phosphatase is required for energy metabolism, growth, differentiation, and infectivity of Trypanosoma cruzi. J. Biol. Chem. 2018, 293, 17402–17417. [Google Scholar] [CrossRef] [PubMed]
- Barisón, M.J.; Rapado, L.N.; Merino, E.F.; Furusho Pral, E.M.; Mantilla, B.S.; Marchese, L.; Nowicki, C.; Silber, A.M.; Cassera, M.B. Metabolomic profiling reveals a finely tuned, starvation-induced metabolic switch in Trypanosoma cruzi epimastigotes. J. Biol. Chem. 2017, 292, 8964–8977. [Google Scholar] [CrossRef] [PubMed]
- Castro, D.P.; Moraes, C.S.; Gonzalez, M.S.; Ratcliffe, N.A.; Azambuja, P.; Garcia, E.S. Trypanosoma cruzi immune response modulation decreases microbiota in Rhodnius prolixus gut and is crucial for parasite survival and development. PLoS ONE 2012, 7, e36591. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sana, A.; Rossi, I.V.; Sabatke, B.; Ramirez, M.I. Extracellular Vesicles Mediate Epimastigogenesis in Trypanosoma cruzi: Strain-Specific Dynamics and Temperature-Dependent Differentiation. Life 2025, 15, 931. https://doi.org/10.3390/life15060931
Sana A, Rossi IV, Sabatke B, Ramirez MI. Extracellular Vesicles Mediate Epimastigogenesis in Trypanosoma cruzi: Strain-Specific Dynamics and Temperature-Dependent Differentiation. Life. 2025; 15(6):931. https://doi.org/10.3390/life15060931
Chicago/Turabian StyleSana, Abel, Izadora Volpato Rossi, Bruna Sabatke, and Marcel Ivan Ramirez. 2025. "Extracellular Vesicles Mediate Epimastigogenesis in Trypanosoma cruzi: Strain-Specific Dynamics and Temperature-Dependent Differentiation" Life 15, no. 6: 931. https://doi.org/10.3390/life15060931
APA StyleSana, A., Rossi, I. V., Sabatke, B., & Ramirez, M. I. (2025). Extracellular Vesicles Mediate Epimastigogenesis in Trypanosoma cruzi: Strain-Specific Dynamics and Temperature-Dependent Differentiation. Life, 15(6), 931. https://doi.org/10.3390/life15060931






