Abstract
Purpose: To explore the association between anxiety levels and health outcomes in long-term breast cancer survivors (LTBCSs) and to identify predictors of anxiety in this population. Methods: A multicenter cross-sectional study was conducted with 80 LTBCSs, categorized into two groups based on their anxiety levels: low anxiety (≤3.4) and high anxiety (≥3.5). The analysis focused on variables assessed at least five years after diagnosis, including sociodemographic and clinical data, mood, cancer-related fatigue (CRF), pain, self-perceived physical fitness, physical activity (PA), and health-related quality of life (HRQoL). ANOVA, Mann–Whitney U, and chi-square tests were conducted, along with correlation and multiple regression analysis. Effect sizes were calculated using Cohen’s d. Results: Among the participants, 46.25% exhibited higher anxiety levels. This group showed significantly worse mood, self-perceived physical fitness, and HRQoL and elevated CRF and pain (p < 0.05). Regression analysis identified “total CRF” (β = 0.51; p < 0.01) and “cognitive functioning” (β = −0.24; p = 0.02) as significant predictors of higher levels of anxiety (r2 adjusted = 0.470). Conclusions: Anxiety significantly impacts multiple dimensions of health in LTBCSs. Total CRF and cognitive functioning are key predictors of anxiety. These findings have direct clinical implications: routine psychological and physical assessments should be integrated into survivorship care to identify individuals at risk and inform targeted interventions to enhance long-term well-being and HRQoL.
1. Introduction
Breast cancer (BC) is the most commonly diagnosed malignancy among women worldwide [1], and advances in early detection and treatment have significantly increased survival rates [2]. As a result, attention has increasingly shifted toward understanding the long-term sequelae experienced by BC survivors, particularly those who have surpassed the five-year mark following diagnosis and primary treatment. These long-term breast cancer survivors (LTBCSs) often face a complex array of persistent physical, emotional, and psychological symptoms that may compromise their health-related quality of life (HRQoL) and overall well-being [3,4].
Among these psychological concerns faced by LTBCSs, anxiety remains one of the most prevalent yet under-characterized conditions [5,6,7]. Despite its clinical significance, it has often been grouped with depression under the broader umbrella of psychological distress [4,8,9]. However, recent evidence suggests that anxiety may involve distinct physiological and behavioral mechanisms [6,7,10,11,12,13] and may also follow a unique trajectory, particularly in response to psychological treatment, exhibiting differential responses to specific therapeutic approaches such as cognitive–behavioral therapy or mindfulness-based interventions [14]. This distinction is further supported by evidence of potential biopsychosocial pathways through which anxiety may influence health outcomes in BC survivors, including neuroendocrine dysregulation, maladaptive health behaviors, and heightened inflammatory responses, which together justify the focused assessment of anxiety in this population [15].
Studies specifically focusing on anxiety in both BC survivors [16,17] and LTBCSs [5,18] have shown that high anxiety levels are associated with poorer coping mechanisms, diminished cognitive functioning, greater cancer-related fatigue (CRF), and reduced HRQoL. Nevertheless, many existing studies do not provide an integrative analysis of factors associated with anxiety in LTBCSs, which limits the design of targeted interventions. Methodological heterogeneity and inconsistent outcome measures further hinder the interpretation and generalization of findings [5,16,17,18]. A clearer understanding of anxiety and its predictors in this population is therefore essential to develop personalized, multidisciplinary care strategies. Recognizing anxiety as a distinct clinical concern may help improve long-term outcomes, as it remains frequently underdiagnosed despite its substantial impact on HRQoL and functional recovery.
Considering the aforementioned, the present study aims to (1) explore the association between anxiety levels and health outcomes in LTBCSs and (2) identify predictors of anxiety in this population ≥ 5 years beyond BC diagnosis. By focusing solely on anxiety, this study seeks to fill a critical gap in survivorship research and support the development of more individualized interventions aimed to improve the long-term emotional and physical well-being of LTBCSs.
2. Materials and Methods
2.1. Study Design and Sample
This study followed a cross-sectional and descriptive design and was conducted in accordance with the Declaration of Helsinki (version 14/2017) [19]. Ethical approval was obtained from the Biomedical Research Ethics Committee of Granada (CEIm) (Ref: 1038-N-16 I.P/26 July 2018). A total of 80 LTBCSs were recruited in 2024 through the oncology department of the San Cecilio University Hospital of Granada and various BC associations in Granada and Málaga. A detailed description of the participant recruitment process is provided in Supplementary Material Figure S1.
Sample size was calculated using G*Power (Version 3.1.9.7), assuming a medium effect size (f = 0.25; Cohen’s d = 0.50), α = 0.05, and power = 0.80. This assumption is supported by studies on anxiety and related outcomes [20,21] and by similar sample size calculation approaches used in cancer survivor populations for other clinical variables [22]. A minimum of 72 participants was required, and 80 were ultimately recruited. Evaluations were conducted either at the Faculty of Health Sciences (University of Granada) or within the facilities of the collaborating associations.
The oncology department was aware of the study’s inclusion and exclusion criteria for women referred from the hospital. When a potential participant was identified, the oncologist informed her about the study and provided our contact number. If she was interested, she voluntarily reached out to us, ensuring data protection. Upon contact, further details were provided via phone, an in-person appointment was scheduled at the university, and a study dossier was offered. Any questions were addressed, and if the participant agreed, she signed the informed consent form before undergoing an on-site assessment lasting approximately 50 min.
For participants from BC associations, a research team member visited the associations to present the study. Interested women were identified by the association and contacted us voluntarily to schedule an assessment. The same protocol used for hospital-referred participants was applied for assessments at the association premises.
The same researcher conducted all assessments, regardless of location. Additionally, one team member was responsible for digitalizing the questionnaires, while another, independently, analyzed the results.
Eligibility criteria included being at least 18 years old, having been diagnosed with stage I–IIIa BC at least five years prior, having no current psychiatric disorders, not undergoing active cancer treatment (radiotherapy, chemotherapy, and/or hormonal therapy), and being Spanish-speaking. Participants using psychotropic medication or those unable to understand or complete the assessments were excluded from the study.
Anxiety levels were measured to classify participants into groups using an adapted version of the Visual Analogue Scale (VAS) for Anxiety (VAS-A). This scale consists of a 10 cm horizontal line ranging from “no anxiety” (0) on the left to “worst possible anxiety” (10) on the right. Although the concept of a VAS adapted to measure anxiety was proposed in 1976 and first applied in 1988 for patients undergoing dental treatment [23], it has since demonstrated utility in various clinical populations, including women with BC [24]. More recent studies have shown a significant correlation between VAS-A scores and those obtained with standardized instruments like the Hospital Anxiety and Depression Scale (HADS) or the State-Trait Anxiety Inventory (STAI), suggesting its validity as a rapid screening tool for anxiety [25,26]. As such, it may serve as an efficient preliminary screening tool for anxiety and potential anxiety symptoms, particularly in clinical contexts where time constraints preclude the use of more comprehensive measures. Therefore, and following similar recommendations for cut-off points [25], participants were classified into two groups: those with low anxiety levels (≤3.4) and those with higher anxiety levels (≥3.5).
2.2. Measures
2.2.1. Primary Outcome
The level of anxiety was evaluated using an adapted version of the VAS. The VAS-A is a 10 cm scale where 0 signifies “no anxiety” and 10 represents the “worst possible anxiety” [23]. Participants were asked to rate their level of anxiety at the time of the assessment. Previous studies have shown this tool to be reliable for assessing anxiety in BC patients [24].
2.2.2. Secondary Outcomes
Demographic and Clinical Data Collection
Structured interviews were conducted using a tailored questionnaire to gather sociodemographic and clinical information. Clinical variables assessed included time since diagnosis, tumor stage, family history of BC, surgical interventions, types of treatment undergone, current medication use, presence of metastasis or recurrence, menopausal status, engagement with psychological or physiotherapy services, and lifestyle habits such as smoking and alcohol consumption.
Mood State
Mood state was assessed using the Scale for Mood Assessment (EVEA), which has demonstrated high reliability, with a Cronbach’s alpha ranging from 0.88 to 0.93 [27]. This instrument consists of 16 items, each rated on a Likert scale from 0 to 10, and evaluates four dimensions: sadness/depression, anxiety, anger/hostility, and happiness. The score for each dimension is obtained by averaging the corresponding item scores, with higher values indicating greater intensity of the respective mood state.
Cancer-Related Fatigue
The Piper Fatigue Scale (PFS), a 22-item instrument, was used to assess CRF across four key dimensions: behavioral severity, affective, sensory, and cognitive/mood. The total score reflects overall CRF intensity, with higher values indicating greater fatigue [28,29]. The reliability of the scale has been confirmed (Cronbach’s alpha = 0.86) [30]. Previous research supports two classification models (Models A and B) for CRF severity [28,29]. In Model A, CRF is categorized as follows: 0 = none, 1–3 = mild, 4–6 = moderate, and 7–10 = severe. Model B defines the categories as: 0 = none, 1–2 = mild, 3–5 = moderate, and 6–10 = severe. Notably, moderate CRF—regardless of the model used—is considered clinically relevant [31]. Patients with moderate to severe CRF should undergo further evaluation to identify any underlying or comorbid conditions requiring medical attention [32].
Pain
Pain intensity was evaluated using the original version of the VAS, a 10 cm scale known for its high reliability with an intraclass correlation coefficient (ICC = 0.97) [33], where 0 signifies “no pain” and 10 represents the “worst possible pain”. Participants were asked to rate the pain in both their affected and unaffected arms at the time of the assessment. For those with bilateral BC where both arms were impacted, the arm deemed “affected” was identified based on these factors: (1) the patient’s subjective pain report when comparing the two arms, (2) the level of surgical intervention, and (3) the presence of lymphedema or other post-surgical complications. Additionally, the Brief Pain Inventory (BPI) short form, with Cronbach’s alpha values ranging from 0.87 to 0.89 [34], was employed to measure pain intensity (severity) through four questions, and its impact on daily life (interference) through seven questions. Higher scores reflected greater pain intensity and more significant interference with daily activities.
Self-Perceived Physical Fitness
Self-perceived physical fitness was assessed using the International Fitness Scale (IFIS), a questionnaire that employs a 5-point Likert scale ranging from 1 (very poor) to 5 (very good). This instrument includes five key items evaluating general fitness, as well as specific components such as cardiorespiratory endurance, muscular strength, speed/agility, and flexibility in comparison to peers. The IFIS has shown moderate reliability, with an average weighted kappa 0.45 [35].
Physical Activity Level
PA was assessed using the Minnesota Leisure Time Physical Activity (MLTPA) questionnaire, which evaluates the average frequency and total hours dedicated to PA over the past week. This tool has shown high reliability, with an ICC of 0.95 [36]. The assessment was based on a predefined list of specific physical activities. To calculate energy expenditure, the reported weekly duration (in hours) of each activity was multiplied by its corresponding metabolic equivalent of task (MET) value [37], which reflects the energy cost of the activity. Higher scores indicate a greater total weekly PA duration. For the main analysis, the total PA value obtained was categorized into three groups based on previously established cutoff points: very low activity (≤3 MET), low activity (3.1–7.4 MET), and sufficiently active (≥7.5 MET) [38,39,40].
Health-Related Quality of Life
HRQoL was evaluated using two validated instruments: the EORTC QLQ-C30 (version 3.0) and its BC-specific module, the QLQ-BR23. These tools have demonstrated reliability, with Cronbach’s alpha values ranging from 0.46 to 0.94 [41]. Responses were recorded on a 4-point Likert scale (1 = not at all, 4 = very much) and subsequently converted to a 0–100 scale. In the interpretation of results, higher scores on functional and global HRQoL scales reflect better health status, whereas higher scores on symptom scales indicate a greater burden of symptoms. Additionally, a summary score for the QLQ-C30 was derived by aggregating 13 scales and items, excluding global health status and financial impact scales. For this summary score, greater values represent better overall HRQoL [42].
2.3. Statistical Analysis
Data analysis was performed using the Statistical Package for the Social Sciences (IBM SPSS Statistics for Windows, version 27.0, Armonk, NY, USA). The significance threshold was set at p < 0.05, with a 95% confidence interval (CI). The Kolmogorov–Smirnov test was applied to assess the normality of all variables, considering p > 0.05 as indicative of normal distribution.
- Normally distributed variables (age, time since diagnosis, and time since first surgery) were analyzed using ANOVA to compare the two anxiety-level groups: low (≤3.4) vs. high (≥3.5). Results are reported as mean ± standard deviation. Post hoc comparisons were adjusted using Bonferroni correction to account for multiple testing.
- Non-normally distributed variables (mood state, non-categorized CRF, pain, self-perceived physical fitness, and HRQoL) were analyzed using the Mann–Whitney U test. Results are also reported as mean ± standard deviation. Bonferroni correction was applied where appropriate to minimize the risk of Type I error due to multiple comparisons.
- Categorical variables, including other demographic, clinical, and medical characteristics, as well as categorized CRF and PA levels (based on predefined cut-off scores) [28,29,38,39,40], were analyzed using chi-square tests and are presented as percentages.
Additionally, effect sizes (Cohen’s d) were classified as follows: negligible (d = 0–0.19), small (d = 0.2–0.49), moderate (d = 0.5–0.79), large (d = 0.8–1.19), and very large (d ≥ 1.20) [43].
As most variables did not meet the normality assumption, Spearman correlation analysis was performed to examine the relationship between anxiety levels (assessed with the VAS-A and analyzed as a continuous, non-categorized variable) and the other study variables. Furthermore, a stepwise multiple regression analysis was conducted to identify factors contributing to long-term anxiety variability. Variables were included in the regression model if they met two conditions: (1) a significant correlation with the dependent variable and (2) a correlation coefficient below 0.70 between independent variables to mitigate collinearity issues [44]. A forward selection approach was employed, incorporating significant predictors sequentially based on their association strength with the dependent variable. At each step, the significance of the linear regression model was evaluated, and standardized β coefficients were calculated for the final model.
3. Results
3.1. Demographic and Clinical Characteristics
No significant differences were observed between the groups in terms of the demographic and clinical characteristics of the 80 LTBCSs, based on their anxiety levels. Following established recommendations for cut-off points [25], participants were classified into two groups using the VAS-A: those with low anxiety levels (53.75%) and those with high anxiety levels (46.25%).
The mean age of participants with low anxiety levels was 48.41 ± 8.05 years, whereas for those with high anxiety levels it was 50.51 ± 8.02 years. Among individuals with low anxiety levels, 44.2% had a university-level education, 25.6% were on sick leave, 20.9% were smokers, 95.3% had received both radiotherapy and chemotherapy, and 60.5% had attended psychological therapy sessions in the past three months. In contrast, within the group of individuals with high anxiety, 24.3% had a university-level education, 51.4% were on sick leave, 27% were smokers, 81.1% had undergone both radiotherapy and chemotherapy, and 67.6% had attended psychological therapy sessions in the past three months. Additional details on demographic and clinical characteristics are provided in Table 1.

Table 1.
Demographic, clinical, and medical characteristics of the groups.
3.2. Mood State
The analysis of mood states, assessed using the EVEA, revealed significant differences between groups. LTBCSs with high anxiety levels reported greater “sadness–depression” and “anger–hostility”, as well as lower “happiness” compared to those with low anxiety levels (U = 310.00 to 765.00; all p < 0.01; d = 0.06 to >1.20). A detailed overview of these findings can be found in Table 2.

Table 2.
Mood state, cancer-related fatigue, pain, self-perceived physical fitness, and physical activity level values between groups.
3.3. Cancer-Related Fatigue
The assessment of PFS domains revealed significant differences between the groups. LTBCSs with high anxiety levels showed higher levels of CRF across all domains compared to those with low anxiety levels (U = 323.50 to 380.00; all p < 0.01; d = 1.11 to > 1.20) (Table 2).
In relation to the two cut-off scores, A and B, the analysis indicated that LTBCSs with high anxiety levels had a significantly greater prevalence of “moderate” to “severe” CRF for both cut-off scores (A = 67.5% and B = 78.3%) than those with low anxiety levels (A = 18.6% and B = 21%) (both p < 0.01). For a visual representation of these differences, refer to Figure 1.
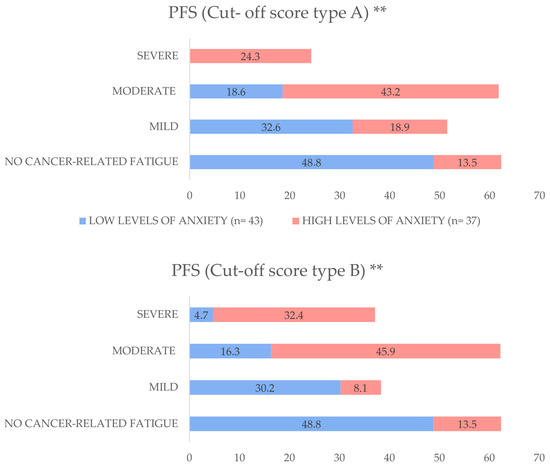
Figure 1.
Distribution of cancer-related fatigue (CRF) severity by anxiety levels among long-term breast cancer survivors, expressed as percentages. Participants were stratified into low anxiety levels (VAS-A ≤ 3.4) and high anxiety levels (VAS-A ≥ 3.5). Fatigue severity was categorized using two cut-off score systems: Cut-off score A (No Fatigue: 0; Mild: 1–3; Moderate: 4–6; Severe: 7–10) and Cut-off score B (No Fatigue: 0; Mild: 1–2; Moderate: 3–5; Severe: 6–10). Between-group comparisons were performed using the Mann–Whitney U test. Abbreviations: PFS: Piper Fatigue Scale, VAS-A: Visual Analogue Scale for Anxiety, n: sample size. ** p < 0.01.
3.4. Pain
The evaluation of pain identified significant differences between the groups in both the VAS and BPI measures. Specifically, LTBCSs with high anxiety levels reported greater pain intensity in both the affected and non-affected arms (VAS), as well as increased pain severity and interference (BPI) compared to those with low anxiety levels (U = 434.00 to 549.50; p < 0.01 to 0.01; d = 0.71 to 0.99) (Table 2).
3.5. Self-Perceived Physical Fitness
The assessment of self-perceived physical fitness, measured using the IFIS, identified significant differences between the groups across all domains. LTBCSs with high anxiety levels reported lower self-perceived “general physical fitness”, “cardiorespiratory endurance”, “muscular strength”, “speed–agility”, and “flexibility” compared to those with low anxiety levels (U = 493.00 to 742.50; p < 0.01 to 0.05; d = 0.13 to 0.73) (Table 2).
3.6. Physical Activity Level
The analysis of between-group differences in MLTPA scores did not reveal significant differences (p > 0.05). However, LTBCSs with high anxiety levels exhibited a higher rate of inactivity (35.1%) compared to those with low anxiety levels (18.6%). Furthermore, only 29.7% of LTBCSs with high anxiety levels met the minimum PA recommendations [38,39,40]. Although this proportion was slightly higher among LTBCSs with low anxiety levels, it remained limited to 37.2% (refer to Table 2). Hence, higher anxiety levels may be associated with lower engagement in physical activity among LTBCSs.
3.7. Health-Related Quality of Life
The analysis of HRQoL, assessed using the QLQ-C30, revealed significant differences between groups. LTBCSs with high anxiety levels had significantly lower scores in “role functioning”, “emotional functioning”, “cognitive functioning”, “social functioning”, “global health status”, and the “summary score”. Additionally, they reported significantly higher scores in all symptom scales and single-item measures, except for “constipation”, compared to LTBCSs with low anxiety levels (U = 316.00 to 634.00; p < 0.01 to 0.04; d = 0.20 to 1.11). No significant differences were found in “physical functioning” between the groups (p > 0.05). See Table 3 for further details.

Table 3.
Health-related quality of life values between groups.
Moreover, significant differences between the groups were observed in the BR23 module. LTBCSs with high anxiety levels had significantly lower scores in “body image” and “sexual functioning”, as well as higher scores in “future perspective” and all symptom scales compared to the other group (U = 388.00 to 680.00; p < 0.01 to 0.01; d = 0.18 to 0.90). No significant differences were found in “sexual enjoyment” between the groups (p > 0.05) (refer to Table 3).
Therefore, considering both the QLQ-C30 and BR23, higher anxiety levels in LTBCSs appears to be linked to poorer HRQoL across functional and symptom domains.
3.8. Correlation Analysis and Multiple Regression Analysis
The Spearman’s correlation analysis revealed significant positive correlations between the level of anxiety and the following variables: PFS: “behavioral/severity”, “affective”, “sensory”, “cognitive”, and “total fatigue score”, QLQ-C30: “fatigue”, “nausea and vomiting”, “pain”, “dyspnea”, “insomnia”, “appetite loss”, and “financial difficulties”, QLQ-BR23: “breast symptoms”, “arm symptoms”, and “upset by hair loss”, VAS: “affected arm” and “non-affected arm”, BPI: “intensity” and “interference” (ρ = 0.231 to 0.587; p < 0.01 to 0.03). Meanwhile, significant negative correlations were observed between the level of anxiety and the following variables: “role functioning”, “emotional functioning”, “cognitive functioning”, “social functioning”, “global health”, and “summary score”, QLQ-BR23: “body image”, “sexual functioning”, and “systemic therapy side effects”, IFIS: “general physical fitness”, “speed/agility”, and “flexibility” (ρ = −0.302 to −0.550; all p < 0.01). In summary, higher anxiety levels are generally associated with greater CRF, symptom burden, and pain, alongside reduced functioning, global HRQoL, and self-perceived physical fitness. The results are presented in Figure 2.

Figure 2.
Spearman’s correlation coefficients between anxiety levels and multiple health-related variables in long-term breast cancer survivors. Anxiety was assessed using the Visual Analogue Scale for Anxiety (VAS-A), analyzed as a continuous (non-categorized) variable. The figure displays the strength and direction of associations with measures of fatigue, pain, self-perceived fitness, and health-related quality of life. Abbreviations: VAS-A: Visual Analogue Scale for Anxiety, PFS: Piper Fatigue Scale, BPI: Brief Pain Inventory, IFIS: International Fitness Scale, QLQ-C30: EORTC Core Quality of Life Questionnaire, QLQ-BR23: Breast Cancer-Specific Module, SCC: Spearman’s Correlation Coefficient. Significant correlation at * p < 0.05; ** p < 0.01.
The final regression model determined that “total CRF” from the PFS (β = 0.051; p = <0.01) and “cognitive functioning” from the QLQ-C30 (β = −0.024; p = 0.02) were significant predictors of high anxiety levels. Collectively, these variables accounted for 47.0% of the variance in anxiety (r2 adjusted = 0.470; p < 0.01 to 0.02) among individuals who were ≥5 years post-cancer diagnosis. See Table 4 for further details.

Table 4.
Summary of Stepwise Multiple Regression Analysis to determine predictors of anxiety using the Visual Analogue Scale for Anxiety (VAS-A).
4. Discussion
The first aim of this study was to explore the association between anxiety levels and health outcomes in LTBCSs. The second aim was to identify factors that influence anxiety in this population. The main findings of this study revealed that 46.25% of LTBCSs exhibited high anxiety levels, while 53.75% had low anxiety levels. Those with high anxiety demonstrated greater impairments in mood, self-perceived physical fitness, and HRQoL, alongside elevated levels of CRF and pain. Additionally, the combination of “total CRF” and “cognitive functioning” accounted for 47.0% of the variance in anxiety among LTBCSs. Although the cut-off point used to define high and low anxiety (3.5 vs. 3.4) may appear narrow, it was derived from a previous validation study using the VAS-A in oncological settings [25], where scores ≥ 3.5 have been shown to reflect clinically relevant anxiety. In our cohort, this threshold revealed consistent and meaningful differences in multiple health domains. Therefore, despite the subtle numerical distinction, the stratification proved useful for identifying LTBCSs at greater risk for symptom burden and poorer health outcomes. However, this cut-off should not be interpreted as a definitive diagnostic threshold. Rather, it may serve as an initial red flag in time-constrained clinical settings, prompting more comprehensive psychological assessments or referral to mental health professionals when anxiety scores exceed 3.5.
4.1. Mood State
In relation to mood state, our findings indicate that LTBCSs with elevated anxiety levels experience significantly higher levels of sadness–depression and anger–hostility, alongside reduced happiness, compared to those with low anxiety levels. This observation is consistent with existing literature, which highlights that LTBCSs often face persistent psychological challenges, including anxiety and depression, even years post-treatment [5,6,7,45]. In this sense, the interplay between anxiety and mood disturbances in LTBCSs can be attributed to several factors. Elevated anxiety may stem from concerns about cancer recurrence, leading to heightened emotional distress [46]. Additionally, the physical and social challenges encountered during survivorship, such as ongoing treatment side effects and changes in social roles, can exacerbate negative mood states [47]. Addressing these psychological concerns is crucial, as unmanaged anxiety can adversely affect overall well-being and hinder the long-term recovery process.
4.2. Cancer-Related Fatigue
As for CRF, it is a common concern in LTBCSs and is closely linked to psychological distress, particularly anxiety and depression [3]. Consistent with previous studies, our findings show that participants with high anxiety levels reported significantly greater CRF across all PFS domains, supporting a bidirectional relationship between fatigue and mood. Shared neurobiological mechanisms—such as HPA axis dysregulation and inflammation—may underlie this association [48]. Notably, the prevalence of moderate to severe CRF was substantially higher among LTBCSs with elevated anxiety, with 67.5% and 78.3% meeting severity thresholds under models A and B, respectively. These results reinforce evidence that anxiety exacerbates fatigue and its functional impact during survivorship [49]. As moderate CRF warrants clinical attention [31], screening for anxiety may be key to improving fatigue management. Future research should investigate targeted interventions, such as cognitive–behavioral therapy or structured exercise, to address anxiety-related CRF and enhance long-term quality of life.
4.3. Pain
Pain is a prevalent symptom among LTBCSs, with studies reporting post-treatment pain in approximately 46% of cases [50,51]. Our results show that those with high anxiety levels report significantly greater pain intensity in both the affected and unaffected arms (VAS), as well as higher pain severity and interference scores (BPI), compared to those with low anxiety. These findings align with evidence linking anxiety to increased pain perception in post-mastectomy women [3]. This relationship may be driven by shared neurobiological pathways, such as anxiety-related amplification of pain through dysregulated dopaminergic activity and overlapping pain–emotion neural circuits [52]. Moreover, the bidirectional interplay between pain and anxiety can create a self-reinforcing cycle of emotional and physical distress. Addressing anxiety may enhance pain management and improve HRQoL in LTBCSs. Integrated interventions targeting both symptoms should be prioritized in survivorship care.
4.4. Self-Perceived Physical Fitness and Physical Activity Levels
With respect to self-perceived physical fitness and PA levels, they are both important components of long-term health outcomes in BC survivors. Our findings indicate that LTBCSs with high anxiety levels perceive their physical fitness as significantly lower across all fitness domains, including cardiorespiratory endurance, muscular strength, speed–agility, and flexibility. These results align with prior research suggesting that psychological distress, particularly anxiety, can negatively impact self-efficacy and perceived physical capacity in cancer survivors [53]. The interplay between anxiety and self-perception may be explained by cognitive distortions common in anxious individuals, leading to underestimation of their physical abilities despite a possible comparable objective performance [54]. Given that self-perceived physical fitness has been identified as a predictor of mortality and serves as a stable indicator of habitual PA levels [55,56], its evaluation may be as relevant as objective assessments in understanding long-term health risks. Moreover, although not specifically in LTBCSs, previous studies have reported strong correlations between self-reported and objectively measured fitness, reinforcing the validity of self-perception as a valuable tool for identifying individuals with lower physical ability and guiding tailored interventions [55,56]. Therefore, considering the possible impact of anxiety on both objectively measured and self-perceived physical fitness could provide a more comprehensive approach in the design of rehabilitation programs, allowing for more personalized and effective interventions in this population.
Although no statistically significant differences in overall MLTPA scores were found, a higher proportion of physically inactive LTBCSs was observed among those with elevated anxiety levels. This trend is consistent with previous studies indicating that anxiety can act as a barrier to engaging in regular PA, potentially due to fear of discomfort, avoidance behaviors, and fatigue-related concerns [10]. Given the well-documented benefits of PA in mitigating anxiety and other cancer-related symptoms [57], targeted interventions focusing on behavioral activation and structured exercise programs may be beneficial in this population. Future research should explore whether personalized psychological and PA interventions can enhance both perceived and actual physical fitness in anxious LTBCSs.
4.5. Health-Related Quality of Life
When it comes to HRQoL using the QLQ-C30, our LTBCSs with high anxiety levels exhibited notably lower scores in different functioning domains, as well as in “global health status” and in the “summary score”. Additionally, these LTBCSs reported significantly higher scores across all symptom scales and single-item measures, with the exception of “constipation”, when compared to LTBCSs with low anxiety levels. These findings are consistent with previous research indicating that anxiety can adversely affect physiological function, treatment adherence, psychological well-being, and overall HRQoL in BC patients and survivors [16,58].
Moreover, significant differences were observed in the BR23 module. LTBCSs experiencing high anxiety levels reported significantly lower scores in “body image” and “sexual functioning”, as well as higher scores in “future perspective” and all symptom scales, compared to their counterparts with low anxiety levels. These results align with a study which highlights that psychological distress, including anxiety, is prevalent among BC survivors and can profoundly impact various aspects of HRQoL, such as body image perception and satisfaction, sexual libido, increased concerns about the future, or other possible physical symptoms [59]. These findings underscore the critical need for comprehensive psychosocial interventions aimed at addressing anxiety and its associated effects on HRQoL in LTBCSs. Implementing routine distress screenings and providing tailored psychological support could be instrumental in enhancing the overall well-being and HRQoL for this population.
4.6. Correlation Analysis and Multiple Regression Analysis
The correlation analysis revealed significant positive associations between anxiety levels and various physical and psychological symptoms, including CRF, pain, dyspnea, insomnia, appetite loss, and financial difficulties. Conversely, we observed significant negative correlations between anxiety levels and factors such as role, emotional, cognitive, social, and sexual functioning, global health status, and self-perceived physical fitness. These findings align with the limited existing studies that have specifically conducted correlation analyses between anxiety and potential predictors in LTBCSs [6,8,60]. To date, most research has focused on descriptive comparisons, analyzing how different variables present depending on anxiety levels, rather than exploring their interrelationships and predictive value. However, our results highlight the importance of addressing these associations, as anxiety in LTBCSs is often linked to a greater symptom burden, including persistent CRF and chronic pain, which can exacerbate distress and impair daily functioning [6,8,60]. Given the scarcity of studies examining these correlations in long-term survivorship, further research is needed to investigate the potential interactions between anxiety and these physical and psychological factors beyond five years after a BC diagnosis.
Finally, our regression analysis revealed that total CRF (from the PFS) and cognitive functioning (from the QLQ-C30) were significant predictors of elevated anxiety levels among LTBCSs, collectively accounting for 47.0% of the variance. These findings suggest that higher CRF levels may increase anxiety, while impaired cognitive functioning also plays a role in heightened anxiety among LTBCSs. This finding aligns with existing literature that identifies various factors influencing anxiety in LTBCSs, though differences in study designs and reported predictors limit direct comparisons [5,6].
For instance, Breidenbach et al. (2022) found that younger age, lower income, and the presence of comorbidities significantly predicted higher anxiety levels in LTBCSs, explaining approximately 20% of the variance at follow-up [6]. Compared to our findings, which account for a larger proportion of variance (47.0%), this suggests that CRF and cognitive functioning may play an even greater role in long-term anxiety than previously recognized, warranting further investigation into their underlying mechanisms. Similarly, Cheng et al. (2021) [5] highlighted the role of coping profiles in predicting long-term anxiety trajectories in BC survivors, particularly emphasizing that maladaptive coping strategies were linked to heightened anxiety levels. Although the study did not report the variance explained by these predictors, its findings reinforce the relevance of psychological and behavioral factors in anxiety regulation [5]. Overall, while previous research has identified various contributors to anxiety in LTBCSs, our study provides novel insight into the strong predictive value of CRF and cognitive functioning. Hence, the 47.0% variance explained underscores the importance of integrating both physical and cognitive assessments in survivorship care, helping to identify individuals at risk and develop more targeted interventions.
4.7. Limitations and Strengths
This study has several limitations that should be acknowledged. First, although the VAS-A provided a rapid and practical method for stratifying anxiety levels, the use of a single-item tool rather than a standardized multidimensional instrument (e.g., HADS, STAI, and the Generalized Anxiety Disorder-7 (GAD-7)) may limit the depth of the anxiety assessment. Nonetheless, the VAS-A has been shown to correlate with more comprehensive scales in diverse populations and may serve as an efficient preliminary screening tool, particularly in clinical settings where time constraints preclude the use of lengthier instruments [25,26]. Moreover, we acknowledge that while our use of the VAS-A is grounded in previous work, it does not replace the depth of standardized multidimensional tools, and future studies should aim to validate these cut-offs further in specific cancer survivor populations. Second, the exclusive inclusion of female and Spanish-speaking LTBCSs limits the generalizability of findings to male survivors and non-Spanish-speaking or ethnically diverse groups. Third, the lack of information regarding pre-existing anxiety levels prior to cancer diagnosis restricts the ability to determine whether the observed anxiety is a result of the cancer experience or a pre-existing condition. Lastly, the cross-sectional nature of the study limits causal inferences, and although the cut-off points used were based on prior research in oncological populations [25], different thresholds might yield alternative results. Longitudinal studies are needed to clarify the directionality of these associations.
Despite its limitations, this study offers novel and clinically relevant insights into the relationship between anxiety and a range of health-related factors—physical, emotional, cognitive, and HRQoL domains—in LTBCSs. To our knowledge, it is one of the few studies to adopt a multidimensional approach that integrates CRF, pain, and self-perceived physical fitness as potential correlates of anxiety in long-term survivorship. The use of previously validated instruments in oncological populations strengthens the methodological robustness of the findings [24,28,29,30,34,41,42], and the regression model—explaining 47.0% of the variance in anxiety—underscores the clinical importance of these associations. Together, these results contribute to the scientific understanding of survivorship care and identify potential targets for individualized interventions aimed at improving both psychological and physical outcomes in LTBCSs.
5. Conclusions
In conclusion, nearly half of LTBCSs (46.25%) experienced high anxiety levels, which were linked to greater impairments in mood, self-perceived physical fitness, HRQoL, CRF, and pain. Total CRF and cognitive functioning significantly predicted anxiety, explaining 47.0% of its variance even five or more years post-diagnosis.
These results underscore the critical interaction between anxiety and key physical and psychological factors in long-term survivorship. Clinically, routine assessment of anxiety, CRF, and cognitive function in LTBCSs is essential for early identification of those at risk. Implementing targeted interventions—such as cognitive–behavioral therapy and exercise programs—could reduce anxiety symptoms and improve overall well-being. Future research should prioritize evaluating multidisciplinary interventions to optimize long-term health outcomes and HRQoL in this population.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/life15060932/s1, Figure S1: Flow diagram for study participants.
Author Contributions
Conceptualization, F.Á.-S., J.M.-L., C.P.-F., and M.F.-M.; Methodology, F.Á.-S., S.A.-A., M.F.-M., and C.P.-F.; Software, F.Á.-S.; Validation, F.Á.-S., J.M.-L., S.A.-A., M.F.-M., and C.P.-F.; Formal analysis, F.Á.-S., M.F.-M., and J.M.-L.; Investigation, F.Á.-S., M.F.-M., S.A.-A., C.P.-F., and C.B.-P.; Resources, F.Á.-S.; Data curation, F.Á.-S. and J.M.-L.; Writing—original draft preparation, F.Á.-S., J.M.-L., C.P.-F., S.A.-A., and M.F.-M.; Writing—review and editing, F.Á.-S., J.M.-L., C.P.-F., S.A.-A., and M.F.-M.; Visualization, F.Á.-S., M.F.-M., and J.M.-L.; Supervision, F.Á.-S., M.F.-M., C.P.-F., and S.A.-A.; Project administration, F.Á.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Biomedical Research Ethics Committee of Granada (CEIm) (1038-N-16 I.P/26 July 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We sincerely thank all long-term breast cancer survivors who willingly participated and contributed to this project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Barrios, C.H. Global challenges in breast cancer detection and treatment. Breast 2022, 62, S3–S6. [Google Scholar] [CrossRef] [PubMed]
- Lovelace, D.L.; McDaniel, L.R.; Golden, D. Long-term effects of breast cancer surgery, treatment, and survivor care. J. Midwifery Women’s Health 2019, 64, 713–724. [Google Scholar] [CrossRef]
- Ren, Y.; Maselko, J.; Tan, X.; Olshan, A.F.; Stover, A.M.; Bennett, A.V.; Andersen, K.K.; Reeve, B.B.; Ekwueme, D.U.; Pisu, M.; et al. Emotional and functional well-being in long-term breast cancer survivorship. Cancer Causes Control. 2024, 35, 1191–1200. [Google Scholar] [CrossRef]
- Cheng, C.-T.; Ho, S.M.Y.; Lai, Y.; Zhang, Q.; Wang, G.L.; Liu, Y.; Li, J.; Chen, M.; Wu, X.; Zhao, L.; et al. Coping profiles predict long-term anxiety trajectory in breast cancer survivors. Support Care Cancer. 2021, 29, 4045–4053. [Google Scholar] [CrossRef]
- Breidenbach, C.; Heidkamp, P.; Hiltrop, K.; Müller, S.; Schneider, A.; Weber, T.; Fischer, M.; Hoffmann, R.; Klein, L.; Wolf, J.; et al. Prevalence and determinants of anxiety and depression in long-term breast cancer survivors. BMC Psychiatry 2022, 22, 101–111. [Google Scholar] [CrossRef]
- Götze, H.; Friedrich, M.; Taubenheim, S.; Müller, C.; Becker, A.; Hoffmann, B.; Lange, F.; Richter, S.; Schmitt, T.; Vogel, M.; et al. Depression and anxiety in long-term survivors 5 and 10 years after cancer diagnosis. Support Care Cancer 2020, 28, 211–220. [Google Scholar] [CrossRef]
- Maass, S.W.M.C.; Boerman, L.M.; Verhaak, P.F.M.; van der Linden, M.; de Vries, J.; Smits, A.; Janssen, M.; Peters, R.; Bakker, T.; Willems, R.; et al. Long-term psychological distress in breast cancer survivors and their matched controls: A cross-sectional study. Maturitas 2019, 130, 6–12. [Google Scholar] [CrossRef]
- De La Torre-Luque, A.; Victoria Cerezo, M.; López, E.; Sibole, J.V. Emotional distress among long-term breast cancer survivors: The role of insomnia and worry. Behav. Psychol. 2020, 28, 533–549. [Google Scholar]
- Ribeiro, F.E.; Palma, M.R.; Silva, D.T.C.; Costa, L.P.; Almeida, V.C.; Santos, R.M.; Oliveira, M.F.; Lima, A.S.; Ferreira, T.J.; Gomes, L.C.; et al. Relationship of anxiety and depression symptoms with the different domains of physical activity in breast cancer survivors. J. Affect. Disord. 2020, 273, 210–214. [Google Scholar] [CrossRef]
- Eysenck, M.W.; Fajkowska, M. Anxiety and depression: Toward overlapping and distinctive features. Cogn. Emot. 2018, 32, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Saunders, R.; Buckman, J.E.J.; Cape, J.; Agnew-Davies, R.; Barkham, M.; Reynolds, S.; Szmukler, G.; Kellett, S.; Kuyken, W.; Byford, S.; et al. Trajectories of depression and anxiety symptom change during psychological therapy. J. Affect. Disord. 2019, 249, 327–335. [Google Scholar] [CrossRef]
- Penninx, B.W.J.H.; Nolen, W.A.; Lamers, F.; Zitman, F.G.; Beekman, A.T.F.; Smit, J.H.; Cuijpers, P.; de Graaf, R.; van Dyck, R.; Spinhoven, P.; et al. Two-year course of depressive and anxiety disorders: Results from the Netherlands Study of Depression and Anxiety (NESDA). J. Affect. Disord. 2011, 133, 76–85. [Google Scholar] [CrossRef]
- Li, J.; Cai, Z.; Li, X.; Wang, Y.; Zhang, L.; Chen, H.; Liu, Q.; Zhao, T.; Sun, W.; Huang, F.; et al. Mindfulness-based therapy versus cognitive behavioral therapy for people with anxiety symptoms: A systematic review and meta-analysis of random controlled trials. Ann. Palliat. Med. 2021, 10, 7596–7612. [Google Scholar] [CrossRef] [PubMed]
- McGregor, B.A.; Antoni, M.H. Psychological intervention and health outcomes among women treated for breast cancer: A review of stress pathways and biological mediators. Brain Behav. Immun. 2009, 23, 159–166. [Google Scholar] [CrossRef]
- Williams, A.M.; Khan, C.P.; Heckler, C.E.; Mustian, K.M.; Peppone, L.J.; Janelsins, M.C.; Morrow, G.R.; Palesh, O.G.; Sprod, L.K.; Mohile, S.G.; et al. Fatigue, anxiety, and quality of life in breast cancer patients compared to non-cancer controls: A nationwide longitudinal analysis. Breast Cancer Res. Treat. 2021, 187, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; Cornelius, V.; Ream, E.; Watson, E.; Smith, J.; Jones, K.; Patel, R.; Green, A.; Clarke, S.; Wright, D.; et al. Anxiety after completion of treatment for early-stage breast cancer: A systematic review to identify candidate predictors and evaluate multivariable model development. Support. Care Cancer 2017, 25, 2321–2333. [Google Scholar] [CrossRef]
- Greer, J.A.; Solis, J.M.; Temel, J.S.; Lennes, I.T.; Pirl, W.F.; Park, E.R.; Lynch, T.J.; Gallagher, E.R.; Maguire, R.; Liao, Z.; et al. Anxiety disorders in long-term survivors of adult cancers. Psychosomatics 2011, 52, 417–423. [Google Scholar] [CrossRef]
- Shrestha, B.; Dunn, L. The Declaration of Helsinki on medical research involving human subjects: A review of seventh revision. J. Nepal Health Res. Counc. 2020, 17, 548–552. [Google Scholar] [CrossRef]
- Sarac, F.S.; Erkal Ilhan, S.; Kutun, S.; Kutluturkan, S. The effect of informative mobile app use on anxiety, distress, and quality of life of women with breast cancer. Eur. J. Breast Health 2024, 20, 207–214. [Google Scholar] [CrossRef]
- Mittal, N.; Tyagi, N.; Parvaiz, N.; Dhalla, N.; Asthana, G. Comparative assessment of stress and anxiety among medical and nonmedical undergraduate students: A cross-sectional study. Indian J. Med. Spec. 2025, 16, 60–64. [Google Scholar] [CrossRef]
- Cantarero-Villanueva, I.; Sanchez-Jimenez, A.; Galiano-Castillo, N.; Diaz-Rodriguez, L.; Martin-Martin, L.; Arroyo-Morales, M. Effectiveness of lumbopelvic exercise in colon cancer survivors: A randomized controlled clinical trial. Med. Sci. Sports Exerc. 2016, 48, 1438–1446. [Google Scholar] [CrossRef] [PubMed]
- Luyk, N.H.; Beck, F.M.; Weaver, J.M. A visual analogue scale in the assessment of dental anxiety. Anesth. Prog. 1988, 35, 121–123. [Google Scholar]
- Aviado-Langer, J. Measuring preoperative anxiety in patients with breast cancer using the visual analog scale. Clin. J. Oncol. Nurs. 2014, 18, 489–491. [Google Scholar] [CrossRef]
- Labaste, F.; Ferré, F.; Combelles, H.; Dupont, C.; Martin, P.; Leroy, A.; Moreau, J.; Bernard, S.; Petit, L.; Dubois, M.; et al. Validation of a visual analogue scale for the evaluation of the postoperative anxiety: A prospective observational study. Nurs. Open 2019, 6, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Widyastuty, A.; Effendy, E.; Amin, M.M. Correlation between visual analogue scale score and Hospital Anxiety Depression Scale-depression score in patients with cervical cancer in the Hospital Vina Cancer, Medan. Open Access Maced. J. Med. Sci. 2019, 7, 2634–2637. [Google Scholar] [CrossRef]
- Sanz, J.; Gutiérrez, S.; García-Vera, M.P. Psychometric properties of the Scale for Mood Assessment (EVEA): A review. Ansiedad Estrés 2014, 20, 27–49. [Google Scholar]
- Piper, B.F.; Dibble, S.L.; Dodd, M.J.; Weiss, M.; Slaughter, R.; Paul, S.M.; Henrichs, M.; McCallum, C.; Fischer, P.; Johnson, M.; et al. The revised Piper Fatigue Scale: Psychometric evaluation in women with breast cancer. Oncol. Nurs. Forum 1998, 25, 677–684. [Google Scholar]
- Stover, A.M.; Reeve, B.B.; Piper, B.F.; Oberst, M.T.; Jacobsen, P.B.; Cella, D.; Wenzel, L.; Snyder, C.; Schipper, H.; Kornblith, A.B.; et al. Deriving clinically meaningful cut-scores for fatigue in a cohort of breast cancer survivors: A Health, Eating, Activity, and Lifestyle (HEAL) Study. Qual. Life Res. 2013, 22, 2279–2292. [Google Scholar] [CrossRef]
- Cantarero-Villanueva, I.; Fernández-Lao, C.; Díaz-Rodríguez, L.; Galiano-Castillo, N.; Arroyo-Morales, M.; Martín-Martín, L.; Pecos-Martín, D. The Piper Fatigue Scale-Revised: Translation and psychometric evaluation in Spanish-speaking breast cancer survivors. Qual. Life Res. 2014, 23, 271–276. [Google Scholar] [CrossRef]
- Berger, A.M.; Mooney, K.; Alvarez-Perez, A.; Breitbart, W.; Carpenter, K.M.; Cella, D.; Cleeland, C.; Dotan, E.; Eisenberger, M.A.; Escalante, C.P.; et al. Cancer-Related Fatigue, version 2.2015. J. Natl. Compr. Cancer Netw. 2015, 13, 1012–1039. [Google Scholar] [CrossRef] [PubMed]
- Fabi, A.; Bhargava, R.; Fatigoni, S.; Mazzotta, M.; Schiavon, G.; Zamagni, C.; Montanari, M.; Pinto, C.; Di Maio, M.; Grassi, L.; et al. Cancer-related fatigue: ESMO Clinical Practice Guidelines for diagnosis and treatment. Ann. Oncol. 2020, 31, 713–723. [Google Scholar] [CrossRef]
- Boonstra, A.M.; Schiphorst Preuper, H.R.; Reneman, M.F.; Post, M.W.; Stewart, R.E. Reliability and validity of the visual analogue scale for disability in patients with chronic musculoskeletal pain. Int. J. Rehabil. Res. 2008, 31, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Badia, X.; Muriel, C.; Gracia, A.; Herdman, M.; Casado, A. Validación española del cuestionario Brief Pain Inventory en pacientes con dolor de causa neoplásica. Med. Clin. 2003, 120, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Gallardo, I.C.; Soriano-Maldonado, A.; Segura-Jiménez, V.; Estévez-López, F.; García-Gómez, A. International Fitness scale (IFIS): Construct validity and reliability in women with fibromyalgia: The al-Ándalus project. Arch. Phys. Med. Rehabil. 2016, 97, 395–404. [Google Scholar] [CrossRef]
- Ruiz Comellas, A.; Pera, G.; Baena Díez, J.M.; González López-Valcárcel, B.; Rodríguez Artalejo, F. Validation of a Spanish short version of the Minnesota leisure time Physical Activity Questionnaire (VREM). Rev. Española Salud Pública 2012, 86, 495–508. [Google Scholar] [CrossRef]
- Herrmann, S.D.; Willis, E.A.; Ainsworth, B.E.; Hartman, T.J.; Lofgren, I.E. 2024 Adult Compendium of Physical Activities: A third update of the energy costs of human activities. J. Sport Health Sci. 2024, 13, 6–12. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; Dempsey, P.C.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Mendes, M.d.A.; da Silva, I.; Ramires, V.; de Souza, B.L.; da Silva, L.L. Metabolic equivalent of task (METs) thresholds as an indicator of physical activity intensity. PLoS ONE 2018, 13, e0200701. [Google Scholar] [CrossRef]
- Matthews, C.E.; Moore, S.C.; Arem, H.; Freedman, N.D.; Berrington de González, A.; Hollenbeck, A.R.; Leitzmann, M.F. Amount and intensity of leisure-time physical activity and lower cancer risk. J. Clin. Oncol. 2020, 38, 686–697. [Google Scholar] [CrossRef]
- Cerezo, O.; Oñate-Ocaña, L.F.; Arrieta-Joffe, P.; García-Pérez, A.; Cruz-Ramos, J.A.; López-Macías, D. Validation of the Mexican-Spanish version of the EORTC QLQ-C30 and BR23 questionnaires to assess health-related quality of life in Mexican women with breast cancer: Quality of life in patients with breast cancer. Eur. J. Cancer Care 2012, 21, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Husson, O.; de Rooij, B.H.; Kieffer, J.; van Leeuwen, M.C.; Mols, F.; van de Poll-Franse, L.V. The EORTC QLQ-C30 summary score as prognostic factor for survival of patients with cancer in the “real-world”: Results from the population-based PROFILES registry. Oncologist 2019, 25, e722–e732. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioural Sciences; Academic Press: New York, NY, USA, 1977. [Google Scholar]
- Vatcheva, K.P.; Lee, M.; McCormick, J.B.; Rahbar, M.H. Multicollinearity in regression analyses conducted in epidemiologic studies. Epidemiology 2016, 6, 227–247. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J.; Ferguson, D.W.; Gill, J.; Paul, J.; Symonds, P. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: A systematic review and meta-analysis. Lancet Oncol. 2013, 14, 721–732. [Google Scholar] [CrossRef]
- Tran, T.X.M.; Jung, S.-Y.; Lee, E.-G.; Kim, H.-J.; Kim, H. Fear of cancer recurrence and its negative impact on health-related quality of life in long-term breast cancer survivors. Cancer Res. Treat. 2022, 54, 1065–1073. [Google Scholar] [CrossRef]
- Keesing, S.; Rosenwax, L.; McNamara, B. The implications of women’s activity limitations and role disruptions during breast cancer survivorship. Women’s Health 2018, 14, 1745505718756381. [Google Scholar] [CrossRef]
- Schmidt, M.E.; Maurer, T.; Behrens, S.; Kneis, S.; Wiskemann, J. Cancer-related fatigue: Towards a more targeted approach based on classification by biomarkers and psychological factors. Int. J. Cancer 2024, 154, 1011–1018. [Google Scholar] [CrossRef]
- Lobefaro, R.; Rota, S.; Porcu, L.; Gatto, M.; Ricciardi, C. Cancer-related fatigue and depression: A monocentric, prospective, cross-sectional study in advanced solid tumors. ESMO Open 2022, 7, 100457. [Google Scholar] [CrossRef]
- Yin, M.; Wang, C.; Gu, K.; Xu, X.; Zheng, Y. Chronic pain and its correlates among long-term breast cancer survivors. J. Cancer Surviv. 2023, 17, 460–467. [Google Scholar] [CrossRef]
- Pérez, C.; Ochoa, D.; Sánchez, N.; García, L.; Martínez, R. Pain in long-term cancer survivors: Prevalence and impact in a cohort composed mostly of breast cancer survivors. Cancers 2024, 16, 1581. [Google Scholar] [CrossRef]
- Aboushaar, N.; Serrano, N. The mutually reinforcing dynamics between pain and stress: Mechanisms, impacts and management strategies. Front. Pain Res. 2024, 5, 1445280. [Google Scholar] [CrossRef] [PubMed]
- Lingens, S.P.; Schulz, F.; Müller, I.; Nissen, C.; Hinz, A. Associations between self-efficacy, distress and anxiety in cancer patient-relative dyads visiting psychosocial cancer support services: Using actor-partner interdependence modelling. PLoS ONE 2021, 16, e0255318. [Google Scholar] [CrossRef]
- Rodrigues, B.; Encantado, J.; Franco, S.; Silva, M.; Santos, L. Psychosocial correlates of physical activity in cancer survivors: A systematic review and meta-analysis. J. Cancer Surviv. 2024. ahead of print. [Google Scholar] [CrossRef]
- Kochman, M.; Kielar, A.; Kasprzak, M.; Nowak, P.; Zielińska, A. The relationship between self-rated health and physical fitness in Polish youth. Healthcare 2023, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Aandstad, A. Self-perceived and self-tested endurance: Associations with objective measures. Percept. Mot. Ski. 2022, 129, 1492–1503. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, K.H.; Campbell, A.M.; Stuiver, M.M.; Pinto, B.M.; Schwartz, A.L. Exercise is medicine in oncology: Engaging clinicians to help patients move through cancer. CA Cancer J. Clin. 2019, 69, 468–484. [Google Scholar] [CrossRef]
- Voskanyan, V.; Marzorati, C.; Sala, D.; Grasso, R.; Pietrobon, R.; van der Heide, I. Correction to: Psychosocial factors associated with quality of life in cancer survivors: Umbrella review. J. Cancer Res. Clin. Oncol. 2024, 150, 408. [Google Scholar] [CrossRef]
- Franzoi, M.A.; Aupomerol, M.; Havas, J.; Pérez, N.; González, M. Investigating sexual health after breast cancer by longitudinal assessment of patient-reported outcomes. ESMO Open 2024, 9, 102236. [Google Scholar] [CrossRef]
- Niedzwiedz, C.L.; Knifton, L.; Robb, K.A.; Stahl, D.; Hann, M. Depression and anxiety among people living with and beyond cancer: A growing clinical and research priority. BMC Cancer 2019, 19, 943–951. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).