1. Introduction
Neuroblastoma is a type of cancer that arises from immature nerve cells, primarily affecting young children. It most commonly develops in the adrenal glands, located above the kidneys, but can also occur in nerve tissues along the spine, chest, abdomen, or pelvis. Neuroblastoma is the most common extracranial solid tumor in children and is known for its heterogeneous behavior, ranging from spontaneous regression to aggressive progression [
1]. The disease can be associated with genetic mutations, such as MYCN oncogene amplification, which is linked to poor prognosis [
2,
3].
SH-SY5Y cells, a cloned subline of a neuroblastoma cell line derived from a metastatic bone tumor, are commonly used as an in vitro model for neuronal function and cell differentiation. They have been widely applied in neuroscience research, including studies on Parkinson’s disease [
4], Alzheimer’s disease, neurotoxicity, ischemia, and amyotrophic lateral sclerosis (ALS) [
5,
6,
7]. Additionally, they serve as a valuable tool for investigating other characteristics of brain cells and neurogenesis [
8].
Radiolabeled compounds are useful as both diagnostic and therapeutic agents. In diagnostics, they play a crucial role in identifying subtle indicators of oncological and neurological disorders [
9]. In treatment, radiopharmaceuticals can be designed with various carriers to damage or eliminate tumor cells [
10]. Some molecules function as both diagnostic and therapeutic agents, a concept known as theranostics [
11]. Similarly, certain elements can exhibit a dual role, as exemplified by radioactive iodine-containing compounds, which can function as either imaging or therapeutic agents, depending on their decay mode, and whose versatility is enabled by radiohalogenation. There are over 30 isotopes of iodine (I), among which Iodine-127 is a stable, non-radioactive isotope. Four of these radionuclides have been adapted for medical use: Iodine-123 and Iodine-124 produce high-quality images, while Iodine-125 and Iodine-131 can be used for therapeutic purposes. Most iodine isotopes are not naturally occurring; for instance, Iodine-131 is produced from the decay of Tellurium-131 through beta emission [
12]. Furthermore, because compounds must be labeled to function as radiopharmaceuticals, radioiodination is a crucial procedure. An effective radioiodination process adds radioactive iodine to a compound or biomolecule without altering its physicochemical or biological properties [
13].
Imidazoles are organic, heterocyclic compounds containing two non-adjacent nitrogen atoms (meta-substituted). Their structural versatility enables wide-ranging pharmaceutical and materials science applications. For instance, benzimidazole derivatives, featuring a benzene ring fused to an imidazole, have shown effectiveness as fungicides, anthelmintics, and agents for antiviral, antitumor, and antihypertensive therapies [
14]. Zeolitic imidazolate frameworks (ZIFs), when combined with bioactive molecules such as amino acids, peptides, proteins, or nucleotides, have also been employed in biosensor development [
15]. One notable imidazole derivative is 6-Iodo-2-(4′-dimethylamino)-phenyl-imidazo[1,2-a]pyridine (IMPY), a heterocyclic compound derived from the thioflavin-T structure. It features a dimethylamino-substituted phenyl group at the 2-position and an iodine atom at the 6-position of the imidazo[1,2-a]pyridine core. The unique structural and chemical characteristics of IMPY make it a valuable scaffold for drug discovery, with potential as a lead compound targeting biological receptors or enzymes such as GABA-A receptors and kinases [
16].
IMPY has been extensively studied as a radiolabeled probe for imaging β-amyloid plaques in Alzheimer’s disease [
17]. The iodine moiety allows for radiolabeling with isotopes such as iodine-123, iodine-125, or iodine-131, facilitating its use in diagnostic imaging [
18]. For example,
18F-labeled styryltriazole and imidazo[1,2-a]pyridine derivatives [
19], as well as
123I-labeled IMPY, have been employed to selectively image β-amyloid plaques [
20], while
125I-labeled variants have shown strong binding affinity to post-mortem Alzheimer’s disease brain tissue, indicating their potential for detecting Tau tangles [
21]. Notably, IMPY derivatives also exhibit good blood–brain barrier permeability, further underscoring their relevance in neuroimaging research. Beyond neurodegenerative applications, IMPY has shown additional therapeutic potential, including antiviral [
22] and anti-angiogenic [
23] properties.
In this study, a novel imidazole-based compound was synthesized through the regioselective formation of three heterocyclic bonds with targeted substitutions at the N-1, C-2, and C-4 positions [
24]. To expand its potential applications, we radiolabeled the compound with
125I, a radionuclide known for its favorable nuclear properties, including a 60-day half-life and gamma emissions ideal for targeted radiotherapy with minimized damage to surrounding healthy tissues. We compared different radiolabeling methods to determine optimal labeling efficiency, and their effects on neuroblastoma cells were evaluated.
2. Materials and Methods
2.1. Compound Syntheses
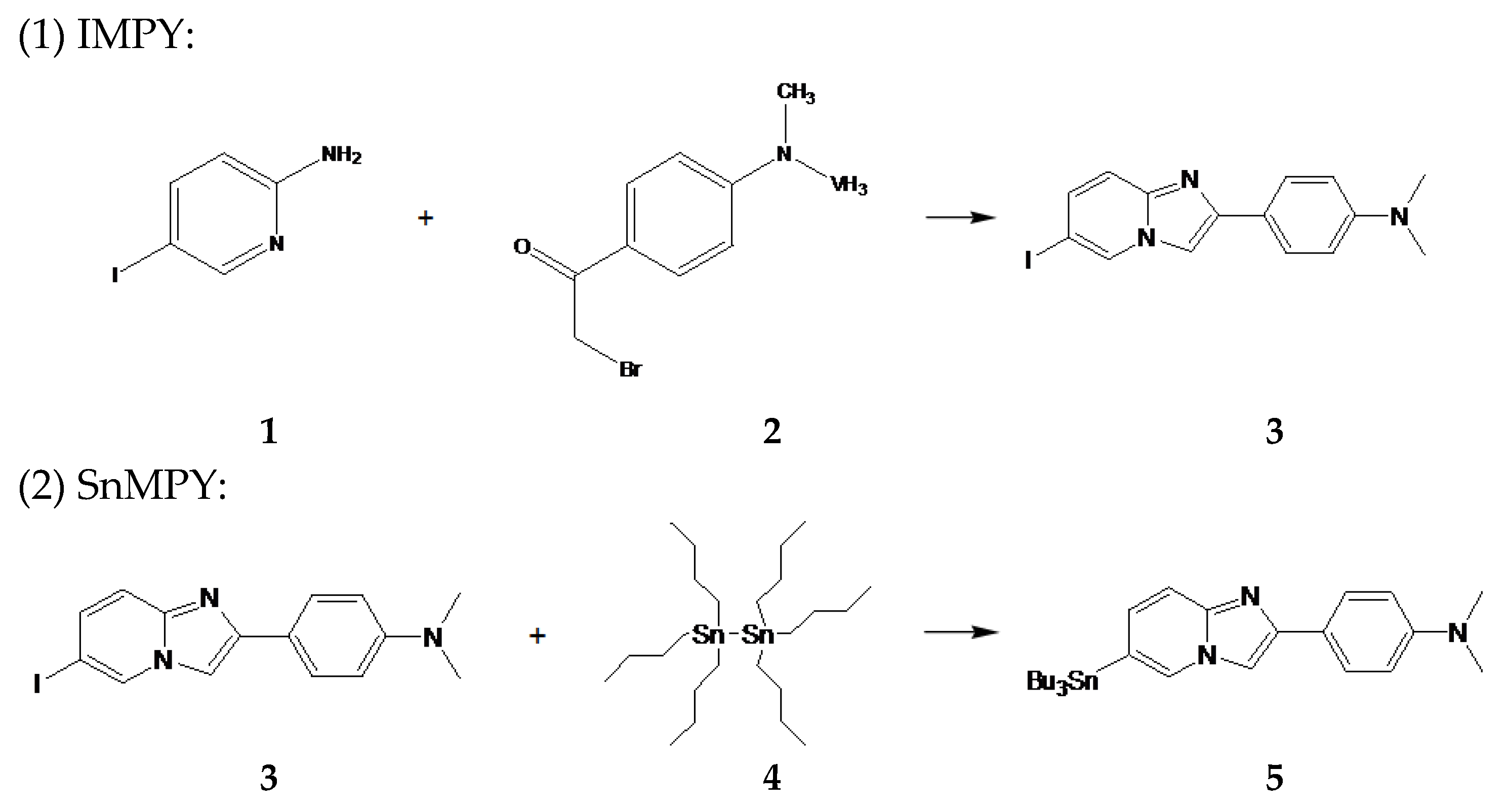
All reagents were purchased from commercial suppliers and used without further purification unless stated otherwise. Column chromatography and thin-layer chromatography (TLC) were performed on silica gel 60 F254 (230–400 mesh, Merck, Darmstadt, Germany) and silica gel 60 glass plates (Merck, Darmstadt, Germany), respectively. Preparative thin-layer chromatography (PTLC) was conducted on silica gel plates with a fluorescent indicator, visualized under 254 nm UV light.
An ethanol (J.T.Baker #8006-05, Landsmeer, The Netherlands) solution containing 5-iodo-2-pyridinamine 1 (100 mg) (IPA, Matrix #001779, Columbia, SC, USA) and 4-(dimethylamino)phenacyl bromide 2 (120 mg) (DMPB, Apollo Scientific 102 #OR3530, Manchester, UK) was refluxed for 2 h, during which time samples were taken at hourly intervals for analysis by TLC (Macherey-Nagel #818133, Rotherham, UK). After cooling, NaHCO3 (Sigma #S5761, St. Louis, MO, USA) was added to the solution, which was then refluxed for another hour. Subsequently, the reaction mixture was absorbed onto silica (0.04–0.063 mm, Macherey-Nagel #815381, Rotherham, UK). The crude product was purified by column chromatography using a solvent mixture of ethyl acetate and n-hexane (1:6) (Ethyl acetate, Sigma #270989: n-Hexane, Sigma #296090, St. Louis, MO, USA) to yield 6-iodo-2-(4′-dimethylamino)-phenyl-imidazo[1,2-a]pyridine 3 (86.1 mg, 47.4%). 1H NMR (200 MHz, CDCl3): δ 2.998 (s, 6H), 6.633 (d, 2H), 7.265 (dd, 1H), 7.381 (d, 1H), 7.643 (s, 1H), 7.788 (d, 2H), 8.302 (s, 1H). 13C NMR (200 MHz, CDCl3): δ 40.415, 98.274, 106.225, 112.401, 117.970, 121.202, 127.060, 130.094, 131.915, 144.085, 146.953, 150.580.
A mixture of 6-iodo-2-(4′-dimethylamino)-phenyl-imidazo[1,2-a]pyridine 3 (200 mg) and Pd(Ph3P)4 (Sigma #216666, St. Louis, MO, USA) in a solution of 1,4-dioxane (Merk #1115097, St. Louis, MO, USA), triethylamine (Sigma #T0886, St. Louis, MO, USA), and (Bu3Sn)2 4 (80 mg) (Alfa Aesar #A12007, Waltham, MA USA) was stirred at 90 °C for 1 h. The solvent was then removed, and the residue was purified by PTLC (ethyl acetate: n-hexane = 1:1 as the developing solvent) to yield SnMPY 5 (52.9 mg, 18.3%). 1H NMR (200 MHz, CDCl3): δ 0.901 (t, 9H), 1.106 (t, 6H), 1.330 (m, 6H), 1.567 (m, 6H), 2.994 (s, 6H), 6.791 (d, 2H), 7.111 (d, 1H), 7.576 (d, 1H), 7.710 (s, 1H), 7.848 (d, 2H), 7.954 (s, 1H). 13C NMR (200 MHz, CDCl3): δ 9.748, 13.617, 27.274, 28.974, 40.476, 105.573, 112.477, 116.605, 121.369, 122.204, 126.953, 129.836, 130.747, 145.511, 145.693, 150.322.
The chemical synthesis reactions and reaction equations of IMPY and SnMPY are shown below (
Scheme 1):
2.2. Structural Analysis of Compounds
The 1H NMR and 13C NMR spectra of the synthesized compounds were recorded on NMR spectrometers (Varian NMR spectrometer Gemini 2000, Palo Alto, CA, USA), with chemical shifts reported in ppm (δ values) and referenced to CDCl3. Mass spectra were obtained using liquid chromatography coupled with mass spectrometry (LC-MS), specifically a Waters 2695 Separations Module with a Waters Micromass ZQ (Milford, MA, USA).
2.3. Radiolabeling
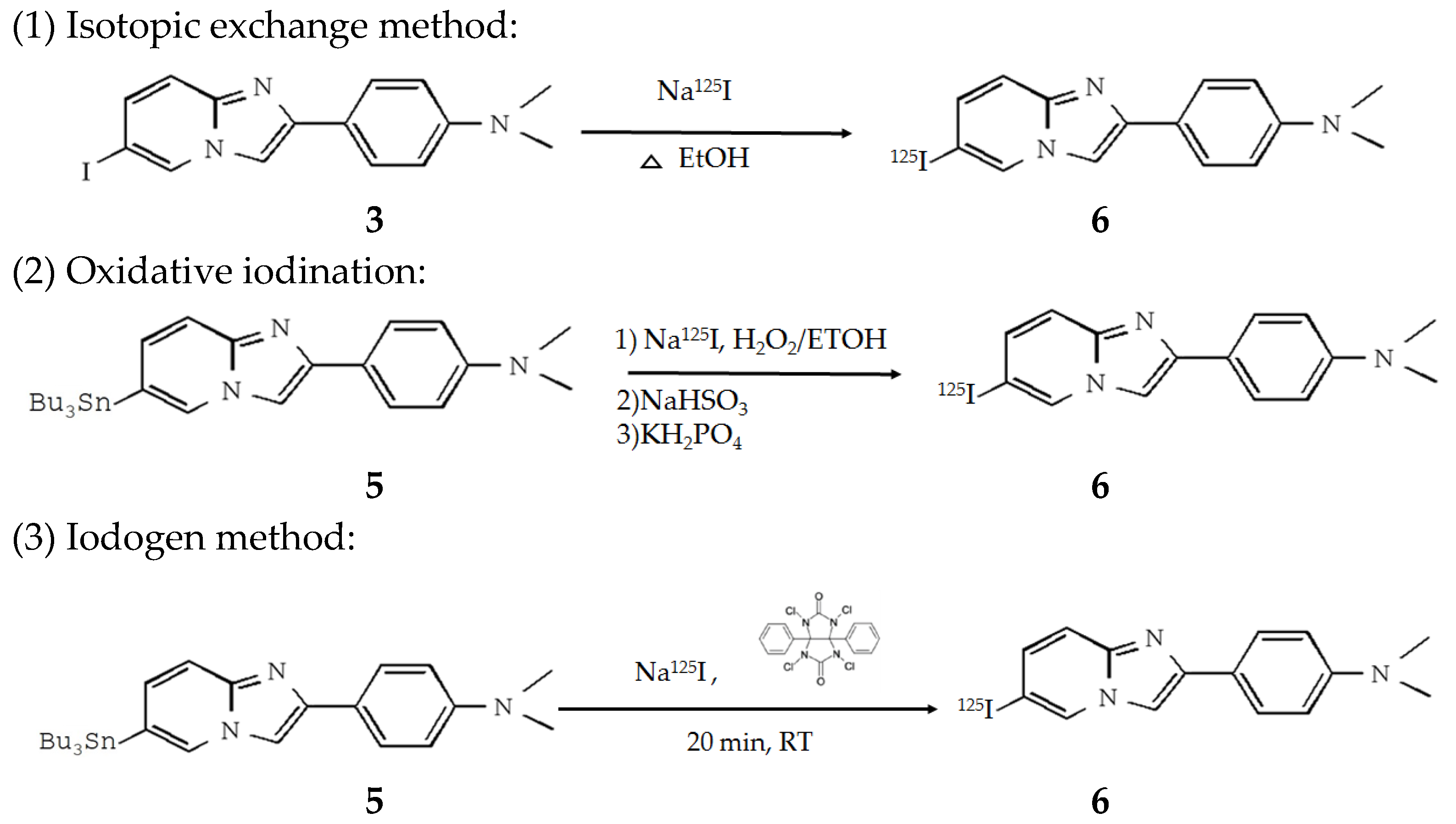
IMPY was labeled with 125I ion using three methods: isotopic exchange, oxidative iodination, and the Iodogen method.
For the isotopic exchange method, IMPY was incubated in a Na125I (I-RB-4, Institute of Isotopes) solution for 2 h, allowing the 125I ion in the solution to exchange with labile iodine in IMPY. After incubation, the mixture was purified using a C18 SPE cartridge (Thermo #60108-302, Waltham, MA, USA) to separate unlabeled 125I ions and IMPY from 125I-IMPY.
For oxidative iodination, 50 μL of 100% ethanol was added to 200 μg of SnMPY, then thoroughly mixed with 300 μL of Na125I (activity approximately 1 mCi). Next, 100 μL of 5% H2O2 solution (prepared by mixing 58.3 μL of water, 25 μL of glacial acetic acid, and 16.7 μL of 30% H2O2) was added, and the mixture was shaken for 15 min. After the reaction, 300 μL of 39% NaHSO3 (Sigma #243973, St. Louis, MO, USA) was added to stop the iodination reaction, followed by the immediate addition of 2 mL of saturated KH2PO4 (Riedel-de Haën #S240697, Seelze, Germany). The resulting solution was passed through a C18 SPE cartridge, washed with 9 mL of water, and then eluted with 2 mL of 40% ethanol to remove impurities. Finally, the desired product, 125I-IMPY, was eluted using 2 mL of 60% ethanol.
For the Iodogen method, 300 μg of SnMPY and 10 μL of phosphate buffer (pH 8.0) were added to a coating tube containing 2 mg of Iodogen (Thermo #28600, Waltham, MA, USA) and mixed. Then, approximately 200 μCi of Na125I was added and mixed. The reaction was allowed to proceed at room temperature for 20 min and then the reaction solution was removed. To terminate the reaction and remove unbound 125I, the Iodogen tube was rinsed with 1 mL of deionized water. The radiolabeled IMPY was then eluted by rinsing the tube with 1 mL of ethanol. A TLC reader (BrightSpec bSCAN-V3, Niel, Belgium) was used to determine the radiochemical conversion and Rf values.
For the method of radiochemical yield (RCY) determination, the dose of iodinated IMPY (DL) is divided by the sum of the dose of iodinated IMPY (DL) and the dose of free 125I ion (DF). The RCY was calculated using the following formula: .
The radiolabeling procedures to obtain
125I-IMPY are shown below (
Scheme 2):
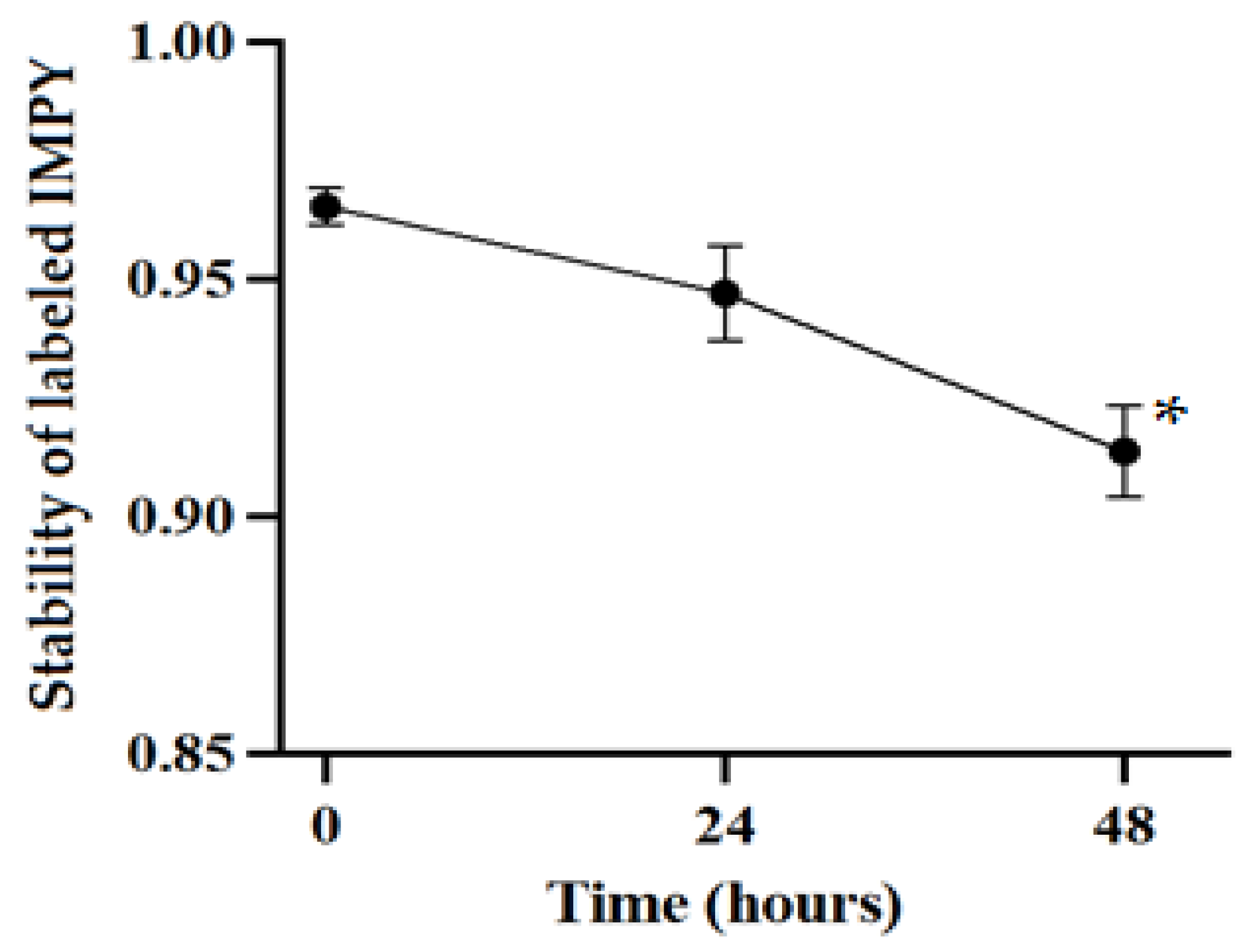
2.4. Radiolabeling Stability Test
After radiolabeling the compound, TLC was used to measure the ratio of iodinated IMPY to free iodine in order to assess the stability of the compound. Stability tests were conducted at 0, 24, and 48 h after preparation, monitoring the ratio of iodinated IMPY to free 125I and calculating its change over time.
2.5. Cell Culture
A neuroblastoma cell line (SH-SY5Y) was cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), L-glutamine (2 mM), streptomycin (100 μg/mL), and penicillin (100 units/mL). The cell line was maintained at 37 °C in a humidified CO2 incubator.
2.6. Cell Viability and Cellular Uptake
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) is a yellow compound that can be catalyzed by succinate dehydrogenase (SDH) in the mitochondria of viable cells, generating purple formazan crystals. By dissolving these formazan crystals in dimethyl sulfoxide (DMSO), their absorbance can be used to evaluate cell viability, making MTT assays a common method for assessing the effects of drugs on cell survival.
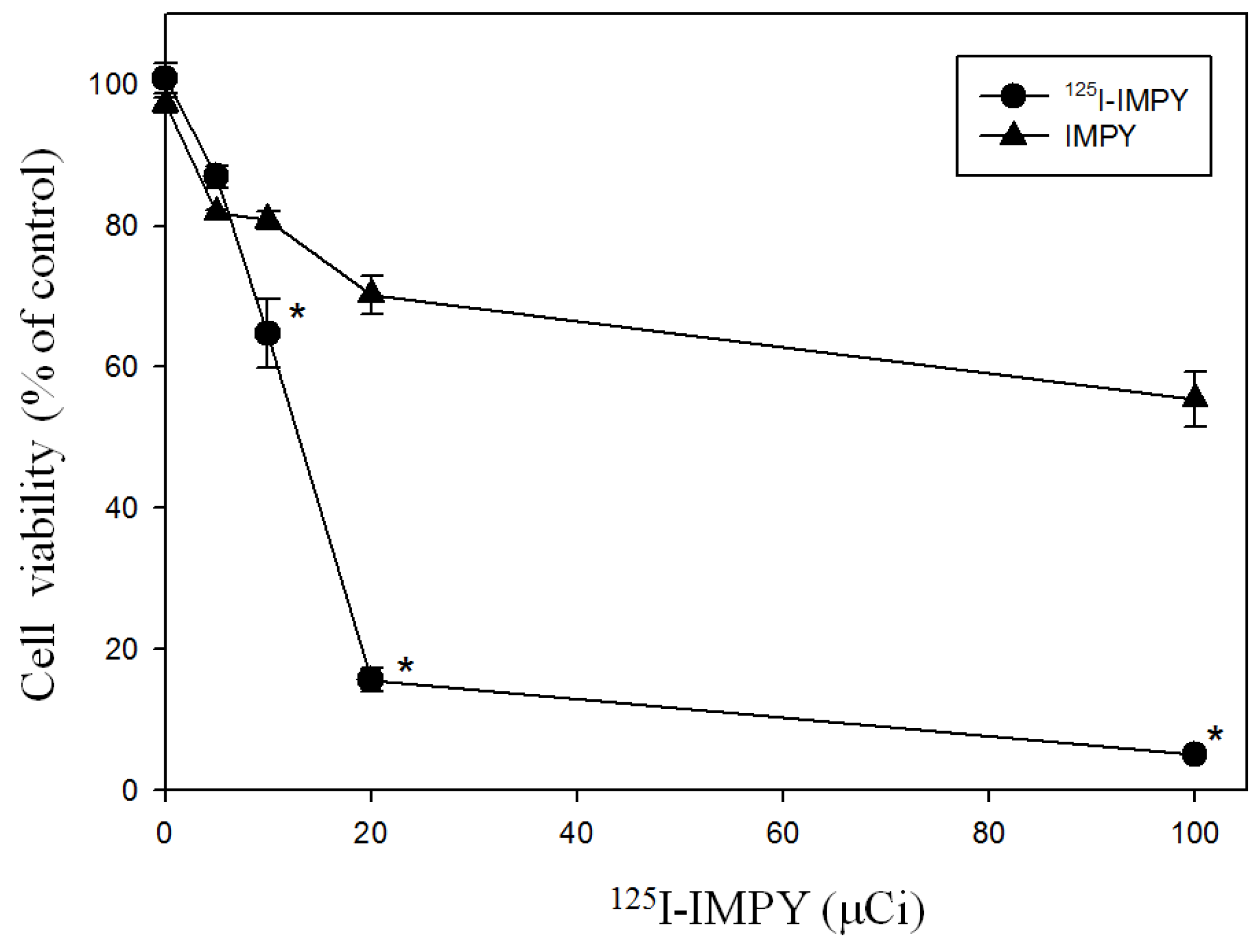
To evaluate the effect of 125I-IMPY on SH-SY5Y cell viability, SH-SY5Y cells (2 × 104 cells per well, 200 μL per well) were seeded into a 96-well plate. After 24 h, the culture medium was replaced, and the cells were incubated with 125I-IMPY at four different doses (5, 10, 20, and 100 μCi) for 24 h. During cell culture, lead plates were used as shielding barriers to separate cells receiving different radiation doses. Following incubation, the medium was removed, and 100 μL of fresh culture medium was added to each well, followed by the addition of 10 μL of 12 mM MTT solution. The plate was incubated at 37 °C in a 5% CO2 incubator for 4 h.
After incubation, 85 μL of the MTT-containing medium was removed, leaving a residual volume of 25 μL. Then, 100 μL of DMSO was added to each well, mixed thoroughly, and incubated at 37 °C in a 5% CO2 incubator for 10 min. The absorbance at 540 nm was then measured using a microplate reader (BioTek Synergy H1, Agilent, Santa Clara, CA, USA).
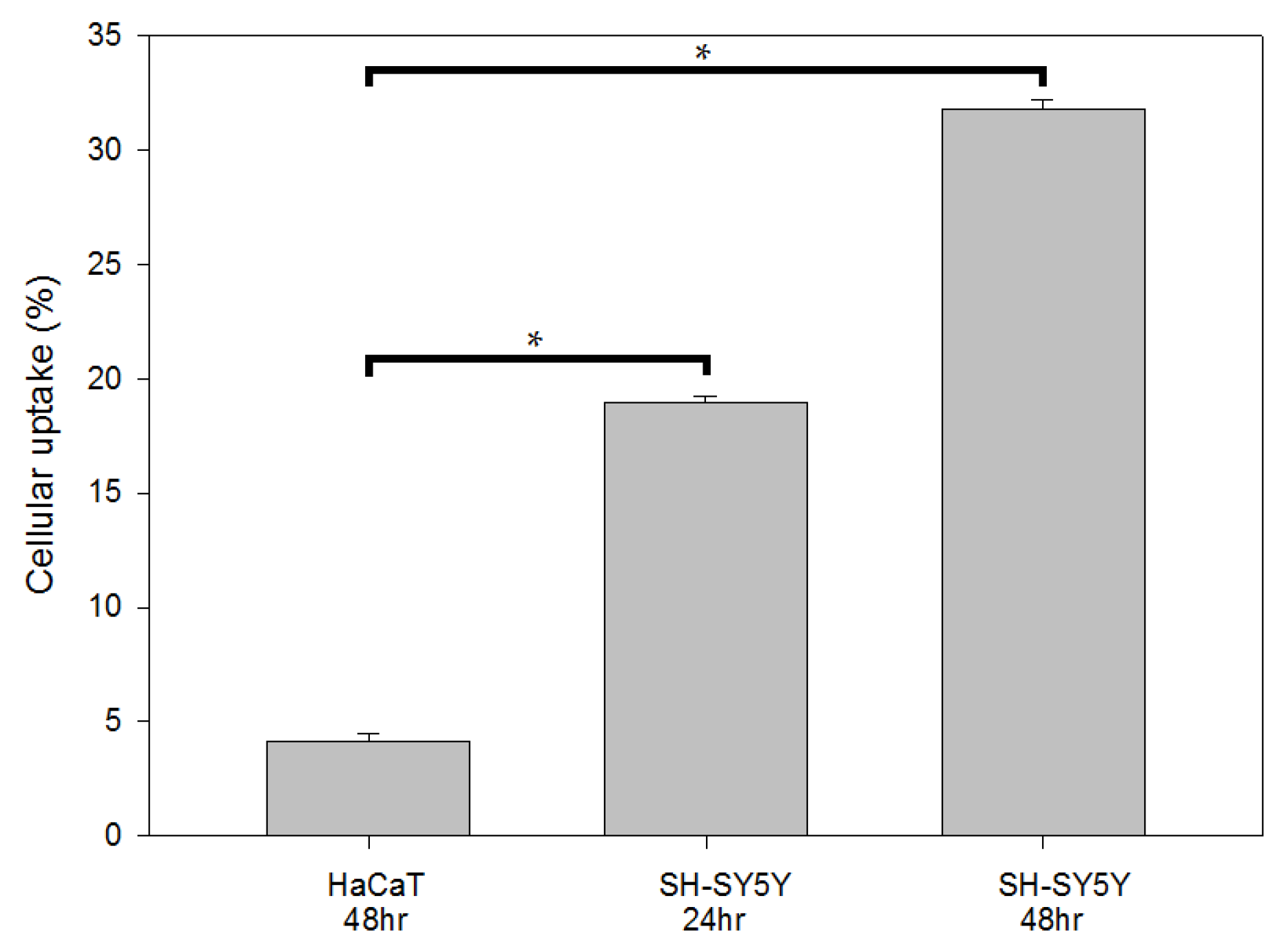
To determine the cellular uptake, cells (1.5 × 105) were seeded overnight in 10 cm dishes and incubated with 2 μCi of 125I-IMPY. After 24 and 48 h of incubation at 37 °C with 5% CO2, the cells were collected, and the isotopic activity was analyzed using a well-type scintillation counter (Scalar Ratemeter SR-7, Nuclear Enterprises Ltd., Edinburgh, UK).
2.7. LDH Assay
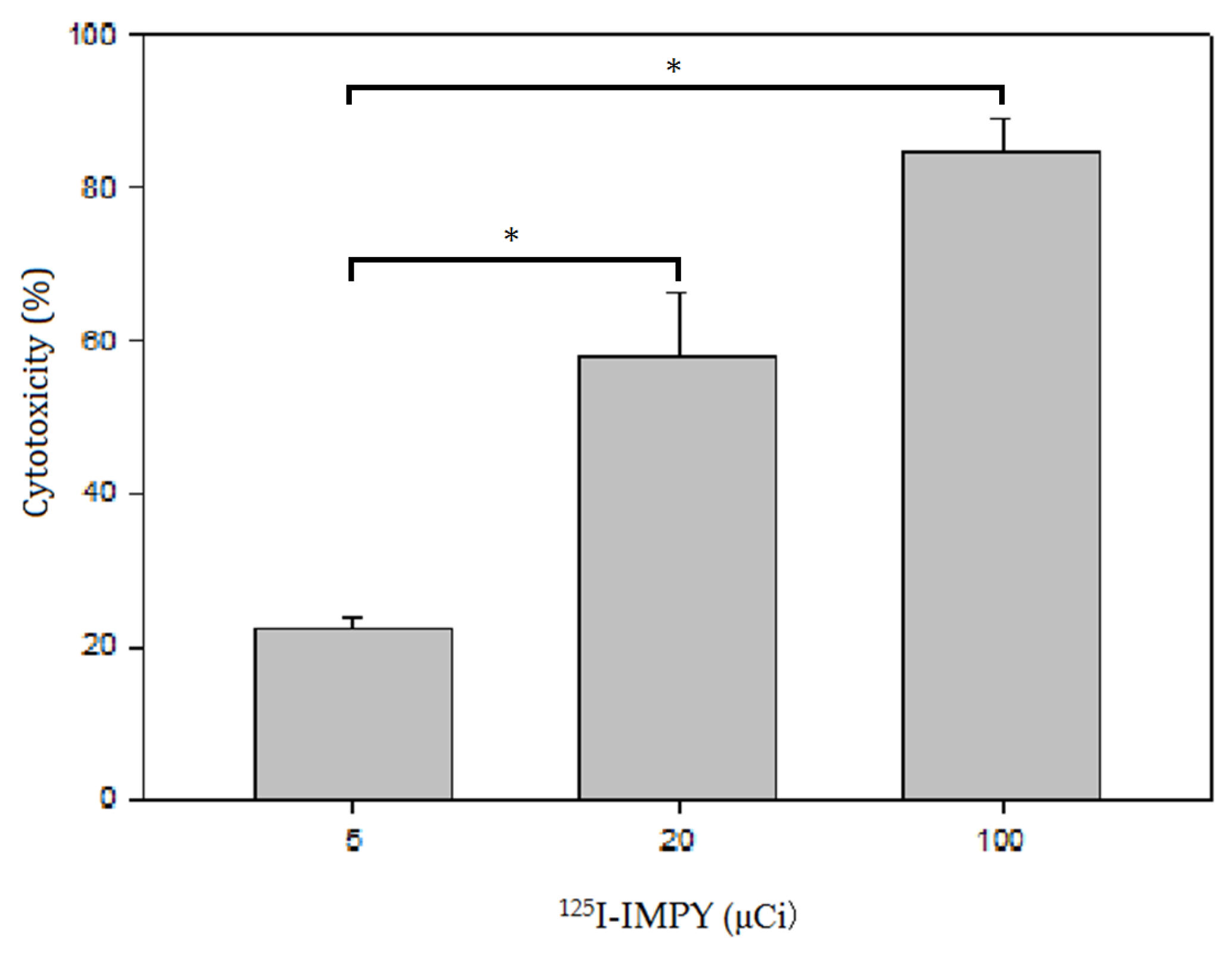
The cell cytotoxicity study was conducted using the lactate dehydrogenase (LDH) leakage assay, which measures LDH released into the culture medium. The assay is based on the conversion of lactate to pyruvate in the presence of LDH, accompanied by the reduction of NAD. The resulting formation of NADH led to a measurable change in absorbance at 340 nm using a microplate reader (BioTek Synergy H1, Agilent, Santa Clara, CA, USA).
2.8. Immunofluorescence Staining
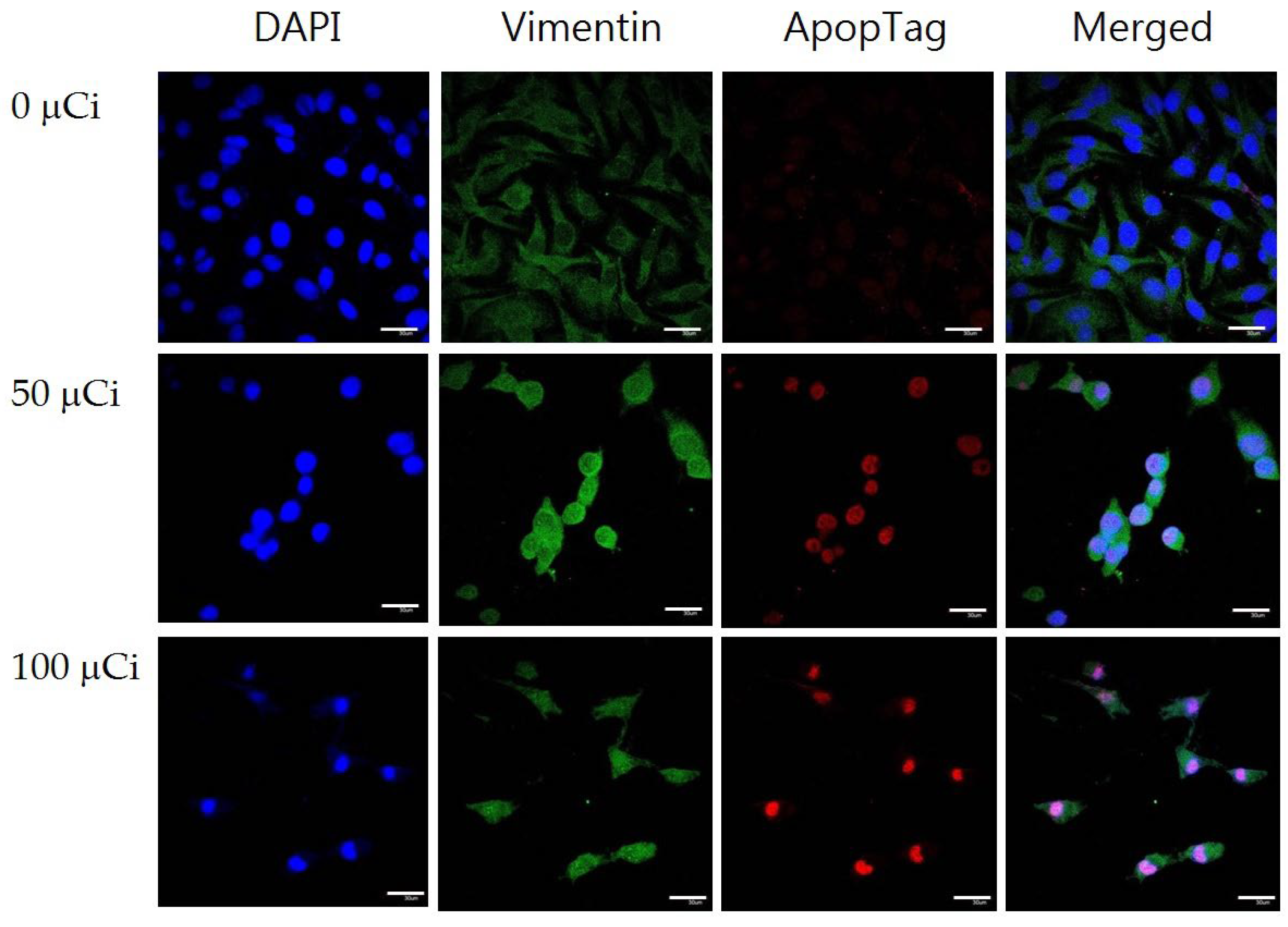
After treating with various concentrations of 125I-IMPY, cells were fixed with cold methanol. The nuclei and cytoskeleton of the cells were stained with 4′-6-diamidino-2-phenylindole (DAPI, Thermo# 62247, Waltham, MA, USA) and vimentin (EPITOMICS #2862-1, Waltham, MA, USA), respectively. In addition, an Apoptosis Detection Kit (ApopTag#S7165, Merk, St. Louis, MO, USA) was used according to the manufacture’ protocol. The DNA break was detected and stained with a rhodamine fluorochrome (FLoid Cell Fluorescence Imaging Station, Invitrogen, Waltham, MA, USA).
2.9. Western Blot
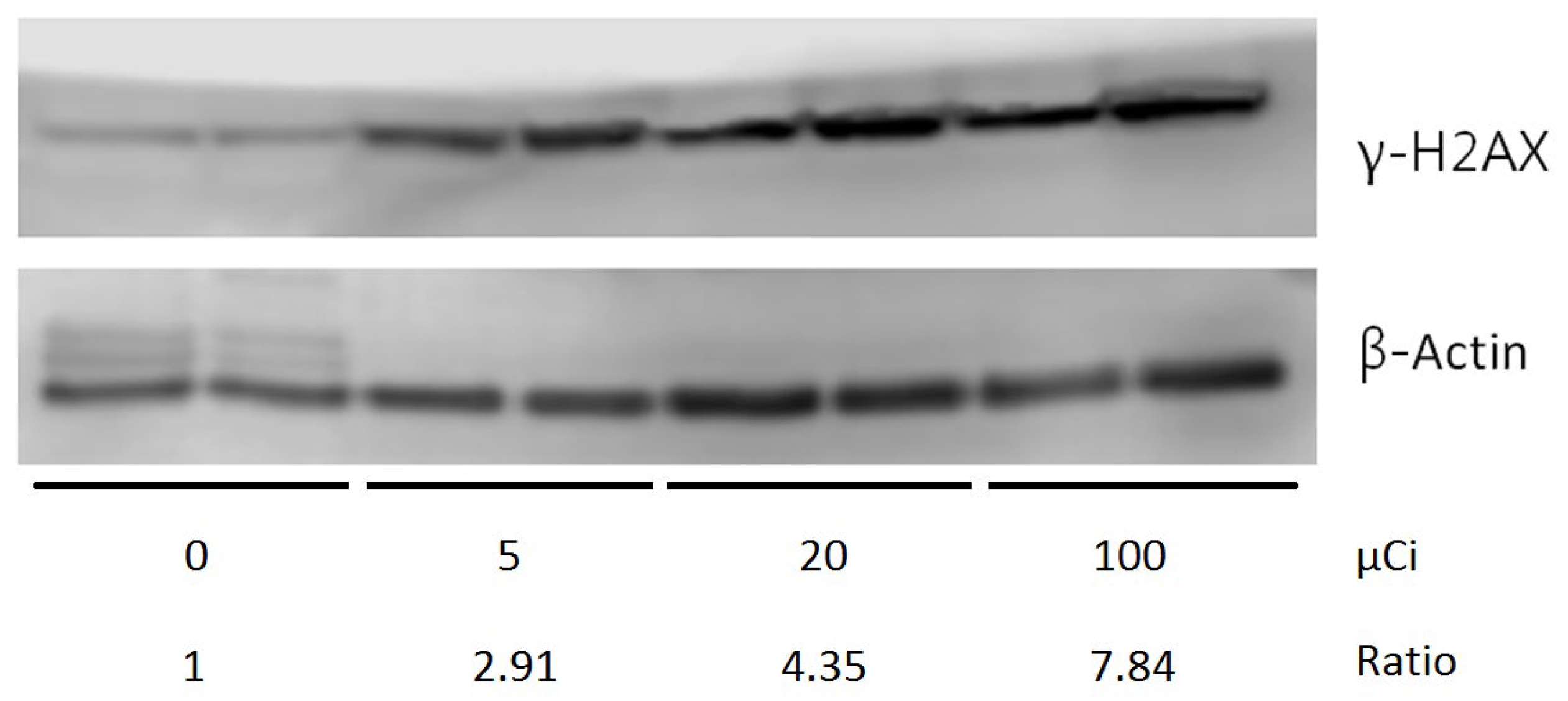
Protein extracts were prepared in RIPA cell lysis buffer. Each protein sample (1 mg/mL, 10 μL) was electrophoresed on a precast gel (NuPAGE® Novex® 4–12% Bis-Tris Gel, 1.5 mm, 10 wells; Invitrogen™, Waltham, MA, USA). Proteins were then transferred from the gel to a polyvinylidene difluoride (PVDF) membrane using a semidry transfer system (Criterion Blotter, Bio-Rad, Hercules, CA, USA) at 100 V for 60 min and subsequently blocked with 5% milk in PBS (pH 7.4) containing 0.05% Tween-20. The membranes were incubated overnight with the primary antibody (γ-H2AX, SAB5600038, Sigma-Aldrich, St. Louis, MO, USA, 1:500 dilution or β-actin, AC-15, Sigma-Aldrich, St. Louis, MO, USA, 1:5000 dilution).
Following rinsing with PBST, the membranes were incubated with HRP-conjugated secondary antibodies (1:10,000 dilution) for 1 h. Protein detection was performed using an enhanced chemiluminescence (ECL) system, and quantitative analysis was carried out using the ImageQuant-TL-7.0 software (GE Healthcare, Amsterdam, The Netherlands).
2.10. Statistical Analysis
p-Values were calculated using the unpaired two-tailed Student’s t-test with MS excel and GraphPad Prism 5.0 (GraphPad, La Jolla, CA, USA). Data were considered statistically significant at p < 0.05.
4. Discussion
As a promising neuroimaging agent in nuclear medicine,
125I-IMPY exhibits high specificity, favorable pharmacokinetics with rapid metabolism, and low uptake by surrounding normal cells. This study aimed to synthesize IMPY, label it with
125I ion using various methods, and evaluate its cytotoxic effects while monitoring cellular changes through fluorescent immunostaining. Three different methods were employed for labeling
125I-IMPY: isotope exchange labeling, oxidative iodination, and Iodogen iodination. The isotope exchange method replaces a non-radioactive iodine atom on an aromatic ring with a radioactive one, while oxidative iodination utilizes radioiododestannylation—both proceeding via electrophilic substitution reactions. In contrast, the Iodogen method follows a nucleophilic substitution reaction [
26]. Although radioiododestannylation is widely used in laboratory settings [
27], its labeling efficiency was significantly lower compared to the Iodogen method, which achieved a radiochemical yield of 96.52%. Even after 48 h, the labeling rate remained at 93.38%, demonstrating excellent stability. Notably, we are the first group to successfully label
125I into IMPY using the Iodogen method, highlighting its superior efficiency. These findings suggest that Iodogen iodination is the most effective method for
125I-IMPY labeling and warrants further exploration for potential applications.
Initially,
123I-IMPY was developed as a SPECT imaging agent for β-amyloid labeling; however, during preclinical trials, its signal-to-noise ratio proved unsatisfactory [
28]. Consequently, the continued development of novel β-amyloid probes led to the removal of
123I-IMPY from the list of potential imaging agents [
28]. Among the available isotopes,
125I is cost-effective, widely available, and associated with relatively few complications [
26]. Therefore, we labeled
125I into IMPY as a potential therapeutic agent for neuroendocrine tumors and evaluated its toxicity in neuroblastoma cells. To assess the effect of
125I-IMPY on SH-SY5Y cell growth, we performed a serial dilution analysis across a range of concentrations. Although
125I-IMPY at lower doses inhibited cell growth, its cytotoxicity remained within the microcurie (µCi) range, indicating relatively low toxicity to these cells. When comparing the
125I-IMPY cell growth inhibition curve, we observed that at doses between 15 and 100 µM, cell viability sharply declined to approximately 60%.
The radiochemical yield results indicate that the Iodogen iodination method achieved the highest yield. In cellular experiments, SH-SY5Y cells exhibited significantly higher uptake rates compared to normal HaCaT cells, with uptake on the second day being markedly higher than that on the first day. In the cytotoxicity assay, SH-SY5Y cells maintained 90% viability at a dose that was approximately half of the IC50 value. This may be attributed to the fact that SH-SY5Y cells are neuronal in origin, while 125I-IMPY is a brain imaging agent designed for regions rich in neuronal cells. Consequently, SH-SY5Y cells were more sensitive to 125I-IMPY, reaching the IC50 at double the tolerable dose. Furthermore, fluorescent immunostaining demonstrated that as the drug concentration increased, SH-SY5Y cell morphology changed, cell numbers decreased, and apoptosis increased.
The phosphorylated form of the histone protein H2AX, known as γ-H2AX, is a well-established biomarker for DNA double-strand breaks (DSBs). Upon the occurrence of DSBs, such as those induced by ionizing radiation or DNA-damaging agents, H2AX is rapidly phosphorylated at the site of damage, leading to the formation of γ-H2AX foci. These foci serve as platforms for the recruitment and localization of DNA repair proteins, thereby playing a critical role in the DNA damage response and repair mechanisms [
29,
30]. Quantifying γ-H2AX levels is widely used to evaluate both the extent of DNA damage and the repair efficiency of cells.
In the context of our study, radiolabeled IMPY may also exert radiopharmaceutical effects, including the induction of DNA damage in tumor cells. This suggests a dual role for radiolabeled IMPY as a tumor-targeting tracer and as a therapeutic agent capable of eliciting cellular responses such as γ-H2AX formation, which is indicative of DNA damage. Further investigation is warranted to explore this potential and to better understand the therapeutic implications of radiolabeled IMPY in cancer treatment.