Behind-the-Scenes Actors in Fertility: A Comprehensive Review of the Female Reproductive Tract Microbiome and Its Clinical Relevance
Abstract
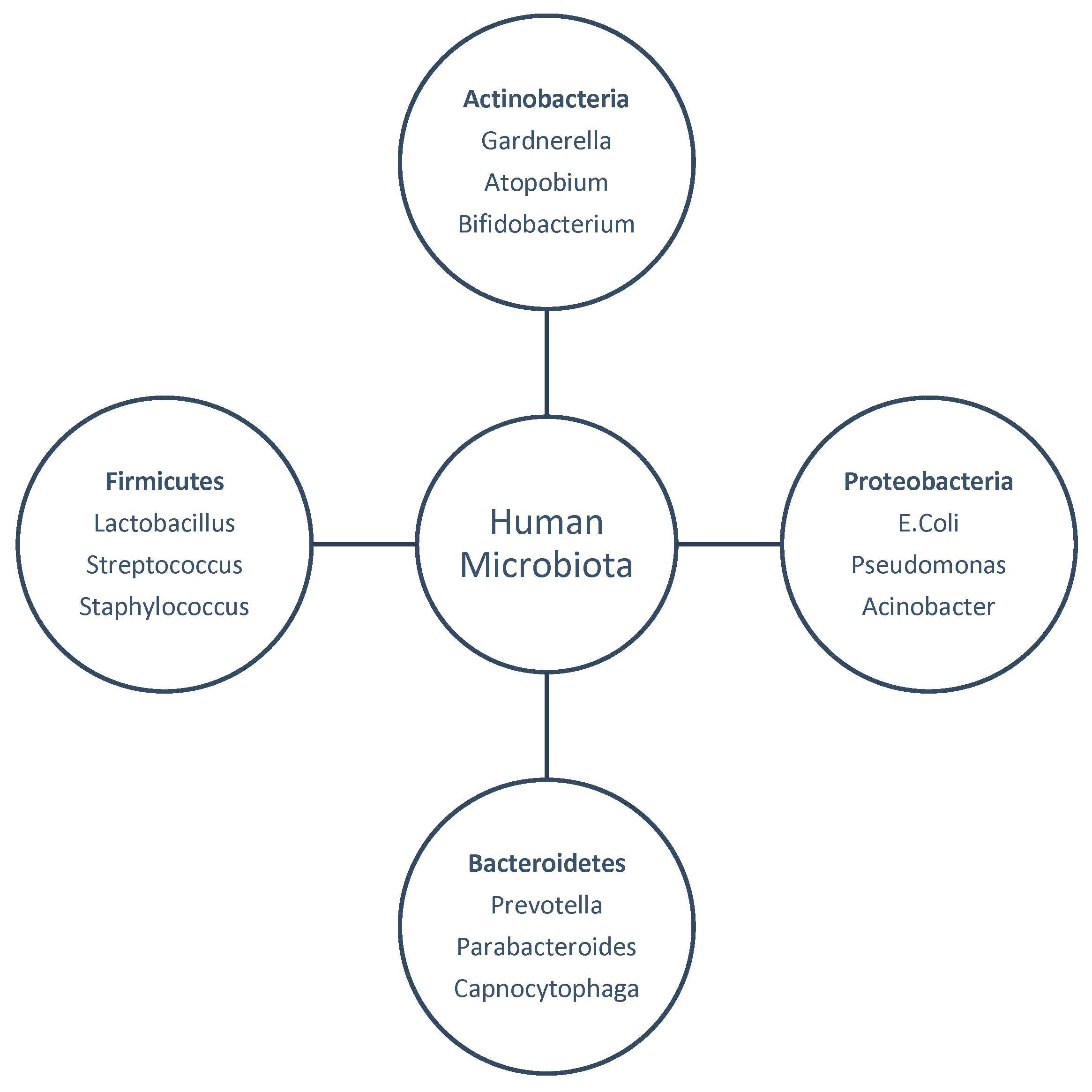
1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Research Questions
2.3. Sampling Methods
2.4. Analytical Techniques
3. Results
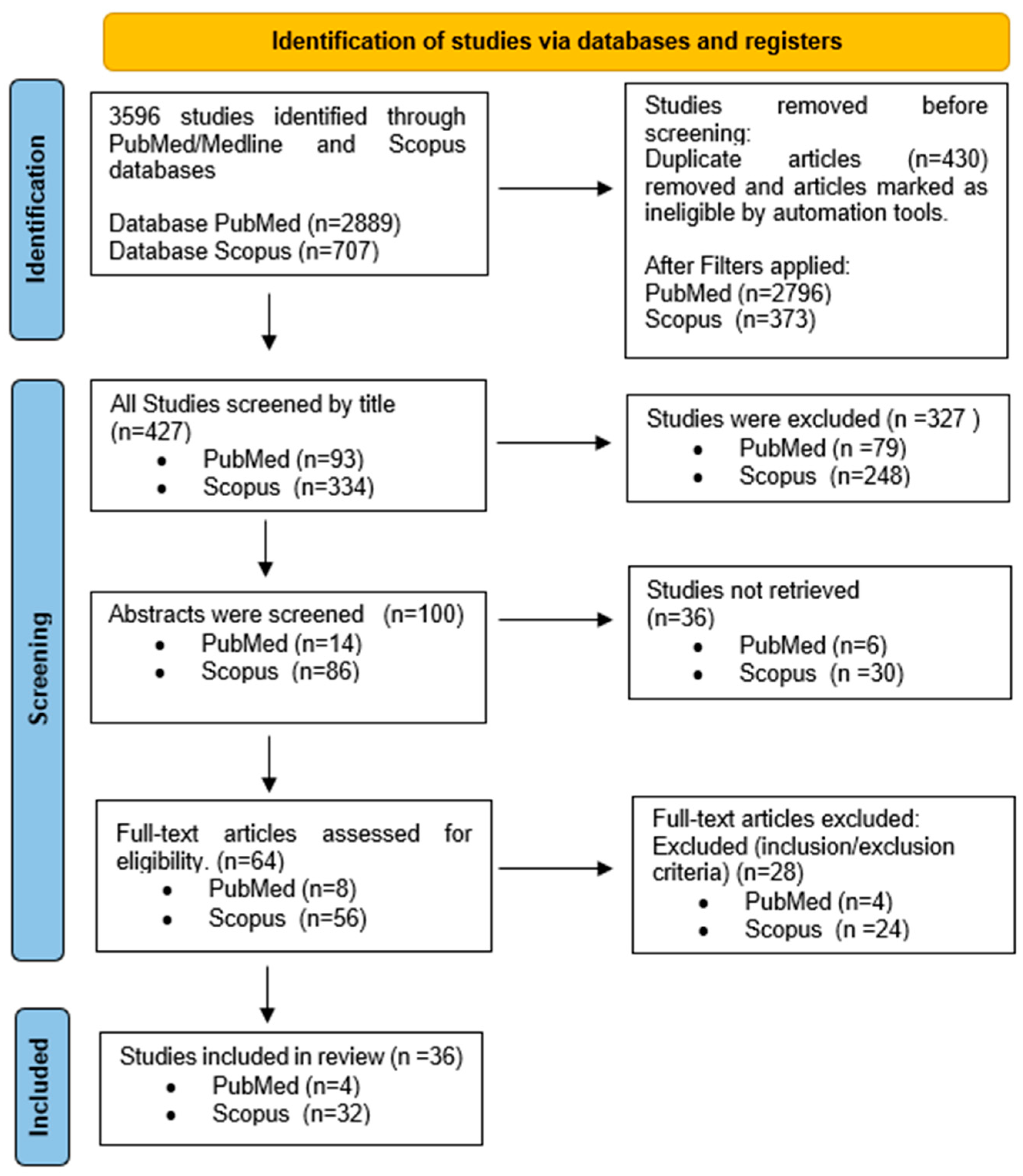
3.1. Study Selection
3.2. Microbiome of the Reproductive Tract in Infertility and ART Outcomes
3.2.1. Vaginal Microbiome and Its Role in Vaginitis/Dysbiosis
3.2.2. Findings from Vaginal Microbiome Related to Recurrent Implantation Failure (RIF)
3.2.3. Findings from Vaginal Microbiome Related to Antibiotic/Probiotic/Vaginal Lactobacillus Supplementation
3.2.4. Findings from Vaginal Microbiome in Relation to IVF
3.3. Cervical Microbiome and Its Implications for Fertility
3.4. Endometrial Microbiome and IVF Success: The Role of Lactobacillus Dominance
3.4.1. Endometrial Microbiome in Relation to Chronic Endometritis
3.4.2. Endometrial Microbiome in Relation to Recurrent Implantation Failure (RIF)
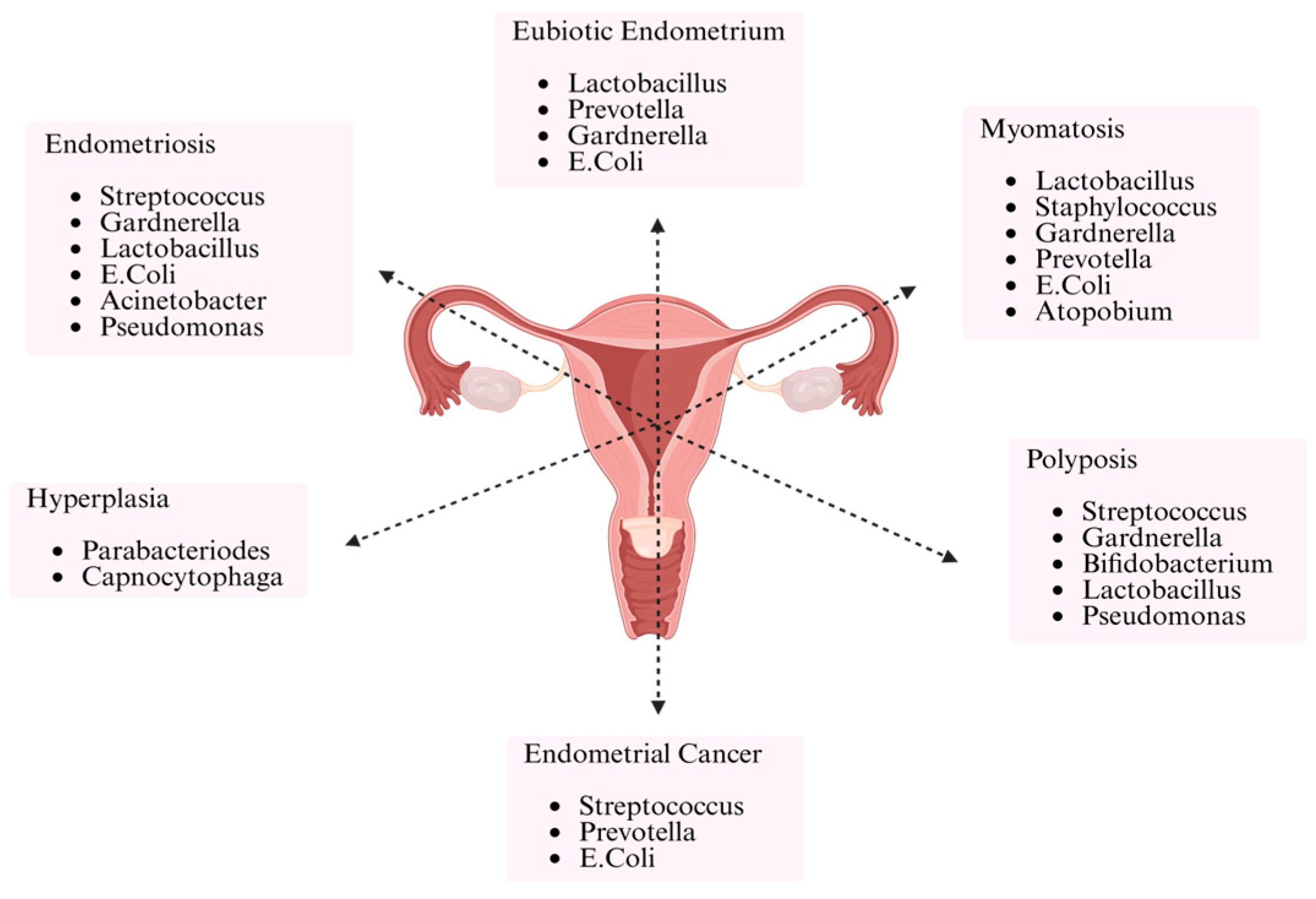
4. Discussion
5. Conclusions
6. Strengths and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marchesi, J.R.; Ravel, J. The vocabulary of microbiome research: A proposal. Microbiome 2015, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the human microbiome. Nutr. Rev. 2012, 70, S38–S44. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Verges, M.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Garcia-Grau, I.; Perez-Villaroya, D.; Gonzalez-Monfort, M.; Bahceci, M.; Barrionuevo, M.J.; Taguchi, S.; Puente, E.; Dimattina, M.; Lim, M.W.; et al. Endometrial microbiota composition is associated with reproductive outcome in infertile patients. Microbiome 2022, 10, 1. [Google Scholar] [CrossRef]
- Kaltsas, A.; Zikopoulos, A.; Vrachnis, D.; Skentou, C.; Symeonidis, E.N.; Dimitriadis, F.; Stavros, S.; Chrisofos, M.; Sofikitis, N.; Vrachnis, N.; et al. Advanced Paternal Age in Focus: Unraveling Its Influence on Assisted Reproductive Technology Outcomes. J. Clin. Med. 2024, 13, 2731. [Google Scholar] [CrossRef]
- Stavros, S.; Panagopoulos, P.; Machairiotis, N.; Potiris, A.; Mavrogianni, D.; Sfakianakis, A.; Drakaki, E.; Christodoulaki, C.; Panagiotopoulos, D.; Sioutis, D.; et al. Association between cytokine polymorphisms and recurrent pregnancy loss: A review of current evidence. Int. J. Gynaecol. Obstet. 2024, 167, 45–57. [Google Scholar] [CrossRef]
- Patronia, M.M.; Potiris, A.; Mavrogianni, D.; Drakaki, E.; Karampitsakos, T.; Machairoudias, P.; Topis, S.; Zikopoulos, A.; Vrachnis, D.; Moustakli, E.; et al. The Expression of microRNAs and Their Involvement in Recurrent Pregnancy Loss. J. Clin. Med. 2024, 13, 3361. [Google Scholar] [CrossRef]
- Elnashar, A.M. Impact of endometrial microbiome on fertility. Middle East Fertil. Soc. J. 2021, 26, 4. [Google Scholar] [CrossRef]
- Toson, B.; Simon, C.; Moreno, I. The Endometrial Microbiome and Its Impact on Human Conception. Int. J. Mol. Sci. 2022, 23, 485. [Google Scholar] [CrossRef]
- Haahr, T.; Jensen, J.S.; Thomsen, L.; Duus, L.; Rygaard, K.; Humaidan, P. Abnormal vaginal microbiota may be associated with poor reproductive outcomes: A prospective study in IVF patients. Hum. Reprod. 2016, 31, 795–803. [Google Scholar] [CrossRef]
- Campisciano, G.; Florian, F.; D’Eustacchio, A.; Stankovic, D.; Ricci, G.; De Seta, F.; Comar, M. Subclinical alteration of the cervical-vaginal microbiome in women with idiopathic infertility. J. Cell Physiol. 2017, 232, 1681–1688. [Google Scholar] [CrossRef]
- Kyono, K.; Hashimoto, T.; Nagai, Y.; Sakuraba, Y. Analysis of endometrial microbiota by 16S ribosomal RNA gene sequencing among infertile patients: A single-center pilot study. Reprod. Med. Biol. 2018, 17, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Amato, V.; Papaleo, E.; Pasciuta, R.; Vigano, P.; Ferrarese, R.; Clementi, N.; Sanchez, A.M.; Quaranta, L.; Burioni, R.; Ambrosi, A.; et al. Differential Composition of Vaginal Microbiome, but Not of Seminal Microbiome, Is Associated with Successful Intrauterine Insemination in Couples with Idiopathic Infertility: A Prospective Observational Study. Open. Forum Infect. Dis. 2020, 7, ofz525. [Google Scholar] [CrossRef]
- Liu, Y.; Ko, E.Y.; Wong, K.K.; Chen, X.; Cheung, W.C.; Law, T.S.; Chung, J.P.; Tsui, S.K.; Li, T.C.; Chim, S.S. Endometrial microbiota in infertile women with and without chronic endometritis as diagnosed using a quantitative and reference range-based method. Fertil. Steril. 2019, 112, 707–717.e701. [Google Scholar] [CrossRef]
- Haahr, T.; Humaidan, P.; Elbaek, H.O.; Alsbjerg, B.; Laursen, R.J.; Rygaard, K.; Johannesen, T.B.; Andersen, P.S.; Ng, K.L.; Jensen, J.S. Vaginal Microbiota and In Vitro Fertilization Outcomes: Development of a Simple Diagnostic Tool to Predict Patients at Risk of a Poor Reproductive Outcome. J. Infect. Dis. 2019, 219, 1809–1817. [Google Scholar] [CrossRef]
- Kitaya, K.; Nagai, Y.; Arai, W.; Sakuraba, Y.; Ishikawa, T. Characterization of Microbiota in Endometrial Fluid and Vaginal Secretions in Infertile Women with Repeated Implantation Failure. Mediat. Inflamm. 2019, 2019, 4893437. [Google Scholar] [CrossRef]
- Bernabeu, A.; Lledo, B.; Diaz, M.C.; Lozano, F.M.; Ruiz, V.; Fuentes, A.; Lopez-Pineda, A.; Moliner, B.; Castillo, J.C.; Ortiz, J.A.; et al. Effect of the vaginal microbiome on the pregnancy rate in women receiving assisted reproductive treatment. J. Assist. Reprod. Genet. 2019, 36, 2111–2119. [Google Scholar] [CrossRef]
- Kong, Y.; Liu, Z.; Shang, Q.; Gao, Y.; Li, X.; Zheng, C.; Deng, X.; Chen, T. The Disordered Vaginal Microbiota Is a Potential Indicator for a Higher Failure of in vitro Fertilization. Front. Med. 2020, 7, 217. [Google Scholar] [CrossRef]
- Vladislavovna, B.V.; Borisovna, K.N.; Olegovna, B.I.; Mikhailovna, S.K.; Evgenievich, P.D.; Vladimirovich, A.M.; Valerievna, D.V. Microbiome of the uterus in women with unsuccessful in vitro fertilization attempts. Int. J. Women’s Health Reprod. Sci. 2020, 8, 423–427. [Google Scholar] [CrossRef]
- Zhao, C.; Wei, Z.; Yang, J.; Zhang, J.; Yu, C.; Yang, A.; Zhang, M.; Zhang, L.; Wang, Y.; Mu, X.; et al. Characterization of the Vaginal Microbiome in Women with Infertility and Its Potential Correlation with Hormone Stimulation during In Vitro Fertilization Surgery. mSystems 2020, 5, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Haahr, T.; Freiesleben, N.C.; Pinborg, A.; Nielsen, H.S.; Hartvig, V.; Mikkelsen, A.L.; Parks, T.; Uldbjerg, N.; Jensen, J.S.; Humaidan, P. Effect of clindamycin and a live biotherapeutic on the reproductive outcomes of IVF patients with abnormal vaginal microbiota: Protocol for a double-blind, placebo-controlled multicentre trial. BMJ Open 2020, 10, e035866. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Martinez, M.D.C.; Bernabeu, A.; Lledo, B.; Carratala-Munuera, C.; Quesada, J.A.; Lozano, F.M.; Ruiz, V.; Morales, R.; Llacer, J.; Ten, J.; et al. Impact of the Vaginal and Endometrial Microbiome Pattern on Assisted Reproduction Outcomes. J. Clin. Med. 2021, 10, 4063. [Google Scholar] [CrossRef]
- Azpiroz, M.A.; Orguilia, L.; Palacio, M.I.; Malpartida, A.; Mayol, S.; Mor, G.; Gutierrez, G. Potential biomarkers of infertility associated with microbiome imbalances. Am. J. Reprod. Immunol. 2021, 86, e13438. [Google Scholar] [CrossRef] [PubMed]
- Ichiyama, T.; Kuroda, K.; Nagai, Y.; Urushiyama, D.; Ohno, M.; Yamaguchi, T.; Nagayoshi, M.; Sakuraba, Y.; Yamasaki, F.; Hata, K.; et al. Analysis of vaginal and endometrial microbiota communities in infertile women with a history of repeated implantation failure. Reprod. Med. Biol. 2021, 20, 334–344. [Google Scholar] [CrossRef]
- Hao, X.; Li, P.; Wu, S.; Tan, J. Association of the Cervical Microbiota with Pregnancy Outcome in a Subfertile Population Undergoing In Vitro Fertilization: A Case-Control Study. Front. Cell. Infect. Microbiol. 2021, 11, 654202. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhou, G.; Wu, L.; Huang, X.; Li, Y.; Luo, B.; Zhu, H.; Huang, W. The Microbial Composition of Lower Genital Tract May Affect the Outcome of in vitro Fertilization-Embryo Transfer. Front. Microbiol. 2021, 12, 729744. [Google Scholar] [CrossRef]
- Eskew, A.M.; Stout, M.J.; Bedrick, B.S.; Riley, J.K.; Herter, B.N.; Gula, H.; Jungheim, E.S.; Wylie, K.M. Association of vaginal bacterial communities and reproductive outcomes with prophylactic antibiotic exposure in a subfertile population undergoing in vitro fertilization: A prospective exploratory study. F&S Sci. 2021, 2, 71–79. [Google Scholar] [CrossRef]
- Karaer, A.; Dogan, B.; Gunal, S.; Tuncay, G.; Arda Duz, S.; Unver, T.; Tecellioglu, N. The vaginal microbiota composition of women undergoing assisted reproduction: A prospective cohort study. BJOG 2021, 128, 2101–2109. [Google Scholar] [CrossRef]
- Chen, W.; Wei, K.; He, X.; Wei, J.; Yang, L.; Li, L.; Chen, T.; Tan, B. Identification of Uterine Microbiota in Infertile Women Receiving in vitro Fertilization with and Without Chronic Endometritis. Front. Cell. Dev. Biol. 2021, 9, 693267. [Google Scholar] [CrossRef]
- Jepsen, I.E.; Saxtorph, M.H.; Englund, A.L.M.; Petersen, K.B.; Wissing, M.L.M.; Hviid, T.V.F.; Macklon, N. Probiotic treatment with specific lactobacilli does not improve an unfavorable vaginal microbiota prior to fertility treatment-A randomized, double-blinded, placebo-controlled trial. Front. Endocrinol. 2022, 13, 1057022. [Google Scholar] [CrossRef]
- Iniesta, S.; Esteban, S.; Armijo, O.; Lobo, S.; Manzano, S.; Espinosa, I.; Cardenas, N.; Bartha, J.L.; Jimenez, E. Ligilactobacillus salivarius PS11610 exerts an effect on the microbial and immunological profile of couples suffering unknown infertility. Am. J. Reprod. Immunol. 2022, 88, e13552. [Google Scholar] [CrossRef] [PubMed]
- Villani, A.; Fontana, A.; Barone, S.; de Stefani, S.; Primiterra, M.; Copetti, M.; Panebianco, C.; Parri, C.; Scianname, N.; Quitadamo, P.A.; et al. Identifying Predictive Bacterial Markers from Cervical Swab Microbiota on Pregnancy Outcome in Woman Undergoing Assisted Reproductive Technologies. J. Clin. Med. 2022, 11, 680. [Google Scholar] [CrossRef] [PubMed]
- Keburiya, L.K.; Smolnikova, V.Y.; Priputnevich, T.V.; Muravieva, V.V.; Gordeev, A.B.; Trofimov, D.Y.; Shubina, E.S.; Kochetkova, T.O.; Rogacheva, M.S.; Kalinina, E.A.; et al. Does the uterine microbiota affect the reproductive outcomes in women with recurrent implantation failures? BMC Women’s Health 2022, 22, 168. [Google Scholar] [CrossRef]
- Ji, L.; Peng, C.; Bao, X. Effect of vaginal flora on clinical outcome of frozen embryo transfer. Front. Cell. Infect. Microbiol. 2022, 12, 987292. [Google Scholar] [CrossRef]
- Tanaka, S.E.; Sakuraba, Y.; Kitaya, K.; Ishikawa, T. Differential Vaginal Microbiota Profiling in Lactic-Acid-Producing Bacteria between Infertile Women with and without Chronic Endometritis. Diagnostics 2022, 12, 878. [Google Scholar] [CrossRef]
- Lull, K.; Saare, M.; Peters, M.; Kakhiani, E.; Zhdanova, A.; Salumets, A.; Boyarsky, K.; Org, E. Differences in microbial profile of endometrial fluid and tissue samples in women with in vitro fertilization failure are driven by Lactobacillus abundance. Acta Obstet. Gynecol. Scand. 2022, 101, 212–220. [Google Scholar] [CrossRef]
- Patel, N.; Patel, N.; Pal, S.; Nathani, N.; Pandit, R.; Patel, M.; Patel, N.; Joshi, C.; Parekh, B. Distinct gut and vaginal microbiota profile in women with recurrent implantation failure and unexplained infertility. BMC Women’s Health 2022, 22, 113. [Google Scholar] [CrossRef] [PubMed]
- Vajpeyee, M.; Tiwari, S.; Yadav, L.B.; Tank, P. Assessment of bacterial diversity associated with assisted reproductive technologies through next-generation sequencing. Middle East Fertil. Soc. J. 2022, 27, 28. [Google Scholar] [CrossRef]
- Chen, P.; Jia, L.; Zhou, Y.; Guo, Y.; Fang, C.; Li, T. Interaction between endometrial microbiota and host gene regulation in recurrent implantation failure. J. Assist. Reprod. Genet. 2022, 39, 2169–2178. [Google Scholar] [CrossRef]
- Bednarska-Czerwinska, A.; Czerwinski, M.; Morawiec, E.; Lach, A.; Ziaja, A.; Kusaj, A.; Straczynska, P.; Sagan, D.; Boron, D.; Grabarek, B.O. Marking the Profile of the Microflora of the Endometrium and Uterine Cervix in Women as a Potential Factor Determining the Effectiveness of In Vitro Fertilization. J. Clin. Med. 2022, 11, 3348. [Google Scholar] [CrossRef]
- Sezer, O.; Soyer Caliskan, C.; Celik, S.; Kilic, S.S.; Kuruoglu, T.; Unluguzel Ustun, G.; Yurtcu, N. Assessment of vaginal and endometrial microbiota by real-time PCR in women with unexplained infertility. J. Obstet. Gynaecol. Res. 2022, 48, 129–139. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, X.; Chen, P.; Wang, Y.; Li, W.; Huang, R. The endometrial microbiota profile influenced pregnancy outcomes in patients with repeated implantation failure: A retrospective study. J. Reprod. Immunol. 2023, 155, 103782. [Google Scholar] [CrossRef]
- Bui, B.N.; van Hoogenhuijze, N.; Viveen, M.; Mol, F.; Teklenburg, G.; de Bruin, J.P.; Besselink, D.; Brentjens, L.S.; Mackens, S.; Rogers, M.R.C.; et al. The endometrial microbiota of women with or without a live birth within 12 months after a first failed IVF/ICSI cycle. Sci. Rep. 2023, 13, 3444. [Google Scholar] [CrossRef]
- Lan, J.; Chen, C. The role of lactic acid bacteria in maintaining vaginal internal environment homeostasis in patients with infertility. Microb. Pathog. 2023, 176, 106004. [Google Scholar] [CrossRef]
- Elahi, Z.; Mokhtaryan, M.; Mahmoodi, S.; Shahroodian, S.; Darbandi, T.; Ghasemi, F.; Ghanavati, R.; Darbandi, A. All Properties of Infertility Microbiome in a Review Article. J. Clin. Lab. Anal. 2025, 39, e25158. [Google Scholar] [CrossRef]
- Henry, C.; Foss, L.; Ahl, H. Gender and entrepreneurship research: A review of methodological approaches. Int. Small Bus. J. 2016, 34, 217–241. [Google Scholar] [CrossRef]
- Joseph, R.J.; Ser, H.L.; Kuai, Y.H.; Tan, L.T.; Arasoo, V.J.T.; Letchumanan, V.; Wang, L.; Pusparajah, P.; Goh, B.H.; Ab Mutalib, N.S.; et al. Finding a Balance in the Vaginal Microbiome: How Do We Treat and Prevent the Occurrence of Bacterial Vaginosis? Diagnostics 2021, 10, 719. [Google Scholar] [CrossRef]
- Oyenihi, A.B.; Haines, R.; Trama, J.; Faro, S.; Mordechai, E.; Adelson, M.E.; Osei Sekyere, J. Molecular characterization of vaginal microbiota using a new 22-species qRT-PCR test to achieve a relative-abundance and species-based diagnosis of bacterial vaginosis. Front. Cell. Infect. Microbiol. 2024, 14, 1409774. [Google Scholar] [CrossRef]
- Panek, M.; Cipcic Paljetak, H.; Baresic, A.; Peric, M.; Matijasic, M.; Lojkic, I.; Vranesic Bender, D.; Krznaric, Z.; Verbanac, D. Methodology challenges in studying human gut microbiota-effects of collection, storage, DNA extraction and next generation sequencing technologies. Sci. Rep. 2018, 8, 5143. [Google Scholar] [CrossRef]
- Talapko, J.; Juzbašić, M.; Matijević, T.; Pustijanac, E.; Bekić, S.; Kotris, I.; Škrlec, I. Candida albicans—The Virulence Factors and Clinical Manifestations of Infection. J. Fungi 2021, 7, 79. [Google Scholar] [CrossRef]
- Zheng, Q.; Sun, T.; Li, X.; Zhu, L. Reproductive tract microbiome dysbiosis associated with gynecological diseases. Front. Cell Infect. Microbiol. 2025, 15, 1519690. [Google Scholar] [CrossRef] [PubMed]
- Medina-Bastidas, D.; Camacho-Arroyo, I.; Garcia-Gomez, E. Current findings in endometrial microbiome: Impact on uterine diseases. Reproduction 2022, 163, R81–R96. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Louwers, Y.V.; Laven, J.S.E.; Schoenmakers, S. Clinical Relevance of Vaginal and Endometrial Microbiome Investigation in Women with Repeated Implantation Failure and Recurrent Pregnancy Loss. Int. J. Mol. Sci. 2024, 25, 622. [Google Scholar] [CrossRef]
- Adapen, C.; Réot, L.; Menu, E. Role of the human vaginal microbiota in the regulation of inflammation and sexually transmitted infection acquisition: Contribution of the non-human primate model to a better understanding? Front. Reprod. Health 2022, 4, 992176. [Google Scholar] [CrossRef]
| Database | Number of Studies Retrieved | Search Strategy |
|---|---|---|
| PubMed/MEDLINE | 2889 | (((microflora) OR (microbiome)) OR (microbiota)) OR (microbiom*)) OR (microbiot*)) AND (((infertility) OR (subfertility)) OR (sterility)) |
| Scopus | 707 | TITLE-ABS-KEY ((microbiome) OR (microbiota) OR (microflora) OR (microbiom*) OR (microbiot*)) AND ((infertility) OR (subfertility) OR (sterility)) |
| Study/Authors | Country | Patient Population | Sample Type | Diagnostic Method |
|---|---|---|---|---|
| Haahr et al. (2016) [10] | Denmark | 30 infertile women undergoing IVF | Vaginal fluid | qPCR, Nugent Score |
| Campisciano et al. (2017) [11] | Italy | 96 idiopathic infertile women, 96 fertile controls | Vaginal and cervical fluid | NGS (16s rRNA V3) |
| Kyono et al. (2018) [12] | Japan | 102 infertile women (79 IVF, 23 non-IVF), 7 fertile controls | Vaginal and endometrial fluid | NGS (16s rRNA V4) |
| Amato et al. (2019) [13] | Italy | 23 infertile couples undergoing IUI | Vaginal and seminal fluid | NGS (16s rRNA V4) |
| Liu, Y., et al. (2019) [14] | China | 130 infertile women (12 with chronic endometritis, 118 without) | Endometrial fluid and biopsy | PCD, PCR, NGS (16s rRNA V4) |
| Haahr et al. (2019) [15] | Denmark | 75 infertile women before embryo transfer | Vaginal fluid | qPCR, NGS (16s rRNA V4) |
| Kitaya et al. (2019) [16] | Japan | 28 infertile women with RIF, 18 women with first IVF | Vaginal and endometrial fluid | NGS (V3–V4 of 16s rRNA) |
| Bernabeu et al. (2019) [17] | Spain | 31 infertile women undergoing ART | Vaginal fluid | NGS (V3–V4 of 16s rRNA) |
| Kong et al. (2020) [18] | China | 475 infertile women (238 pregnant, 237 not pregnant after IVF) | Vaginal fluid | PCR (V4 of 16s rRNA) |
| Vladislavnova et al. (2020) [19] | Russia | 22 women with >2 IVF failures, 20 healthy controls | Endometrial fluid | NGS (V3–V4 of 16s rRNA) |
| Zhao et al. (2020) [20] | China | 22 women with >2 IVF failures, 20 healthy controls | Endometrial fluid | NGS (V3–V4 of 16s rRNA) |
| Haahr et al. (2020) [21] | Denmark | 111 infertile women undergoing IVF | Cervical fluid | NGS qPCR |
| Diaz-Martinez et al. (2021) [22] | Spain | 48 infertile women prior to ARTs | Vaginal and cervical fluid | NGS V3–V4 16sRNA |
| Azpiroz et al. (2021) [23] | Argentina | 287 infertile women with multiple IVF attempts, 20 fertile controls | 287 infertile women with multiple IVF attempts and 20 fertile controls | NGS miRNA PCR |
| Ichiyama et al. (2021) [24] | Japan | 89 infertile women with RIF, 17 fertile women | Cervical and vaginal fluid | NGS 16sRNA |
| Hao et al. (2021) [25] | China | 124 infertile women undergoing IVF | Vaginal and seminal fluid | NGS (16s rRNA V4) |
| Wang et al. (2021) [26] | China | 150 infertile women prior to their first IVF-ET | Vaginal and cervical fluid | NGS (16s rRNA V4) |
| Eskew et al. (2021) [27] | USA | 27 infertile women with RIF, 12 non-IVF controls | Cervical fluid | NGS (16s rRNA) |
| Karaer et al. (2021) [28] | Turkey | 223 infertile women prior to ART | Vaginal and seminal fluid | NGS (16s rRNA V4) |
| Chen et al. (2021) [29] | China | 94 infertile women (25 with chronic endometritis, 69 without) | Endometrial fluid | NGS (V4 of 16s rRNA) |
| Moreno et al. (2022) [4] | Spain | 345 infertile women before embryo transfer | Endometrial fluid | NGS (16s rRNA V4) |
| Jepsen et al. (2022) [30] | Denmark | 74 infertile women before ART | Vaginal fluid | NGS (16s rRNA V4) |
| Iniesta et al. (2022) [31] | Spain | 17 infertile couples | Vaginal, seminal, endometrial fluid, and plasma | NGS (16s rRNA V4) |
| Villani et al. (2022) [32] | Italy | 88 infertile women | Cervical smear | NGS (V3–V4 of 16s rRNA) |
| Keburiya et al. (2022) [33] | Russia | 130 infertile women | Endometrial fluid and cervical smear | NGS (V3–V4 of 16s rRNA) |
| Ji et al. (2022) [34] | China | 229 infertile women undergoing frozen embryo transfer | Vaginal smear | NGS (16s rRNA V4) |
| Tanaka et al. (2022) [35] | Japan | 123 infertile women with and without chronic endometritis | Endometrial biopsy and vaginal fluid | NGS (16s rRNA V4) |
| Lull et al. (2022) [36] | Estonia | 25 infertile women with first IVF failure | Endometrial fluid and biopsy | NGS (V3–V4 of 16s rRNA) |
| Patel et al. (2022) [37] | India | 20 women with unexplained infertility, 11 fertile controls | Vaginal and fecal fluid | NGS (16s rRNA V2–V3) |
| Vajpeyee et al. (2022) [38] | India | 197 infertile couples before IVF | Vaginal, follicular, endometrial, and seminal fluid | NGS (16s rRNA V4) |
| Chen et al. (2022) [39] | China | 75 women with RIF and 36 healthy controls | Endometrial fluid | NGS (16s rRNA V4) |
| Bednarska-Czerwinksa et al. (2022) [40] | Poland | 142 infertile women before IVF | Endometrial and cervical fluid | NGS (16s rRNA V4) |
| Sezer et al. (2022) [41] | Turkey | 26 infertile women, 26 healthy controls | Vaginal and endometrial smear | Real-time PCR |
| Zou et al. (2023) [42] | China | 141 infertile women with RIF | Vaginal fluid | NGS 16s rRNA |
| Bui et al. (2023) [43] | The Netherlands | 141 infertile women with first IVF/ICSI cycle | Vaginal fluid | NGS V1–V2 16sRNA |
| Lan et al. (2023) [44] | China | 100 infertile women undergoing IVF and 50 healthy controls | Cervical fluid | Calfrolferia Gram-negative anaerobic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papakonstantinou, A.; Moustakli, E.; Potiris, A.; Zikopoulos, A.; Tsarna, E.; Christodoulaki, C.; Tsakiridis, I.; Dagklis, T.; Panagopoulos, P.; Drakakis, P.; et al. Behind-the-Scenes Actors in Fertility: A Comprehensive Review of the Female Reproductive Tract Microbiome and Its Clinical Relevance. Life 2025, 15, 916. https://doi.org/10.3390/life15060916
Papakonstantinou A, Moustakli E, Potiris A, Zikopoulos A, Tsarna E, Christodoulaki C, Tsakiridis I, Dagklis T, Panagopoulos P, Drakakis P, et al. Behind-the-Scenes Actors in Fertility: A Comprehensive Review of the Female Reproductive Tract Microbiome and Its Clinical Relevance. Life. 2025; 15(6):916. https://doi.org/10.3390/life15060916
Chicago/Turabian StylePapakonstantinou, Anthi, Efthalia Moustakli, Anastasios Potiris, Athanasios Zikopoulos, Ermioni Tsarna, Chrysi Christodoulaki, Ioannis Tsakiridis, Themistoklis Dagklis, Periklis Panagopoulos, Peter Drakakis, and et al. 2025. "Behind-the-Scenes Actors in Fertility: A Comprehensive Review of the Female Reproductive Tract Microbiome and Its Clinical Relevance" Life 15, no. 6: 916. https://doi.org/10.3390/life15060916
APA StylePapakonstantinou, A., Moustakli, E., Potiris, A., Zikopoulos, A., Tsarna, E., Christodoulaki, C., Tsakiridis, I., Dagklis, T., Panagopoulos, P., Drakakis, P., & Stavros, S. (2025). Behind-the-Scenes Actors in Fertility: A Comprehensive Review of the Female Reproductive Tract Microbiome and Its Clinical Relevance. Life, 15(6), 916. https://doi.org/10.3390/life15060916









