Neonatal Health Following IVF: Own Versus Donor Material in Singleton and Multiple Pregnancies
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample and Data Collection
2.2. Outcome Measures
2.3. Statistical Analysis
3. Results
3.1. Singletons Conceived Through IVF Donor vs. Autologous Material
3.2. Multiples Conceived Through IVF Donor vs. Autologous Material
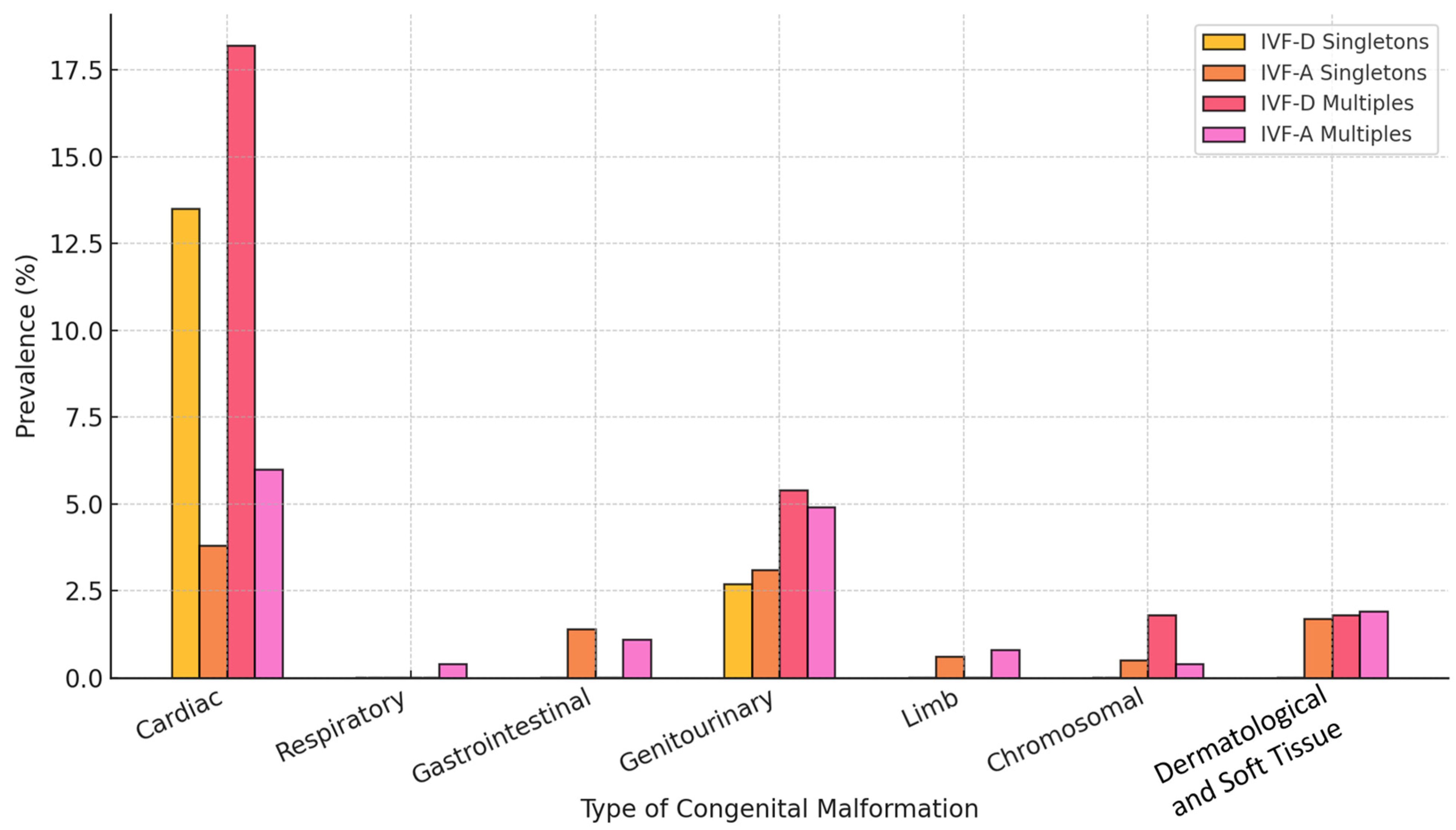
3.3. Prevalence and Distribution of Congenital Malformations (Singletons vs. Multiples)
4. Discussion
4.1. Key Findings
4.2. Comparison with Existing Literature
4.3. Explanation of Results
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lutjen, P.; Trounson, A.; Leeton, J.; Findlay, J.; Wood, C.; Renou, P. The establishment and maintenance of pregnancy using in vitro fertilization and embryo donation in a patient with primary ovarian failure. Nature 1984, 307, 174–175. [Google Scholar] [CrossRef]
- Melnick, A.P.; Rosenwaks, Z. Oocyte donation: Insights gleaned and future challenges. Fertil. Steril. 2018, 110, 988–993. [Google Scholar]
- ESHRE Working Group on Reproductive Donation; Kirkman-Brown, J.; Calhaz-Jorge, C.; Dancet, E.A.F.; Lundin, K.; Martins, M.; Tilleman, K.; Thorn, P.; Vermeulen, N.; Frith, L. Good practice recommendations for information provision for those involved in reproductive donation†. Hum. Reprod. Open 2022, 2022, hoac001. [Google Scholar]
- Allan, S.; Balaban, B.; Banker, M.; Buster, J.; Horton, M.; Miller, K.; Mocanu, E.; Ory, S.; Pai, H.; VanderPoel, S.; et al. International Federation of Fertility Societies’ Surveillance (IFFS) 2019: Global Trends in Reproductive Policy and Practice, 8th Edition. Glob. Reprod. Health 2019, 4, e29. [Google Scholar]
- Malchau, S.S.; Loft, A.; Larsen, E.C.; Aaris Henningsen, A.-K.; Rasmussen, S.; Andersen, A.N.; Pinborg, A. Perinatal outcomes in 375 children born after oocyte donation: A Danish national cohort study. Fertil. Steril. 2013, 99, 1637–1643.e3. [Google Scholar]
- Kamath, M.S.; Antonisamy, B.; Mascarenhas, M.; Sunkara, S.K. High-risk of preterm birth and low birth weight after oocyte donation IVF: Analysis of 133,785 live births. Reprod. Biomed. Online 2017, 35, 318–324. [Google Scholar] [PubMed]
- Boulet, S.L.; Kawwass, J.F.; Crawford, S.; Davies, M.J.; Kissin, D.M. Preterm Birth and Small Size for Gestational Age in Singleton, In Vitro Fertilization Births Using Donor Oocytes. Am. J. Epidemiol. 2018, 187, 1642–1650. [Google Scholar] [PubMed]
- Yu, B.; Vega, M.; Zaghi, S.; Fritz, R.; Jindal, S.; Buyuk, E. Comparison of perinatal outcomes following frozen embryo transfer cycles using autologous versus donor oocytes in women 40 to 43 years old: Analysis of SART CORS data. J. Assist. Reprod. Genet. 2018, 35, 2025–2029. [Google Scholar]
- Schwartz, K.M.; Boulet, S.L.; Kawwass, J.F.; Kissin, D.M. Perinatal outcomes among young donor oocyte recipients. Hum. Reprod. 2019, 34, 2533–2540. [Google Scholar]
- Monfort, S.; Orellana, C.; Oltra, S.; Rosello, M.; Caro-Llopis, A.; Martinez, F. Prevalence of pathogenic copy number variants among children conceived by donor oocyte. Sci. Rep. 2021, 11, 6752. [Google Scholar] [CrossRef]
- McCoy, D.E.; Haig, D.; Kotler, J. Egg donation and gestational surrogacy: Pregnancy is riskier with an unrelated embryo. Early Hum. Dev. 2024, 196, 106072. [Google Scholar] [CrossRef] [PubMed]
- Oberg, A.S.; VanderWeele, T.J.; Almqvist, C.; Hernandez-Diaz, S. Pregnancy complications following fertility treatment-disentangling the role of multiple gestation. Int. J. Epidemiol. 2018, 47, 1333–1342. [Google Scholar] [CrossRef]
- Grantz, K.L.; Kawakita, T.; Lu, Y.-L.; Newman, R.; Berghella, V.; Caughey, A. SMFM Special Statement: State of the science on multifetal gestations: Unique considerations and importance. Am. J. Obstet. Gynecol. 2019, 221, B2–B12. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, L.; Zhang, P.; Sun, Y.; Ma, C.; Li, Y. Risk of birth defects in children conceived with assisted reproductive technology: A meta-analysis. Medicine 2022, 101, e32405. [Google Scholar] [CrossRef] [PubMed]
- Kogan, E.A.; Rudenko, E.E.; Demura, T.A.; Zharkov, N.V.; Trifonova, N.S.; Zhukova, E.V.; Aleksandrov, L.S.; Bayanova, S.N. Structural, immunohistochemical and molecular features of placentas and placental sites after in vitro fertilization with donor eggs (surrogate motherhood). Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 238, 68–72. [Google Scholar] [CrossRef]
- Gundogan, F.; Bianchi, D.W.; Scherjon, S.A.; Roberts, D.J. Placental pathology in egg donor pregnancies. Fertil. Steril. 2010, 93, 397–404. [Google Scholar] [CrossRef]
- Modest, A.M.; Johnson, K.M.; Karumanchi, S.A.; Resetkova, N.; Young, B.C.; Fox, M.P.; Wise, L.A.; Hacker, M.R. Risk of ischemic placental disease is increased following in vitro fertilization with oocyte donation: A retrospective cohort study. J. Assist. Reprod. Genet. 2019, 36, 1917–1926. [Google Scholar] [CrossRef]
- Levron, Y.; Dviri, M.; Segol, I.; Yerushalmi, G.M.; Hourvitz, A.; Orvieto, R.; Mazaki-Tovi, S.; Yinon, Y. The “immunologic theory” of preeclampsia revisited: A lesson from donor oocyte gestations. Am. J. Obstet. Gynecol. 2014, 211, 383.e1-5. [Google Scholar] [CrossRef]
- Choux, C.; Petazzi, P.; Sanchez-Delgado, M.; Hernandez Mora, J.R.; Monteagudo, A.; Sagot, P.; Monk, D.; Fauque, P. The hypomethylation of imprinted genes in IVF/ICSI placenta samples is associated with concomitant changes in histone modifications. Epigenetics 2020, 15, 1386–1395. [Google Scholar] [CrossRef]
- Choux, C.; Binquet, C.; Carmignac, V.; Bruno, C.; Chapusot, C.; Barberet, J.; Lamotte, M.; Sagot, P.; Bourc’his, D.; Fauque, P. The epigenetic control of transposable elements and imprinted genes in newborns is affected by the mode of conception: ART versus spontaneous conception without underlying infertility. Hum. Reprod. Oxf. Engl. 2018, 33, 331–340. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Le, F.; Wang, N.; Zhang, F.; Luo, Y.; Lou, Y.; Hu, M.; Wang, L.; Thurston, L.M.; et al. Alteration in the expression of the renin-angiotensin system in the myocardium of mice conceived by in vitro fertilization. Biol. Reprod. 2018, 99, 1276–1288. [Google Scholar] [CrossRef] [PubMed]
- Zdanowicz, J.A.; Yildrim, G.; Fonseca, A.; Hecher, K.; Tavares de Sousa, M. Is There a Cumulative Effect for Congenital Heart Defects in Monochorionic Twins after Assisted Reproduction?—A Retrospective Analysis at a Tertiary Referral Center. Geburtshilfe Frauenheilkd 2024, 84, 274–281. [Google Scholar]
- Tomimatsu, T.; Mimura, K.; Matsuzaki, S.; Endo, M.; Kumasawa, K.; Kimura, T. Preeclampsia: Maternal Systemic Vascular Disorder Caused by Generalized Endothelial Dysfunction Due to Placental Antiangiogenic Factors. Int. J. Mol. Sci. 2019, 20, 4246. [Google Scholar] [CrossRef]
- Wang, C.; Lv, H.; Ling, X.; Li, H.; Diao, F.; Dai, J.; Du, J.; Chen, T.; Xi, Q.; Zhao, Y.; et al. Association of assisted reproductive technology, germline de novo mutations and congenital heart defects in a prospective birth cohort study. Cell Res. 2021, 31, 919–928. [Google Scholar]
- Scherrer, U.; Rexhaj, E.; Allemann, Y.; Sartori, C.; Rimoldi, S.F. Cardiovascular dysfunction in children conceived by assisted reproductive technologies. Eur. Heart J. 2015, 36, 1583–1589. [Google Scholar] [CrossRef] [PubMed]
- von Wolff, M.; Haaf, T. In Vitro Fertilization Technology and Child Health. Dtsch. Arztebl. Int. 2020, 117, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Razak, A.; Florendo-Chin, A.; Banfield, L.; Abdul Wahab, M.G.; McDonald, S.; Shah, P.S.; Mukerji, A. Pregnancy-induced hypertension and neonatal outcomes: A systematic review and meta-analysis. J. Perinatol. 2018, 38, 46–53. [Google Scholar]
- Ge, J.; Gu, X.; Jiang, S.; Yang, L.; Li, X.; Jiang, S.; Jia, B.; Chen, C.; Cao, Y.; Lee, S.; et al. Impact of hypertensive disorders of pregnancy on neonatal outcomes among infants born at 24+0–31+6 weeks’ gestation in China: A multicenter cohort study. Front. Pediatr. 2023, 11, 1005383. [Google Scholar]
- Kim, H.-R.; Lee, B.K. Outcomes of singleton preterm very low birth weight infants born to mothers with pregnancy-induced hypertension. Sci. Rep. 2023, 13, 6100. [Google Scholar]
- Hirsch, A.; Peled, T.; Schlesinger, S.; Sela, H.Y.; Grisaru-Granovsky, S.; Rottenstreich, M. Impact of gestational diabetes mellitus on neonatal outcomes in small for gestational age infants: A multicenter retrospective study. Arch. Gynecol. Obstet. 2024, 310, 685–693. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Zhang, D. Maternal diabetes mellitus and risk of neonatal respiratory distress syndrome: A meta-analysis. Acta Diabetol. 2019, 56, 729–740. [Google Scholar] [PubMed]
- Tefera, M.; Assefa, N.; Mengistie, B.; Abrham, A.; Teji, K.; Worku, T. Elective Cesarean Section on Term Pregnancies Has a High Risk for Neonatal Respiratory Morbidity in Developed Countries: A Systematic Review and Meta-Analysis. Front. Pediatr. 2020, 8, 286. [Google Scholar] [CrossRef] [PubMed]
- Barker, P.M.; Olver, R.E. Invited Review: Clearance of lung liquid during the perinatal period. J. Appl. Physiol. 2002, 93, 1542–1548. [Google Scholar] [PubMed]
- Ramachandrappa, A.; Jain, L. Elective Cesarean Section: Its Impact on Neonatal Respiratory Outcome. Clin. Perinatol. 2008, 35, 373–393. [Google Scholar]
| Singleton Pregnancies | IVF Donor Material (IVF-D) | IVF Autologous Material (IVF-A) | p-Value (IVF-D vs. IVF-A) | Unadjusted OR (95% CI) | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Maternal age | ||||||
| <35 | 5/670 | 13.5 | 282/670 | 44.5 | p < 0.001 * | 1.63 (0.68–2.59) |
| ≥35 | 32/670 | 86.5 | 351/670 | 55.5 | ||
| Pregnancy-induced arterial hypertension | 14/639 | 37.8 | 60/639 | 10 | p < 0.001* | 1.7 (0.98–2.42) |
| Gestational diabetes | 10/639 | 27 | 81/639 | 13.5 | p = 0.027* | 0.86 (0.1–1.63) |
| Multiple Pregnancies | IVF Donor Material (IVF-D) | IVF Autologous Material (IVF-A) | p-Value (IVF-D vs. IVF-A) | Unadjusted OR (95% CI) | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Maternal age | ||||||
| <35 | 9/159 | 33.3 | 69/159 | 52.3 | p = 0.056 | 0.78 (−0.08–1.65) |
| ≥35 | 18/159 | 66.6 | 63/159 | 47.7 | ||
| Pregnancy-induced arterial hypertension | 7/140 | 26 | 11/140 | 9.7 | p = 0.032 | 1.17 (0.11–2.23) |
| Gestational diabetes | 2/140 | 7.4 | 14/140 | 12.4 | p = 0.86 | −0.57 (−2.11–0.97) |
| Singletons (n = 670) | IVF Donor Material (IVF-D) n = 37 | IVF Autologous Material (IVF-A) n = 633 | ||||
|---|---|---|---|---|---|---|
| Median (IQR) | Range | Median (IQR) | Range | |||
| Birthweight, grams | 3130 (590) | 920–4000 | 3190 (640) | 590–4680 | ||
| Gestational age, weeks | 38 (3) | 28–40 | 38 (1) | 25–42 | ||
| Apgar score at 1 min | 9 (1) | 3–10 | 9 (1) | 1–10 | ||
| Apgar score at 5 min | 7(1) | 3–8 | 8 (1) | 1–8 | ||
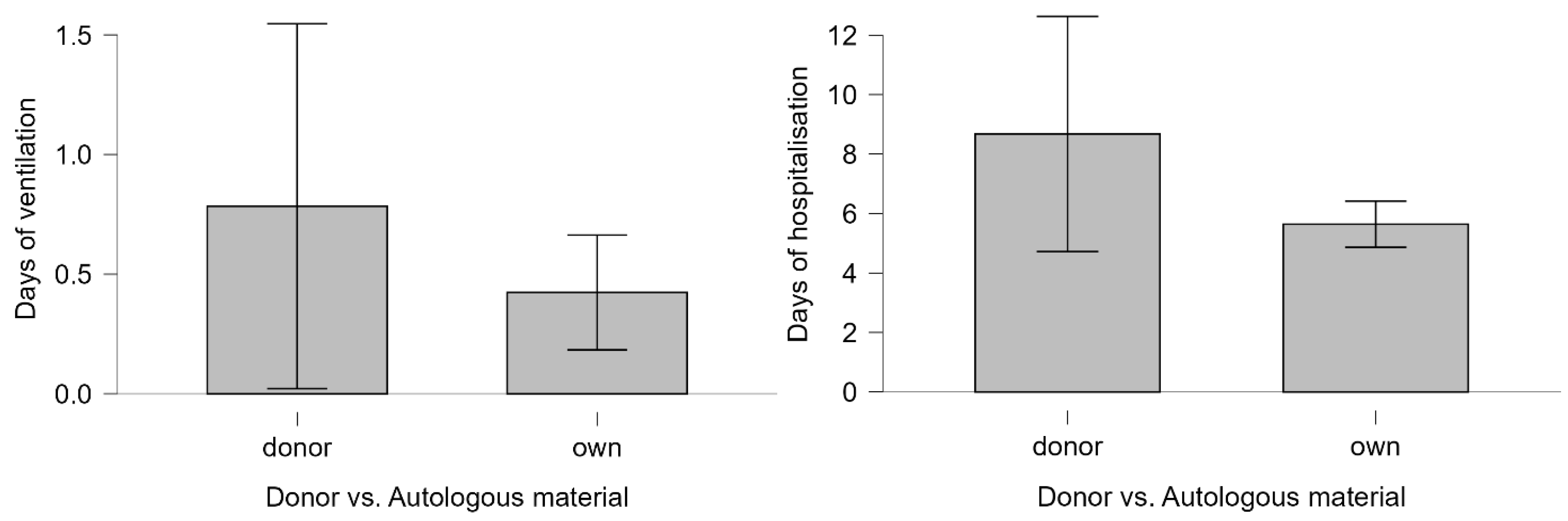
| Days of ventilation | 0 (0) | 0–10 | 0 (0) | 0–56 | ||
| Days of hospitalization | 3 (5) | 3–49 | 3 (1) | 2–100 | ||
| n | % | n | % | p-value | Unadjusted OR (95% CI) | |
| Apgar 1′ | ||||||
| ≤7 | 6/670 | 16.2 | 53/670 | 8.4 | 0.097 | 2.11 (0.84–5.3) |
| >7 | 31/670 | 83.8 | 580/670 | 91.6 | ||
| Apgar 5′ | ||||||
| ≤7 | 5/670 | 13.5 | 25/670 | 4 | 0.02 | 3.8 (1.36–10.57) |
| >7 | 32/670 | 86.5 | 608/670 | 96 | ||
| Asphyxia | 6/670 | 16.2 | 53/670 | 8.4 | 0.097 | 2.11 (0.84–5.3) |
| Preterm birth (<37 weeks) | 12/670 | 32.4 | 118/670 | 18.6 | 0.038 | 2.09 (1.02–4.29) |
| Low birthweight (<2500 g) | 8/670 | 21.6 | 72/670 | 11.4 | 0.062 | 2.14 (0.94–4.88) |
| Macrosomia (≥4000 g) | 1/670 | 2.7 | 34/670 | 5.4 | 0.087 | 0.48 (0.06–3.67) |
| Ventilation required | 6/670 | 16.2 | 27/670 | 4.3 | 0.007 | 4.34 (1.67–11.29) |
| Days of ventilation | ||||||
| >2 | 4/670 | 10.8 | 24/670 | 3.8 | 0.062 | 3.07 (1–9.37) |
| ≤2 | 33/670 | 89.2 | 609/670 | 96.2 | ||
| Days of hospitalization | ||||||
| >3 | 18/670 | 48.6 | 177/670 | 28 | 0.008 | 2.44 (1.25–4.75) |
| ≤3 | 19/670 | 51.4 | 456/670 | 72 | ||
| Congenital malformations | 6/670 | 16.2 | 71/670 | 11.2 | 0.24 | 1.53 (0.61–3.8) |
| Neonatal death | 1/670 | 2.7 | 3/670 | 0.5 | 0.2 | 5.83 (0.59–57.48) |
| Multiples (n = 318) | IVF Donor Material (IVF-D) n = 55 | IVF Autologous Material (IVF-A) n = 263 | ||||||
|---|---|---|---|---|---|---|---|---|
| Median (IQR) | Range | Median (IQR) | Range | |||||
| Birthweight, grams | 2030 (540) | 700–3800 | 2360 (1090) | 600–4400 | ||||
| Gestational age, weeks | 34 (2) | 27–39 | 36 (5) | 25–39 | ||||
| Apgar score at 1 min | 7 (1.5) | 4–10 | 8 (2) | 1–10 | ||||
| Apgar score at 5 min | 6 (2) | 4–8 | 7 (2) | 1–8 | ||||
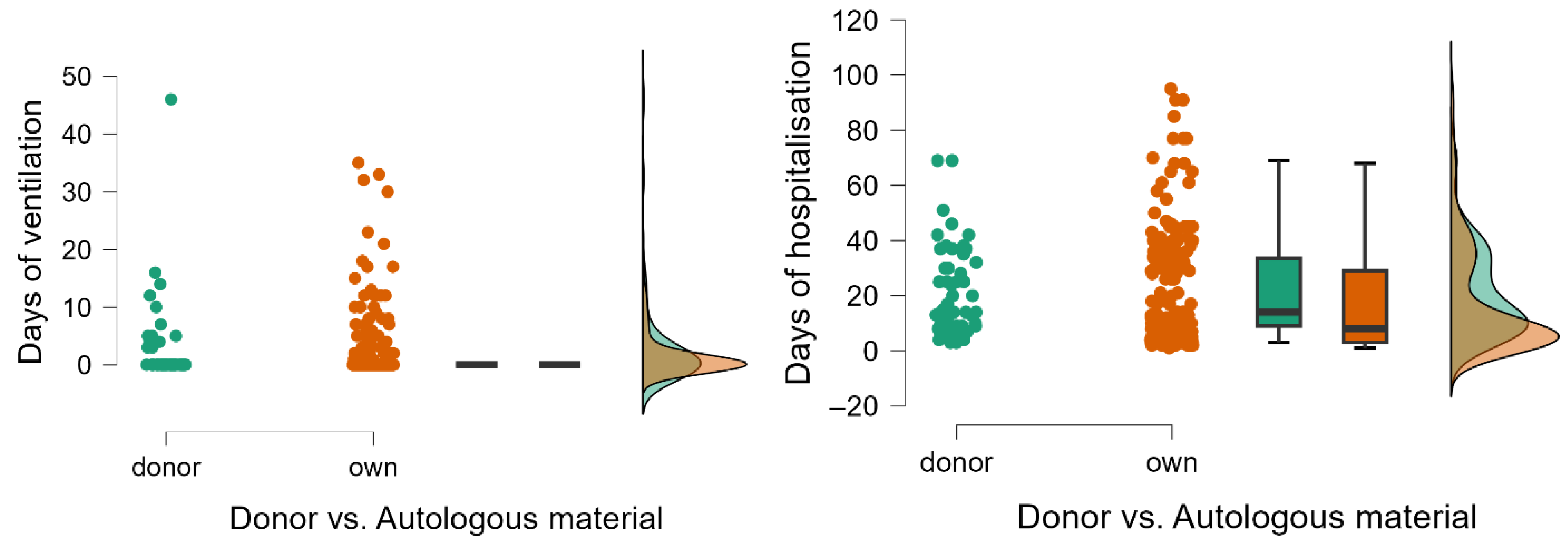
| Days of ventilation | 0 (0) | 0–46 | 0 (0) | 0–35 | ||||
| Days of hospitalization | 14 (24.5) | 3–69 | 8 (26) | 1–95 | ||||
| n | % | n | % | p-value | Unadjusted OR (95% CI) | |||
| Apgar 1′ | ||||||||
| ≤7 | 35/318 | 63.6 | 89/318 | 33.8 | <0.001 | 3.42 (1.86–6.27) | ||
| >7 | 20/318 | 36.4 | 174/318 | 66.2 | ||||
| Apgar 5′ | ||||||||
| ≤7 | 15/318 | 27.3 | 49/318 | 18.6 | 0.1 | 1.63 (0.83–3.2) | ||
| >7 | 40/318 | 72.7 | 214/318 | 81.4 | ||||
| Asphyxia | 35/318 | 63.6 | 89/318 | 33.8 | <0.001 | 3.42 (1.86–6.27) | ||
| Preterm birth (<37 weeks) | 50/318 | 90.9 | 179/318 | 68 | <0.001 | 4.69 (1.8–12.19) | ||
| Low birthweight (<2500 g) | 47/318 | 85.5 | 150/318 | 57 | <0.001 | 4.42 (2.01–9.73) | ||
| Macrosomia (≥4000 g) | 0/318 | 0 | 4/318 | 1.5 | 1 | 0.52 (0.02–9.78) | ||
| Ventilation required | 13/318 | 23.6 | 64/318 | 24.3 | 0.6 | 0.96 (0.48–1.9) | ||
| Days of ventilation | ||||||||
| >2 | 13/318 | 23.6 | 41/318 | 15.6 | 0.1 | 1.67 (0.82–3.39) | ||
| ≤2 | 42/318 | 76.4 | 222/318 | 84.4 | ||||
| Days of hospitalization | ||||||||
| >3 | 53/318 | 96.4 | 189/318 | 71.9 | <0.001 * | 10.37 (2.46–43.66) | ||
| ≤3 | 2/318 | 3.6 | 74/318 | 28.1 | ||||
| Congenital malformations | 15/318 | 27.3 | 41/318 | 15.6 | 0.034 * | 2.03 (1.02–4.01) | ||
| Neonatal death | 1/318 | 1.8 | 5/318 | 1.9 | 0.68 | 0.95 (0.1–8.34) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niculae, L.E.; Tocariu, R.; Archir, E.-D.; Niculae, A.-Ș.; Coricovac, A.-M.; Comandașu, D.-E.; Petca, A.; Brătilă, E. Neonatal Health Following IVF: Own Versus Donor Material in Singleton and Multiple Pregnancies. Life 2025, 15, 578. https://doi.org/10.3390/life15040578
Niculae LE, Tocariu R, Archir E-D, Niculae A-Ș, Coricovac A-M, Comandașu D-E, Petca A, Brătilă E. Neonatal Health Following IVF: Own Versus Donor Material in Singleton and Multiple Pregnancies. Life. 2025; 15(4):578. https://doi.org/10.3390/life15040578
Chicago/Turabian StyleNiculae, Lucia Elena, Raluca Tocariu, Evelyn-Denise Archir, Alexandru-Ștefan Niculae, Anca-Magdalena Coricovac, Diana-Elena Comandașu, Aida Petca, and Elvira Brătilă. 2025. "Neonatal Health Following IVF: Own Versus Donor Material in Singleton and Multiple Pregnancies" Life 15, no. 4: 578. https://doi.org/10.3390/life15040578
APA StyleNiculae, L. E., Tocariu, R., Archir, E.-D., Niculae, A.-Ș., Coricovac, A.-M., Comandașu, D.-E., Petca, A., & Brătilă, E. (2025). Neonatal Health Following IVF: Own Versus Donor Material in Singleton and Multiple Pregnancies. Life, 15(4), 578. https://doi.org/10.3390/life15040578








