Abstract
Chronic kidney disease (CKD) is a widespread condition with significant cardiovascular risks and a progression to end-stage kidney failure. In recent years, increasing attention has been paid to the role of dietary interventions as a factor capable of influencing disease trajectory. This review summarizes the current observational and interventional evidence on various dietary approaches in patients with CKD and kidney transplants (KTs), including Mediterranean, plant-based, and low-protein diets. A balanced Mediterranean diet, rich in fruits, vegetables, whole grains, fish, and unsaturated fats, shows promises in improving the prognosis for CKD patients. Plant-based diets, which emphasize legumes, vegetables, and grains while minimizing animal protein, improve blood pressure and the glycemic and lipid control. Low-protein diets (LPDs), typically providing less than 0.6 g/kg/day of protein, may reduce the CKD progression and nitrogen burden, further delaying the initiation of dialysis. In conclusion, diets represent a valuable and underutilized therapeutic strategy in the management of CKD and KTs, influencing disease progression and patient outcomes.
1. Introduction
Chronic kidney disease (CKD) is a well demonstrated pandemic syndrome affecting about 10% of the population worldwide [1]. Its onset, at the individual level, forecasts a striking risk for future cardiovascular (CV) events, kidney failure ((KF), the most advanced and dangerous phase of CKD), and overall mortality. These concepts have been well demonstrated over the past decades so that the following step was an attempt to limit the dimension of the problem. To date, significant progress has been made. At least three novel drug classes (MRA, SGLT2i, and HIF inhibitors but also GLP1-RA and ERA) have been implemented, through the conduction of phase III clinical trials, and have shown to confer protection against the aforementioned events when added to the standard of care [2,3,4]. The number of randomized studies also grew in the past few years and is going to grow in the future as well. In keeping with this, the risk stratification of patients with CKD has also been faced with an evaluation of the different prognoses by age categories, as well as by sex categories [5,6]. So, this generates the question of whether we have reached the sufficient protection of our CKD patients managed by nephrologists. In this case, in our opinion the answer is definitely no! Many problems and poor data still remain. It is emblematic that about 70% of CKD patients followed by nephrologists for at least one year still have proteinuria above its normal range and, thus, a high cardiorenal residual risk [7]. Similarly, even though the past clinical trials showed a nephroprotection provided by the novel drug classes, the absolute risk of cardiorenal events in the treatment groups is too far from being abolished. Hence, a continuous effort is needed. One old, often forgotten, therapeutic tool is represented by diet. It is demonstrated that diet is included in the lifestyle characteristics that mostly influence future prognoses, including in CKD patients [8]. In fact, a balanced diet composed of vegetables, meat, fish, and antioxidants (i.e., the Mediterranean diet) may provide a better prognosis also in patients with CKD [9]. Very few studies are available on dietary habits in kidney transplant (KT) recipients and on the interaction between diet and other variables (i.e., age, gender, and metabolic parameters) in influencing future prognoses. Moreover, randomized clinical trials (RCTs) have failed to demonstrate the value of a low-protein diet on kidney outcomes, with several concerns raised about the appropriateness of randomized studies for investigating this area [10]. Herein, with this review, we aim to summarize the current observational and intervention evidence regarding dietary approaches in patients with CKD or KTs, both of which require optimal individual risk management. The present review offers a comprehensive overview that bridges nutritional strategies and molecular insights, with a particular focus on the modulation of gut-derived metabolites, such as short-chain fatty acids (SCFAs), which may represent a mechanistic link between diet and disease progression. The relevant literature was identified through a non-systematic search of electronic databases (PubMed, Google Scholar, and Scopus), using combinations of keywords, including “chronic kidney disease”, “kidney transplant”, “mediterranean diet”, “low-protein diet”, “alkaline diet”, and “gut microbiota”. Articles were selected based on their relevance to the topic, and findings were synthesized thematically.
2. Biomarkers Associated with Diet
Since ancient times, as the father of medicine Hippocrates stated “Let food be thy medicine and medicine be thy food”, diet has played an undeniable role in lifestyle quality and the general health condition. Nowadays, in the specific management of CKD, nutritional treatment is recommended by the 2020 Kidney Disease Outcome Quality Initiative (KDOQI) guidelines in addition to pharmacological therapy [8]. But how and how much does diet positively influence the progression of CKD? More than 100 years ago, Addis first described the relationship between protein intake and urea excretion rates and suggested the restriction of protein intake in patients with CKD to minimize the “work overload” (and subsequent “exhaustion”) of surviving nephrons [11], posing the bases for the study of the link between diet and kidney function. Chronic kidney disease is known to cause the severe impairment of different systems, including the acid–base equilibrium, electrolyte and water balance, calcium–phosphorus metabolism, and hormonal systems [12,13], leading to oxidative stress and chronic low-grade inflammation [14].
In particular, the occurrence of metabolic complications of CKD was studied by Moranne and colleagues over 1038 CKD patients from the NephroTest cohort not on dialysis: it has been demonstrated that the prevalence of metabolic acidosis increased from 2% to 39% as the eGFR decreased from 60 to 90 mL/min/1.73 m2 to a value < 20 mL/min/1.73 m2, requiring a regular screening for metabolic disorders as the eGFR decreases below 40 mL/min/1.73 m2 [15]. In fact, metabolic acidosis is a consequence of the decreased overall renal excretion of acid ions, leading to a compensatory increase in acid excretion per nephron, which in turn may promote tubulointerstitial injury [16].
Acidosis actives the renin–angiotensin–aldosterone system (RAAS) with a following increased synthesis of aldosterone and increased production of endothelin, both associated with the worsening of the prognosis in terms of the progression of CKD and mortality [17]. Moreover, long-term consequences of metabolic acidosis include bone demineralization, muscular mass loss, and insulin resistance [18,19]. Thus, as a modifiable risk factor for CKD progression [20], the treatment of acidosis is needed. In order to obtain a reduction in metabolic acidosis, the KDOQI guidelines recommend an alkali treatment consisting of the oral administration of sodium citrate or sodium bicarbonate to patients with serum bicarbonate levels below 22 mmol/L [21]. However, this pharmacological intervention presents some side effects due to the sodium overload and since sodium citrate can increase gastric aluminum absorption and sodium bicarbonate can cause bloating and flatulence. Moreover, sodium contains compounds that can enhance water retention, contributing to arterial hypertension [22].
Diet has been shown to be a valid and personalized approach for this purpose since its composition influences the net endogenous acid production (NEAP, mEq/d) and, therefore, the acid–base balance of our body. NEAP results from the differences in the amounts of fixed acid (nonvolatile acid) and alkali precursors in the diet [23], and it may be measured by urine (net acid excretion, NAE) or estimated by dietary intake equations (Table 1). The potential renal acid load (PRAL) is the measurement of the capacity of the acid or alkali production of any food, and it is calculated with an equation including the amount of protein, phosphorus, potassium, magnesium, and calcium in a food [24].

Table 1.
NEAP and PRAL equations. NAE, net acid excretion. NEAP, net endogenous acid production. OAes, organic acid excretions. PRAL, potential renal acid load.
Foods rich in proteins and phosphorus (i.e., meat, fish, eggs, and cheese) have a positive PRAL > 0 and are the main sources of fixed acids (amino acids) that favor the generation of hydrogen ions that increases the NEAP, while fruits and vegetables have high contents of potassium salts (negative PRAL) with alkalizing potential, favoring the production of bases and balancing hydrogen excess. Increased NEAP (which occurs in the Western diet) determines a chronic low-grade metabolic acidosis state, together with an increased risk for obesity, metabolic disease, and kidney damage [25].
The prognostic role of NEAP and the PRAL has been tested in a survival analysis to investigate their association with kidney disease. Analyzing 632 participants with CKD from the cohort from the AASK trial (African American Study of Kidney Disease and Hypertension), Scialla and colleagues have demonstrated the association between a higher NEAP dietary pattern and a faster worsening of kidney function (slope of measured GFR), especially among patients without proteinuria; while the association with renal events (doubling of creatinine or kidney failure) was not statistically significant [16]. On the other hand, a study including 642 kidney transplant recipients has demonstrated the association of a high dietary acid load with the risk of the doubling of serum creatinine or graft failure [26].
Among the variables needed to plan individualized nutrition plans for patients with CKD, there are biomarkers that directly indicate the dietary intake [27]. Among these, serum C-reactive protein and serum albumin are primarily indicators of the overall nutritional status or inflammation rather than direct indicators of a specific dietary intake. The former is considered as a good marker of cardiovascular disease; the latter has been used for decades as an indicator of malnutrition in stable conditions. Serum pre-albumin has been proposed as a more favorable nutritional marker due to its shorter half-life compared to albumin, potentially reflecting more acute changes in the nutritional state, but its levels are also significantly influenced by inflammation [28]. These laboratory measurements are part of the nutritional assessment process aimed at identifying nutritional abnormalities most frequently observed in patients with CKD: protein energy wasting syndrome, sarcopenia [29], and obesity [30].
Several laboratory measurements indicate metabolic derangements that are significantly influenced by the dietary composition, particularly in the context of CKD: hyperkaliemia and hyperphosphatasemia are both associated with a high morbidity and mortality in CKD [31] and blood bicarbonate and pH are key biomarkers for metabolic acidosis. Screening for other micronutrient deficiencies (trace elements and vitamins) may be performed based on clinical suspicion. For instance, as suggested in KDIGO guidelines [32], in patients with anemia and CKD, nephrologists should perform a basic set of measurements: complete blood count, ferritin, transferrin saturation, folate, and vitamin B12. In fact, a deficiency of these micronutrients can arise from multiple causes, including an inadequate dietary intake [33].
3. Evidence of the Benefits of Diet on the Progression of Chronic Kidney Disease
The Western diet is typically characterized by a high intake of saturated fats and sodium, which constitutes a high dietary acid load (50–100 mEq/daily) [34]. When kidneys cannot eliminate all of the dietary acid load due to reductions in kidney function, a state of metabolic acidosis may develop. The main dietetic schemes used to reduce the dietary acid load and to treat metabolic acidosis in CKD include the Mediterranean diet, “hypoproteic diet” and plant-based diet.
3.1. The Mediterranean Diet, the Possible Proper Dietary Style for Mild to Moderate Severity CKD”
The term Mediterranean diet commonly refers to the dietary pattern of populations living on the Mediterranean Sea coast (southern Italy, Greece, and southern Europe), which is characterized by a regular consumption of high alkaline-forming foods (i.e., fresh fruits, vegetables, legumes, cereals, and nuts), a moderate intake of fish and poultry, and a low intake of eggs, red meat, sweets, and dairy products [35]. The primary source of fat in the Mediterranean diet (>35% fat) is represented by olive oil, especially extra virgin olive oil (EVOO), which contains a high amount of mono- and poly-unsaturated fatty acids [36], while the moderate amount of saturated fats derives from meat and eggs. The Mediterranean diet is particularly suitable for patients with early to moderate stages of CKD, especially with cardiovascular risk factors such as diabetes, hypertension, or dyslipidemia.
The adherence to the Mediterranean diet can be assessed using specific scoring tools, such as the Mediterranean Diet Score (MDS) and the Alternate Mediterranean Diet Score (aMed), which assign scores based on the frequency of the consumption of specific food groups [37,38].
Several cohort studies demonstrated the beneficial and protective effect of the Mediterranean diet related to mortality [38], cardiovascular complications [39], and chronic kidney disease [9]. In fact, fruits and vegetables not only are important sources of nutrients, such as potassium, vitamins, and dietary fibers, but also contain phenolic compounds (especially flavonoids) which show significant bioactive antioxidant and anti-inflammatory properties [40]. Fruits are generally good sources of potassium and phosphorus, though the levels vary among different types. Bananas are particularly rich in potassium, providing about 358 mg per 100 g, while avocados offer even more at around 485 mg. Dried fruits, such as dates and apricots, have very high potassium contents—656 mg and 1162 mg per 100 g, respectively—due to their low water content. Common fruits like oranges, apples, and grapes contain moderate potassium levels, ranging from 107 to 191 mg. Phosphorus levels in fruits tend to be lower than potassium. For example, bananas contain about 22 mg of phosphorus, while apples have around 11 mg. Avocados and dried fruits again stand out, with avocados containing approximately 52 mg of phosphorus and dried apricots up to 71 mg. In general, fresh fruits provide essential nutrients and can contribute significantly to the daily potassium intake, though they are less significant sources of phosphorus compared to other food groups like dairy, meat, and legumes [41]. Also wine (in particular red wine), which is regularly consumed during meals in the Mediterranean Area and has an abundant content of resveratrol polyphenol, has been reported to have nephroprotective effects with increased kidney filtration rates [42].
Interestingly, an analysis of aMed scores calculated from the dietary data of 2403 participants with CKD from the Chronic Renal Insufficiency Cohort (CRIC) demonstrated that a higher adherence to the Mediterranean diet (higher aMed score) was associated with a lower risk of CKD progression (HR 0.75, 95% CI 0.62–0.90) compared to lower aMed scores [43].
A similar protective impact on kidney function was demonstrated by an RCT confronting Mediterranean dietary patterns versus the low-fat diet (<30% fat) in 1002 patients with type 2 diabetes mellitus and coronary heart disease from the CORDIOPREV cohort: the eGFR decline was significantly slower in the group treated with the Mediterranean diet (p = 0.04), especially in patients with a mildly impaired eGFR (p = 0.002) [44]. Moreover, a longitudinal study using three big cohorts including 3.316.633 person-years of follow-up revealed the association between the aMed and a reduced risk of developing kidney stones (HR 0.72, p-value < 0.001): in fact the adherence to the diet contributes to a lower urinary excretion of sodium and a higher excretion of water, citrate, acids, and magnesium, with protective effects against kidney stones [45]. Likewise, the adherence to the Mediterranean diet has been demonstrated to be associated with a better kidney graft outcome: in a study including 632 kidney transplant recipients, the aMed was inversely associated with graft failure (HR 0.68), kidney function decline (HR 0.68), and graft loss (HR 0.74), and this protective effect was more pronounced in patients with more proteinuria and with a more recent transplantation [46]. More specifically, typical Mediterranean protein sources, such as legumes and nuts, have demonstrated to be protective against CKD development, while red meat consumption is associated with CKD risk [47]. Additionally, an increased intake of fruits and vegetables has shown significant improvements in metabolic acidosis among CKD patients, although not to the same extent as oral sodium bicarbonate [48].
Clinical studies have also been performed on olive oil, the keystone of the Mediterranean diet, which has shown to possess healthy properties because of the content of polyphenols (tyrosol, hydroxytyrosol, oleuropein, secoirodoids, and lignans) [49] and mono-unsaturated fatty acids [36]. A meta-analysis of 32 cohort studies conducted by Schwingshackl and colleagues demonstrated that mono-unsaturated fatty acids from olive oil are associated with a reduced risk of stroke, CV events, and mortality [36]. A small pilot study conducted in Italy on a group of 27 patients with stage I-IV CKD revealed that the daily consumption of 40 mL of EVOO for 9 weeks was significant for the improvement of the eGFR (p-value 0.04) and serum concentration of albumin (p-value 0.021) and the reduction in the serum concentration of triglycerides (0.016) and uric acid (p-value 0.049) [50].
Moreover, the high intake of fibers derived from the diet improves the integrity of tight junctions in the colonic epithelium through short-chain fatty acid production and provides the modulation of the intestinal microbiota composition toward eubiosis, leading to protective effects against obesity, diabetes, cardiovascular disease, inflammation, and cancer, along with immune regulation [51]. This is of particular importance in KT recipients who are chronically exposed to immunosuppressive therapy, which is associated with an increased risk of developing hyperlipidemia, hypertension, hyperglycemia, and weight gain [52].
Despite the demonstrated advantages of a Mediterranean diet, it shows significant limitations: a diet full of fruits and vegetables is not recommended for patients with advanced stages of CKD, due to the higher risk of hyperkalemia and hyperphosphatemia; therefore, serum potassium and phosphorus levels need to be checked frequently. For a safe intake, nutritional requirements of potassium and phosphorus may be individualized according to serum levels of electrolytes, and practical counseling should be provided [53].
3.2. The Low-Protein Diet: An Improved CKD Progression with a Concern of Malnutrition
The low-protein diet or a very-low-protein diet have been associated with a slower progression to KF without considerable negative effects of the patient’s nutritional status in different controlled trials [54]. KDOQI guidelines suggest a low-protein diet (0.55–0.60 g/kg/day) or a very-low-protein diet (0.28–0.43 g/kg/day) with keto-analogs to reduce eGFR declines in patients with stage 3–5 CKD without diabetes and to help reduce the accumulation of nitrogenous products [55]. The rationale of a low-protein diet (LPD) is that while CKD progresses, patients accumulate nitrogen-containing products derived from protein catabolism, which progressively cause uremia and its related complications [55]. Acids derived from protein metabolism and acid retention due to kidney disease result in chronic metabolic acidosis. Moreover, a higher protein intake increases the glomerular filtration rate (GFR) through the elevation of intraglomerular pressure; overtime, this mechanism generates glomerular hyperfiltration leading to increased urinary albumin excretion [56]. Proteinuria contributes to glomerulosclerosis progression through the inhibition of podocyte regeneration [57] and can induce tubular cell apoptosis resulting in tubular atrophy [58]. Hence, it is postulated that an LPD can slow CKD progression and symptoms by reducing nitrogen waste products and lowering glomerular hyperfiltration. CKD-mineral bone disorder can also benefit from an LPD because animal proteins are a major source of phosphorous [59], thus decreasing the long-term vascular calcification.
A cost-benefit analysis of an Italian group demonstrated that a very-low-protein diet, VLPD, can delay the dialysis initiation in patients from the age of 70 years without negative effects, also providing economic advantages [60]. The LPD can also be supplemented with keto-analogs of essential amino acids, which are mandatory when a VLPD is followed to prevent malnutrition [61]. If there is an adherence to a restricted low-protein diet, keto-analogs of essential amino acids can transfer themselves into essential amino acids through the “transamination effect”, using circulating amino groups. Through this path, they can reduce the risk of malnutrition without implementing a nitrogen burden [62]. In a retrospective study published in 2023 including more than 1000 patients [63], results showed that, as compared to an LPD only, an LPD with a keto-analogs supplementation was significantly associated with a slower decline of kidney function and a lower rate of dialysis initiation. In another recent study based on the dataset of the Chang Gung Medical System of Taiwan [62], the purpose was to assess whether a low-protein diet supplemented with a keto-analog (sLPD) at a dosage of 0.6 g/kg body weight per day could decrease the risk of dialysis among patients with stage 4 CKD. Patients who subsequently underwent a ketosteril treatment (the most used keto-analog of essential amino acids) were categorized into two groups based on whether they continued the ketosteril treatment for more than three months or not. Over a one-year follow-up period, the group that continued the treatment demonstrated a significantly lower incidence of new-onset KF requiring maintenance dialysis compared to the discontinuation group. These results are encouraging; however, more research is needed to draw definitive conclusions on whether to choose a VLPD supplemented with keto-analogs (sVLPD) or an LPD only. In this regard, in another randomized controlled study [64], Bellizzi et al. compared a standard LPD to an sVLPD in patients affected by stage 4–5 CKD. Results showed patients’ poor adherence to a protein restriction diet; furthermore, there was no additional advantage of an sVLPD when compared to an LPD. In any case, in 2020 the KDOQI Guideline for Nutrition [8], a probable overall benefit of a protein restriction diet plus the supplementation of keto-analogs on RRT/renal survival in patients with stage 3–5 CKD is presumed. If we focus on patients who received a kidney transplant, an appropriate management of post-transplant diet is of major importance to improve allograft survival and outcomes [65]. A high-protein diet can induce glomerular hyperfiltration and increase the allograft’s workload, thus leading to a low nephron mass and glomerulomegaly. Overtime, this condition may result in focal segmental glomerulosclerosis [66]. Even though the literature based on a low-protein diet in KT patients is still poor. Bernardi et al. [67] demonstrated that KT recipients who were compliant with a moderate intake of protein (0.8 g/kg), sodium, and lipids maintained a stabilized kidney function during a 12 years observation when compared to non-compliant patients. The Plant-Dominant Low-Protein Diet (PLADO), which consists of an LPD with at least 50% of plant-dominant sources, has been proposed to ameliorate CKD progression both in KT and non-KT patients, and among its benefits, preventions of harmful effects of meats, a favorable effect on the microbiome, and oxidative stress reductions have been underlined [65,68]. Surely, patients and clinicians must be aware of the increased potassium load, and this diet should be thoughtfully applied to patients with advanced CKD [68]. In a recent review of dietary approaches to KT recipients [69], the potential positive impact of a vegetarian diet with a smaller animal protein intake is outlined: phosphorous from plants is less absorbed, and lower phosphoremia can be an advantage in the context of graft disfunction because of a lesser impact on vascular calcification. In the first period after the transplant though, patients often tend to exhibit hypophosphatemia [70]. Another aspect that needs to be emphasized is that KT recipients’ nutritional assessments must be carefully carried out, especially in the first year after transplantation when they tend to gain weight. For this reason, diets with a higher protein intake and low glycemic index to induce satiety sensations and weight loss are being studied [71].
What we can assume from the literature is that among patients in their early time after transplantation and patients with chronic allograft disfunction, diet management can substantially differ. Still, the overall evidence of the advantage of an LPD in slowing CKD progression and lowering the nitrogen burden is now established, and the importance of the adherence to an LPD must be stressed during clinical evaluations. Patients affected by stage 3–5 CKD should be informed by clinicians of the overall benefits of a low-protein diet, and their nutritional state should be assessed to avoid sarcopenia and malnutrition. Further studies and new criteria are needed to define whether to prescribe an LPD or an sVLPD in stage 3–5 CKD patients. Regarding kidney transplant recipients, further trials are necessary to define the appropriate protein intake, depending on graft function; the risk of diabetes; obesity; and side effects of immunosuppressive therapies, and the preservation of graft function should be considered.
3.3. The Plant-Based Diet: Slowing CKD Progression with the Benefits of Plants
Recently, a great interest on plant-based diets has developed worldwide. A rising body of evidence suggests that the increased consumption of plant-based foods is associated with several health benefits [72]. In fact, plant-based diets have been shown to be effective in improving blood pressure (BP), dyslipidemia, glycemic control, the body mass index (BMI), and the acid–base balance, thus lowering the risk of complications such as diabetes [73], cardiovascular disease [74], and mortality [75]. The term “plant-based” refers to a dietary pattern that focuses on foods derived primarily from plants, while limiting—but not necessarily eliminating—animal-based products. This more flexible dietary pattern differs from the traditional vegetarian diet, which is defined by the exclusion of meat and fish [76]. The plant-based diet is particularly indicated for patients in the early to moderato stages of CKD without a high risk of hyperkalemia and who prefer a vegetarian lifestyle.
Several studies documented favorable associations of plant-based diets with CKD outcomes, including the incidence (i.e., the development of albuminuria and/or eGFR declines) and progression of CKD [47,77,78]. Existing clinical trials’ data have demonstrated that the partial replacement of animal proteins with plant proteins reduces albuminuria [79,80,81]. Moreover, a recent systematic review suggested that a vegetarian diet improves renal functions in patients with CKD [82].
Three examples of plant-based dietary patterns that have been specifically designed for CKD patients not on dialysis are the alkaline diet [83] (an LPD with only 30% of proteins from animal origins), the Plant-Dominant Low-Protein Diet (PLADO) [68], and the Plant-Focused Nutrition in CKD and Diabetes Diet (PLAFOND) [84], all consisting of a protein intake of 0.6–0.8 g/kg/day from at least 50% plant-based sources.
The first condition on which the plant-based diets can demonstrate their effectiveness is the control of acidosis, one of the principal concerns in the late stages of CKD. Since the consumption of proteins leads to a higher production of acid and the consumption of fruit and vegetables leads to a higher production of alkali species, the so-called “alkaline diet” (AD) represents a nutritional approach that could be helpful for CKD metabolic acidosis management [85], as demonstrated by a series of trials. In an RCT involving 71 patients with stage 4 CKD, patients assigned to a diet including a higher fruits and vegetables intake over the course of one year had higher levels of plasma CO2 and lower urinary markers of kidney injury [48]. Another RCT involving 108 patients with stage 3 CKD confirmed similar effects of the fruit and vegetable intake on metabolic acidosis: the daily administration of 2 to 4 cups of fruits and vegetables over a 3-year period resulted in higher CO2 levels, a lower excretion of net acid, lower urinary albumin–creatinine ratios, and preserved kidney function [86]. Plant-based diets have demonstrated their efficacy in reducing inflammation and oxidative stress. Their consumption lead to a reduced generation of uremic toxins (i.e., trimethylamine n-oxide, p-cresyl sulfate, and indoxyl sulfate) [87,88] as demonstrated in two trials, respectively, on hemodialysis and non-dialysis-dependent CKD patients [89,90]. It is worth considering how, on the contrary, animal protein can promote proteolytic fermentation and subsequently results in increased indoles and phenols in the intestinal tract, decreased insulin sensitivity, and increased oxidative stress [91,92,93,94]. In addition, in the NHANES III cohort including 14,543 participants, it was observed that the dietary fiber intake was negatively associated with serum C-reactive protein (CRP) levels, such that each 10 g/day increase in the total fiber intake was associated with an 11% and 38% decline in the odds of elevated serum CRP levels in the CKD and non-CKD groups, respectively [51]
Several RCTs have shown the benefits of plant-based diets in lowering systolic BP [95,96]. The Dietary Approach to Stop Hypertension (DASH) trial showed that the DASH diet (a largely plant-based diet) reduced BP by 5.5 mmHg compared to the control diet [97]. A meta-analysis performed using data from seven RCTs involving 313 participants confirmed similar benefits: a mean reduction in systolic BP by 4.8 mmHg was observed in patients adhering to vegetarian diets compared to omnivorous diets [98]. Through various pathways, different components of vegetarian diets contribute to a lower BP in CKD, directly or indirectly. First, unprocessed plant-based foods contain less sodium than processed or animal-based foods. A lower sodium intake can prevent and control hypertension and lower albuminuria levels, as demonstrated by two meta-analyses including, respectively, dialysis patients and kidney transplant recipients [99] and patients with stage 1–4 CKD [100]. Second, the dietary fiber intake improves hypertension by its effect on arterial contraction, influencing the angiotensin-converting enzyme (ACE) activity and retaining electrolytes (such as potassium and magnesium) in its matrix [8,101]. In addition, plant-based diets may be effective for the prevention and treatment of diabetes mellitus. During digestion, soluble dietary fibers increase viscosity, resulting in the trapping of carbohydrates, slowing the absorption of glucose, and lowering the postprandial glycemia. A dietary fiber intake, in addition to clinical benefits already reported in Section 3.1, is also able to delay gastric emptying, improving insulin sensitivity and producing greater satiety [84,102].
It is useful to underline that a common concern with plant-based diets is the over consumption or over-accumulation of minerals, such as potassium, phosphorus, and sodium, that are contraindicated in CKD patients. Despite the fact that these minerals tend to be in higher abundances in vegetables, they tend to be less bioavailable compared to in animal-based foods due to a reduced absorption of naturally found minerals from plant sources. In fact, potassium contained in fruits and vegetables is typically ingested with non-chloride anions and bicarbonate that promote an increased urinary potassium excretion [103]. However, when hyperkalemia occurs, possible treatments include fighting constipation and binding potassium in the colon with the use of oral binders or increasing the potassium excretion in the urine with the prescription of drugs, such as diuretics or SGLT2i. [104]. Both of the newer potassium binders (patiromer and sodium zirconium cyclosilicate SZC) have shown to lower potassium levels in hyperkalemic CKD patients, even in those receiving RAAS inhibitors [105,106]. These newer agents have shown improved safety profiles: sodium and calcium polystyrene sulfonate (SPS) binders were effective but associated with potentially serious adverse events for the digestive system, such as intestinal necrosis. The most common gastrointestinal events reported with patiromer were mild to moderate constipation, diarrhea, and nausea, each occurring in approximately 4% of patients [106]. Studies have shown that SZC can rapidly reduce serum potassium to normal levels within 48 h, so physicians can prescribe it in cases of acute hyperkalemia [107]. Moreover, as reported in the APPETIZE study, both patiromer and SZC outperformed the sodium and calcium polystyrene sulfonate binder on overall palatability, an essential feature of a drug for optimal compliance [108].
Phosphorus from plant-based foods has also proven to be less bioavailable (20–40% bioavailability) compared to animal foods and processed foods, which mostly have phosphorus in the form of caseins (40–60% bioavailability) and food additives (~100% bioavailability), respectively [95,96]. The reduced phosphorus bioavailability from plant-based foods is due to the phosphorus primarily bound to phytate, which is poorly absorbed by the gastrointestinal tract given humans’ lack of phytase enzymes in the gut [95,97,98,99,100].
Potential drawbacks and limitations to a plant-based diet must be considered. Generally, plant-based diets are considered to be healthier; however, this dietary pattern is typically deficient in vitamin B12, of which animal-based diets are instead rich in. In the setting of a plant-based diet, taking supplements of vitamin B12 is essential for people at risk of deficiency, including those following vegan diets [109] and patients with CKD who follow low-protein diets, have a compromised absorption of nutrients, and take medications (e.g., metformin and proton-pump inhibitors) that can reduce the assimilation of vitamin B12 [110]. Another limitation is the potential nutritional inadequacy of plant-based diets in people with CKD, who are more predisposed to malnutrition–wasting conditions (protein-energy wasting), that have adverse impacts on health and survival [111]. Protein sources considered as high-quality are so defined if they contain easily digestible and absorbable essential amino acids and in quantities adequate to support human growth [112].
The currently recommended method for evaluating dietary protein quality is the amino acid scoring system, which considers most animal proteins and soy proteins to be complete protein sources [112]. Although individual plant-based proteins (with the exception of soy protein) have insufficient levels of one or more indispensable amino acid, the consumption of different sources of plant-based proteins can help to reach the adequate intake of indispensable amino acids, providing health benefits [113]. Several studies in experimental animal models [114,115,116] and in humans [117] have shown that plant-based diets are indeed nutritionally adequate in CKD. For example, it was reported that vegetarian diets with very low protein contents (0.3 g/kg/day) supplemented with keto-analogs provided an adequate nutritional status (i.e., serum albumin levels and the BMI remained stable over 29.6 months) [118]. Additionally, a systematic review including 141 observational and interventional studies from Europe, South-East Asia, and North America reported that the average protein intake was lower in patients consuming plant-based diets compared to those consuming animal-based diets, but the overall dietary protein intake was well within the recommended intake levels for both groups, and the dietary energy intake was comparable among those receiving plant-based vs. animal-based diets [119]. Limited studies among non-CKD [120] and CKD populations [121] have reported that a higher consumption of fruit and vegetables was correlated with a reduced risk of sarcopenia. Future studies evaluating the impact of animal-based diets vs. plant-based diets on muscle heath are needed, with a consideration of the energy intake and overall diet quality.
In order to optimize a plant-based diet intake, it is appropriate for nephrologists to provide CKD patients with some advice: the consumption of vegetables with a negative PRAL (kale, broccoli, Brussels sprouts, cabbage, onions, garlic, celery, zucchini, lettuce, cucumber, radish, bell pepper, rocket, and sprouted seeds); the inclusion of two portions (about 250 g per day) of those vegetables in two meals per day; and two portions of fruit (about 300 g per day) according to their serum potassium content. Some vegetables are naturally rich in potassium, and spinach is among the top sources. It provides about 558 mg of potassium and 49 mg of phosphorus per 100 g. Potatoes—especially when boiled—offer approximately 379 mg of potassium and 44 mg of phosphorus per 100 g. Cooked beet greens are exceptionally high in potassium, delivering around 909 mg, along with 73 mg of phosphorus. Vegetables that are moderate in their potassium content (between 150 and 200 mg per 100 g) include cauliflower, carrots, cabbage, broccoli, and zucchini. Low-potassium vegetables (less than 150 mg per 100 g) include the following: cucumbers, with about 147 mg of potassium and 24 mg of phosphorus; iceberg lettuce, with around 141 mg of potassium and 20 mg of phosphorus; and onions, with about 146 mg of potassium and 29 mg of phosphorus. For patients with hyperkalemia and hyperphosphatemia, soaking and boiling can help lower the potassium and phosphorus in foods [122]. The consumption of legumes, such as lentils, beans, and chickpeas, as an alternative source of protein instead of meat is recommended because of their low PRAL, as well as the consumption of meal/grain foods such as bread, breakfast cereals, rice, and pasta. However, the consumption of these foods should be adjusted to avoid exceeding the daily caloric intake (30–35 kcal/kg/day in CKD patients) [123,124] and, as a consequence, weight gain [18].
5. Molecular Aspects of Research Around Diet and Kidney Transplants
Despite improvements in the care of KT recipients, outcomes remain suboptimal, especially for long-term graft survival. The KT outcome is affected by numerous immunological and donor-related factors. However, the lifestyle and dietary regimen are strong drivers of comorbidities, including cardiovascular disease, obesity, and diabetes [69]. Even though the Mediterranean and Dietary Adherence to Stop Hypertension (DASH) dietary patterns have been demonstrated to be the most advantageous dietary patterns for KT recipients [69], very little molecular evidence has been described.
In a recent study, Heldal et al. reported a statistically significant association between death-censored long-term kidney graft loss and patterns of systemic inflammation in the early period after kidney transplantation, including fibrogenesis activity and metabolic and general/vascular inflammation [187].
Interestingly, fibrogenesis activity markers, like growth differentiation factor 15 (GDF-15) and Cathepsin S, have been described as reduced in subjects who followed the Mediterranean or Healthy Nordic Diet [188].
Several metabolic pathways can be impaired after a KT, leading to pathological states like obesity, hyperglycemia, dyslipidemia, and hypertension [189]; nevertheless, still very little has been described about dietary regimen effects. Among possible biomarkers, resistin, a hormone secreted from adipose tissue, has been correlated with metabolic disorders, atherosclerosis, and inflammation [190]. Data reported by Nagy and colleagues have shown associations between serum resistin levels and clinical outcomes, such as mortality and graft loss, in a large KT recipient cohort [191]. Serum resistin levels have also been positively correlated with saturated fat intake and inversely associated with an adherence to the Mediterranean diet [192].
The immune response towards the allogeneic organ contributes to low-grade persistent inflammation in KT recipients, which may be mirrored at the systemic level and increase mortality risks [193]. C-reactive protein (CRP) is a molecule largely associated with inflammation and cardiovascular disease [194], and over the past few decades its consistently high concentration in plasma and sera in KT recipients has been described as a biomarker for renal rejection [195]. In a systematic review and meta-analysis of randomized controlled trials published by Koleman et al., they recapitulate the recent evidence on the effects of different dietary patterns on inflammatory and immune-related biomarkers in humans, and CRP has been shown to be significantly diminished in subjects who followed the Mediterranean, DASH, or vegetarian/vegan diet [196].
Cytokines such as Interleukin-8 (IL-8), Interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) are markers of inflammation, and increased levels of these cytokines in urine and serum have been significantly associated with allograft rejections in KT patients [197]. Furthermore, Interferon-gamma inducible protein-10 (IP-10/CXCL10) and Monocyte Chemoattractant Protein-1 (MCP-1/CCL2) are chemokines that play a role in the alloimmune response against kidney allografts, and high urinary levels have been associated with rejection [198]. Intriguingly, several studies have shown that the Mediterranean regimen appeared to be the most prominent dietary pattern associated with reductions in inflammatory biomarkers, such as IL-8, IL-6, TNF-α, IFN-ɣ, and MCP-1 [196]. IL-18, a pro-inflammatory cytokine associated with a greater risk of cardiovascular events, graft failure and mortality, has also been described to be significantly reduced after a long-term adherence to the Mediterranean diet + EVOO [199].
Additionally, while transplantation leads to metabolic alterations in the immune system, especially on T cells [200], the nutritional approach, like caloric restriction or intermittent fasting, can deeply remodel immune cell niches [201]. Nevertheless, if and how specific/personalized dietary patterns can be effective in improving graft tolerance and all the comorbidities needs to be further investigated on a molecular level.
Recently, more and more studies are also reporting a correlation between KT outcomes and the gut microbiota, since the latter has been shown to play a role in alloimmunity, infections, and the immunosuppressant and antibiotics metabolism [202]; beneficial effects seem to come from a dietary fiber intake [203].
In KT recipients, dysbiosis—affected by immunosuppressive therapies, antibiotics, lifestyle, and diet—alters the gut microbiota composition, typically increasing Firmicutes, Proteobacteria, and Verrucomicrobia while reducing Bacteroidetes and Actinobacteria [204,205,206,207]. The microbial shift impairs the intestinal barrier, increasing permeability and bacterial translocation [208,209]. This activates pro-inflammatory pathways, including the cytokine release (IL-1, IL-6, IL-18) and Th1/Th17 cell differentiation, while reducing regulatory T cells and promoting graft rejection via immune cross-reactivity [210]. Dysbiosis is associated with acute rejections, infections, fibrosis, diarrhea, altered drug levels, and a reduced SCFA production [211]. Gut-derived uremic toxins (e.g., TMAO, p-cresyl sulfate) further exacerbate renal inflammation and damage [212].
SCFAs exert anti-inflammatory and metabolic effects via G-protein-coupled receptors (GPR41, GPR43, GPR109A), supporting glucose regulation, atherosclerosis prevention, and immune tolerance [211,213,214]. Conversely, TMAO promotes atherogenesis, monocyte activation, inflammasome signaling, an impaired cholesterol metabolism, and an increased thrombosis risk [215].
Diet strongly influences microbial health: Western and high-protein diets promote dysbiosis and inflammation by reducing SCFA-producing bacteria (e.g., Roseburia), lowering fecal butyrate levels, and increasing TMAO [216,217,218]. Conversely, plant-based, DASH, and Mediterranean diets lower the Firmicutes/Bacteroidetes ratio; increase SCFAs; reduce TMAO; and improve blood pressure, lipid profiles, and the immune balance [219,220,221,222,223].
The gut microbiota should be considered a new frontier to modulate and personalize therapies and nutritional approaches to improve graft survival and patients’ quality of life.
All aspects considered need further studies to clarify the molecular aspects and define biomarkers linking dietary regimens and outcomes in the transplant patient. In addition, an appropriate stratification based on immunosuppressive therapies is required.
6. Lifestyle Differences Between Men and Women with Chronic Kidney Disease: The Role of Diet
Sex-specific differences in genetics, physiology, and immunology as well as gender factors (i.e., how the individual self-identifies and behaves) influence the kidney pathophysiology and disease course. Globally, the prevalence of CKD-ND according to the eGFR is greater in women than men, while there are conflicting data about the impact of sex-based factors on the progression of CKD. The variability of these data could reflect differences in the kidney disease etiology and outcomes [224].
A meta-analysis from J. Neugarten et al. based on 68 studies and a total of 11,345 patients with CKD from different causes observed a more rapid decline in renal function with time in men than women. However, this analysis cannot assess whether this result is independent from other covariates, such as diet, blood pressure, or serum lipid levels [225].
In the univariate analysis of The Modification of Diet in Renal Disease Study, a prospective multicenter study, male gender was identified as a risk factor for a more rapid progression of the eGFR decline in 840 primarily nondiabetic subjects with CKD. However, the multivariate analysis showed that only proteinuria, high-density lipoprotein (HDL) levels, and BP are independent risk factors for a worse renal outcome [226].
More recently, Minutolo et al., with their multicohort study considering 2335 patients affected by moderate to advanced CKD, confirmed the impact of sex on the progression of kidney disease, reporting a 50% higher risk of progression in men than in women [5].
Even data from the initial 10 years of the Swedish Renal Registry–Chronic Kidney Disease, a nationwide, population-based inception registry of CKD G3b-G5 patients, reported an overall rate of CKD progression of 19.6% per year, with men showing a higher risk of progression and of death, especially from cardiovascular disease [227].
The biological mechanisms at the basis of men’s faster decline of the eGFR is not known. Among the more reliable theories there is the possible impact of sex-based differences in oxidative stress, nitrogen oxide metabolism, and sex steroids [228].
Animal studies, for example, have shown a protective and anti-inflammatory activity of estrogens on podocytes. However, Swartling O. et al. found no differences between the decline of the eGFR in women than in men before and after the mean menopausal age in Sweden [229].
All these data could be affected by an epidemiological bias as confirmed by data from population-based studies that have shown that women are more represented than men in earlier stages of CKD [230].
Minutolo et al. suggested that the fastest renal progression in men could, in part, be related to higher levels of proteinuria in men compared with women [5].
What we would speculate is that even diet could have affected the rate of the progression of the kidney damage. Gender differences on dietary choices impact the protein burden and the creation of a pro-inflammatory environment, with men preferring a high-protein diet and women preferring an antioxidant-rich diet. However, more studies need to be performed.
7. Social, Cultural, and Economic Factors Influencing Dietary Adherence
Dietary adherence is fundamental for slowing the progression of CKD, managing uremic symptoms, and preventing complications such as electrolyte imbalances and cardiovascular disease [8]. However, it is strongly influenced by social, cultural, and economic factors. Family and social support can facilitate adherence, particularly in patients who struggle with motivation or meal preparation. When family eating habits align with CKD dietary restrictions, adherence becomes less challenging. Changes in lifestyle may be required to stick to regular meal schedules and avoid fasting routines. Also, the patient’s cultural background must be taken into consideration for appropriate communication to improve understanding and compliance: traditional foods may provide high contents of sodium, potassium, and phosphorus and may conflict with dietary restrictions; religious practices or dietary laws (e.g., halal and vegetarianism) may be adjusted to prioritize nutrition advice; and if a language barrier is present, it must be addressed to avoid misinterpretation and confusion. In low-resource settings, access to fresh and affordable foods should not be taken for granted: renal-friendly foods are often more expensive than processed alternatives and many patients may find it difficult to afford recommended foods, such as fresh fruits and vegetables. Food supply constraints, such as living in food deserts with limited transportation possibilities, further restrict access to suitable dietary choices.
Social determinants of health play a crucial role in dietary adherence, and addressing these determinants is essential for improving patient outcomes.
In our digital era, technology can be a useful tool in dietary management: there are mobile applications that can remind users of eating schedules or that reinforce positive behaviors towards food by texting messages to patients. Online groups with other patients can be an opportunity to exchange recipes or to find solutions for overcoming barriers to adhering to the diet or to agree on group workouts. Telemedicine could increase the patient’s adherence to the diet by online follow-up appointments or telephone calls. Technology can also assist nephrologists in detecting and monitoring sarcopenia in CKD patients: there are imaging techniques (such as ultrasound or computed tomography) that allow for a non-invasive and thorough evaluation of muscle quality, including the assessment of ectopic fat infiltration [231].
8. Conclusions
Managing diets is a therapeutic tool that can influence the prognosis of CKD patients (Figure 1 and Table 2). Several dietary patterns have been shown to offer health benefits to CKD patients; therefore, an individualized dietary plan should be developed based on each patient’s nutritional needs and food preferences. Given the progressive and chronic nature of CKD, a periodic reassessment is essential to further personalize the dietary plan to the patient’s evolving clinical needs and to ensure nutritional adequacy. Moreover, nephrologists should educate patients on lifestyle modifications as a strategy to delay CKD progression and improve cardiovascular outcomes, alongside blood pressure control and the use of new drug classes. By integrating clinical and molecular perspectives, this review may serve as a foundation for future research and dietary interventions in nephrology.
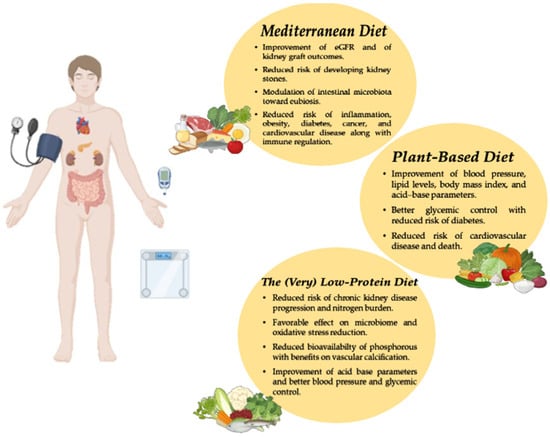
Figure 1.
Impact of Mediterranean, plant-based, and low-protein diets in improving kidney disease outcomes.

Table 2.
The main diets for CKD patients.
Author Contributions
Conceptualization, L.H. and M.P.; investigation, L.H., G.B., C.R., E.G. and S.B.; writing—original draft preparation, L.H., G.B., C.R., E.G., S.B. and M.P.; writing—review and editing, L.H., G.B., C.R., E.G., S.B., G.P. (Giuliana Papalia), G.P. (Gemma Patella), M.E.L., G.Z., O.B., I.C. and M.P.; visualization, L.H., G.B., C.R., E.G., S.B., G.P. (Giuliana Papalia), G.P. (Gemma Patella), M.E.L., G.Z., O.B., I.C. and M.P.; supervision, L.H. and M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BP | blood pressure |
| CKD | chronic kidney disease |
| CRP | C-reactive protein |
| CV | cardiovascular |
| DASH | Dietary Adherence to Stop Hypertension |
| EVOO | extra virgin olive oil |
| GFR | glomerular filtration rate |
| IL-8, IL-6 | Interleukin-8, Interleukin-6 |
| IP-10 | Interferon-gamma inducible protein-10 |
| KT | kidney transplant |
| LPD | low-protein diet |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| NEAP | net endogenous acid production |
| NODAT | new-onset diabetes after transplant |
| PLADO | Plant-Dominant Low-Protein Diet |
| PLAFOND | Plant-Focused Nutrition in CKD and Diabetes Diet |
| PRAL | potential renal acid load |
| SCFAs | short-chain fatty acids |
| sVLPD | VLPD supplemented with keto-analogs |
| TNF-α | tumor necrosis factor-α |
| VLPD | very-low-protein diet |
References
- Jager, K.J.; Kovesdy, C.; Langham, R.; Rosenberg, M.; Jha, V.; Zoccali, C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int. 2019, 96, 1048–1050. [Google Scholar] [CrossRef]
- Capelli, I.; Gasperoni, L.; Ruggeri, M.; Donati, G.; Baraldi, O.; Sorrenti, G.; Caletti, M.T.; Aiello, V.; Cianciolo, G.; La Manna, G. New mineralocorticoid receptor antagonists: Update on their use in chronic kidney disease and heart failure. J. Nephrol. 2020, 33, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Salah, H.M.; Al’Aref, S.J.; Khan, M.S.; Al-Hawwas, M.; Vallurupalli, S.; Mehta, J.L.; Mounsey, J.P.; Greene, S.J.; McGuire, D.K.; Lopes, R.D.; et al. Effect of sodium-glucose cotransporter 2 inhibitors on cardiovascular and kidney outcomes-Systematic review and meta-analysis of randomized placebo-controlled trials. Am. Heart J. 2021, 232, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.; Del Vecchio, L. Hypoxia-Inducible Factor-Prolyl Hydroxyl Domain Inhibitors: From Theoretical Superiority to Clinical Noninferiority Compared with Current ESAs? J. Am. Soc. Nephrol. 2022, 33, 1966–1979. [Google Scholar] [CrossRef] [PubMed]
- Minutolo, R.; Gabbai, F.B.; Chiodini, P.; Provenzano, M.; Borrelli, S.; Garofalo, C.; Bellizzi, V.; Russo, D.; Conte, G.; De Nicola, L.; et al. Sex Differences in the Progression of CKD Among Older Patients: Pooled Analysis of 4 Cohort Studies. Am. J. Kidney Dis. 2020, 75, 30–38. [Google Scholar] [CrossRef]
- Fernandez-Fernandez, B.; Mahillo, I.; Sanchez-Rodriguez, J.; Carriazo, S.; Sanz, A.B.; Sanchez-Niño, M.D.; Ortiz, A. Gender, Albuminuria and Chronic Kidney Disease Progression in Treated Diabetic Kidney Disease. J. Clin. Med. 2020, 9, 1611. [Google Scholar] [CrossRef]
- Minutolo, R.; Gabbai, F.B.; Provenzano, M.; Chiodini, P.; Borrelli, S.; Garofalo, C.; Sasso, F.C.; Santoro, D.; Bellizzi, V.; Conte, G.; et al. Cardiorenal prognosis by residual proteinuria level in diabetic chronic kidney disease: Pooled analysis of four cohort studies. Nephrol. Dial. Transplant. 2018, 33, 1942–1949. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.-J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76 (Suppl. S1), S1–S107. [Google Scholar] [CrossRef]
- Chauveau, P.; Koppe, L.; Combe, C.; Lasseur, C.; Trolonge, S.; Aparicio, M. Vegetarian diets and chronic kidney disease. Nephrol. Dial. Transplant. 2019, 34, 199–207. [Google Scholar] [CrossRef]
- Torreggiani, M.; Wang, A.Y.-M.; Fois, A.; Piccoli, G.B. Personalized Low-Protein Diet Prescription in CKD Population: Merging Evidence From Randomized Trials With Observational Data. Semin. Nephrol. 2023, 43, 151402. [Google Scholar] [CrossRef]
- Piccoli, G.B. The heritage of Thomas Addis: Why do nephrologists still love glomerulonephritis? J. Nephrol. 2022, 35, 1059–1060. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Napoletano, A.; Provenzano, M.; Garofalo, C.; Bini, C.; Comai, G.; La Manna, G. Mineral Bone Disorders in Kidney Disease Patients: The Ever-Current Topic. Int. J. Mol. Sci. 2022, 23, 12223. [Google Scholar] [CrossRef]
- Melamed, M.L.; Raphael, K.L. Metabolic Acidosis in CKD: A Review of Recent Findings. Kidney Med. 2021, 3, 267–277. [Google Scholar] [CrossRef]
- Ebert, T.; Pawelzik, S.-C.; Witasp, A.; Arefin, S.; Hobson, S.; Kublickiene, K.; Shiels, P.G.; Bäck, M.; Stenvinkel, P. Inflammation and Premature Ageing in Chronic Kidney Disease. Toxins 2020, 12, 227. [Google Scholar] [CrossRef] [PubMed]
- Moranne, O.; Froissart, M.; Rossert, J.; Gauci, C.; Boffa, J.-J.; Haymann, J.P.; M’rad, M.B.; Jacquot, C.; Houillier, P.; Stengel, B.; et al. Timing of onset of CKD-related metabolic complications. J. Am. Soc. Nephrol. 2009, 20, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Scialla, J.J.; Appel, L.J.; Astor, B.C.; Miller, E.R.; Beddhu, S.; Woodward, M.; Parekh, R.S.; Anderson, C.A.M.; African American Study of Kidney Disease and Hypertension Study Group. Net endogenous acid production is associated with a faster decline in GFR in African Americans. Kidney Int. 2012, 82, 106–112. [Google Scholar] [CrossRef]
- Agapitov, A.V.; Haynes, W.G. Role of endothelin in cardiovascular disease. J. Renin Angiotensin Aldosterone Syst. 2002, 3, 1–15. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Wilson Jones, G.; Di Lauro, M.; Pietroboni Zaitseva, A.; Ramadori, L.; Celotto, R.; Mitterhofer, A.P.; Di Daniele, N. Nutritional Approaches for the Management of Metabolic Acidosis in Chronic Kidney Disease. Nutrients 2021, 13, 2534. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.S.; Kozan, P.; Samocha-Bonet, D. The role of dietary acid load and mild metabolic acidosis in insulin resistance in humans. Biochimie 2016, 124, 171–177. [Google Scholar] [CrossRef]
- Shah, S.N.; Abramowitz, M.; Hostetter, T.H.; Melamed, M.L. Serum bicarbonate levels and the progression of kidney disease: A cohort study. Am. J. Kidney Dis. 2009, 54, 270–277. [Google Scholar] [CrossRef]
- Chapter 3: Management of progression and complications of CKD. Kidney Int. Suppl. 2013, 3, 73–90. [CrossRef]
- Yari, Z.; Mirmiran, P. Alkaline Diet: A Novel Nutritional Strategy in Chronic Kidney Disease? Iran. J. Kidney Dis. 2018, 12, 204–208. [Google Scholar] [PubMed]
- Frassetto, L.A.; Lanham-New, S.A.; Macdonald, H.M.; Remer, T.; Sebastian, A.; Tucker, K.L.; Tylavsky, F.A. Standardizing terminology for estimating the diet-dependent net acid load to the metabolic system. J. Nutr. 2007, 137, 1491–1492. [Google Scholar] [CrossRef]
- Remer, T.; Dimitriou, T.; Manz, F. Dietary potential renal acid load and renal net acid excretion in healthy, free-living children and adolescents. Am. J. Clin. Nutr. 2003, 77, 1255–1260. [Google Scholar] [CrossRef]
- Frassetto, L.A.; Todd, K.M.; Morris, R.C.; Sebastian, A. Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am. J. Clin. Nutr. 1998, 68, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.M.H.; Gomes-Neto, A.W.; Osté, M.C.J.; van den Berg, E.; Kootstra-Ros, J.E.; Sanders, J.S.F.; Berger, S.P.; Carrero, J.J.; De Borst, M.H.; Navis, G.J.; et al. Net Endogenous Acid Excretion and Kidney Allograft Outcomes. Clin. J. Am. Soc. Nephrol. 2021, 16, 1398–1406. [Google Scholar] [CrossRef]
- Kistler, B.M.; Moore, L.W.; Benner, D.; Biruete, A.; Boaz, M.; Brunori, G.; Chen, J.; Drechsler, C.; Guebre-Egziabher, F.; Hensley, M.K.; et al. The International Society of Renal Nutrition and Metabolism Commentary on the National Kidney Foundation and Academy of Nutrition and Dietetics KDOQI Clinical Practice Guideline for Nutrition in Chronic Kidney Disease. J. Ren. Nutr. 2021, 31, 116–120.e1. [Google Scholar] [CrossRef] [PubMed]
- Keller, U. Nutritional Laboratory Markers in Malnutrition. J. Clin. Med. 2019, 8, 775. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Furth, S.L.; Zoccali, C.; World Kidney Day Steering Committee. Obesity and kidney disease: Hidden consequences of the epidemic. J. Nephrol. 2017, 30, 1–10. [Google Scholar] [CrossRef]
- Hougen, I.; Leon, S.J.; Whitlock, R.; Rigatto, C.; Komenda, P.; Bohm, C.; Tangri, N. Hyperkalemia and its Association With Mortality, Cardiovascular Events, Hospitalizations, and Intensive Care Unit Admissions in a Population-Based Retrospective Cohort. Kidney Int. Rep. 2021, 6, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Drüeke, T.B.; Parfrey, P.S. Summary of the KDIGO guideline on anemia and comment: Reading between the (guide)line(s). Kidney Int. 2012, 82, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Green, R.; Datta Mitra, A. Megaloblastic Anemias: Nutritional and Other Causes. Med. Clin. N. Am. 2017, 101, 297–317. [Google Scholar] [CrossRef]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef]
- Finicelli, M.; Di Salle, A.; Galderisi, U.; Peluso, G. The Mediterranean Diet: An Update of the Clinical Trials. Nutrients 2022, 14, 2956. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated fatty acids, olive oil and health status: A systematic review and meta-analysis of cohort studies. Lipids Health Dis. 2014, 13, 154. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C. Dietary patterns: A Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 559–568. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Cao, Y.-L.; Lin, J.-H.; Hammes, H.-P.; Zhang, C. Flavonoids in Treatment of Chronic Kidney Disease. Molecules 2022, 27, 2365. [Google Scholar] [CrossRef]
- Grembecka, M.; Szefer, P. Comparative assessment of essential and heavy metals in fruits from different geographical origins. Environ. Monit. Assess. 2013, 185, 9139–9160. [Google Scholar] [CrossRef] [PubMed]
- Den Hartogh, D.J.; Tsiani, E. Health Benefits of Resveratrol in Kidney Disease: Evidence from In Vitro and In Vivo Studies. Nutrients 2019, 11, 1624. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.A.; Coresh, J.; Anderson, C.A.M.; Appel, L.J.; Grams, M.E.; Crews, D.C.; Mills, K.T.; He, J.; Scialla, J.; Rahman, M.; et al. Adherence to Healthy Dietary Patterns and Risk of CKD Progression and All-Cause Mortality: Findings From the CRIC (Chronic Renal Insufficiency Cohort) Study. Am. J. Kidney Dis. 2021, 77, 235–244. [Google Scholar] [CrossRef]
- Podadera-Herreros, A.; Alcala-Diaz, J.F.; Gutierrez-Mariscal, F.M.; Jimenez-Torres, J.; de la Cruz-Ares, S.; Arenas-de Larriva, A.P.; Cardelo, M.P.; Torres-Peña, J.D.; Luque, R.M.; Ordovas, J.M.; et al. Long-term consumption of a mediterranean diet or a low-fat diet on kidney function in coronary heart disease patients: The CORDIOPREV randomized controlled trial. Clin. Nutr. 2022, 41, 552–559. [Google Scholar] [CrossRef]
- Rodriguez, A.; Curhan, G.C.; Gambaro, G.; Taylor, E.N.; Ferraro, P.M. Mediterranean diet adherence and risk of incident kidney stones. Am. J. Clin. Nutr. 2020, 111, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Neto, A.W.; Osté, M.C.J.; Sotomayor, C.G.; van den Berg, E.; Geleijnse, J.M.; Berger, S.P.; Gans, R.O.B.; Bakker, S.J.L.; Navis, G.J. Mediterranean Style Diet and Kidney Function Loss in Kidney Transplant Recipients. Clin. J. Am. Soc. Nephrol. 2020, 15, 238–246. [Google Scholar] [CrossRef]
- Haring, B.; Selvin, E.; Liang, M.; Coresh, J.; Grams, M.E.; Petruski-Ivleva, N.; Steffen, L.M.; Rebholz, C.M. Dietary Protein Sources and Risk for Incident Chronic Kidney Disease: Results From the Atherosclerosis Risk in Communities (ARIC) Study. J. Ren. Nutr. 2017, 27, 233–242. [Google Scholar] [CrossRef]
- Goraya, N.; Simoni, J.; Jo, C.-H.; Wesson, D.E. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin. J. Am. Soc. Nephrol. 2013, 8, 371–381. [Google Scholar] [CrossRef]
- Rodríguez-López, P.; Lozano-Sanchez, J.; Borrás-Linares, I.; Emanuelli, T.; Menéndez, J.A.; Segura-Carretero, A. Structure-Biological Activity Relationships of Extra-Virgin Olive Oil Phenolic Compounds: Health Properties and Bioavailability. Antioxidants 2020, 9, 685. [Google Scholar] [CrossRef]
- Romani, A.; Bernini, R.; Noce, A.; Urciuoli, S.; Di Lauro, M.; Pietroboni Zaitseva, A.; Marrone, G.; Di Daniele, N. Potential Beneficial Effects of Extra Virgin Olive Oils Characterized by High Content in Minor Polar Compounds in Nephropathic Patients: A Pilot Study. Molecules 2020, 25, 4757. [Google Scholar] [CrossRef]
- Krishnamurthy, V.M.R.; Wei, G.; Baird, B.C.; Murtaugh, M.; Chonchol, M.B.; Raphael, K.L.; Greene, T.; Beddhu, S. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int. 2012, 81, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Lo, A. Immunosuppression and metabolic syndrome in renal transplant recipients. Metab. Syndr. Relat. Disord. 2004, 2, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, A.; Caverni-Muñoz, A.; González García, E. Mediterranean Diet and Chronic Kidney Disease (CKD): A Practical Approach. Nutrients 2022, 15, 97. [Google Scholar] [CrossRef]
- Rhee, C.M.; Ahmadi, S.-F.; Kovesdy, C.P.; Kalantar-Zadeh, K. Low-protein diet for conservative management of chronic kidney disease: A systematic review and meta-analysis of controlled trials. J. Cachexia Sarcopenia Muscle 2018, 9, 235–245. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Fouque, D. Nutritional Management of Chronic Kidney Disease. N. Engl. J. Med. 2018, 378, 584–585. [Google Scholar] [CrossRef]
- Ko, G.J.; Obi, Y.; Tortorici, A.R.; Kalantar-Zadeh, K. Dietary protein intake and chronic kidney disease. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 77–85. [Google Scholar] [CrossRef]
- Carney, E.F. Glomerular disease: Albuminuria inhibits podocyte regeneration. Nat. Rev. Nephrol. 2013, 9, 554. [Google Scholar] [CrossRef]
- Peired, A.; Angelotti, M.L.; Ronconi, E.; la Marca, G.; Mazzinghi, B.; Sisti, A.; Lombardi, D.; Giocaliere, E.; Della Bona, M.; Villanelli, F.; et al. Proteinuria impairs podocyte regeneration by sequestering retinoic acid. J. Am. Soc. Nephrol. 2013, 24, 1756–1768. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, A.; Kalantar-Zadeh, K. Management of natural and added dietary phosphorus burden in kidney disease. Semin. Nephrol. 2013, 33, 180–190. [Google Scholar] [CrossRef]
- Scalone, L.; Borghetti, F.; Brunori, G.; Viola, B.F.; Brancati, B.; Sottini, L.; Mantovani, L.G.; Cancarini, G. Cost-benefit analysis of supplemented very low-protein diet versus dialysis in elderly CKD5 patients. Nephrol. Dial. Transpl. 2010, 25, 907–913. [Google Scholar] [CrossRef]
- Cupisti, A.; Bolasco, P. Keto-analogues and essential aminoacids and other supplements in the conservative management of chronic kidney disease. Panminerva Med. 2017, 59, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.-L.; Fan, P.-C.; Chen, J.-J.; Kuo, G.; Hsiao, C.-C.; Chen, C.-Y.; Tu, Y.-R.; Hsu, H.-H.; Chen, Y.-C.; Chang, C.-H. Ketoanalogues Supplemental Low Protein Diet Safely Decreases Short-Term Risk of Dialysis among CKD Stage 4 Patients. Nutrients 2022, 14, 4020. [Google Scholar] [CrossRef] [PubMed]
- Ariyanopparut, S.; Metta, K.; Avihingsanon, Y.; Eiam-Ong, S.; Kittiskulnam, P. The role of a low protein diet supplemented with ketoanalogues on kidney progression in pre-dialysis chronic kidney disease patients. Sci. Rep. 2023, 13, 15459. [Google Scholar] [CrossRef] [PubMed]
- Bellizzi, V.; Signoriello, S.; Minutolo, R.; Di Iorio, B.; Nazzaro, P.; Garofalo, C.; Calella, P.; Chiodini, P.; De Nicola, L.; ERIKA Study Group Investigators of the Italian Society of Nephrology-Conservative Therapy of CKD Work Group. No additional benefit of prescribing a very low-protein diet in patients with advanced chronic kidney disease under regular nephrology care: A pragmatic, randomized, controlled trial. Am. J. Clin. Nutr. 2022, 115, 1404–1417. [Google Scholar] [CrossRef]
- Tantisattamo, E.; Kalantar-Zadeh, K.; Molnar, M.Z. Nutritional and dietary interventions to prolong renal allograft survival after kidney transplantation. Curr. Opin. Nephrol. Hypertens. 2022, 31, 6–17. [Google Scholar] [CrossRef]
- Tantisattamo, E.; Dafoe, D.C.; Reddy, U.G.; Ichii, H.; Rhee, C.M.; Streja, E.; Landman, J.; Kalantar-Zadeh, K. Current Management of Patients With Acquired Solitary Kidney. Kidney Int. Rep. 2019, 4, 1205–1218. [Google Scholar] [CrossRef]
- Bernardi, A.; Biasia, F.; Pati, T.; Piva, M.; D’Angelo, A.; Bucciante, G. Long-term protein intake control in kidney transplant recipients: Effect in kidney graft function and in nutritional status. Am. J. Kidney Dis. 2003, 41 (Suppl. S1), S146–S152. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Joshi, S.; Schlueter, R.; Cooke, J.; Brown-Tortorici, A.; Donnelly, M.; Schulman, S.; Lau, W.-L.; Rhee, C.M.; Streja, E.; et al. Plant-Dominant Low-Protein Diet for Conservative Management of Chronic Kidney Disease. Nutrients 2020, 12, 1931. [Google Scholar] [CrossRef]
- Cyrino, L.G.; Galpern, J.; Moore, L.; Borgi, L.; Riella, L.V. A Narrative Review of Dietary Approaches for Kidney Transplant Patients. Kidney Int. Rep. 2021, 6, 1764–1774. [Google Scholar] [CrossRef]
- van Londen, M.; Aarts, B.M.; Deetman, P.E.; van der Weijden, J.; Eisenga, M.F.; Navis, G.; Bakker, S.J.L.; de Borst, M.H.; NIGRAM Consortium. Post-Transplant Hypophosphatemia and the Risk of Death-Censored Graft Failure and Mortality after Kidney Transplantation. Clin. J. Am. Soc. Nephrol. 2017, 12, 1301–1310. [Google Scholar] [CrossRef]
- Pedrollo, E.F.; Nicoletto, B.B.; Carpes, L.S.; de Freitas, J.d.M.C.; Buboltz, J.R.; Forte, C.C.; Bauer, A.C.; Manfro, R.C.; Souza, G.C.; Leitão, C.B. Effect of an intensive nutrition intervention of a high protein and low glycemic-index diet on weight of kidney transplant recipients: Study protocol for a randomized clinical trial. Trials 2017, 18, 413. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Medawar, E.; Huhn, S.; Villringer, A.; Veronica Witte, A. The effects of plant-based diets on the body and the brain: A systematic review. Transl. Psychiatry 2019, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Satija, A.; Bhupathiraju, S.N.; Rimm, E.B.; Spiegelman, D.; Chiuve, S.E.; Borgi, L.; Willett, W.C.; Manson, J.E.; Sun, Q.; Hu, F.B. Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies. PLoS Med. 2016, 13, e1002039. [Google Scholar] [CrossRef]
- Satija, A.; Bhupathiraju, S.N.; Spiegelman, D.; Chiuve, S.E.; Manson, J.E.; Willett, W.; Rexrode, K.M.; Rimm, E.B.; Hu, F.B. Healthful and Unhealthful Plant-Based Diets and the Risk of Coronary Heart Disease in U.S. Adults. J. Am. Coll. Cardiol. 2017, 70, 411–422. [Google Scholar] [CrossRef]
- Baden, M.Y.; Liu, G.; Satija, A.; Li, Y.; Sun, Q.; Fung, T.T.; Rimm, E.B.; Willett, W.C.; Hu, F.B.; Bhupathiraju, S.N. Changes in Plant-Based Diet Quality and Total and Cause-Specific Mortality. Circulation 2019, 140, 979–991. [Google Scholar] [CrossRef]
- Hargreaves, S.M.; Rosenfeld, D.L.; Moreira, A.V.B.; Zandonadi, R.P. Plant-based and vegetarian diets: An overview and definition of these dietary patterns. Eur. J. Nutr. 2023, 62, 1109–1121. [Google Scholar] [CrossRef] [PubMed]
- Yuzbashian, E.; Asghari, G.; Mirmiran, P.; Hosseini, F.-S.; Azizi, F. Associations of dietary macronutrients with glomerular filtration rate and kidney dysfunction: Tehran lipid and glucose study. J. Nephrol. 2015, 28, 173–180. [Google Scholar] [CrossRef]
- Nettleton, J.A.; Steffen, L.M.; Palmas, W.; Burke, G.L.; Jacobs, D.R. Associations between microalbuminuria and animal foods, plant foods, and dietary patterns in the Multiethnic Study of Atherosclerosis. Am. J. Clin. Nutr. 2008, 87, 1825–1836. [Google Scholar] [CrossRef]
- Azadbakht, L.; Esmaillzadeh, A. Soy-protein consumption and kidney-related biomarkers among type 2 diabetics: A crossover, randomized clinical trial. J. Ren. Nutr. 2009, 19, 479–486. [Google Scholar] [CrossRef]
- Teixeira, S.R.; Tappenden, K.A.; Carson, L.; Jones, R.; Prabhudesai, M.; Marshall, W.P.; Erdman, J.W. Isolated soy protein consumption reduces urinary albumin excretion and improves the serum lipid profile in men with type 2 diabetes mellitus and nephropathy. J. Nutr. 2004, 134, 1874–1880. [Google Scholar] [CrossRef]
- Azadbakht, L.; Atabak, S.; Esmaillzadeh, A. Soy protein intake, cardiorenal indices, and C-reactive protein in type 2 diabetes with nephropathy: A longitudinal randomized clinical trial. Diabetes Care 2008, 31, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Świątek, Ł.; Jeske, J.; Miedziaszczyk, M.; Idasiak-Piechocka, I. The impact of a vegetarian diet on chronic kidney disease (CKD) progression—A systematic review. BMC Nephrol. 2023, 24, 168. [Google Scholar] [CrossRef] [PubMed]
- Passey, C. Reducing the Dietary Acid Load: How a More Alkaline Diet Benefits Patients With Chronic Kidney Disease. J. Ren. Nutr. 2017, 27, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Rhee, C.M.; Joshi, S.; Brown-Tortorici, A.; Kramer, H.M. Medical nutrition therapy using plant-focused low-protein meal plans for management of chronic kidney disease in diabetes. Curr. Opin. Nephrol. Hypertens. 2022, 31, 26–35. [Google Scholar] [CrossRef]
- Rodrigues Neto Angéloco, L.; Arces de Souza, G.C.; Almeida Romão, E.; Garcia Chiarello, P. Alkaline Diet and Metabolic Acidosis: Practical Approaches to the Nutritional Management of Chronic Kidney Disease. J. Ren. Nutr. 2018, 28, 215–220. [Google Scholar] [CrossRef]
- Goraya, N.; Simoni, J.; Jo, C.-H.; Wesson, D.E. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int. 2014, 86, 1031–1038. [Google Scholar] [CrossRef]
- Cases, A.; Cigarrán-Guldrís, S.; Mas, S.; Gonzalez-Parra, E. Vegetable-Based Diets for Chronic Kidney Disease? It Is Time to Reconsider. Nutrients 2019, 11, 1263. [Google Scholar] [CrossRef]
- Koppe, L.; Fouque, D.; Soulage, C.O. The Role of Gut Microbiota and Diet on Uremic Retention Solutes Production in the Context of Chronic Kidney Disease. Toxins 2018, 10, 155. [Google Scholar] [CrossRef]
- Marzocco, S.; Dal Piaz, F.; Di Micco, L.; Torraca, S.; Sirico, M.L.; Tartaglia, D.; Autore, G.; Di Iorio, B. Very low protein diet reduces indoxyl sulfate levels in chronic kidney disease. Blood Purif. 2013, 35, 196–201. [Google Scholar] [CrossRef]
- Sirich, T.L.; Plummer, N.S.; Gardner, C.D.; Hostetter, T.H.; Meyer, T.W. Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 1603–1610. [Google Scholar] [CrossRef]
- Montemurno, E.; Cosola, C.; Dalfino, G.; Daidone, G.; De Angelis, M.; Gobbetti, M.; Gesualdo, L. What would you like to eat, Mr CKD Microbiota? A Mediterranean Diet, please! Kidney Blood Press. Res. 2014, 39, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, A.; D’Alessandro, C.; Gesualdo, L.; Cosola, C.; Gallieni, M.; Egidi, M.F.; Fusaro, M. Non-Traditional Aspects of Renal Diets: Focus on Fiber, Alkali and Vitamin K1 Intake. Nutrients 2017, 9, 444. [Google Scholar] [CrossRef]
- Evenepoel, P.; Claus, D.; Geypens, B.; Hiele, M.; Geboes, K.; Rutgeerts, P.; Ghoos, Y. Amount and fate of egg protein escaping assimilation in the small intestine of humans. Am. J. Physiol. 1999, 277, G935–G943. [Google Scholar] [CrossRef] [PubMed]
- Evenepoel, P.; Meijers, B.K. Dietary fiber and protein: Nutritional therapy in chronic kidney disease and beyond. Kidney Int. 2012, 81, 227–229. [Google Scholar] [CrossRef]
- Rouse, I.L.; Beilin, L.J.; Armstrong, B.K.; Vandongen, R. Blood-pressure-lowering effect of a vegetarian diet: Controlled trial in normotensive subjects. Lancet 1983, 1, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Margetts, B.M.; Beilin, L.J.; Vandongen, R.; Armstrong, B.K. Vegetarian diet in mild hypertension: A randomised controlled trial. Br. Med. J. (Clin. Res. Ed.) 1986, 293, 1468–1471. [Google Scholar] [CrossRef]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Nishimura, K.; Barnard, N.D.; Takegami, M.; Watanabe, M.; Sekikawa, A.; Okamura, T.; Miyamoto, Y. Vegetarian diets and blood pressure: A meta-analysis. JAMA Intern. Med. 2014, 174, 577–587. [Google Scholar] [CrossRef]
- McMahon, E.J.; Campbell, K.L.; Bauer, J.D.; Mudge, D.W.; Kelly, J.T. Altered dietary salt intake for people with chronic kidney disease. Cochrane Database Syst. Rev. 2021, 6, CD010070. [Google Scholar] [CrossRef]
- Garofalo, C.; Borrelli, S.; Provenzano, M.; De Stefano, T.; Vita, C.; Chiodini, P.; Minutolo, R.; De Nicola, L.; Conte, G. Dietary Salt Restriction in Chronic Kidney Disease: A Meta-Analysis of Randomized Clinical Trials. Nutrients 2018, 10, 732. [Google Scholar] [CrossRef]
- Sánchez-Muniz, F.J. Dietary fibre and cardiovascular health. Nutr. Hosp. 2012, 27, 31–45. [Google Scholar] [PubMed]
- Tuttle, K.R.; Bakris, G.L.; Bilous, R.W.; Chiang, J.L.; De Boer, I.H.; Goldstein-Fuchs, J.; Hirsch, I.B.; Kalantar-Zadeh, K.; Narva, A.S.; Navaneethan, S.D.; et al. Diabetic Kidney Disease: A Report From an ADA Consensus Conference. Diabetes Care 2014, 37, 2864–2883. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, E.J.; Donnelly, S.M.; Ethier, J.H.; Quaggin, S.E.; Kaiser, U.B.; Vasuvattakul, S.; Kamel, K.S.; Halperin, M.L. Modulation of the secretion of potassium by accompanying anions in humans. Kidney Int. 1991, 39, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F.; Clegg, D.J. Hyperkalemia treatment standard. Nephrol. Dial. Transplant. 2024, 39, 1097–1104. [Google Scholar] [CrossRef]
- Spinowitz, B.S.; Fishbane, S.; Pergola, P.E.; Roger, S.D.; Lerma, E.V.; Butler, J.; von Haehling, S.; Adler, S.H.; Zhao, J.; Singh, B.; et al. Sodium Zirconium Cyclosilicate among Individuals with Hyperkalemia: A 12-Month Phase 3 Study. Clin. J. Am. Soc. Nephrol. 2019, 14, 798–809. [Google Scholar] [CrossRef]
- Weir, M.R.; Bakris, G.L.; Bushinsky, D.A.; Mayo, M.R.; Garza, D.; Stasiv, Y.; Wittes, J.; Christ-Schmidt, H.; Berman, L.; Pitt, B.; et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N. Engl. J. Med. 2015, 372, 211–221. [Google Scholar] [CrossRef]
- Kosiborod, M.; Rasmussen, H.S.; Lavin, P.; Qunibi, W.Y.; Spinowitz, B.; Packham, D.; Roger, S.D.; Yang, A.; Lerma, E.; Singh, B. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: The HARMONIZE randomized clinical trial. JAMA 2014, 312, 2223–2233. [Google Scholar] [CrossRef]
- Wheeler, D.C.; Søndergaard, H.; Gwynn, C.; Hedman, K.; Hedberg, J.; Allum, A.; Chung, H.-L.; Någård, M.; Stjernlöf, G.; Wittbrodt, E.; et al. Randomised, blinded, cross-over evaluation of the palatability of and preference for different potassium binders in participants with chronic hyperkalaemia in the USA, Canada and Europe: The APPETIZE study. BMJ Open 2024, 14, e074954. [Google Scholar] [CrossRef]
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J. Acad. Nutr. Diet. 2016, 116, 1970–1980. [Google Scholar] [CrossRef]
- Zarantonello, D.; Brunori, G. The Role of Plant-Based Diets in Preventing and Mitigating Chronic Kidney Disease: More Light than Shadows. J. Clin. Med. 2023, 12, 6137. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Ikizler, T.A.; Block, G.; Avram, M.M.; Kopple, J.D. Malnutrition-inflammation complex syndrome in dialysis patients: Causes and consequences. Am. J. Kidney Dis. 2003, 42, 864–881. [Google Scholar] [CrossRef] [PubMed]
- Hertzler, S.R.; Lieblein-Boff, J.C.; Weiler, M.; Allgeier, C. Plant Proteins: Assessing Their Nutritional Quality and Effects on Health and Physical Function. Nutrients 2020, 12, 3704. [Google Scholar] [CrossRef]
- Young, V.; Pellett, P. Plant proteins in relation to human protein and amino acid nutrition. Am. J. Clin. Nutr. 1994, 59, 1203S–1212S. [Google Scholar] [CrossRef] [PubMed]
- Moe, S.M.; Chen, N.X.; Seifert, M.F.; Sinders, R.M.; Duan, D.; Chen, X.; Liang, Y.; Radcliff, J.S.; White, K.E.; Gattone, V.H. A rat model of chronic kidney disease-mineral bone disorder. Kidney Int. 2009, 75, 176–184. [Google Scholar] [CrossRef]
- Ogborn, M.R.; Bankovic-Calic, N.; Shoesmith, C.; Buist, R.; Peeling, J. Soy protein modification of rat polycystic kidney disease. Am. J. Physiol.-Ren. Physiol. 1998, 274, F541–F549. [Google Scholar] [CrossRef]
- Trujillo, J.; Ramírez, V.; Pérez, J.; Torre-Villalvazo, I.; Torres, N.; Tovar, A.R.; Muñoz, R.M.; Uribe, N.; Gamba, G.; Bobadilla, N.A. Renal protection by a soy diet in obese Zucker rats is associated with restoration of nitric oxide generation. Am. J. Physiol.-Ren. Physiol. 2005, 288, F108–F116. [Google Scholar] [CrossRef] [PubMed]
- Mitch, W.E.; Remuzzi, G. Diets for patients with chronic kidney disease, should we reconsider? BMC Nephrol. 2016, 17, 80. [Google Scholar] [CrossRef]
- Kelly, J.T.; Palmer, S.C.; Wai, S.N.; Ruospo, M.; Carrero, J.-J.; Campbell, K.L.; Strippoli, G.F.M. Healthy Dietary Patterns and Risk of Mortality and ESRD in CKD: A Meta-Analysis of Cohort Studies. Clin. J. Am. Soc. Nephrol. 2017, 12, 272–279. [Google Scholar] [CrossRef]
- Neufingerl, N.; Eilander, A. Nutrient Intake and Status in Adults Consuming Plant-Based Diets Compared to Meat-Eaters: A Systematic Review. Nutrients 2021, 14, 29. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.; Kye, S.; Chung, Y.-S.; Kim, K.-M. Association of vegetables and fruits consumption with sarcopenia in older adults: The Fourth Korea National Health and Nutrition Examination Survey. Age Ageing 2015, 44, 96–102. [Google Scholar] [CrossRef]
- Inoshita, H.; Asaoka, D.; Matsuno, K.; Yanagisawa, N.; Suzuki, Y.; Miyauchi, K. Cross-Sectional Study on the Association between Dietary Patterns and Sarcopenia in Elderly Patients with Chronic Kidney Disease Receiving Conservative Treatment. Nutrients 2023, 15, 4994. [Google Scholar] [CrossRef] [PubMed]
- de Abreu, D.B.V.; Picard, K.; Klein, M.R.S.T.; Gadas, O.M.; Richard, C.; Barreto Silva, M.I. Soaking to Reduce Potassium and Phosphorus Content of Foods. J. Ren. Nutr. 2023, 33, 165–171. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US) Subcommittee on the Tenth Edition of the Recommended Dietary Allowances. Recommended Dietary Allowances, 10th ed.; The National Academies Collection: Reports Funded by National Institutes of Health; National Academies Press (US): Washington, DC, USA, 1989; ISBN 978-0-309-04633-6. [Google Scholar]
- I. Adult guidelines. Am. J. Kidney Dis. 2000, 35, S17–S104. [CrossRef] [PubMed]
- Nolte Fong, J.V.; Moore, L.W. Nutrition Trends in Kidney Transplant Recipients: The Importance of Dietary Monitoring and Need for Evidence-Based Recommendations. Front. Med. 2018, 5, 302. [Google Scholar] [CrossRef]
- Chadban, S.J.; Ahn, C.; Axelrod, D.A.; Foster, B.J.; Kasiske, B.L.; Kher, V.; Kumar, D.; Oberbauer, R.; Pascual, J.; Pilmore, H.L.; et al. KDIGO Clinical Practice Guideline on the Evaluation and Management of Candidates for Kidney Transplantation. Transplantation 2020, 104 (Suppl. S1), S11–S103. [Google Scholar] [CrossRef]
- Rebholz, C.M.; Crews, D.C.; Grams, M.E.; Steffen, L.M.; Levey, A.S.; Miller, E.R.; Appel, L.J.; Coresh, J. DASH (Dietary Approaches to Stop Hypertension) Diet and Risk of Subsequent Kidney Disease. Am. J. Kidney Dis. 2016, 68, 853–861. [Google Scholar] [CrossRef]
- Kurnatowska, I.; Małyska, A.; Wysocka, K.; Mazur, K.; Krawczyk, J.; Nowicki, M. Long-Term Effect of Body Mass Index Changes on Graft Damage Markers in Patients After Kidney Transplantation. Ann. Transpl. 2016, 21, 626–631. [Google Scholar] [CrossRef]
- Martins, C.; Pecoits-Filho, R.; Riella, M.C. Nutrition for the post–renal transplant recipients. Transplant. Proc. 2004, 36, 1650–1654. [Google Scholar] [CrossRef]
- Pecoits-Filho, R.; Lindholm, B.; Stenvinkel, P. The malnutrition, inflammation, and atherosclerosis (MIA) syndrome—The heart of the matter. Nephrol. Dial. Transplant. 2002, 17 (Suppl. S11), 28–31. [Google Scholar] [CrossRef]
- Chang, S.H.; Coates, P.T.H.; McDonald, S.P. Effects of Body Mass Index at Transplant on Outcomes of Kidney Transplantation. Transplantation 2007, 84, 981–987. [Google Scholar] [CrossRef]
- Lafranca, J.A.; IJzermans, J.N.; Betjes, M.G.; Dor, F.J. Erratum: Body mass index and outcome in renal transplant recipients: A systematic review and meta-analysis. BMC Med. 2015, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Whittier, F.C.; Evans, D.H.; Dutton, S.; Ross, G.; Luger, A.; Nolph, K.D.; Bauer, J.H.; Brooks, C.S.; Moore, H. Nutrition in Renal Transplantation. Am. J. Kidney Dis. 1985, 6, 405–411. [Google Scholar] [CrossRef]
- Van Den Ham, E.C.H.; Kooman, J.P.; Van Hooff, J.P. Nutritional Considerations in Renal Transplant Patients. Blood Purif. 2002, 20, 139–144. [Google Scholar] [CrossRef]
- Kasiske, B.; Cosio, F.G.; Beto, J.; Bolton, K.; Chavers, B.M.; Grimm, R.; Levin, A.; Masri, B.; Parekh, R.; Wanner, C.; et al. Clinical practice guidelines for managing dyslipidemias in kidney transplant patients: A report from the Managing Dyslipidemias in Chronic Kidney Disease Work Group of the National Kidney Foundation Kidney Disease Outcomes Quality Initiative. Am. J. Transplant. 2004, 4, 13–53. [Google Scholar] [CrossRef]
- Guida, B.; Trio, R.; Laccetti, R.; Nastasi, A.; Salvi, E.; Perrino, N.R.; Caputo, C.; Rotaia, E.; Federico, S.; Sabbatini, M. Role of dietary intervention on metabolic abnormalities and nutritional status after renal transplantation. Nephrol. Dial. Transplant. 2007, 22, 3304–3310. [Google Scholar] [CrossRef] [PubMed]
- Workeneh, B.; Moore, L.W.; Nolte Fong, J.V.; Shypailo, R.; Gaber, A.O.; Mitch, W.E. Successful Kidney Transplantation Is Associated With Weight Gain From Truncal Obesity and Insulin Resistance. J. Ren. Nutr. 2019, 29, 548–555. [Google Scholar] [CrossRef]
- Netto, M.C.A.S.; Alves-Filho, G.; Mazzali, M. Nutritional Status and Body Composition in Patients Early After Renal Transplantation. Transplant. Proc. 2012, 44, 2366–2368. [Google Scholar] [CrossRef] [PubMed]
- Sabbatini, M.; Ferreri, L.; Pisani, A.; Capuano, I.; Morgillo, M.; Memoli, A.; Riccio, E.; Guida, B. Nutritional management in renal transplant recipients: A transplant team opportunity to improve graft survival. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 319–324. [Google Scholar] [CrossRef]
- Sasaki, H.; Suzuki, A.; Kusaka, M.; Fukami, N.; Shiroki, R.; Itoh, M.; Takahashi, H.; Uenishi, K.; Hoshinaga, K. Nutritional Status in Japanese Renal Transplant Recipients With Long-term Graft Survival. Transplant. Proc. 2015, 47, 367–372. [Google Scholar] [CrossRef]
- Heaf, J.; Jakobsen, U.; Tvedegaard, E.; Kanstrup, I.-L.; Fogh-Andersen, N. Dietary habits and nutritional status of renal transplant patients. J. Ren. Nutr. 2004, 14, 20–25. [Google Scholar] [CrossRef]
- Baum, C.L. Weight Gain and Cardiovascular Risk After Organ Transplantation. J. Parenter. Enter. Nutr. 2001, 25, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Elster, E.A.; Leeser, D.B.; Morrissette, C.; Pepek, J.M.; Quiko, A.; Hale, D.A.; Chamberlain, C.; Salaita, C.; Kirk, A.D.; Mannon, R.B. Obesity following kidney transplantation and steroid avoidance immunosuppression. Clin. Transplant. 2008, 22, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Conte, C.; Secchi, A. Post-transplantation diabetes in kidney transplant recipients: An update on management and prevention. Acta Diabetol. 2018, 55, 763–779. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.M.C.; Moura, Á.E.F.; Gonçalves, L.; Pinheiro, L.S.F.; Pinheiro, F.M.L.; Esmeraldo, R.M. Post-Transplantation Weight Gain: Prevalence and the Impact of Steroid-Free Therapy. Transplant. Proc. 2014, 46, 1735–1740. [Google Scholar] [CrossRef]
- Fougeray, S.; Loriot, M.-A.; Nicaud, V.; Legendre, C.; Thervet, E.; Pallet, N. Increased Body Mass Index After Kidney Transplantation in Activating Transcription Factor 6 Single Polymorphism Gene Carriers. Transplant. Proc. 2011, 43, 3418–3422. [Google Scholar] [CrossRef]
- Thoma, B.; Grover, V.K.; Shoker, A. Prevalence of weight gain in patients with better renal transplant function. Clin. Nephrol. 2006, 65, 408–414. [Google Scholar] [CrossRef]
- Eckardt, K.-U.; Kasiske, B.L.; Zeier, M.G. Special Issue: KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients. Am. J. Transplant. 2009, 9, S1–S155. [Google Scholar] [CrossRef]
- Kluch, M.; Kurnatowska, I.; Matera, K.; Łokieć, K.; Puzio, T.; Czkwianianc, E.; Grzelak, P. Nutrition Trends in Patients Over the Long Term After Kidney Transplantation. Transplant. Proc. 2020, 52, 2357–2362. [Google Scholar] [CrossRef]
- Henggeler, C.K.; Plank, L.D.; Ryan, K.J.; Gilchrist, E.L.; Casas, J.M.; Lloyd, L.E.; Mash, L.E.; McLellan, S.L.; Robb, J.M.; Collins, M.G. A Randomized Controlled Trial of an Intensive Nutrition Intervention Versus Standard Nutrition Care to Avoid Excess Weight Gain After Kidney Transplantation: The INTENT Trial. J. Ren. Nutr. 2018, 28, 340–351. [Google Scholar] [CrossRef]
- Patel, M.G. The effect of dietary intervention on weight gains after renal transplantation. J. Ren. Nutr. 1998, 8, 137–141. [Google Scholar] [CrossRef]
- Chadban, S.; Chan, M.; Fry, K.; Patwardhan, A.; Ryan, C.; Trevillian, P.; Westgarth, F. Nutritional management of overweight and obesity in adult kidney transplant recipients. Nephrology 2010, 15, S22–S55. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.; Jardine, A.; Andrews, P. Renal Association Clinical Practice Guideline on Post-operative Care of the Kidney Transplant Recipient. Nephron Clin. Pr. 2011, 118, c311–c347. [Google Scholar] [CrossRef]
- Yates, C.J.; Fourlanos, S.; Hjelmesæth, J.; Colman, P.G.; Cohney, S.J. New-Onset Diabetes After Kidney Transplantation—Changes and Challenges. Am. J. Transplant. 2012, 12, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Jenssen, T.; Hartmann, A. Emerging treatments for post-transplantation diabetes mellitus. Nat. Rev. Nephrol. 2015, 11, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, F.; Friman, S.; Scheuermann, E.; Rostaing, L.; Jenssen, T.; Campistol, J.M.; Uchida, K.; Pescovitz, M.D.; Marchetti, P.; Tuncer, M.; et al. Results of an International, Randomized Trial Comparing Glucose Metabolism Disorders and Outcome with Cyclosporine Versus Tacrolimus. Am. J. Transplant. 2007, 7, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Midtvedt, K.; Hjelmesæth, J.; Hartmann, A.; Lund, K.; Paulsen, D.; Egeland, T.; Jenssen, T. Insulin Resistance after Renal Transplantation: The Effect of Steroid Dose Reduction and Withdrawal. J. Am. Soc. Nephrol. 2004, 15, 3233–3239. [Google Scholar] [CrossRef]
- Woodle, E.S.; First, M.R.; Pirsch, J.; Shihab, F.; Gaber, A.O.; Van Veldhuisen, P. A Prospective, Randomized, Double-Blind, Placebo-Controlled Multicenter Trial Comparing Early (7 Day) Corticosteroid Cessation Versus Long-Term, Low-Dose Corticosteroid Therapy. Ann. Surg. 2008, 248, 564–577. [Google Scholar] [CrossRef]
- Hjelmesæth, J.; Hartmann, A.; Leivestad, T.; Holdaas, H.; Sagedal, S.; Olstad, M.; Jenssen, T. The impact of early-diagnosed new-onset post-transplantation diabetes mellitus on survival and major cardiac events. Kidney Int. 2006, 69, 588–595. [Google Scholar] [CrossRef]
- Cole, E.H.; Johnston, O.; Rose, C.L.; Gill, J.S. Impact of Acute Rejection and New-Onset Diabetes on Long-Term Transplant Graft and Patient Survival. Clin. J. Am. Soc. Nephrol. 2008, 3, 814–821. [Google Scholar] [CrossRef]
- Jenssen, T.; Hartmann, A. Post-transplant diabetes mellitus in patients with solid organ transplants. Nat. Rev. Endocrinol. 2019, 15, 172–188. [Google Scholar] [CrossRef]
- Nafar, M.; Noori, N.; Jalali-Farahani, S.; Hosseinpanah, F.; Poorrezagholi, F.; Ahmadpoor, P.; Samadian, F.; Firouzan, A.; Einollahi, B. Mediterranean diets are associated with a lower incidence of metabolic syndrome one year following renal transplantation. Kidney Int. 2009, 76, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Osté, M.C.J.; Corpeleijn, E.; Navis, G.J.; Keyzer, C.A.; Soedamah-Muthu, S.S.; Van Den Berg, E.; Postmus, D.; De Borst, M.H.; Kromhout, D.; Bakker, S.J.L. Mediterranean style diet is associated with low risk of new-onset diabetes after renal transplantation. BMJ Open Diabetes Res. Care 2017, 5, e000283. [Google Scholar] [CrossRef]
- Ichimaru, N.; Nakazawa, S.; Yamanaka, K.; Kakuta, Y.; Abe, T.; Kaimori, J.-Y.; Imamura, R.; Nonomura, N.; Takahara, S. Adherence to Dietary Recommendations in Maintenance Phase Kidney Transplant Patients. Transplant. Proc. 2016, 48, 890–892. [Google Scholar] [CrossRef]
- Lin, I.-H.; Wong, T.-C.; Nien, S.-W.; Chou, Y.-T.; Chiang, Y.-J.; Wang, H.-H.; Yang, S.-H. Dietary Compliance Among Renal Transplant Recipients: A Single-Center Study in Taiwan. Transplant. Proc. 2019, 51, 1325–1330. [Google Scholar] [CrossRef]
- Opinion of the Scientific Panel on Dietetic products, nutrition and allergies [NDA] related to the Tolerable Upper Intake Level of Boron (Sodium Borate and Boric Acid). EFSA J. 2004, 80, 1–22. [CrossRef]
- Curtis, J.J.; Luke, R.G.; Jones, P.; Diethelm, A.G. Hypertension in cyclosporine-treated renal transplant recipients is sodium dependent. Am. J. Med. 1988, 85, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Osté, M.C.J.; Gomes-Neto, A.W.; Corpeleijn, E.; Gans, R.O.B.; De Borst, M.H.; Van Den Berg, E.; Soedamah-Muthu, S.S.; Kromhout, D.; Navis, G.J.; Bakker, S.J.L. Dietary Approach to Stop Hypertension (DASH) diet and risk of renal function decline and all-cause mortality in renal transplant recipients. Am. J. Transplant. 2018, 18, 2523–2533. [Google Scholar] [CrossRef]
- Chadban, S.; Chan, M.; Fry, K.; Patwardhan, A.; Ryan, C.; Trevillian, P.; Westgarth, F. Protein requirement in adult kidney transplant recipients. Nephrology 2010, 15, S68–S71. [Google Scholar] [CrossRef] [PubMed]
- Said, M.Y.; Deetman, P.E.; De Vries, A.P.J.; Zelle, D.M.; Gans, R.O.B.; Navis, G.; Joosten, M.M.; Bakker, S.J.L. Causal path analyses of the association of protein intake with risk of mortality and graft failure in renal transplant recipients. Clin. Transplant. 2015, 29, 447–457. [Google Scholar] [CrossRef]
- Van Den Berg, E.; Engberink, M.F.; Brink, E.J.; Van Baak, M.A.; Gans, R.O.B.; Navis, G.; Bakker, S.J.L. Dietary protein, blood pressure and renal function in renal transplant recipients. Br. J. Nutr. 2013, 109, 1463–1470. [Google Scholar] [CrossRef]
- Deetman, P.E.; Said, M.Y.; Kromhout, D.; Dullaart, R.P.F.; Kootstra-Ros, J.E.; Sanders, J.-S.F.; Seelen, M.A.J.; Gans, R.O.B.; Navis, G.; Joosten, M.M.; et al. Urinary Urea Excretion and Long-term Outcome After Renal Transplantation. Transplantation 2015, 99, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, A.; Biasia, F.; Piva, M.; Poluzzi, P.; Senesi, G.; Scaramuzzo, P.; Garizzo, O.; Stoppa, F.; Cavallaro, B.; Bassini, S.; et al. Dietary protein intake and nutritional status in patients with renal transplant. Clin. Nephrol. 2000, 53, 3–5. [Google Scholar]
- Coburn, P.D.J.W. Renal osteodystrophy. Kidney Int. 1980, 17, 677–693. [Google Scholar] [CrossRef][Green Version]
- Merhi, B.; Shireman, T.; Carpenter, M.A.; Kusek, J.W.; Jacques, P.; Pfeffer, M.; Rao, M.; Foster, M.C.; Kim, S.J.; Pesavento, T.E.; et al. Serum Phosphorus and Risk of Cardiovascular Disease, All-Cause Mortality, or Graft Failure in Kidney Transplant Recipients: An Ancillary Study of the FAVORIT Trial Cohort. Am. J. Kidney Dis. 2017, 70, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Kasiske, B.L.; Vazquez, M.A.; Harmon, W.E.; Brown, R.S.; Danovitch, G.M.; Gaston, R.S.; Roth, D.; Scandling, J.D.; Singer, G.G. Recommendations for the outpatient surveillance of renal transplant recipients. American Society of Transplantation. J. Am. Soc. Nephrol. 2000, 11 (Suppl. S15), S1–S86. [Google Scholar] [CrossRef]
- Ryan, K.J.; Casas, J.M.S.; Mash, L.E.; McLellan, S.L.; Lloyd, L.E.; Stinear, J.W.; Plank, L.D.; Collins, M.G. The effect of intensive nutrition interventions on weight gain after kidney transplantation: Protocol of a randomised controlled trial. BMC Nephrol. 2014, 15, 148. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Stoler, S.T.; Chan, M.; Chadban, S.J. Nutrition in the Management of Kidney Transplant Recipients. J. Ren. Nutr. 2023, 33, S67–S72. [Google Scholar] [CrossRef]
- Chadban, S.J.; Singer, J.; Coates, P.T. That sinking gut feeling: Is transplant-induced dysbiosis contributing to allograft outcomes? Kidney Int. 2023, 103, 454–457. [Google Scholar] [CrossRef]
- Singer, J.; Li, Y.J.; Ying, T.; Aouad, L.J.; Gracey, D.M.; Wyburn, K.; Macia, L.; Wu, H.; Chadban, S.J. Protocol for a pilot single-centre, parallel-arm, randomised controlled trial of dietary inulin to improve gut health in solid organ transplantation: The DIGEST study. BMJ Open 2021, 11, e049184. [Google Scholar] [CrossRef]
- Chan, M.; Patwardhan, A.; Ryan, C.; Trevillian, P.; Chadban, S.; Westgarth, F.; Fry, K. Evidence-based Guidelines for the Nutritional Management of Adult Kidney Transplant Recipients. J. Ren. Nutr. 2011, 21, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, G.; Zelle, D.M.; Navis, G.J.; Dijkema, D.; Bemelman, F.J.; Bakker, S.J.L.; Corpeleijn, E. Lifestyle intervention to improve quality of life and prevent weight gain after renal transplantation: Design of the Active Care after Transplantation (ACT) randomized controlled trial. BMC Nephrol. 2017, 18, 296. [Google Scholar] [CrossRef] [PubMed]
- Skerrett, P.J.; Willett, W.C. Essentials of Healthy Eating: A Guide. J. Midwife. Womens Health 2010, 55, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Sabbatini, M.; Garofalo, G.; Borrelli, S.; Vitale, S.; Capone, D.; Russo, L.; Pisani, A.; Carrano, R.; Gallo, R.; Federico, S.; et al. Efficacy of a reduced pill burden on therapeutic adherence to calcineurin inhibitors in renal transplant recipients: An observational study. Patient Prefer. Adherence 2014, 8, 7–813. [Google Scholar] [CrossRef]
- Cianciaruso, B.; Pota, A.; Pisani, A.; Torraca, S.; Annecchini, R.; Lombardi, P.; Capuano, A.; Nazzaro, P.; Bellizzi, V.; Sabbatini, M. Metabolic effects of two low protein diets in chronic kidney disease stage 4-5—A randomized controlled trial. Nephrol. Dial. Transplant. 2007, 23, 636–644. [Google Scholar] [CrossRef]
- Heldal, T.F.; Åsberg, A.; Ueland, T.; Reisæter, A.V.; Pischke, S.E.; Mollnes, T.E.; Aukrust, P.; Reinholt, F.; Hartmann, A.; Heldal, K.; et al. Systemic inflammation early after kidney transplantation is associated with long-term graft loss: A cohort study. Front. Immunol. 2023, 14, 1253991. [Google Scholar] [CrossRef]
- Sotos-Prieto, M.; Maroto-Rodriguez, J.; Ortolá, R.; Martinez-Gomez, D.; García-Esquinas, E.; Buño-Soto, A.; Rodríguez-Artalejo, F. Association between a Mediterranean lifestyle and growth differentiation factor 15: The seniors ENRICA-2 cohort. Free Radic. Biol. Med. 2023, 195, 192–198. [Google Scholar] [CrossRef]
- Lin, L.; Tan, W.; Pan, X.; Tian, E.; Wu, Z.; Yang, J. Metabolic Syndrome-Related Kidney Injury: A Review and Update. Front. Endocrinol. 2022, 13, 904001. [Google Scholar] [CrossRef]
- Reilly, M.P.; Lehrke, M.; Wolfe, M.L.; Rohatgi, A.; Lazar, M.A.; Rader, D.J. Resistin Is an Inflammatory Marker of Atherosclerosis in Humans. Circulation 2005, 111, 932–939. [Google Scholar] [CrossRef]
- Nagy, K.; Ujszaszi, A.; Czira, M.E.; Remport, A.; Kovesdy, C.P.; Mathe, Z.; Rhee, C.M.; Mucsi, I.; Molnar, M.Z. Association between serum resistin level and outcomes in kidney transplant recipients. Transpl. Int. 2016, 29, 352–361. [Google Scholar] [CrossRef]
- Cabrera De León, A.; Almeida González, D.; González Hernández, A.; Domínguez Coello, S.; Marrugat, J.; Juan Alemán Sánchez, J.; Brito Díaz, B.; Marcelino Rodríguez, I.; Pérez, M.D.C.R. Relationships between Serum Resistin and Fat Intake, Serum Lipid Concentrations and Adiposity in the General Population. J. Atheroscler. Thromb. 2014, 21, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Heldal, T.F.; Åsberg, A.; Ueland, T.; Reisæter, A.V.; Pischke, S.E.; Mollnes, T.E.; Aukrust, P.; Hartmann, A.; Heldal, K.; Jenssen, T. Inflammation in the early phase after kidney transplantation is associated with increased long-term all-cause mortality. Am. J. Transplant. 2022, 22, 2016–2027. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Yao, Y. The Clinical Significance and Potential Role of C-Reactive Protein in Chronic Inflammatory and Neurodegenerative Diseases. Front. Immunol. 2018, 9, 1302. [Google Scholar] [CrossRef]
- Ozdemir, N.F.; Elsurer, R.; Ibis, A.; Arat, Z.; Haberal, M. Serum C-Reactive Protein Surge in Renal Transplant Recipients: Link With Allograft Survival. Transplant. Proc. 2007, 39, 934–937. [Google Scholar] [CrossRef]
- Koelman, L.; Egea Rodrigues, C.; Aleksandrova, K. Effects of Dietary Patterns on Biomarkers of Inflammation and Immune Responses: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2022, 13, 101–115. [Google Scholar] [CrossRef]
- Waiser, J.; Budde, K.; Katalinic, A.; Kuerzdorfer, M.; Riess, R.; Neumayer, H.H. Interleukin-6 expression after renal transplantation. Nephrol. Dial. Transplant. 1997, 12, 753–759. [Google Scholar] [CrossRef]
- Raza, A.; Firasat, S.; Khaliq, S.; Aziz, T.; Mubarak, M.; Naqvi, S.A.A.; Mehdi, S.Q.; Rizvi, S.A.-H.; Abid, A. The association of urinary interferon-gamma inducible protein-10 (IP10/CXCL10) levels with kidney allograft rejection. Inflamm. Res. 2017, 66, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N.; Carpenter, M.A.; Weiner, D.E.; Levey, A.S.; Pfeffer, M.; Kusek, J.W.; Cai, J.; Hunsicker, L.G.; Park, M.; Bennett, M.; et al. Urine Injury Biomarkers and Risk of Adverse Outcomes in Recipients of Prevalent Kidney Transplants: The Folic Acid for Vascular Outcome Reduction in Transplantation Trial. J. Am. Soc. Nephrol. 2016, 27, 2109–2121. [Google Scholar] [CrossRef]
- Domínguez-Amorocho, O.; Takiishi, T.; Cunha, F.F.D.; Câmara, N.O.S. Immunometabolism: A target for the comprehension of immune response toward transplantation. World J. Transplant. 2019, 9, 27–34. [Google Scholar] [CrossRef]
- Procaccini, C.; De Candia, P.; Russo, C.; De Rosa, G.; Lepore, M.T.; Colamatteo, A.; Matarese, G. Caloric restriction for the immunometabolic control of human health. Cardiovasc. Res. 2024, 119, 2787–2800. [Google Scholar] [CrossRef]
- García-Martínez, Y.; Borriello, M.; Capolongo, G.; Ingrosso, D.; Perna, A.F. The Gut Microbiota in Kidney Transplantation: A Target for Personalized Therapy? Biology 2023, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Qin, X.; Yang, C.; Sabatino, A.; Kelly, J.T.; Avesani, C.M.; Carrero, J.J. Fiber intake and health in people with chronic kidney disease. Clin. Kidney J. 2022, 15, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Ranjan, R.; McGee, H.S.; Andropolis, K.E.; Panchal, D.V.; Hajjiri, Z.; Brennan, D.C.; Finn, P.W.; Perkins, D.L. Urinary microbiome of kidney transplant patients reveals dysbiosis with potential for antibiotic resistance. Transl. Res. 2017, 181, 59–70. [Google Scholar] [CrossRef]
- Sharma, A.; Giorgakis, E. Gut microbiome dysbiosis in the setting of solid organ transplantation: What we have gleaned from human and animal studies. World J. Transplant. 2022, 12, 157–162. [Google Scholar] [CrossRef]
- Yılmaz, G.; Saygılı, S.; Ağbaş, A.; Karabağ Yılmaz, E.; Variş, A.; Canpolat, N. Pediatric kidney transplant recipients are at an increased risk for dysbiosis. Front. Microbiol. 2025, 16, 1499813. [Google Scholar] [CrossRef]
- Winichakoon, P.; Chaiwarith, R.; Chattipakorn, N.; Chattipakorn, S.C. Impact of gut microbiota on kidney transplantation. Transplant. Rev. 2022, 36, 100668. [Google Scholar] [CrossRef]
- Wang, W.; Xu, S.; Ren, Z.; Jiang, J.; Zheng, S. Gut microbiota and allogeneic transplantation. J. Transl. Med. 2015, 13, 275. [Google Scholar] [CrossRef]
- Anders, H.-J.; Andersen, K.; Stecher, B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int. 2013, 83, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Bromberg, J.S.; Fricke, W.F.; Brinkman, C.C.; Simon, T.; Mongodin, E.F. Microbiota—Implications for immunity and transplantation. Nat. Rev. Nephrol. 2015, 11, 342–353. [Google Scholar] [CrossRef]
- Salvadori, M.; Tsalouchos, A. The Microbiota and Kidney Transplantation: Influence on the Graft. 2021. Available online: https://www.emjreviews.com/urology/article/the-microbiota-and-kidney-transplantation-influence-on-the-graft-j180121/ (accessed on 22 May 2025).
- Ardalan, M.; Vahed, S.Z. Gut microbiota and renal transplant outcome. Biomed. Pharmacother. 2017, 90, 229–236. [Google Scholar] [CrossRef]
- Ichimura, A.; Hasegawa, S.; Kasubuchi, M.; Kimura, I. Free fatty acid receptors as therapeutic targets for the treatment of diabetes. Front. Pharmacol. 2014, 5, 236. [Google Scholar] [CrossRef] [PubMed]
- Lukasova, M.; Hanson, J.; Tunaru, S.; Offermanns, S. Nicotinic acid (niacin): New lipid-independent mechanisms of action and therapeutic potentials. Trends Pharmacol. Sci. 2011, 32, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, X.; Yang, F.; Zhao, R.; Pan, X.; Liang, J.; Tian, L.; Li, X.; Liu, L.; Xing, Y.; et al. Gut Microbiota-Dependent Marker TMAO in Promoting Cardiovascular Disease: Inflammation Mechanism, Clinical Prognostic, and Potential as a Therapeutic Target. Front. Pharmacol. 2019, 10, 1360. [Google Scholar] [CrossRef]
- Russell, W.R.; Gratz, S.W.; Duncan, S.H.; Holtrop, G.; Ince, J.; Scobbie, L.; Duncan, G.; Johnstone, A.M.; Lobley, G.E.; Wallace, R.J.; et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am. J. Clin. Nutr. 2011, 93, 1062–1072. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Crimarco, A.; Springfield, S.; Petlura, C.; Streaty, T.; Cunanan, K.; Lee, J.; Fielding-Singh, P.; Carter, M.M.; Topf, M.A.; Wastyk, H.C.; et al. A randomized crossover trial on the effect of plant-based compared with animal-based meat on trimethylamine-N-oxide and cardiovascular disease risk factors in generally healthy adults: Study With Appetizing Plantfood-Meat Eating Alternative Trial (SWAP-MEAT). Am. J. Clin. Nutr. 2020, 112, 1188–1199. [Google Scholar] [CrossRef]
- Soldán, M.; Argalášová, Ľ.; Hadvinová, L.; Galileo, B.; Babjaková, J. The Effect of Dietary Types on Gut Microbiota Composition and Development of Non-Communicable Diseases: A Narrative Review. Nutrients 2024, 16, 3134. [Google Scholar] [CrossRef]
- Okunlola, F.O.; Okunlola, A.R.; Adetuyi, B.O.; Soliman, M.E.S.; Alexiou, A.; Papadakis, M.; Fawzy, M.N.; El-Saber Batiha, G. Beyond the gut: Unraveling the multifaceted influence of microbiome on cardiovascular health. Clin. Nutr. ESPEN 2025, 67, 71–89. [Google Scholar] [CrossRef]
- Diao, Z.; Molludi, J.; Latef Fateh, H.; Moradi, S. Comparison of the low-calorie DASH diet and a low-calorie diet on serum TMAO concentrations and gut microbiota composition of adults with overweight/obesity: A randomized control trial. Int. J. Food Sci. Nutr. 2024, 75, 207–220. [Google Scholar] [CrossRef]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Neugarten, J.; Acharya, A.; Silbiger, S.R. Effect of Gender on the Progression of Nondiabetic Renal Disease: A Meta-Analysis. J. Am. Soc. Nephrol. 2000, 11, 319–329. [Google Scholar] [CrossRef]
- Klahr, S.; Breyer, J.A.; Beck, G.J.; Dennis, V.W.; Hartman, J.A.; Roth, D.; Steinman, T.I.; Wang, S.R.; Yamamoto, M.E. Dietary protein restriction, blood pressure control, and the progression of polycystic kidney disease. Modification of Diet in Renal Disease Study Group. J. Am. Soc. Nephrol. 1995, 5, 2037–2047. [Google Scholar] [CrossRef]
- Swartling, O.; Rydell, H.; Stendahl, M.; Segelmark, M.; Trolle Lagerros, Y.; Evans, M. CKD Progression and Mortality Among Men and Women: A Nationwide Study in Sweden. Am. J. Kidney Dis. 2021, 78, 190–199.e1. [Google Scholar] [CrossRef]
- Carrero, J.J.; Hecking, M.; Chesnaye, N.C.; Jager, K.J. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 151–164. [Google Scholar] [CrossRef]
- Catanuto, P.; Doublier, S.; Lupia, E.; Fornoni, A.; Berho, M.; Karl, M.; Striker, G.E.; Xia, X.; Elliot, S. 17 β-estradiol and tamoxifen upregulate estrogen receptor β expression and control podocyte signaling pathways in a model of type 2 diabetes. Kidney Int. 2009, 75, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, A.; Sola, K.H.; Brismar, T.B.; Lindholm, B.; Stenvinkel, P.; Avesani, C.M. Making the invisible visible: Imaging techniques for assessing muscle mass and muscle quality in chronic kidney disease. Clin. Kidney J. 2024, 17, sfae028. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).