AI-Based Treatment Recommendations Enhance Speed and Accuracy in Bacteremia Management: A Comparative Study of Molecular and Phenotypic Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population
2.3. Data Acquisition and Description
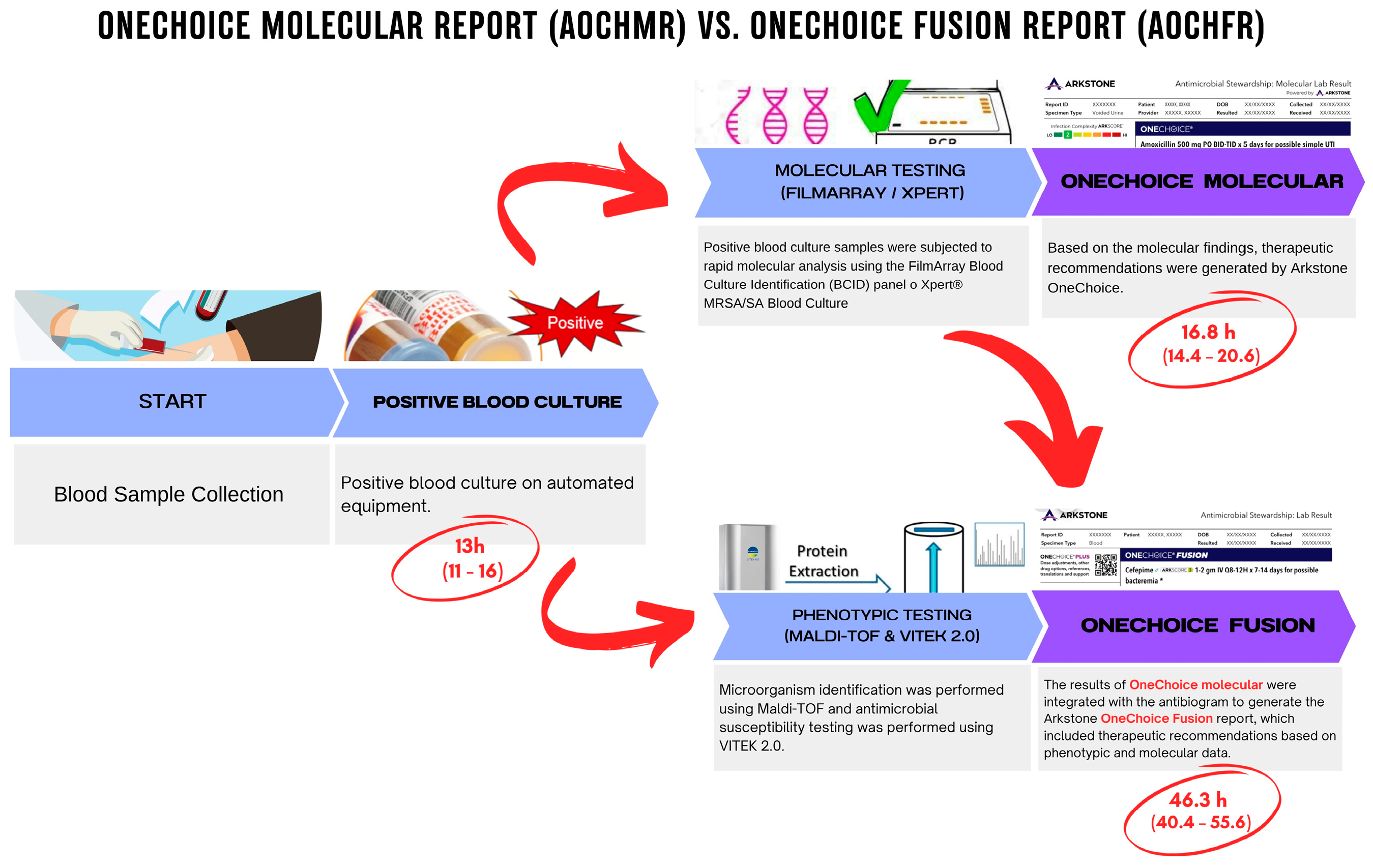
2.4. Study Procedures and Tools/Instruments/Materials/Equipment Molecular Testing Procedures
- Sample Preparation: A 200 µL aliquot of positive blood culture was prepared for analysis. The sample was mixed with a lysis buffer to release nucleic acids.
- FilmArray/GeneXpert Assay: The prepared sample was loaded into the FilmArray or GeneXpert cartridge and inserted into the instrument. The system performed automated nucleic acid extraction, amplification, and detection, providing results within approximately 2 h.
- Pathogen Identification and Resistance Detection: The system identified pathogens and detected antimicrobial resistance genes, generating an AOCHMR with therapeutic recommendations based solely on molecular findings.
Phenotypic Testing Procedures
- Culture and Isolation: Positive blood culture samples were streaked onto agar plates and incubated at 37 °C for 18–24 h. Colonies were examined for morphological characteristics.
- MALDI-TOF Identification: A single colony was applied to a MALDI-TOF target plate, overlaid with a matrix solution, and analyzed by the mass spectrometer. The system matched the obtained spectra to a reference database for organism identification.
- VITEK 2.0 AST: Isolated organisms were suspended in saline to a McFarland standard of 0.5 and loaded into the VITEK 2.0 system for AST. The system provided results within 8–12 h, which were used to refine AOCHFR therapeutic recommendations.
2.5. Data Preparation
2.6. Data Analysis
2.7. Statistical Techniques
- Concordance Analysis**: Cohen’s Kappa was used to measure the agreement between the therapeutic recommendations of AOCHMR and AOCHFR. This analysis provided insight into the consistency and reliability of the molecular-only versus combined molecular and phenotypic approaches.
- Regression Analysis: Poisson regression was employed to analyze factors influencing concordance between AOCHMR and AOCHFR recommendations. This included controlling for potential confounders such as age, gender, number of positive vials, time differences, and specific bacteriological factors. Poisson regression was chosen based on its suitability for modeling count data and the presence of overdispersion in the outcome variable.
- Time Comparison: A paired non-parametric test (Mann–Whitney U) was conducted to evaluate the time efficiency of AOCHMR versus AOCHFR recommendations. The time difference in hours between the two systems was analyzed to provide insights into the potential clinical advantages of each diagnostic approach.
2.8. Ethical Considerations
3. Results
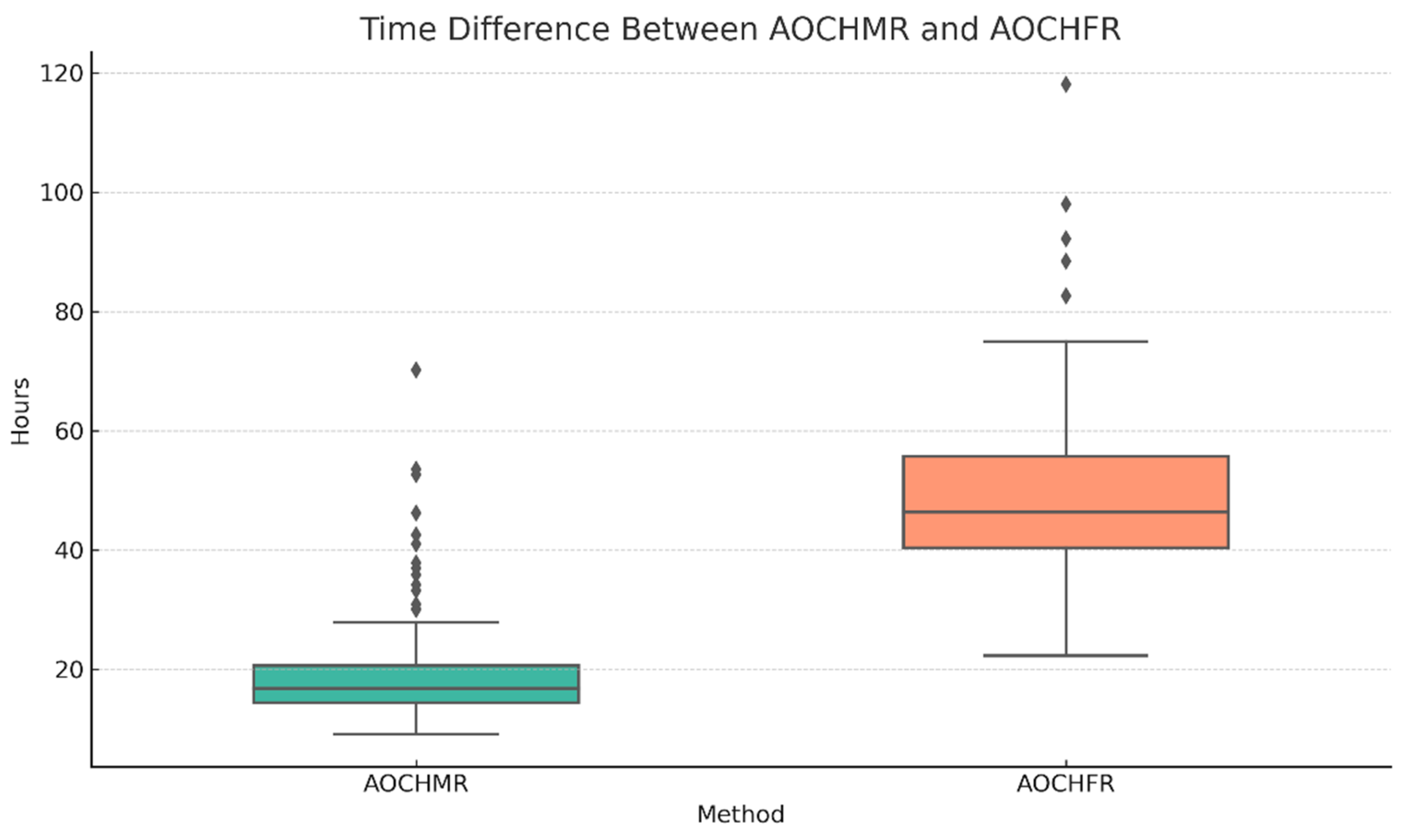
3.1. Time to Recommendation
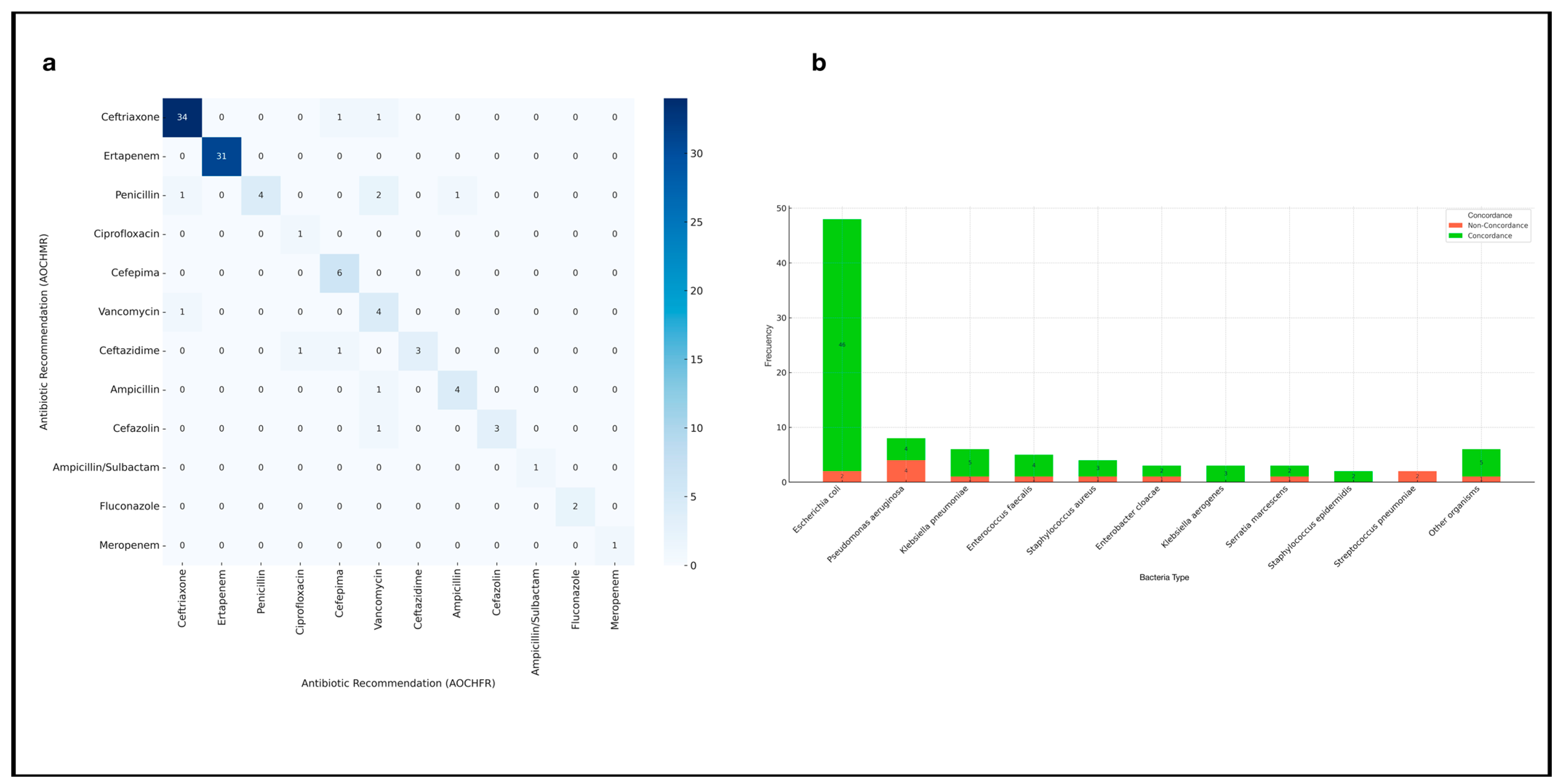
3.2. Concordance of Therapeutic Recommendations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| ML | Machine learning |
| CDSS | Clinical decision support system |
| BSIs | Bloodstream infections |
| AMR | Antimicrobial resistance |
| AOCHMR | Arkstone’s OneChoice Molecular report |
| AOCHFR | Arkstone’s OneChoice Fusion report |
References
- Kern, W.V.; Rieg, S. Burden of bacterial bloodstream infection—A brief update on epidemiology and significance of multidrug-resistant pathogens. Clin. Microbiol. Infect. 2020, 26, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Munford, R.S. Severe sepsis and septic shock: The role of gram-negative bacteremia. Annu. Rev. Pathol. Mech. Dis. 2006, 1, 467–496. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for the Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Rondon, C.; Garcia, C.; Krapp, F.; Machaca, I.; Olivera, M.; Fernández, V.; Villegas, M.; Vilcapoma, P.; Casapia, M.; Concha-Velasco, F.; et al. Antibiotic point prevalence survey and antimicrobial resistance in hospitalized patients across Peruvian reference hospitals. J. Infect. Public Health 2023, 16, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Bonine, N.G.; Berger, A.; Altincatal, A.; Wang, R.; Bhagnani, T.; Gillard, P.; Lodise, T. Impact of delayed appropriate antibiotic therapy on patient outcomes by antibiotic resistance status from serious gram-negative bacterial infections. Am. J. Med. 2019, 132, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Carrara, E.; Pfeffer, I.; Zusman, O.; Leibovici, L.; Paul, M. Determinants of inappropriate empirical antibiotic treatment: Systematic review and meta-analysis. Int. J. Antimicrob. Agents 2018, 51, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Bryan, C.S. Clinical implications of positive blood cultures. Clin. Microbiol. Rev. 1989, 2, 329–353. [Google Scholar] [CrossRef]
- Timbrook, T.; Morton, J.; McConeghy, K.; Caffrey, A.; Mylonakis, E.; LaPlante, K. The Effect of Molecular Rapid Diagnostic Testing on Clinical Outcomes in Bloodstream Infections: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2016, 64, ciw649. [Google Scholar] [CrossRef]
- Holma, T.; Torvikoski, J.; Friberg, N.; Nevalainen, A.; Tarkka, E.; Antikainen, J.; Martelin, J.J. Evaluation and utility of the next-generation FilmArray Blood Culture Identification panel for rapid molecular detection of pathogens and resistance genes. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 345–354. [Google Scholar]
- Mwaigwisya, S.; Assiri, R.A.M.; O’Grady, J. Emerging commercial molecular tests for the diagnosis of bloodstream infection. Expert Rev. Mol. Diagn. 2015, 15, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Poole, S.; Kidd, S.P.; Saeed, K. A review of novel technologies and techniques associated with identification of bloodstream infection etiologies and rapid antimicrobial genotypic and quantitative phenotypic determination. Expert Rev. Mol. Diagn. 2018, 18, 833–846. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, V.; Salimans, L.; Bedenić, B.; Cartuyvels, R.; Barišić, I.; Gyssens, I.C. The Clinical Impact of Rapid Molecular Microbiological Diagnostics for Pathogen and Resistance Gene Identification in Patients With Sepsis: A Systematic Review. Open Forum. Infect. Dis. 2020, 7, ofaa352. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tjandra, K.C.; Ram-Mohan, N.; Abe, R.; Hashemi, M.M. Diagnosis of bloodstream infections: An evolution of technologies towards accurate and rapid identification and antibiotic susceptibility testing. Antibiotics 2022, 11, 511. [Google Scholar] [CrossRef]
- Wu, L.; Xia, D.; Xu, K. Multi-Clinical Factors Combined with an Artificial Intelligence Algorithm Diagnosis Model for HIV-Infected People with Bloodstream Infection. Infect. Drug Resist. 2023, 16, 2701–2712. [Google Scholar] [CrossRef] [PubMed]
- Patel, R. Molecular Approaches to Bacterial Identification and Susceptibility Testing in the Clinical Microbiology Laboratory. J. Mol. Diagn. 2020, 22, 299–308. [Google Scholar]
- InfectionControl.tips. Artificial Intelligence in Antimicrobial Stewardship. 2020. Available online: https://infectioncontrol.tips/2020/06/22/artificial-intelligence-antimicrobial-stewardship/ (accessed on 20 January 2025).
- Presidential Advisory Council on Combating Antibiotic-Resistant Bacteria (PACCARB). Meeting Summary. In Proceedings of the 23rd Public Meeting of the Presidential Advisory Council on Combating Antibiotic-Resistant Bacteria, Virtual, 23–24 March 2023; Available online: https://health.gov/sites/oash/files/2025-01/paccarb-meeting-summary-march-2023.pdf (accessed on 20 January 2025).
- De Angelis, G.; Falcone, M.; Tiseo, G.; Tumbarello, M.; Venditti, M. Emerging Role of Artificial Intelligence in Optimizing Antimicrobial Stewardship. Front. Med. 2020, 7, 591157. [Google Scholar]
- Pennisi, F.; Pinto, A.; Ricciardi, G.E.; Signorelli, C.; Gianfredi, V. The Role of Artificial Intelligence and Machine Learning Models in Antimicrobial Stewardship in Public Health: A Narrative Review. Antibiotics 2025, 14, 134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sarantopoulos, A.; Mastori Kourmpani, C.; Yokarasa, A.L.; Makamanzi, C.; Antoniou, P.; Spernovasilis, N.; Tsioutis, C. Artificial Intelligence in Infectious Disease Clinical Practice: An Overview of Gaps, Opportunities, and Limitations. Trop. Med. Infect. Dis. 2024, 9, 228. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Macesic, N.; Polubriaginof, F.; Tatonetti, N.P. Machine learning: Novel bioinformatics approaches for combating antimicrobial resistance. Curr. Opin. Infect. Dis. 2017, 30, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Giovagnorio, F.; De Vito, A.; Madeddu, G.; Parisi, S.G.; Geremia, N. Resistance in Pseudomonas aeruginosa: A Narrative Review of Antibiogram Interpretation and Emerging Treatments. Antibiotics 2023, 12, 1621. [Google Scholar] [CrossRef] [PubMed]
- Lapin, J.S.; Smith, R.D.; Hornback, K.M.; Johnson, J.K.; Claeys, K.C. From bottle to bedside: Implementation considerations and antimicrobial stewardship considerations for bloodstream infection rapid diagnostic testing. Pharmacotherapy 2023, 43, 847–863. [Google Scholar] [CrossRef] [PubMed]
- Keskilidou, E.; Meletis, G.; Vasilaki, O.; Kagkalou, G.; Mantzana, P.; Kachrimanidou, M.; Protonotariou, E.; Skoura, L. Evaluation of the filmarray blood culture identification panel on diagnosis of bacteremias in an MDRO-endemic hospital environment. Diagn. Microbiol. Infect. Dis. 2025, 111, 116592. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.F.; Gookin, B.A.; Rogers, C.G. Evaluation of the FilmArray Blood Culture Identification (BCID) panel in a community hospital setting. J. Clin. Microbiol. 2017, 55, 248–254. [Google Scholar]
- Parta, M.; Goebel, M.; Thomas, J.; Matloobi, M.; Stager, C.; Musher, D.M. Impact of an assay that enables rapid determination of Staphylococcus species and their drug susceptibility on the treatment of patients with positive blood culture results. Infect. Control Hosp. Epidemiol. 2010, 31, 1043–1048. [Google Scholar] [CrossRef]
- Wenzler, E.; Timbrook, T.T.; Wong, J.R.; Hurst, J.M.; MacVane, S.H. Implementation and optimization of molecular rapid diagnostic tests for bloodstream infections. Am. J. Health Syst. Pharm. 2018, 75, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.M.; Newton, D.; Kunapuli, A.; Gandhi, T.N.; Washer, L.L.; Isip, J.; Collins, C.D.; Nagel, J.L. Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin. Infect. Dis. 2013, 57, 1237–1245. [Google Scholar] [CrossRef]
- Bauer, K.A.; West, J.E.; Balada-Llasat, J.M.; Pancholi, P.; Stevenson, K.B.; Goff, D.A. An antimicrobial stewardship program’s impact with rapid polymerase chain reaction methicillin-resistant Staphylococcus aureus/S. aureus blood culture test in patients with S. aureus bacteremia. Clin. Infect. Dis. 2010, 51, 1074–1080. [Google Scholar] [CrossRef]
- Perez, K.K.; Olsen, R.J.; Musick, W.L.; Cernoch, P.L.; Davis, J.R.; Land, G.A.; Peterson, L.E.; Musser, J.M. Integrating rapid pathogen identification and antimicrobial stewardship significantly decreases hospital costs. Arch. Pathol. Lab. Med. 2013, 137, 1247–1254. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Zimmer, S.M. Infectious Diseases Society of America Guidance on the Treatment of Antimicrobial-Resistant Gram-Negative Infections. Clin. Infect. Dis. 2022, 74, 444–478. [Google Scholar] [CrossRef]
- Claeys, K.C.; Bonomo, R.A.; Papp-Wallace, K.M. Challenges in antimicrobial susceptibility testing: From standard methods to molecular detection and interpretation of resistance mechanisms. Expert Rev. Anti-Infect. Ther. 2022, 20, 1263–1276. [Google Scholar]
- Banerjee, R.; Sinha, M.; Ray, P. Recent advances in molecular diagnosis of bloodstream infections. J. Med. Microbiol. 2020, 69, 1476–1487. [Google Scholar]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances, and emerging therapeutics. Sig. Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef]
- Tatli-Kis, T.; Yildirim, S.; Bicmen, C.; Kirakli, C. Early detection of bacteremia pathogens with rapid molecular diagnostic tests and evaluation of effect on intensive care patient management. Diagn. Microbiol. Infect. Dis. 2024, 110, 116424. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Leanse, L.G.; Feng, Y. Artificial intelligence and machine learning assisted drug delivery for effective treatment of infectious diseases. Adv. Drug Deliv. Rev. 2021, 178, 113922. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, D.; Zhang, X.; Zhang, X. Artificial intelligence applications in the diagnosis and treatment of bacterial infections. Front. Microbiol. 2024, 15, 1449844. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blechman, S.E.; Wright, E.S. Applications of Machine Learning on Electronic Health Record Data to Combat Antibiotic Resistance. J. Infect. Dis. 2024, 230, 1073–1082. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giacobbe, D.R.; Marelli, C.; Guastavino, S.; Mora, S.; Rosso, N.; Signori, A.; Campi, C.; Giacomini, M.; Bassetti, M. Explainable and Interpretable Machine Learning for Antimicrobial Stewardship: Opportunities and Challenges. Clin. Ther. 2024, 46, 474–480. [Google Scholar] [CrossRef] [PubMed]
| Variable | Total (n = 117) | Non-Concordance (n = 23) | Concordance (n = 94) | p-Value |
|---|---|---|---|---|
| Demographics and Clinical Characteristics | ||||
| 67 (45–79) | 69 (45–79) | 65.5 (46–80) | 0.898 a |
| 68 (58.12) | 13 (56.52) | 55 (58.51) | 0.862 b |
| 2 (2–4) | 2 (2–4) | 2 (2–4) | 0.999 a |
| 2 (1–2) | 2 (1–2) | 2 (1–2) | 0.822 a |
| 13 (11–16) | 13 (12–16) | 13 (11–16) | 0.439 a |
| Bacteriological and Molecular Results | - | |||
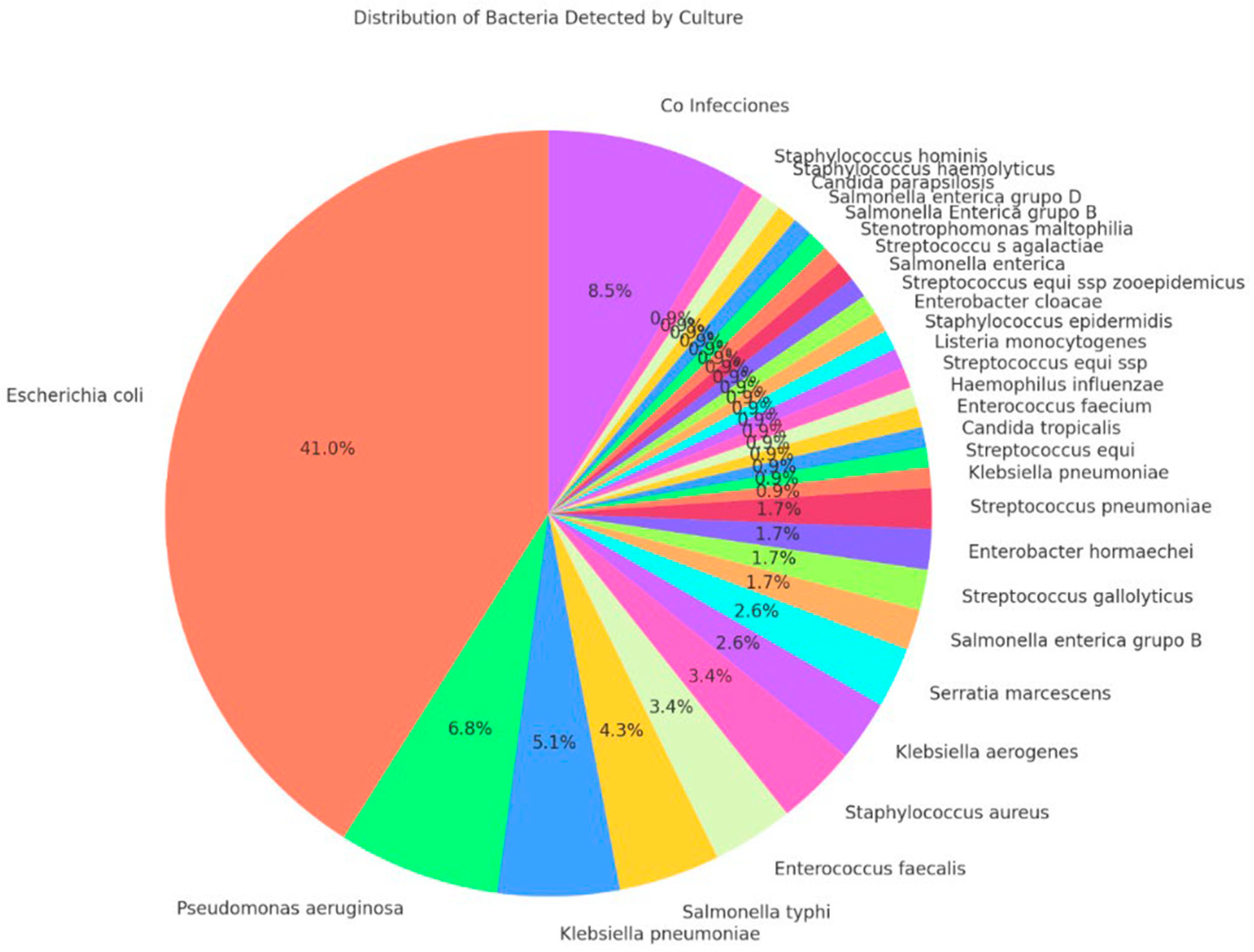
| 117 (100.0) | 23 (100.0) | 94 (100.0) | |
| 117 (100.0) | 23 (100.0) | 94 (100.0) | |
| 101 (86.32) | 16 (69.56) | 85 (90.42) | 0.027 b |
| 101 (86.32) | 16 (69.56) | 85 (90.42) | 0.011 b |
| Time comparison | ||||
| 16.81 (14.38–20.58) | 18.02 (15.98–20.33) | 16.62 (14.17–20.68) | 0.434 a |
| 46.32 (40.41–55.69) | 47.83 (42.92–66.95) | 45.84 (39.85–54.25) | 0.111 a |
| 28.43 (22.93–34.89) | 29.57 (23.85–43.68) | 28.09 (22.61–34.42) | 0.246 a |
| Concordance of Therapeutic Recommendations | ||||
| 94 (80.34) | - | - | - |
| 57 (48.71) | 4 (17.39) | 53 (56.38) | 0.002 b |
| Variable | cPR (95% CI) | p-Value | aPR (95% CI) | p-Value |
|---|---|---|---|---|
| Age | 0.999 (0.996–1.003) | 0.88 | - | |
| Male gender | 1.016 (0.845–1.221) | 0.86 | - | |
| Blood culture bottles collected per patient | 0.82 | - | ||
| - Two | Reference | |||
| - Four | 0.977 (0.802–1.190) | |||
| Positive blood culture bottles per patient | - | |||
| - Two | 0.918 (0.758–1.113) | 0.38 | ||
| - Three | 1.200 (1.047–1.374) | <0.01 | 0.954 (0.858–1.060) | 0.38 |
| - Four | 1.066 (0.815–1.395) | 0.63 | ||
| Time to result of molecular (hours) | 1.001 (0.993–1.008) | 0.76 | - | |
| Time to result of phenotype | 0.997 (0.991–1.002) | 0.32 | - | |
| Bacteria detected by conventional culture | ||||
| - Escherichia coli | 0.958 (0.903–1.016) | 0.15 | ||
| - Salmonella spp. | 0.800 (0.586–1.092) | 0.16 | ||
| - Klebsiella spp. | 0.888 (0.704–1.120) | 0.32 | ||
| - Pseudomonas aeruginosa | 0.500 (0.249–1.002) | 0.05 | 0.545 (0.272–1.091) | 0.08 |
| - Streptococcus spp. | 0.375 (0.152–0.920) | 0.03 | 0.408 (0.169–0.988) | 0.04 |
| - Polymicrobial | 0.750 (0.501–1.120) | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez de la Torre, J.C.; Frenkel, A.; Chavez-Lencinas, C.; Rendon, A.; Cáceres, J.A.; Alvarado, L.; Hueda-Zavaleta, M. AI-Based Treatment Recommendations Enhance Speed and Accuracy in Bacteremia Management: A Comparative Study of Molecular and Phenotypic Data. Life 2025, 15, 864. https://doi.org/10.3390/life15060864
Gomez de la Torre JC, Frenkel A, Chavez-Lencinas C, Rendon A, Cáceres JA, Alvarado L, Hueda-Zavaleta M. AI-Based Treatment Recommendations Enhance Speed and Accuracy in Bacteremia Management: A Comparative Study of Molecular and Phenotypic Data. Life. 2025; 15(6):864. https://doi.org/10.3390/life15060864
Chicago/Turabian StyleGomez de la Torre, Juan C., Ari Frenkel, Carlos Chavez-Lencinas, Alicia Rendon, José Alonso Cáceres, Luis Alvarado, and Miguel Hueda-Zavaleta. 2025. "AI-Based Treatment Recommendations Enhance Speed and Accuracy in Bacteremia Management: A Comparative Study of Molecular and Phenotypic Data" Life 15, no. 6: 864. https://doi.org/10.3390/life15060864
APA StyleGomez de la Torre, J. C., Frenkel, A., Chavez-Lencinas, C., Rendon, A., Cáceres, J. A., Alvarado, L., & Hueda-Zavaleta, M. (2025). AI-Based Treatment Recommendations Enhance Speed and Accuracy in Bacteremia Management: A Comparative Study of Molecular and Phenotypic Data. Life, 15(6), 864. https://doi.org/10.3390/life15060864








